Abstract
The mannanase gene of B. circulans NT 6.7 was cloned and expressed in an Escherichia coli expression system. The B. circulans NT 6.7 mannanase gene consists of 1,083 nucleotides encoding a 360-amino acid residue long polypeptide, belonging to glycoside hydrolase family 26. The full-length mannanase gene including its native signal sequence was cloned into the vector pET21d and expressed in E. coli BL21 (DE3). β-Mannanase activities in the culture supernatant and crude cell extract were 37.10 and 515 U per ml, respectively, with most of the activity in the cell extract attributed to the periplasmic fraction. In contrast, expression of mannanase was much lower when using the B. circulans NT 6.7 mannanase gene without its signal sequence. The optimum temperature of recombinant β-mannanase activity was 50°C and the optimum pH was 6.0. The enzyme was very specific for β-mannan substrates with a preference for galactomannan. Hydrolysis products of locust bean gum were various mannooligosaccharides including mannohexaose, mannopentaose, mannotetraose, mannotriose and mannobiose, while mannose could not be detected. In conclusion, this expression system is efficient for the secretory production of recombinant β-mannanase from B. circulans NT 6.7, which shows good characteristics for various applications.
Keywords: β-mannanase, Bacillus circulans NT 6.7, Expression, Escherichia coli, Mannooligosacharides
Background
Endo-1,4-β-D-mannanase or β-mannanase (EC 3.2.1.78) is endohydrolase, which catalyzes the random hydrolysis of the β-1,4-D mannopyranosyl linkage in the main chain of mannan-based polysaccharides (Puls 1997). According to the Carbohydrate Active Enzyme database CAZy (http://www.cazy.org), which is based on sequence similarity and hydrophobic cluster analysis, β-mannanase are classified into various families of glycoside hydrolase (GH) i.e., mostly to GH families 5 and 26 and a few members of GH113 (Dhawan and Kaur 2007; Zhang et al. 2008). β-Mannanases are produced by wide range of organisms and it has been widely used in various industrial applications (Araujo and Ward 1990; Dutta et al. 1997; Lee et al. 2003; McCutchen et al. 1996; Puchar et al. 2004). Moreover, there is increasing interest in the application of β-mannanases for the production of mannooligosaccharides (MOS), which have prebiotic properties and thus are of interest for the feed and food industry (Biggs and Parsons 2007; Gibson et al. 2005; Rastall et al. 2005; Smith et al. 2010).
Copra meal is one of the natural sources that contain high amount of galactomannan (Balasubramaniam 1976). In Thailand, 25 million metric ton per year copra meal waste was produced from coconut oil industry (Index Mundi 2014). It has been used as a feed ingredient but its digestibility was limited (Chauhan et al. 2012). Because of the high galactomannan content so the copra meal is an interested substrate for mannooligosaccharides production.
Bacillus circulans NT 6.7 was isolated from soil taken from a coconut factory from Nakornpathom province in Thailand. Its β-mannanases could hydrolyze defatted copra meal to mannooligosaccharides which have the prebiotic property making it interesting for industrial applications (Phothichitto et al. 2006). However, the production levels in the wild-type organism are not sufficient for the envisaged applications, and therefore gene expression, by which large amounts of an individual enzyme can be produced in a suitable host strain, is of interest for large scale production and application of this particular enzyme.
Escherichia coli is one of most successful expression systems used in biotechnology, showing many advantages for recombinant protein production including well-established and easy operation systems, high yields, and a wealth of molecular tools, to name a few. Recombinant β-mannanases have been successfully produced by E. coli systems (Chen et al. 2008; Ethier et al. 1998; Li et al. 2008; Songsiriritthigul et al. 2010; Yang et al. 2009), however, most of these systems were used for intracellular expression and did not success in secretion of the recombinant enzyme.
Our current study shows that recombinant β-mannanases from B. circulans NT 6.7, showing high stability and specificity, can be efficiently expressed and secreted in suitable E. coli-based expression systems
Results and discussion
Cloning and sequencing of the mannanase gene from B. circulansNT 6.7
The mannanase gene of B. circulans NT 6.7 was obtained by using PCR cloning. Gene sequencing showed that the B. circulans NT 6.7 mannanase gene consists of a total 1,083 bp (GenBank Accession No. JF724077), encoding a 360-amino acid polypeptide. The nucleotide sequence of the B. circulans NT 6.7 mannanase gene showed the highest identity (99%) with the β-mannanase gene from Bacillus amyloliquefaciens strain CICC 23260 (GQ589479.1) and Bacillus subtilis strain A33 (DQ269473.1). The amino acid sequence of the B. circulans NT 6.7 mannanase showed 99% identity with the β-mannanase from B. subtilis (ABB91433.1), and this latter enzyme was classified into glycoside hydrolase family 26 (GH26) based on sequence identity (Altschul et al. 1997). Furthermore, the mannanase sequence was analyzed by SignalP 4.0 (Petersen et al. 2011) and 24 N-terminal amino acid residues (residue number 1–24) were predicted as a signal sequence driving secretion (Petersen et al. 2011).
B. circulans NT 6.7 produces an extracellular β-mannanase, which can be employed for several applications (Phothichitto et al. 2006). Even though this enzyme was found to be useful for industrial applications the yields in β-mannanase activity were, however, found to be too low when using the natural source of this enzyme for its production. Hence, heterologous expression was selected for improvement of the production and efficiency of B. circulans NT 6.7 β-mannanase. Previous partial sequencing of the mannanase gene from B. circulans NT 6.7 β-mannanase showed that it was classified into GH26 family (Lee et al. 2003). This was corroborated by using primers for full-length gene designed from published GH26 mannanase genes of various Bacillus spp. This resulted in the successful cloning of the full-length B. circulans NT 6.7 mannanase gene, and confirmed that this enzyme in fact belongs to GH26 (Sakulsirirat 2008).
Expression of the mannanase gene
The full-length mannanase gene of B. circulans NT 6.7 including its native signal sequence was cloned into pET21d, and the gene could be successfully expressed in E. coli BL21 (DE3) driven by the T7 RNA polymerase promoter. After induction with various concentrations of IPTG, β-mannanase activity was measured in both culture supernatant and cell lysate fractions. Highest β-mannanase activity was found in cultures that induced with 1.0 mM IPTG, especially in the extracellular fraction, while the level of β-mannanase activity in the cell lysate fractions was similar regardless of the IPTG concentration used. Moreover, intracellular β-mannanase activity decreased after incubation of more than 16 h without the increasing of the extracellular β-mannanase activity. Hence, the optimal expression conditions for mannanase gene expression were induction by 1.0 mM IPTG and 16 h of incubation at 18°C after induction. When using this conditions, E. coli BL21 (DE3) harboring pET21d with mannanase gene yielded extracellular and intracellular β-mannanase activities of 37.1 U per ml of crude culture supernatant and 515 U per ml of crude cell extract, respectively, corresponding to a volumetric activity of 37,100 and 515,000 U per liter of culture medium. Most of the intracellular recombinant β-mannanase was found in the periplasmic space of the cells (Table 1). Both intracellular and extracellular β-mannanase showed an identical size that was about 40 kDa on the SDS-PAGE, and also showed activity by zymogram analysis on the substrate gel (Figure 1).
Table 1.
The yield of recombinant β-mannanase in different cell compartments of E. coli (from 100 ml culture)
| Cell compartments | Activity | Protein concentration | Specific activity |
|---|---|---|---|
| (U per ml) | (mg per ml) | (u per mg) | |
| Periplasmic space | 497.62 | 0.03 | 16,587.33 |
| Cell lysate | 45.14 | 0.05 | 902.50 |
Figure 1.
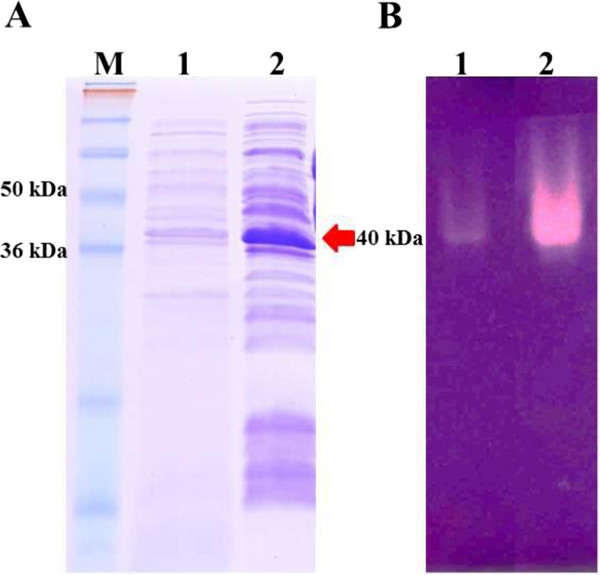
SDS-PAGE and zymogram analysis of recombinant β-mannanase from E. coli expression system. (A) SDS-PAGE : lane M, protein marker (Invitrogen, USA); lane 1, crude culture supernatant; lane 2, crude cell lysate. (B) zymogram analysis: lane 1, crude culture supernatant; lane 2, crude cell lysate.
The B. circulans NT 6.7 mannanase gene without its signal sequence was also cloned into pET21d using the primers Man6.7 F2 (CGGGCCATGGCACACCGTTTATCCCGT) and Man6.7R, and expressed under identical conditions as above. The results obtained showed that recombinant β-mannanase without its signal peptide was hardly secreted regardless of the IPTG concentration used, and intracellular β-mannanase activity was also decreased by a factor of approximately 50, yielding only 9.72 U per ml cell lysate.
The mannanase gene was cloned into the pET21d vector and expressed in E. coli BL21 (DE3), a successful standard and commercial expression system (Nguyen et al. 2012). The full-length mannanase gene was cloned into the vector under the T7 promoter without additional signal sequences that are often used in E. coli for secretion to the periplasm or extracellular environment (Choi and Lee 2004; Mergulhão et al. 2005). Interestingly, recombinant β-mannanase was secreted into the culture media by this system when induce with appropriate IPTG concentrations, and most of the activity in the fraction termed intracellular was localized in the periplasm, suggesting that the native Bacillus signal peptide was recognized by the translocation system of E. coli and the recombinant was transported out of the cytosol. We also cloned and expressed the mature mannanase gene without its signal sequence using the same system. The enzyme without signal peptide was not secreted at any IPTG concentration used. In addition, intracellular β-mannanase activity was decreased by more than 50 times, indicating that the efficiency of expression was much lower for this construct. The signal sequence of residues 1 to 24 (MLKKLAVCLSIVLLLLGAASP ISA) predicted by SignalP 4.0 (Petersen et al. 2011) shows structural features that have been found as common for other signal sequences as well in that it is composed of a hydrophobic H-domain rich in Ala, Val and Leu, preceeded by a short, positively charged N-domain, which is here shown in bold (Choi and Lee 2004).
This expression system can efficiently drive the production of secreted recombinant β-mannanase from B. circulans NT 6.7. Secretory production of an enzyme in E. coli offers various advantages including less contamination with host proteins (and hence facilitated purification of the recombinant enzyme), less proteolytic degradation of the target protein and possible increased yield of folded protein because of chaperones found in the periplasm of E. coli (Choi and Lee 2004; Mergulhão et al. 2005). Although most of the recombinant β-mannanase was found in the periplasmic space, the enzyme activity of extracellular recombinant β-mannanase alone was almost 20 times higher than that found that of β-mannanase from the natural source B. circulans NT 6.7 at the same production scale. Furthermore, overall enzyme activity of this recombinant β-mannanase was about 10 times higher than the yields of recombinant β-mannanase from B. licheniformis DSM13, a β-mannanase in the same GH26 family, which was produced with a different E. coli expression system using the OmpA signal sequence (Songsiriritthigul et al. 2010). Our results also showed efficient secretion when compared with the recombinant β-mannanase B. circulans CGMCC 1416 and B. circulans CGMCC 1554, recombinant enzymes from family GH5 that were produced in similar expression systems (Li et al. 2008; Yang et al. 2009). β-Mannanase from B. circulans NT 6.7 was classified into GH26 whereas β-mannanase from of B. circulans CGMCC 1415 and B. circulans CGMCC 1554 were in GH5. The amino acid sequence of β-mannanase was difference between GH26 and GH5. Moreover, there was different in cloned gene including their signal sequences. So, these may cause the different in secretion of the recombinant enzymes from the same expression system.
Characterization of recombinant β-mannanase
The effects of temperature on enzyme activity were determined for both the extracellular and intracellular recombinant β-mannanase. The optimum temperature was measured using the standard assay while varying the temperature from 30°C to 80°C. The optimum temperature of both fractions of the recombinant β-mannanase was 50°C for the 60 minute assay (Figure 2A).
Figure 2.
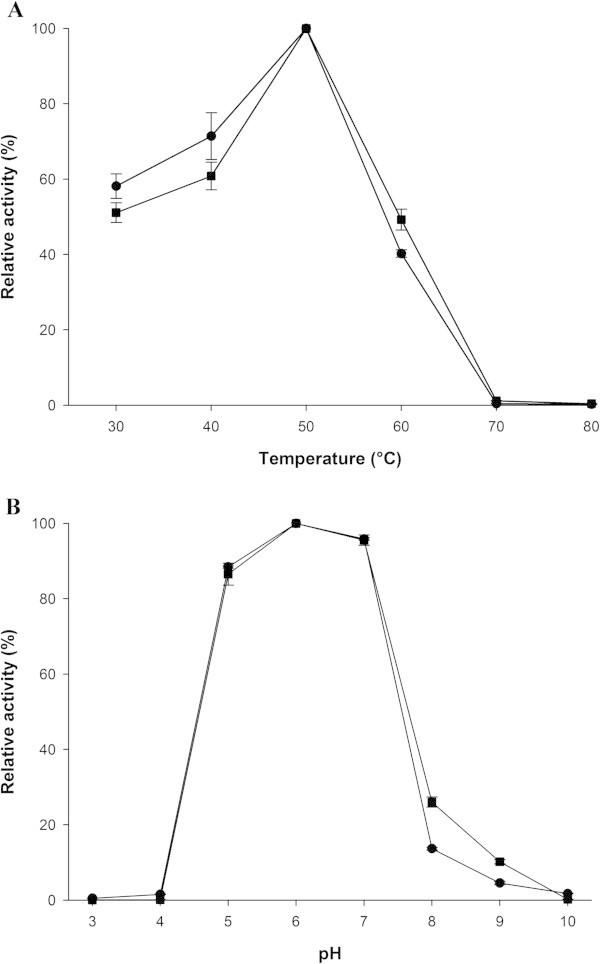
Effect of temperature (A) and pH (B) on recombinant β-mannanase activity. Extracellular (–●–) and intracellular (–■–) enzymes were used for these experiment, and results are expressed as relative activity. (100% relative activity of extracellular and intracellular enzymes were 37.10 and 515.00 U per ml, respectively).
The effects of the pH value on β-mannanase activity were determined for both the extracellular and intracellular recombinant enzyme using the standard assay and varying the pH from 3.0 to 10.0. For both fractions, the optimum pH β-mannanase activity was 6.0, and recombinant β-mannanase was very active in pH range of 5.0-7.0 with more than 80% relative activity (Figure 2B).
The recombinant β-mannanase showed its highest activity on LBG followed by konjac glucomannan. This enzyme also hydrolyzed copra meal and defatted copra meal, other mannan-based substrates from natural product that vary in their degree of substitution with side chains. No activity was found on α-mannan, xylan and cellulose. These results indicate that this recombinant β-mannanase is very specific and exhibits activity only on β-mannan substrates with the preference for galactomannan (Table 2).
Table 2.
Substrate specificity of recombinant β-mannanase from B. circulans NT 6.7
| Substrate | Relative activity (%) | |
|---|---|---|
| Extracellular enzyme | Intracellular enzyme | |
| Locust bean gum (LBG) | 100.00 | 100.00 |
| Konjac glucomannan | 61.89 | 68.35 |
| Ivory nut mannan | 50.50 | 53.83 |
| Defatted copra meal | 3.26 | 0.60 |
| Copra meal | 0.36 | 0.15 |
| α-mannan | 0.00 | 0.00 |
| Xylan from oat spelts | 0.00 | 0.00 |
| Carboxymethylcellulose | 0.00 | 0.00 |
The effect of various metal ions and EDTA on recombinant β-mannanase activity was tested by adding 1 mM of the selected metal ions or EDTA to the enzyme reactions. Their effect on both extracellular and intracellular recombinant β-mannanase activity is shown in Table 3. β-Mannanase activity was slightly enhanced by approximately 5% by Co+ ions. Li+, Ca2+, Mg2+, Mn2+ and Zn2+ ions had minor effects on enzyme activity with the relative activities of more than 90% in the presence of these ions. Cu2+ ion had a moderate effect on the enzyme activity, while Fe2+ ion and EDTA strongly inhibited β-mannanase activity by 60% and 65%, respectively.
Table 3.
Effect of various metal ions and EDTA on the activity of recombinant β-mannanase from B. circulans NT 6.7
| Metal ions/Chemical | Relative activity (%) | |
|---|---|---|
| Extracellular enzyme | Intracellular enzyme | |
| Control | 100.00 | 100.00 |
| Co+ | 104.74 | 106.32 |
| Ca+ | 95.45 | 97.29 |
| Zn2+ | 97.50 | 92.24 |
| Mn2+ | 95.26 | 95.65 |
| Mg2+ | 94.32 | 95.46 |
| Li+ | 96.82 | 97.70 |
| Cu2+ | 79.05 | 64.08 |
| Fe2+ | 40.12 | 39.18 |
| EDTA | 32.00 | 37.95 |
For the hydrolysis of LBG, both of extracellular and intracellular recombinant β-mannanase showed an identical hydrolysis pattern. After 6 h of hydrolysis, 10 mg LBG was hydrolyzed to be various mannooligosaccharide products consisting of mannohexaose, mannopentaose, mannotetraose, mannotriose and mannobiose, with mannotriose as the main reaction product (Table 4). No mannose was formed under these conditions, and even when extending the incubation time to 24 h mannose could not be detected in the hydrolysis mixture.
Table 4.
Mannooligosaccharide products of LBG hydrolysis with recombinant β-mannanase from B. circulans NT 6.7 at 6 h of incubation
| Mannooligosaccharides | Amount of hydrolysis product (mg) | |
|---|---|---|
| Extracellular enzyme | Intracellular enzyme | |
| Mannohexaose (M6) and mannopentaose (M5) | 0.063 | 0.051 |
| Mannotetraose (M4) | 0.109 | 0.149 |
| Mannotriose (M3) | 0.346 | 0.376 |
| Mannobiose (M2) | 0.237 | 0.288 |
HPLC analysis of hydrolysis products of defined mannooligosaccharide substrates showed that the recombinant β-mannanase hydrolyzed mannohexaose, mannopentaose and mannotetraose into smaller molecules but was not active on mannotriose and mannobiose (Table 5). Mannohexaose and mannopentaose were hydrolyzed to be mannotetraose, mannotriose, mannobiose after 0.5 h of incubation and even mannose formed after an extended incubation for 6 h. At 6 h of incubation, almost 90% of mannohexaose and 70% of mannopentaose were hydrolyzed. Moreover, the recombinant enzyme hydrolyzed mannotetraose into smaller oligomers including mannose after 6 h of incubation, and the concentration of these products further increased after 24 h of incubation.
Table 5.
Mannooligosaccharide products of the hydrolysis of mannohexaose, mannopentaose and mannotetraose with recombinant β-mannanase from B. circulans NT 6.7 at 6 h of incubation
| Substrates | Amount of hydrolysis product (mg) | |||||
|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | M6 | |
| Mannohexaose (M6) | 0.048 | 0.995 | 1.207 | 1.517 | 0.000 | - |
| Mannopentaose (M5) | 0.068 | 1.148 | 1.475 | 0.532 | - | - |
| Mannotetraose (M4) | 0.040 | 0.074 | 0.089 | - | - | - |
Both of extracellular and intracellular recombinant β-mannanase from B. circulans NT 6.7 showed the same characteristics including protein size, temperature and pH optima for activity, substrate specificity, effects of ion on activity as well as the hydrolysis patterns obtained after LBG hydrolysis. Moreover, the recombinant, heterologously produced β-mannanase also has the same enzyme properties as the wild type enzyme (Pangsri 2014). The temperature and pH optima of both of wild type and recombinant β-mannanase from B. circulans NT 6.7 were 50°C and 6.0. Both of recombinant and wild type enzyme was active on various mannan substrates especially LBG, a high-molecular-mass galactomannan. The results of substrate specificity showed that this recombinant enzyme is specific for the hydrolysis of β-1,4-mannosyl linkages without activity on structurally related polysaccharides such as xylan or cellulose as same as wild type β-mannanase from B. circulans NT 6.7. There were the difference in enzyme properties such as the optimum temperature and pH, enzyme stability and also specificity in both of native and recombinant β-mannanases that have characterized from various organisms depended on the source of enzymes. This recombinant β-mannanase from B. circulans NT 6.7 had high enzyme activity at the appropriate conditions for industrial application. It also had high specificity on mannan with the preference for galactomannan that was very advantage on the mannooligosaccharide production.
HPLC analysis of hydrolysis products confirmed that recombinant β-mannanase from B. circulans NT 6.7 hydrolyzes the mannan backbone in a random fashion, releasing oligosaccharides of different size, and mannooligosaccharides of two to six mannose units could be identified after LBG hydrolysis, while no free mannose was detected by HPLC analysis. Various mannooligosaccharide substrates (M4 to M6) were again cleaved in a random fashion, since a range of different products was observed. The smaller mannooligosaccharides M3 and M2 when used as pure substrates were not hydrolyzed by the enzyme, indicating that the active site must accommodate at least four mannose units for catalysis. Altogether, these results suggested that this this recombinant β-mannanase had the effective hydrolysis on various β-mannan substrates as wild type enzyme from B. circulans NT 6.7 which could be used for the production of prebiotic mannooligosaccharides from natural resources of high galactomannan content such as copra meal or LBG.
Conclusions
This study has shown an efficient expression system for the secretory production of β-mannanase gene from B. circuans NT 6.7. Furthermore, the recombinant β-mannanase has properties including its optimum temperature and pH, high stability and the effective hydrolysis of mannan substrates that are promising for large-scale production.
Methods
Bacterial strains, plasmids and media
B. circulans NT 6.7 was cultured in Luria-Bertani (LB) medium at 37°C with shaking at 200 rpm for isolation of genomic DNA. E. coli DH5α and E. coli BL21 (DE3) were used as molecular cloning host and expression host, respectively. E. coli strains were cultured in LB medium containing 100 μg/ml ampicillin at 37°C with shaking at 125 rpm. The plasmid pGEM-T Easy (Promega, USA) was used for cloning and sequencing experiments, and the plasmid pET-21d(+) (Novagen, Germany) was used as expression vector in E. coli BL21 (DE3).
Construction of recombinant plasmids
Genomic DNA of B. circulans NT 6.7 was extracted by using the illustra™ bacteria genomicPrep Mini Spin Kit (GE Healthcare, UK). Primers F1 (ATGCTTAAAAAGTTAGC AGTCTGYCT) and R1 (TTATTCCGCGATCGGCGTCAA) were designed based on the conserved nucleotide sequence of β-mannanase from Bacillus GH26. The amplification was performed by using Dream® Taq DNA polymerase (Fermentas, USA) and the reaction cycle consisted of initial denaturation at 95°C for 5 min followed by 30 cycles of 95°C for 1 min, 55°C for 1 min and 72°C for 2 min, and the final extension at 72°C for 5 min. The amplification product was ligated into the pGEM-T Easy vector and transformed into E. coli DH5α for sequencing.
Primers Man6.7 F (GCGGCCATGGCTATGCTTAAAAAGTTAGCA) with the NcoI recognition site and Man6.7R (CCGGCTCGAGTTCCGCGATCGGCGT) with the XhoI recognition site were designed based on the result of sequencing analysis. The PCR reaction contained approximately 100 ng of B. circulans NT 6.7 genomic DNA as template, 10 pmol of each primer, 0.2 mM each dNTP, 2.5 mM MgCl2, 1× buffer and 1U Phusion High-Fidelity DNA polymerase (New England Biolabs, UK). Amplification condition consisted of initial denaturation at 98°C for 3 min followed by 30 cycles of 98°C for 30 s, 60°C for 30 s and 72°C for 45 s, and the final extension at 72°C for 5 min. The amplification product was purified by using the illustra™ GFX PCR DNA and Gel Band Purification Kit (GE Healthcare, UK) and digested with NcoI and XhoI restriction enzymes. The digestion product was ligated into the NcoI-XhoI site of pET21d and subsequently transformed into E. coli BL21 (DE3). Positive clones were confirmed by colony-PCR and sequencing.
Expression of mannanase
For the recombinant expression of the mannanase gene, overnight cultures of E. coli BL21 (DE3) carrying the respective expression plasmid were inoculated into 100 ml of LB medium with 100 μg/ml of ampicillin. The cultures were incubated at 37°C and 200 rpm until the OD600 reached 1.0 and then induced with adding IPTG to a final concentration of 0.1, 0.5 and 1.0 mM. After IPTG induction, the cultivation was carried out at 18°C with 200 rpm for 16, 18 and 20 h. Both of culture supernatant and cell pellet were collected after cultivation. Cells were harvested by centrifugation at 8,000 rpm, 4°C for 30 min. The cell pellet was washed twice with 50 mM potassium phosphate buffer pH 6.0 and then resuspended in the same buffer. Cells were disrupted three times by glass bead stirring with 5000 rpm for 30 seconds. Cell-free extracts were obtained by centrifugation at 13,000 rpm for 30 min and used for subsequent analysis.
Enzyme assay and protein determination
The standard β-mannanase activity assay consisted of 100 μl of 1% locust bean gum (LBG) in 50 mM potassium phosphate buffer pH 6.0 and 100 μl of enzyme solution. The reaction mixture was incubated at 50°C for 60 min. The amount of reducing sugar released was subsequently determined by the dinitrosalicyclic acid (DNS) method using D-mannose as the standard (Miller 1959). One unit (U) of β-mannanase activity was defined as the amount of enzyme producing 1 μmol of mannose equivalents per minute under the assay conditions.
Protein concentrations were determined by the Bradford method using Protein Assay Reagent (BioRad, Austria) with bovine serum albumin as the standard.
Protein localization, protein electrophoresis and zymogram analysis
For localization of recombinant β-mannanase within the cell, the periplasmic and the cell lysate fraction were separated following the method by Songsiriritthigul et al. (2010). For extraction of the periplasmic content, cells were resuspended in spheroplast buffer [100 mM Tris–HCl, pH 8.0, 0.5 mM EDTA, 0.58 M sucrose, and 20 μg/ml phenylmethylsulfonyl fluorides (PMSF)]. After incubation for 5 min on ice, cells were collected and re-suspended in water containing 1 mM MgCl2. The supernatant was then collected by centrifugation as the periplasmic fraction. Subsequently, cells were washed and resuspended in lysis buffer (50 mM Tris–HCl and 0.5 mM EDTA). After centrifugation, the supernatant was collected as the cell lysate fraction. Proteins in each fraction were analyzed by protein electrophoresis.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed using 15% (w/v) polyacrylamide gels. Protein bands were visualized by coomassie brilliant blue staining.
Zymogram analysis of recombinant β-mannanase was performed by a gel activity assay using LBG as substrate. Both of extracellular and intracellular recombinant fractions were run on 15% (w/v) native polyacrylamide gels. After electrophoresis, the native gel was placed on a substrate gel and incubated at 50°C for 1 h. Then, the substrate gel was stained with Congo red solution, destained with 1 M sodium chloride and background stained with 5% acetic acid. Enzyme activity on the substrate gel was visualized as a clear zone against a blue background.
Effect of temperature and pH on β-mannanase activity
The optimum temperature of β-mannanase activity was determined using the standard assay with 1% LBG in 50 mM potassium phosphate buffer pH 6.0 as substrate at the temperature range of 30-60°C.
The optimum pH of β-mannanase activity was determined using the standard assay with 1% LBG in the pH range of 3.0-10.0 at the 50°C.
Substrate specificity
The substrate specificity of recombinant β-mannanase was examined by measuring the enzyme activity under standard assay condition using various substrates including LBG, konjac glucomannan, ivory nut mannan, copra meal, defatted copra meal, α-mannan from yeast cell walls, xylan from oat spelts and carboxymethylcellulose (CMC).
Effect of various metal ions and EDTA on β-mannanase activity
The effect of metal ions on β-mannanase activity was determined by measuring the activity of the enzyme in the presence of various metal ions including Li+, Ca2+, Cu2+, Fe2+, Mg2+, Mn2+, Zn2+ and Co+, each at final concentration of 1 mM using standard assay condition. EDTA was employed at a similar concentration.
Analysis of hydrolysis products of recombinant β-mannanase
For the hydrolysis study, 1% of LBG and the 1% of mannooligosaccharides mannobiose, mannotriose, mannotriose, mannotetraose, mannopentaose and mannohexaose were hydrolyzed by recombinant β-mannanase. In the hydrolysis reaction, 1 ml (10 mg) of substrates were incubated with 10 U of recombinant enzyme in 50 mM potassium phosphate buffer pH 6.0 (2 ml of total volume) at 50°C for 0, 0.5, 1, 3, 6, 12 and 24 h. The reactions were stopped by boiling for 5 min. The hydrolysis products were then analyzed by high pressure liquid chromatography (HPLC) using an Aminex-HPX42C column (Bio-rad, USA) with authentic mannooligosaccharides (Megazyme, Ireland) as standard.
Acknowledgement
This research was funded by Center for Advanced Studies in Agriculture and Food, Institute for Advanced Studies, Kasetsart University, Thailand.
Footnotes
Competing interests
Authors declare that they have no competing interests.
Authors’ contributions
YP drafted the manuscript. All authors participated, read and approved the final manuscript.
Contributor Information
Yotthachai Piwpankaew, Email: yotthachai.p@gmail.com.
Supa Sakulsirirat, Email: bankbyp@hotmail.com.
Sunee Nitisinprasert, Email: fagisnn@ku.ac.th.
Thu-Ha Nguyen, Email: thu-ha.nguyen@boku.ac.at.
Dietmar Haltrich, Email: dietmar.haltrich@boku.ac.at.
Suttipun Keawsompong, Email: fagisuk@ku.ac.th.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo A, Ward OP. Hemicellulases of Bacillus species: preliminary comparative studies on production and properties of mannanases and galactanases. J Appl Bacteriol. 1990;68:253–261. doi: 10.1111/j.1365-2672.1990.tb02572.x. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam K. Polysaccharides of the kernel of maturing and mature coconuts. J Food Sci. 1976;41:1370–1373. doi: 10.1111/j.1365-2621.1976.tb01174.x. [DOI] [Google Scholar]
- Biggs P, Parsons CM. The effects of several oligosaccharides on true amino acid digestibility and true metabolizable energy in cecectomized and conventional roosters. Poult Sci. 2007;86(6):1161–1165. doi: 10.1093/ps/86.6.1161. [DOI] [PubMed] [Google Scholar]
- Chauhan PS, Puri N, Sharma P, Gupta N. Mannanases: microbial sources, production, properties and potential biotechnological applications. Appl Microbiol Biotechnol. 2012;93:1817–1830. doi: 10.1007/s00253-012-3887-5. [DOI] [PubMed] [Google Scholar]
- Chen X, Lu W, Cao Y, Li D. Prokaryotic expression, purification and characterization of Aspergillus sulphureus β-mannanase and site-directed mutagenesis of the catalytic residues. Appl Biochem Biotechnol. 2008;149:139–144. doi: 10.1007/s12010-007-8037-7. [DOI] [PubMed] [Google Scholar]
- Choi JH, Lee SY. Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol. 2004;64:625–635. doi: 10.1007/s00253-004-1559-9. [DOI] [PubMed] [Google Scholar]
- Dhawan S, Kaur J. Microbial mannanases: an overview of production and applications. Crit Rev Biotechnol. 2007;27(4):197–216. doi: 10.1080/07388550701775919. [DOI] [PubMed] [Google Scholar]
- Dutta S, Bradford JK, Nevins JD. Endo-betamannanase activity present in cell wall extracts of lettuce endosperm prior to radicle emergence. Plant Physiol. 1997;113:155–161. doi: 10.1104/pp.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier N, Talbot G, Sygusch J. Gene cloning, DNA sequencing and expression of thermostable β-mannanase from Bacillus stearothermophilus. Appl Environ Microbiol. 1998;64(11):4428–4432. doi: 10.1128/aem.64.11.4428-4432.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, McCartney AL, Rastall RA. Prebiotics and resistance to gastrointestinal infections. Br J Nutr. 2005;93(Suppl 1):31–34. doi: 10.1079/BJN20041343. [DOI] [PubMed] [Google Scholar]
- IndexMundi country reports, Massachusetts. 2014. [Google Scholar]
- Lee JT, Bailey CA, Cartwright AL. Betamannanase ameliorates viscosity-associated depression of growth in broiler chickens fed guar germ and hull fractions. Poult Sci. 2003;82:1925–1931. doi: 10.1093/ps/82.12.1925. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang P, Meng K, Wang Y, Luo H, Wu N, Fan Y, Yao B. Gene cloning, expression, and characterization of a novel β-mannanase from Bacillus circulans CGMCC 1416. J Microbiol Biotechnol. 2008;18(1):160–166. [PubMed] [Google Scholar]
- McCutchen MC, Duffaud DG, Leduc P, Peterson HAR, Tayal A, Khan AS, Kelly MR. Characterization of extremely thermostable enzymatic breakers (α-1,6-galactosidase and β-1,4 mannanase) from the hyperthermophilic bacterium Thermotoga neapolitana 5068 for hydrolysis of guar gum. Biotechnol Bioeng. 1996;52:332–339. doi: 10.1002/(SICI)1097-0290(19961020)52:2<332::AID-BIT13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Mergulhão FJM, Summers DK, Monteiroa GA. Recombinant protein secretion in Escherichia coli. Biotechnol Adv. 2005;23:177–202. doi: 10.1016/j.biotechadv.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Nguyen HA, Nguyen TH, Křen V, Eijsink VGH, Haltrich D, Peterbauer CK. Heterologous expression and characterization of an N-acetyl-β-D-hexosaminidase from Lactococcus lactis ssp. lactis IL1403. J Agric Food Chem. 2012;60:3275–3281. doi: 10.1021/jf204915e. [DOI] [PubMed] [Google Scholar]
- Pangsri P. Purification and characterization of mannanase from Bacillus circulans NT 6.7 and its application in mannooligosaccharides preparation. Kasetsart University: Ph.D. Thesis; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, Von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Phothichitto K, Nitisinprasert S, Keawsompong S. Isolation, screening and identification of mannanase producing microorganisms. Kasetsart J (Nat Sci) 2006;40(Suppl):26–38. [Google Scholar]
- Puchar V, Vrsanska M, Svoboda P, Pohl J, Ogel BZ, Biely P. Purification and characterization of two forms of endo-beta-1,4-mannanase from a thermotolerant fungus, Aspergillus fumigatus IMI 385708 (formerly Thermomyces lanuginosus IMI 158749) Biochim Biophys Acta. 2004;1674:239–250. doi: 10.1016/j.bbagen.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Puls J. Chemistry and biochemistry of hemicelluloses-relationship between hemicellulose structure and enzymes required for hydrolysis. Macromol Symp. 1997;120:183–196. doi: 10.1002/masy.19971200119. [DOI] [Google Scholar]
- Rastall RA, Gibson GR, Gill HS, Guarner F, Klaenhammer TR, Pot B, Reid G, Rowland IR, Sanders ME. Modulation of the microbial ecology of the human colon by probiotics, prebiotics and synbiotics to enhance human health: an overview of enabling science and potential applications. FEMS Microbiol Ecol. 2005;52(2):145–152. doi: 10.1016/j.femsec.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Sakulsirirat S. Cloning and structural analysis of genes encoding mannanase from Bacillus amyloliquefaciens NT6.3 and Bacillus circulans NT6.7. Kasetsart University: M.Sc. Thesis; 2008. [Google Scholar]
- Smith DL, Jr, Nagy TR, Wilson LS, Dong S, Barnes S, Allison DB. The effect of mannan oligosaccharide supplementation on body weight gain and fat accrual in C57Bl/6 J Mice. Obesity (Silver Spring) 2010;18(5):995–999. doi: 10.1038/oby.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songsiriritthigul C, Buranabanyat B, Haltrich D, Yamabhai M. Efficient recombinant expression and secretion of a thermostable GH26 mannan endo-1,4-β-mannosidase from Bacillus licheniformis in Escherichia coli. Microb Cell Fac. 2010;9(20):1–13. doi: 10.1186/1475-2859-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Li Y, Wang Y, Meng K, Luo H, Yuan T, Bai Y, Zhan Z, Yao B. A novel β-mannanase with high specific activity from Bacillus circulans CGMCC1554: gene cloning, expression and enzymatic characterization. Appl Biochem Biotechnol. 2009;159:85–94. doi: 10.1007/s12010-008-8364-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ju J, Peng H, Gao F, Zhou C, Zeng Y, Xue Y, Li Y, Henrissat B, Gao GF, Ma Y. Biochemical and structural characterization of the intracellular mannanase AaManA of Alicyclobacillus acidocaldarius reveals a novel glycoside hydrolase family belonging to clan GH-A. J Biol Chem. 2008;283(46):31551–31558. doi: 10.1074/jbc.M803409200. [DOI] [PubMed] [Google Scholar]


