Abstract
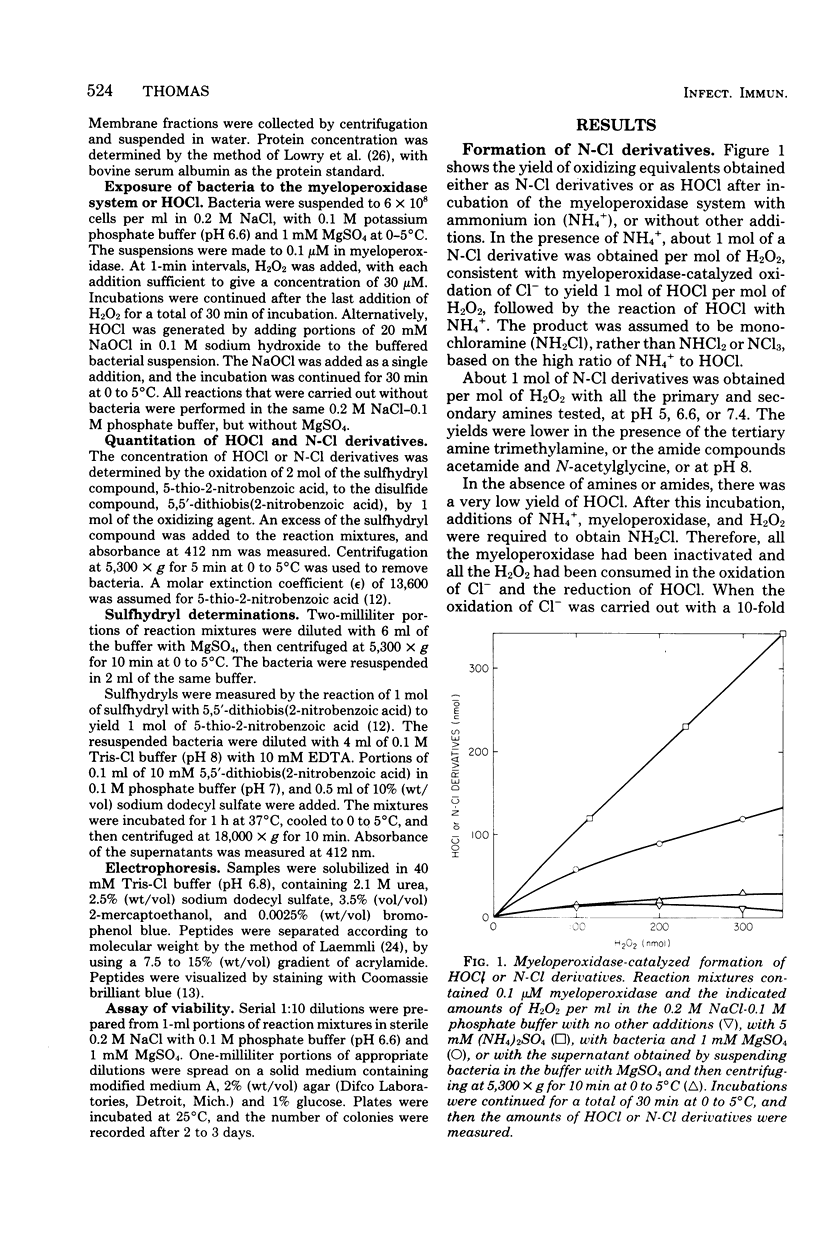
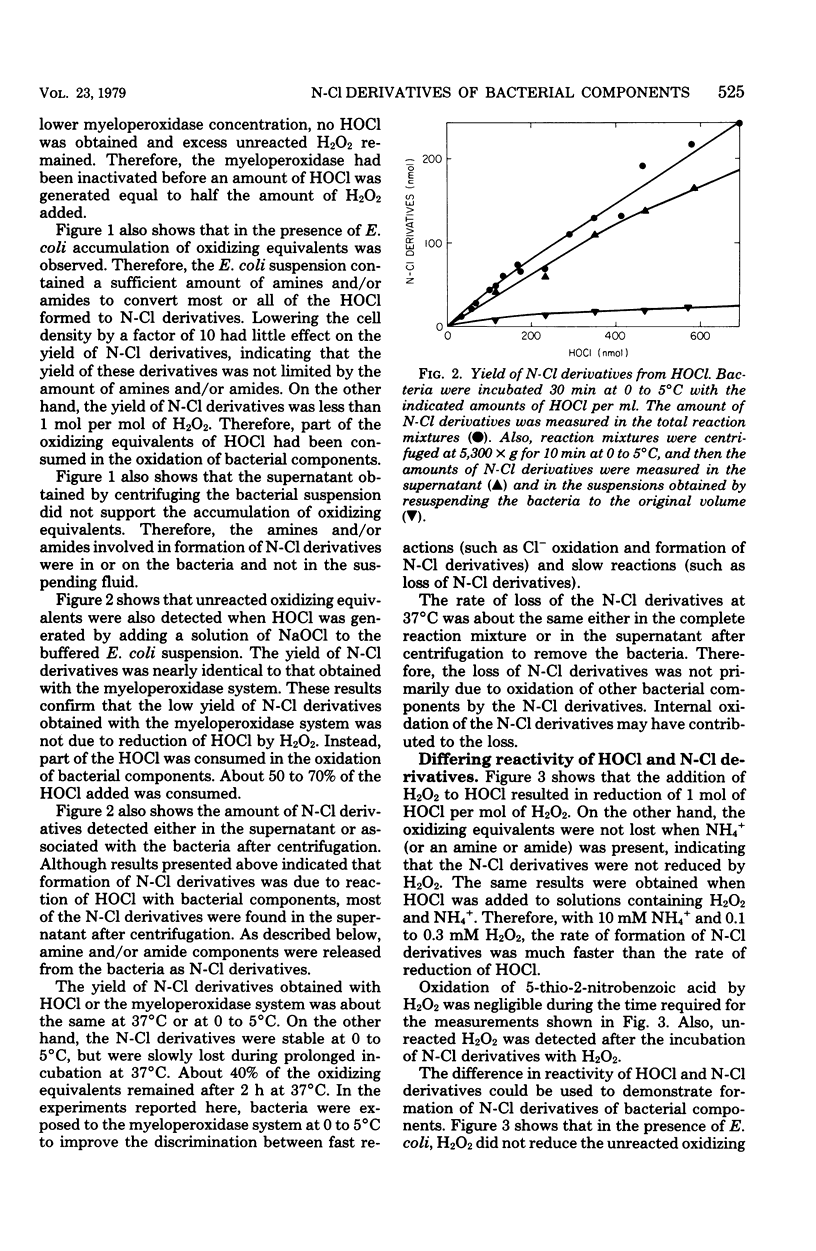
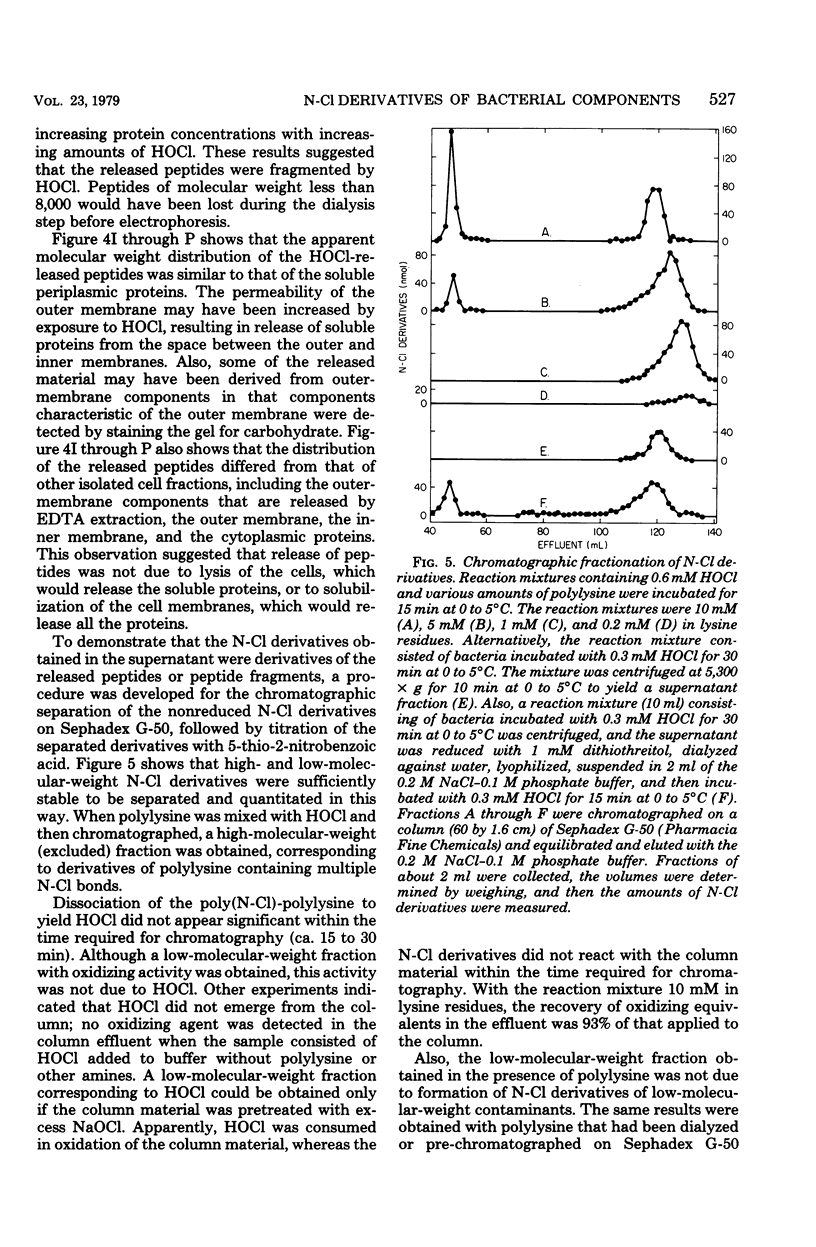
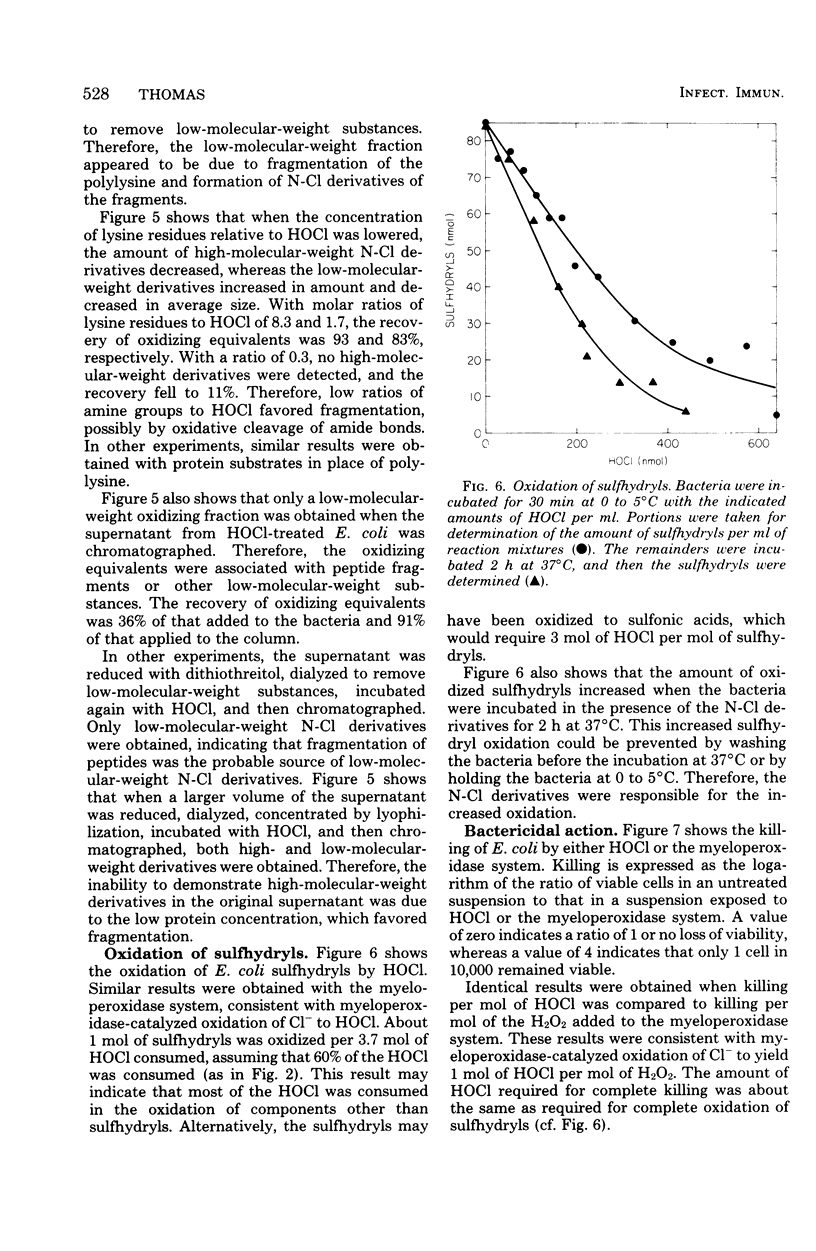
In the presence of Escherichia coli, myeloperoxidase-catalyzed oxidation of chloride ion resulted in formation of long-lived chloramine and/or chloramide derivatives of bacterial components. The same amount of these nitrogen-chlorine (N-Cl) derivatives was obtained with either hypochlorous acid (HOCl) or the myeloperoxidase system, indicating that myeloperoxidase catalyzed the oxidation of chloride to HOCl. Identical killing was obtained with HOCl or the myeloperoxidase system. About 30 to 50% of the oxidizing equivalents of HOCl were detected as N-Cl derivatives of peptides or peptide fragments that were released from the bacteria. The apparent molecular weight distribution of the peptides decreased with increasing amounts of HOCl, suggesting that peptides were fragmented by oxidative cleavage of chloramide derivatives of peptide bonds. The remaining 50 to 70% of the oxidizing equivalents of HOCl were rapidly consumed in peptide bond cleavage or the oxidation of other bacterial components. There was a close correspondence between the oxidation of bacterial sulfhydryls and bactericidal action. The N-Cl derivatives were lost and the oxidation of bacterial sulfhydryls increased over a period of several h at 37 degrees C. These changes were accompanied by increased killing. The increase in sulfhydryl oxidation and killing could be prevented by washing the bacteria to remove the N-Cl derivatives. Therefore, the N-Cl derivatives could oxidize bacterial components long after the myeloperoxidase-catalyzed oxidation of chloride was complete.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem. 1977 Oct 17;80(1):209–214. doi: 10.1111/j.1432-1033.1977.tb11873.x. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. J., Karnovsky M. L. Degranulation of leukocytes in chronic granulomatous disease. J Clin Invest. 1969 Jan;48(1):187–192. doi: 10.1172/JCI105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L. Molecular basis for functional disorders of phagocytes. J Pediatr. 1974 Mar;84(3):317–327. doi: 10.1016/s0022-3476(74)80711-0. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G., Castle W. B. Oxidant injury of caucasian glucose-6-phosphate dehydrogenase-deficient red blood cells by phagocytosing leukocytes during infection. J Clin Invest. 1971 Dec;50(12):2466–2473. doi: 10.1172/JCI106747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. W. Studies on the reaction between sodium hypochlorite and proteins: 1. Physico-chemical study of the course of the reaction. Biochem J. 1947;41(3):337–342. [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Peroxidase-mediated mammalian cell cytotoxicity. J Exp Med. 1973 Jul 1;138(1):318–323. doi: 10.1084/jem.138.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Harrison J. E., Watson B. D., Schultz J. Myeloperoxidase and singlet oxygen: a reappraisal. FEBS Lett. 1978 Aug 15;92(2):327–332. doi: 10.1016/0014-5793(78)80780-7. [DOI] [PubMed] [Google Scholar]
- Held A. M., Hurst J. K. Ambiguity associated with use of singlet oxygen trapping agents in myeloperoxidase-catalyzed oxidations. Biochem Biophys Res Commun. 1978 Apr 14;81(3):878–885. doi: 10.1016/0006-291x(78)91433-x. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Knox W. E., Stumpf P. K., Green D. E., Auerbach V. H. The Inhibition of Sulfhydryl Enzymes as the Basis of the Bactericidal Action of Chlorine. J Bacteriol. 1948 Apr;55(4):451–458. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Piatt J. F., Cheema J. S., O'Brien P. J. Peroxidase catalyzed singlet oxygen formation from hydrogen peroxide. FEBS Lett. 1977 Mar 1;74(2):251–254. doi: 10.1016/0014-5793(77)80857-0. [DOI] [PubMed] [Google Scholar]
- Quie P. G., Mills E. L., Holmes B. Molecular events during phagocytosis by human neutrophils. Prog Hematol. 1977;10:193–210. [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Formation of singlet oxygen by the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1977 Jul 25;252(14):4803–4810. [PubMed] [Google Scholar]
- Sbarra A. J., Paul B. B., Jacobs A. A., Strauss R. R., Mitchell G. W., Jr Role of the phagocyte in host-parasite interactions. 38. Metabolic activities of the phagocyte as related to antimicrobial action. J Reticuloendothel Soc. 1972 Aug;12(2):109–126. [PubMed] [Google Scholar]
- Sbarra A. J., Selvaraj R. J., Paul B. B., Poskitt P. K., Mitchell G. W., Jr, Louis F., Asbell M. A. Granulocyte biochemistry and a hydrogen peroxide-dependent microbicidal system. Prog Clin Biol Res. 1977;13:29–48. [PubMed] [Google Scholar]
- Selvaraj R. J., Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Oxidative peptide cleavage and decarboxylation by the MPO-H2O2-Cl- antimicrobial system. Infect Immun. 1974 Feb;9(2):255–260. doi: 10.1128/iai.9.2.255-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R. M., Hager L. P. The chloroperoxidase-catalyzed oxidation of thiols and disulfides to sulfenyl chlorides. Biochemistry. 1974 Dec 3;13(25):5069–5073. doi: 10.1021/bi00722a001. [DOI] [PubMed] [Google Scholar]
- Stelmaszyńska T., Zgliczyński J. M. Myeloperoxidase of human neutrophilic granulocytes as chlorinating enzyme. Eur J Biochem. 1974 Jun 1;45(1):305–312. doi: 10.1111/j.1432-1033.1974.tb03555.x. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXVII. Myeloperoxidase-H(2)O(2)-Cl-Mediated Aldehyde Formation and Its Relationship to Antimicrobial Activity. Infect Immun. 1971 Apr;3(4):595–602. doi: 10.1128/iai.3.4.595-602.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Cofactor role of iodide in peroxidase antimicrobial action against Escherichia coli. Antimicrob Agents Chemother. 1978 Jun;13(6):1000–1005. doi: 10.1128/aac.13.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun. 1978 May;20(2):456–463. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Oxidation of Escherichia coli sulfhydryl components by the peroxidase-hydrogen peroxide-iodide antimicrobial system. Antimicrob Agents Chemother. 1978 Jun;13(6):1006–1010. doi: 10.1128/aac.13.6.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Susceptibility of Escherichia coli to bactericidal action of lactoperoxidase, peroxide, and iodide or thiocyanate. Antimicrob Agents Chemother. 1978 Feb;13(2):261–265. doi: 10.1128/aac.13.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A., Drachman R. H. Phagocytosis: The normal process and its clinically significant abnormalities. Pediatr Clin North Am. 1974 Aug;21(3):551–569. doi: 10.1016/s0031-3955(16)33024-3. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. I. Resolution of antibacterial and enzymatic activities. J Bacteriol. 1966 Feb;91(2):750–754. doi: 10.1128/jb.91.2.750-754.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgliczynski J. M., Selvaraj R. J., Paul B. B., Stelmaszynska T., Poskitt P. K., Sbarra A. J. Chlorination by the myeloperoxidase-H2O2-Cl- antimicrobial system at acid and neutral pH. Proc Soc Exp Biol Med. 1977 Mar;154(3):418–422. doi: 10.3181/00379727-154-39684. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T. Chlorinating ability of human phagocytosing leucocytes. Eur J Biochem. 1975 Aug 1;56(1):157–162. doi: 10.1111/j.1432-1033.1975.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Domański J., Ostrowski W. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim Biophys Acta. 1971 Jun 16;235(3):419–424. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Ostrowiski W., Naskalski J., Sznajd J. Myeloperoxidase of human leukaemic leucocytes. Oxidation of amino acids in the presence of hydrogen peroxide. Eur J Biochem. 1968 May;4(4):540–547. doi: 10.1111/j.1432-1033.1968.tb00246.x. [DOI] [PubMed] [Google Scholar]



