Abstract
Purpose
Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease that can affect the central nervous system. Neuropsychiatric symptoms are found in 25–70% of patients. Using diffusion tensor imaging (DTI) various studies have reported changes in white matter integrity in SLE patients with neuropsychiatric symptoms (NPSLE patients). The purpose of this study was to investigate, if regional changes in white matter integrity can also be detected in SLE patients without neuropsychiatric symptoms (non-NPSLE patients).
Methods
Applying DTI and tract based spatial statistics (TBSS) we investigated 19 NPSLE patients, 19 non-NPSLE and 18 healthy controls. Groups were matched for age and sex. Image pre-processing was performed using FSL, following the TBSS pipeline (eddy current correction, estimation of fractional anisotropy (FA), normalization, skeletonization of the group mean FA image). A general linear model with threshold-free cluster enhancement was used to assess significant differences between the three groups.
Results
Statistical analyses revealed several regions of decreased prefrontal white matter integrity (decreased FA) in both groups of SLE patients. The changes found in the non-NPSLE patients (as compared to healthy controls) overlapped with those in the NPSLE patients, but were not as pronounced.
Conclusions
Our data suggest that changes in regional white matter integrity, in terms of a decrease in FA, are present not only in NPSLE patients, but also in non-NPSLE patients, though to a lesser degree. We also demonstrate that the way statistical maps are corrected for multiple comparisons has a profound influence on whether alterations in white matter integrity in non-NPSLE patients are deemed significant.
Keywords: Systemic lupus erythematosus, Diffusion tensor imaging, White matter
Abbreviations: ACR, American College of Rheumatology; CNS, central nervous system; dMRI, diffusion MRI; DTI, diffusion tensor imaging; FA, fractional anisotropy; MRI, magnetic resonance imaging; SLE, systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SLICC, systemic lupus erythematosus International Collaborating Clinics; SD, standard deviation; SVM, support vector machine; TBSS, tract based spatial statistics; TFCE, threshold free cluster enhancement; VBM, voxel based morphometry
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory, immune-mediated disease, which affects 0.05–0.1% of the general population (Somers et al., 2014; Bernatsky et al., 2007). Neuropsychiatric symptoms have been reported to occur in 25–70% of patients with SLE (Brey et al., 2002; Monastero et al., 2001) and are associated with increased morbidity and mortality (Sibley et al., 1992; Gladman et al., 1996). Magnetic resonance imaging (MRI) has become part of the routine clinical work up in SLE patients presenting with neuropsychiatric symptoms (NPSLE patients) (Appenzeller et al., 2008a,b; Steup-Beekman et al., 2013), helping to diagnose or exclude cerebral pathologies such as edema, hemorrhage, central thrombosis, or stroke.
Using quantitative analysis methods, changes in brain chemistry (Sundgren et al., 2005; Appenzeller et al., 2007a), regional cerebral blood flow (Shen et al., 1999; Oda et al., 2005; Wang et al., 2012) and cerebral glucose utilization (Otte et al., 1997) have been demonstrated in NPSLE patients. Structural brain imaging methods, such as voxel based morphometry (VBM), cortical thickness analysis and diffusion tensor imaging (DTI), have also been applied to investigate differences in regional gray and white matter in SLE patients and healthy controls (HCs) (Appenzeller et al., 2007a; Jung et al., 2010; Emmer et al., 2010; Welsh et al., 2007). Regions of altered brain structure, in terms of decreases in regional gray matter volume, cortical thickness or fractional anisotropy (FA) have been demonstrated in NPSLE patients, but less frequently in non-NPSLE patients (Appenzeller et al., 2007a; Jung et al., 2010; Jung et al., 2010).
The distinction between NPSLE and non-NPSLE is clinical, based on the American College of Rheumatology (ACR) case definitions. In clinical practice brain imaging is often not part of the routine work up of non-NPSLE, possibly leading to an underestimation of CNS disease. Some authors, using MR imaging, have found evidence for small emboli, cerebral infarctions, hemosiderin deposits, and dilated perivascular spaces in up to 60% of non-NPSLE patients, strongly suggesting cerebral involvement in at least some of these patients (Cotton et al., 2004). Along these lines spectroscopy studies have reported decreased NAA levels, indicative of axonal dysfunction/damage, in SLE patients with active disease, independently of CNS manifestation (Sundgren et al., 2005; Appenzeller et al., 2005; Cagnoli et al., 2013). On the other hand some of the studies using advanced statistical imaging methods, such as VBM and DTI, have detected reduced gray or white matter integrity in NPSLE patients only, but not in non-NPSLE patients, suggesting that non-NPSLE patients with respect to gray and white matter structure are more similar to HCs than to NPSLE patients (Appenzeller et al., 2007b; Jung et al., 2010a,b). For example, Jung et al. investigating NPSLE patients and non-NPSLE patients could identify regions of decreased white matter integrity (decreased FA values) in NPSLE patients only (Jung et al., 2010b). However, in statistical neuroimaging other factors than “true” differences (of outcome parameters, e.g. gray matter density/volume, FA values, etc.), such as group size, acquisition parameters, preprocessing steps and the way multiple comparisons are accounted for play a role for whether differences are considered and reported as significant or not.
The aim of this study was to further investigate white matter integrity in NPSLE and non-NPSLE in a cohort of 19 NPSLE patients, 19 non-NPSLE patients and 18 HCs. We used FSL and the TBSS pipeline (tract based spatial statistics) to preprocess and statistically analyze our data performing both univariate and multivariate statistical tests. We hypothesized that the pattern of altered white matter integrity in non-NPSLE patients does not substantially differ from that seen in NPSLE patients. We also sought to specifically investigate the impact of alternative ways to correct for multiple comparisons that are frequently used in statistical brain imaging. We hypothesized that the way statistical maps are created has a profound influence on whether non-NPSLE patients have significant alterations in white matter integrity.
2. Material and methods
2.1. Subjects
The study comprised 19 patients with acute NPSLE, defined as one or more neuropsychiatric manifestations within 2 weeks prior to inclusion in the study, and of 19 patients with SLE, without current or past history of neuropsychiatric symptoms (non-NPSLE), as well as of 18 HCs. In a recently published VBM study, we had reported differences in regional gray matter volume between NPSLE patients, non-NPSLE patients and HCs (Cagnoli et al., 2012). Some of these patients/subjects had also undergone DTI and took part in current study. There were 19 female patients (out of 19) in the NPSLE group, 18 female patients (out of 19) in the non-NPSLE group, and 16 females (out of 18) in the HC group. The gender ratios did not differ significantly between groups (Fisher's exact test, p = 0.32).
The classification of NPSLE was based on the 19 ACR case definitions (American College of Rheumatology, 1999). In order to participate in the study, individuals needed to fulfill 4 or more of the ACR classification criteria (Tan et al., 1982) and, in order to qualify as an NPSLE patient, present within 2 weeks of the initial neuropsychiatric event. Patients in the non-NPSLE group were required to have no prior history of neuropsychiatric manifestations regardless of attribution. All participants were recruited from the Department of Internal Medicine, Division of Rheumatology, and the Department of Emergency Medicine at the University of Michigan. Patients with previous/known drug abuse, alcohol abuse, diabetes, stroke and/or renal insufficiency were excluded. Groups were matched for age and sex. For details on patient groups and clinical manifestation see Tables 1 and 2. The study had been approved of by the IRB of the University of Michigan (HUM00000714) and informed consent had been obtained from the patients prior to study inclusion.
Table 1.
Demographic data and disease severity.
| NPSLE patients n = 19 | Non-NPSLE patients n = 19 |
HC n = 18 |
Significance | |
|---|---|---|---|---|
| Range mean/SD |
Range mean/SD |
Range mean/SD |
p value | |
| Age | 22–67 39.1/11.7 |
21–60 38.0/11.1 |
18–61 37.6/13.5 |
Kruskal–Wallis test p = 0.99 |
| Female patients | 19 | 18 | 16 | Fisher's exact test p = 0.32 |
| Ethnicity |
|
2/19 AA 17/19 White |
1/18 Asian 17/18 White |
Fisher's exact test p = 0.072 |
| Age at disease onset (years) | 12–65 33.4/13.0 |
20–55 33.3/10.8 |
n/a | Mann–Whitney U test p > 0.99 |
| Disease duration (years) | 0.1–16 5.7/6.3 |
1.0–15 4.7/4.8 |
n/a | Mann–Whitney U test p = 0.95 |
| SLEDAI | 1–24 10.8/5.5 |
0–8 2.0/2.2 |
n/a | Mann–Whitney U test p < 0.0001 |
| SLICC | 0–2 0 68/0.67 |
0–3 0.47/0.84 |
n/a | Mann–Whitney U test p = 0.18 |
| Mini Mental | 22–30 27.8/2.6 |
24–30 28/1.5 |
n/a | Mann–Whitney U test p = 0.60 |
AA = African American; HC = healthy control; NPSLE = neuropsychiatric systemic lupus erythematosus; SD = standard deviation; SLE = systemic lupus erythematosus; SLEDAI = Systemic Lupus Erythematosus Disease Activity Index; SLICC = Systemic Lupus Erythematosus International Collaborating Clinics; SD = standard deviation.
Table 2.
Differences in regional fractional anisotropy (TFCE corrected).
| Comparison of groups | Cluster size |
Coordinates |
Smallest p value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| NPSLE < HC | |||||
| 7291 | 24 | 25 | 1 | 0.008 | |
| Non-NPSLE < HC | |||||
| 2660 | –27 | 37 | 2 | 0.024 | |
| 550 | –17 | 0 | 38 | 0.038 | |
| 258 | 18 | 34 | 12 | 0.039 | |
| NPSLE < non-NPSLE | |||||
| − | − | − | − | − | |
HC = healthy control, NPSLE = neuropsychiatric systemic lupus erythematosus; non-NPSLE = systemic lupus erythematosus without neuropsychiatric symptoms. TFCE = threshold free cluster enhancement. Per-voxel p values, corrected for multiple comparisons, were computed using TFCE and permutation based statistics. Coordinates refer to voxels with smallest p values within a cluster. Unlike cluster-based statistics, per-voxel testing can lead to many small clusters of significant voxels. For brevity, only the ones larger than 100 voxels are reported.
All patients and HCs were submitted to a standardized clinical and neurological examination including medical history (age, gender, ethnicity, age at disease onset, disease duration, etc.), physical examination standard laboratory assessment and a Mini Mental State examination (Folstein et al., 1975). SLE and NPSLE patients underwent required laboratory tests to determine disease activity by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) (Bombardier et al., 1992) as well as antiphospholipid antibody status. SLE was considered active with a SLEDAI score >6. We chose this higher SLEDAI level to minimize the inclusion of patients who have persistently active serologies (anti-DNA antibodies, low complements and /or leukopenia) without clinical evidence of disease activity.
Disease duration was defined as the time between the diagnosis of SLE and the day of the MRI. CNS manifestations were divided into central and peripheral following ACR case definitions. Cumulative damage in both patient groups was assessed using the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SLICC) (Gladman et al., 1996) at the time of MRI acquisition. With respect to age at disease onset, disease duration, SLEDAI, SLICC and Mini-mental scores, non-NPSLE patients and NPSLE patients were compared using the Mann–Whitney U test. Fisher's exact test was applied to compare gender and ethnic distribution in the three groups (non-NPSLE, NPSLE and HCs). For details see Table 1.
Patients and HCs with MRI pathologies that affected white matter integrity, such as cerebral infarcts or confluent white matter lesions, were excluded from the study. Other incidental findings that did not affect white matter did not lead to an exclusion (for a list of incidental findings see Section 3.2). Small, unspecific white matter lesions were found in both patients and HCs (see Results), they were not considered pathological per se, as they are found in up to 45% of healthy people (Fazekas et al., 1988; Schmidt et al., 1992), and did not lead to an exclusion of the subject.
2.2. Data acquisition, pre-processing and analysis
MR imaging was performed at a 3 T MR scanner (Achieva, Philips, Best, Netherlands). To rule out gross abnormalities such as acute or old infarcts, hemorrhage, focal lesions or pathological contrast enhancement and for any additional lesions not related to SLE, T2-w, FLAIR, T1-weighted images pre and post contrast administration were acquired in the same session and evaluated by an experienced neuroradiologist. Lesion burden in form of white matter T2/FLAIR hyper-intensive lesions was defined as mild (1–5 small white matter lesions), moderate (5–10 white matter lesions), or severe (>10 white matter lesions). Atrophy was visually evaluated as none, mild, or severe.
dMRI images (15 gradient directions at b = 800 s/mm2, plus one b = 0 image) of 19 NPSLE patients, 19 non-NPSLE and 18 healthy controls (HCs) were acquired. Image pre-processing and statistical analyses were performed using FSL 5, following the standard TBSS pipeline: eddy current correction, linear regression of the diffusion tensor model, FA estimation and non-linear normalization (FNIRT) were followed by skeletonization of the group mean FA image. Thresholding at FA > 0.2 to restrict analysis to the white matter led to 122,477 mean skeleton voxels, to which data from individual subjects was projected. A general linear model was used to assess significant differences between the three groups (all SLE vs. HC; NPSLE vs. HC; non-NPSLE vs. HC); age was included as a covariate of no interest.
To specifically investigate the impact of correcting for multiple comparisons we applied three different methods. First we applied threshold free cluster enhancement (TFCE), an alternative approach for controlling family wise error, implemented and recommended in version 5 of FSL (Salimi-Khorshidi et al., 2011; Smith and Nichols, 2009). TFCE is a voxel based method; it resembles cluster-based analysis in that it boosts the confidence in the significance of differences that are observed consistently over large spatial neighborhoods, but avoids having to select an initial cluster-forming threshold (for more details see below). Secondly we applied the method used in the paper by Jung et al. to facilitate comparison, thresholding the t-statistic images from a General Linear Model at t > 3.0, and testing the resulting clusters for statistical significance based on 10,000 Monte Carlo permutations for each group difference. We also performed the same procedure applying an initial voxel threshold of t > 2.0, to further elucidate the impact of this initial threshold on cluster statistics.
We finally performed a multivariate analysis, based on support vector machines (SVMs). Specifically, we were interested whether we could train a classifier that could differentiate between the three groups by identifying patterns of altered FA maps in SLE patients. Even if classification accuracy is below the level that would be required for computer-aided detection of the disease, classification rates that are significantly greater than random chance confirm that significant differences exist between the successfully classified groups. Besides in cases where only one of the two alternative methods for controlling family-wise errors indicates a significant difference between two groups, successful classification can serve as an additional confirmation that this effect can be attributed to improved sensitivity, rather than to a false positive detection. For classification, we used projected FA values at all 122,477 skeleton voxels as a feature vector. We averaged the FA values of each subject in the training dataset over the skeleton and used linear regression on the resulting averages to estimate the effect of age. The resulting slope and intercept were used to age-correct all feature values. In order to reduce the impact of voxels less relevant to the classification task, each feature was weighted by its F score, as it would be used in a two-sample F test. Classification accuracy was estimated by training a linear SVM on the resulting vectors in a leave-one-out manner; care was taken to exclude test data from linear regression and feature weighting via the F score. Multivariate analysis was performed within the shogun toolbox (http://shogun-toolbox.org/). We used permutation testing to check if accuracies were significantly higher than random chance. To this end, we built up a null distribution by computing leave-one-out estimates of the accuracy of classifiers that were trained on the same data with permuted labels. Overall our analyses comprised the following steps:
-
1
The threshold free cluster enhancement (TFCE) method as implemented in FSL 5 which is based on pre-processing the t-statistic images using a nonlinear image transformation that boosts maxima with support from a spatial neighborhood before performing a voxel-based permutation test to correct for multiple comparisons.
-
2
A cluster based method where group FA differences are calculated by thresholding the t-statistic images at a cluster-forming threshold of t > 3.0 and controlling for multiple comparisons on a cluster level using permutation methods with FSL's Randomize, running 10,000 Monte Carlo permutations for each of the group differences.
-
3
The same cluster-based method as in 3 with an initial cluster-forming threshold of t > 2.0.
-
4
A multivariate analysis to train a classifier that could differentiate between the three pairs of groups by identifying patterns of altered FA maps.
3. Results
3.1. Patients
Nineteen NPSLE and 19 non-NPSLE patients, as well as 18 HCs participated in the study. The three groups were matched by age and gender and did not differ with respect to ethnicity (for details see Table 1). NPSLE patients showed significantly higher disease activity as determined by the SLEDAI score (NPSLE: mean = 10.8 ± 5.5, non-NPSLE: 2.0 ± 2.2, p < 0.0001). There were no significant differences between age of disease onset (p > 0.99), disease duration (p = 0.95), and SLICC scores (p = 0.18) between the two SLE groups. Mini-mental scores were within normal ranges in both SLE groups with no significant differences (p = 0.60). For details see Table 1.
3.2. Conventional MRI analysis
On the conventional MRI a mild lesion burden was seen in 36.8% (7/19), a moderate in 21.1% (4/19), and a severe in 10.5% (2/19) in the NPSLE patients. A mild lesion burden was seen in 42.1% (8/19) of the non-NPSLE patients, and a moderate lesion burden was seen in 15.8% (3/19) of the patients. In the HC group 33.3% of study subjects (6/18) had a mild lesion burden, 1 subject (5.6%) had a moderate and 1 subject (5.6%) had a severe lesion burden. There were no significant differences in lesion load between the three groups: non-NPSLE vs. HC, p = 0.55 (Fisher's exact test); NPSLE vs. HC: p = 0.43, non-NPSLE vs. NPSLE: p = 0.61.
Mild atrophy was present in 8 of the 19 NPSLE patients and in 5 of the 19 non-NPSLE patients. A few incidental findings were found such as a small pineal cyst present in one NPSLE patient and a small meningioma in another NPSLE patient, while a small venous angioma was present in one non-NPSLE. A few incidental findings were also found in the HCs; 4 venous angiomas and one right carotid artery aneurysm.
3.3. Voxel based analysis with threshold free cluster enhancement
The results of our statistical analyses are summarized in Tables 2, 3 and 4, as well as Figs. 1 and 2. Analysis with TFCE revealed several regions of decreased FA in prefrontal white matter in SLE patients, both with and without neuropsychiatric symptoms. In total in NPSLE patients 7429 skeleton voxels (at the 1 mm3 resolution used by TBSS) showed significantly decreased FA values as compared to HCs. Non-NPSLE patients had decreased FA values in 3520 voxels. However, when we directly compared NPSLE and non-NPSLE patients there were no significant differences between the two groups.
Table 3.
Differences in regional fractional anisotropy (cluster corrected with voxel threshold t > 3.0).
| Comparison of groups | Cluster size |
Coordinates |
p value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| NPSLE < HC | |||||
| 123 | –24 | 31 | 5 | 0.010 | |
| 110 | 24 | 26 | 2 | 0.012 | |
| Non-NPSLE < HC | |||||
| − | − | − | − | − | |
| NPSLE < non-NPSLE | |||||
| − | − | − | − | − | |
HC = healthy control, NPSLE = neuropsychiatric systemic lupus erythematosus; non-NPSLE = systemic lupus erythematosus without neuropsychiatric symptoms. In a first step voxels were thresholded at t > 3.0; permutation-based cluster statistics were then computed to obtain a multiple comparison corrected p values for each cluster. Coordinates refer to voxels with largest values within a cluster.
Table 4.
Differences in regional fractional anisotropy (cluster corrected with voxel threshold t > 2.0).
| Comparison of groups | Cluster size |
Coordinates |
p value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| NPSLE < HC | |||||
| 1683 | 24 | 26 | 2 | 0.001 | |
| 1382 | –24 | 31 | 5 | 0.002 | |
| Non-NPSLE < HC | |||||
| 536 | –6 | 26 | –1 | 0.027 | |
| NPSLE < non-NPSLE | |||||
| − | − | − | − | − | |
HC = healthy control, NPSLE = neuropsychiatric systemic lupus erythematosus; non-NPSLE = systemic lupus erythematosus without neuropsychiatric symptoms. In a first step voxels were thresholded at t > 2.0; permutation-based cluster statistics were then computed to obtain a multiple comparison corrected p values for each cluster. Coordinates refer to voxels with largest t values within a cluster.
Fig. 1.
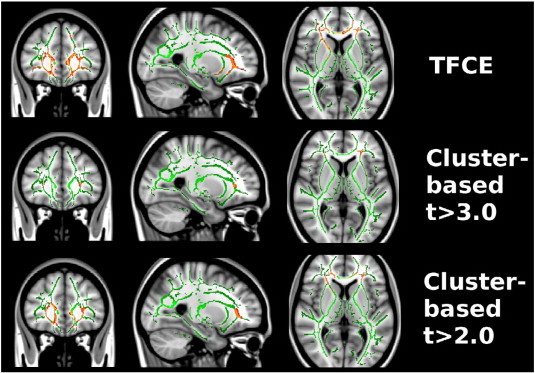
NPSLE patients compared to healthy controls. A significant reduction in regional fractional anisotropy in prefrontal white matter was found in NPSLE patients as compared to healthy controls (red voxels). Compared to TFCE or cluster-based analysis with cluster forming threshold t > 2.0, the partial extent of the reduction is strongly underrepresented with cluster threshold t > 3.0.
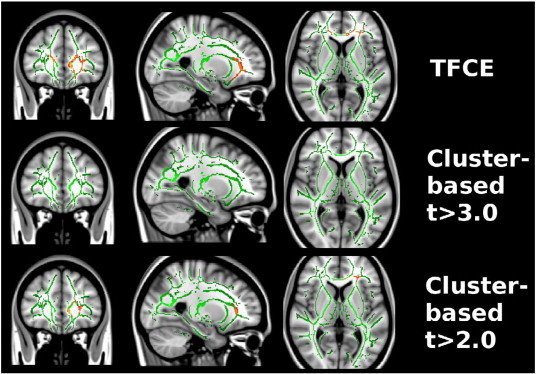
Fig. 2.
Non-NPSLE patients compared to healthy controls. A significant reduction in regional fractional anisotropy in prefrontal white matter was found in non-NPSLE patients (red voxels). No significant differences were found with cluster threshold t > 3.0.
3.4. Cluster based analysis
Statistical analyses with cluster forming threshold t > 3.0 revealed two regions, with 123 and 110 voxels in the prefrontal white matter in each hemisphere, of decreased FA in NPSLE patients as compared to HCs (p = 0.01 and p = 0.012). No significant clusters were found when comparing non-NPSLE patients to HCs or to NPSLE patients. Thus, we observed a greatly reduced sensitivity of cluster-based analysis as compared to TFCE. We hypothesized that this might be caused by the fact that the large clusters found by TFCE are broken down into smaller pieces by the arbitrary cluster-forming threshold, and that those individual pieces are no longer significant. We then repeated cluster-based analysis with a reduced threshold of t > 2.0, which indicated spatially much more extended regions of significant FA change in NPSLE, as compared to HCs (two clusters with 1683 voxel, p = 0.001, and 1382 voxels, p = 0.002), and re-introduced one cluster with significantly lower FA values (536 voxels, p = 0.027) in non-NPSLEs as compared HCs. For more details see Tables 2 and 3 and Figs. 1 and 2.
3.5. Multivariate analysis
Classification analyses revealed a better than chance classification when testing all SLE patients vs. HCs (79%, p = 0.001), NPSLE patients vs. HCs (70%, p = 0.026), and non-NPSLE patients vs. HCs (70%, p = 0.020). We employed a permutation-based test, which helps to guard against accidental double dipping and corrects for imbalanced class sizes (e.g. when classifying all SLEs vs. HCs, an accuracy significantly higher than 67% was tested). Using the same method, it was not possible to classify NPSLE vs. non-NPSLE patients (34% accuracy).
4. Discussion
In this study we applied DTI, using both univariate and multivariate analysis methods, to determine differences in white matter integrity between SLE patients, with and without neuropsychiatric symptoms, and a group of HCs. Applying voxel based statistics and using the TFCE method to correct for multiple comparisons we found decreased FA predominantly in prefrontal white matter in both NPSLE and non-NPSLE patients as compared to HCs; we found no significant differences between the two SLE groups. However as compared to the HC group NPSLE patients displayed about twice as many voxels with significantly reduced FA values as the non-NPSLE group. The implications of our study are twofold: non-NPSLE patients do show alteration in white matter integrity, although to a lesser degree than NPSLE patients. Secondly for the categorical decision whether non-NPSLE patients are more like NPSLE patients or more like HCs the choice of the statistical test, specifically the correction for multiple comparisons plays a critical role.
Our data are in line with other studies describing diminished white matter integrity in NPSLE patients (Emmer et al., 2010; Jung et al., 2010b; Zivadinov et al., 2013), suggesting that directed diffusion is reduced in this patient group, which can possibly be attributed to local pathologies or a combination of these. Possible mechanisms that have been suggested are damage to the myelin, i.e. local demyelination, damage to gray matter resulting in secondary axonal loss in the white matter, or secondary involvement of the white matter due to repeated small vessel vasculitis and acute vessel wall inflammation (Bosma et al., 2000). Such inflammation of the small vessels of the brain might then lead to vasculopathy and cause focal hypoperfusion or microinfarcts that cause subtle changes, not visible on conventional MRI, but detectable by DWI or DTI (Hughes et al., 2007; Luyendijk et al., 2011).
Furthermore our data suggest that non-NPSLE patients, although clinically unaffected with respect to neuropsychiatric symptoms, also display alterations in white matter structure, in terms of decreased FA values in the prefrontal cortex, however to a lesser degree than NPSLE patients. Non-NPSLE patients displayed about half as many voxels of significantly reduced FA as NPSLE patients.
Emmer et al. were the first to apply DTI and TBSS in a cohort of 12 SLE patients and 28 HCs, reporting decreased FA values in various regions such as the inferior fronto-occipital fasciculus, the fasciculus uncinatus and the posterior limb of the internal capsule (corticospinal tract), as well as in the anterior thalamic radiation (Emmer et al., 2010). Importantly only 7 of these patients fulfilled the ACR criteria for NPSLE, so that it is likely that non-NPSLE patients also contributed to the group differences. Investigating a study sample comparable to ours with respect to sample size and mean age, and applying the same methodological approach, with a cluster forming threshold of t > 3.0 Jung et al. could identify regions of decreased FA only in NPSLE patients, but not in non-NPSLE patients (Jung et al., 2010b). Furthermore two regions showed decreased FA values in NPSLE patients as compared to non-NPSLE patients, i.e. the left superior longitudinal fasciculus and the corpus callosum. Importantly, when we applied the same correction (for multiple comparisons) method as in (Jung et al., 2010b) our results were similar to those reported by Jung et al., i.e. we saw no differences between non-NPSLE patients and HCs. For the following reasons, we believe that the more profound differences found by TFCE are genuine, rather than false positives: first, we could achieve similar results when reducing the cluster-forming threshold. We note that there is no systematic or “correct” way of setting this threshold, and that it is one of the main advantages of TFCE that it does not require such a threshold. Its increased sensitivity compared to competing methods is one of the benefits claimed by TFCE (Smith and Nichols, 2009); this claim is further substantiated by the fact that multivariate analyses, using SVMs and the leave-one-out method, were able to classify the two classes (non-NPSLE and HC) with better-than-chance accuracy. Given the rather diffuse patterns of altered FA in SLE, regardless of neuropsychiatric symptoms, we did not expect a classifier based on localized features to achieve highly accurate classification results. Rather, our motivation to use features closely related to the quantities used in statistical testing was to reaffirm the significant differences found by TFCE. Indeed, the accuracy to differentiate between the two SLE groups and HCs was (as expected) modest, but still significantly better than random chance, (non-NPSLE patients − HCs: 70%; and NPSLE patients − HCs: 70%). Differentiation between the two SLE groups was not possible (NPSLE patients – non-NPSLE patients: 34%). Against this background, we tend to think that the results revealed by the TFCE method are valid and that reductions in white matter integrity can be observed in both NPSLE patients and in non-NPSLE patients. However, apart from the thresholding procedure other possible differences between previous studies and the current might explain, why so far no significant differences in the non-NPSLE have been reported; these include local patient characteristics and differences in scanning parameters. While we were not able to identify features that made our sample somewhat special, the current study is the first to apply the TBSS algorithm on DTI data that were collected on a 3 T scanner, with 15 gradient directions and thus might have caught subtle differences that escaped other scanning protocols. Importantly the lesion load seen in this study is comparable to that seen in previous studies showing a higher percentage of patients with a severe lesion load in the NPSLE group, suggesting that both SLE groups are representative.
Different pathogenic pathways have been proposed for neuropsychiatric manifestations in SLE and current research tries to identify mechanisms that make SLE patients prone to CNS involvement. Often neuropsychiatric symptoms occur early in the disease (NPSLE), which for some authors speaks for an immune-mediated pathogenesis (Steup-Beekman et al., 2013). As such CNS involvement might results from a specific antibody profile (Emmer et al., 2010; Matus et al., 2007; DeGiorgio et al., 2001), possibly associated with a leakage of the blood–brain-barrier (Huerta et al., 2006) or it might more generally reflect a higher disease activity as compared to non-NPSLE. Given that with respect to CNS involvement HCs and NPSLE patients are two poles of one spectrum, ranging from healthy to severely ill, our data suggest that non-NPSLE patients fall somewhere in between. In support of this notion white matter lesions, as determined by conventional neuroradiological assessment have been described to occur in up to 60% of non-NPSLE patients (Cotton et al., 2004) and cognitive dysfunction has been reported to be present in 30% of non-NPSLE patients (Monastero et al., 2001; Jung et al., 2012), both strongly suggesting cerebral involvement despite the absence of neuropsychiatric pathologies fulfilling ACR case definitions. One interesting question is how many of the non-NPSLE patients in our cohort would have shown cognitive dysfunction as determined by standardized neuropsychological testing. This issue was not fully addressed, and can be seen as a limitation; as such the Mini-Mental questionnaire with normal scores in both SLE groups is not sensitive to detect subtle, but clinically relevant dysfunctions.
The pattern seen in this study in both NPSLE and non-NPSLE very much resembled each other; the other two studies applying DTI and TBSS however found partially overlapping, partially different regions of diminished white matter integrity. All three studies could identify prefrontal areas and parts of the internal capsula, on the other hand none of the studies found FA decreases in the occipital lobe. Overall it appears that SLE is associated with a rather diffuse pattern of decreased FA. Against this background it will be of importance to further DTI studies to subclassify the study samples (i.e. NPSLE patients only with one symptom, e.g. psychosis, acute confusional state) in order to identify more symptom specific FA correlates.
There are a couple of limitations that need to be pointed out. Although great care was taken to come up with study samples, that did not differ in group size, age, and ethnicity, and despite SLE group performance in the MMSE within normal ranges, and despite no significant differences in white matter lesion burden, we cannot rule out that some of the differences seen in FA maps are associated with differences in behavioral or biological parameters that had not been assessed within the study. The characterization and work up of patients and HCs in future studies will need to include more parameters (e.g. neuropsychological performance, mood) to further assess the neurobiological correlates of FA alterations. Secondly small white matter lesions were found in 8 out of 18 HCs (44%). Small white matter lesions have been reported in up to 45% of healthy people (Fazekas et al., 1988; Schmidt et al., 1992), possibly associated with a higher risk of experiencing a stroke later in life. As such it might be argued that the control group was not ideal. However, HCs were healthy at the time point of scanning with no history/evidence of cardiovascular risk factors or specific white matter disease (e.g. leucodystrophies). Including only HCs, that had been determined post hoc to have absolutely no lesions, could have induced a bias, possibly going along with false positive FA differences between groups not related to the SLE status. Importantly white matter lesions and clusters of FA difference were not systematically found in the same regions, supporting the notion that small white matter lesions occur independently from SLE associated decreases in FA values.
5. Summary
Our data demonstrate changes in white matter integrity in terms of decreased FA values, predominantly in the prefrontal cortex suggesting cerebral involvement in NPSLE patients and to a lesser degree in non-NPSLE patients. These findings go beyond the unspecific white matter lesions reported to be present in up to 60% of these patients group. Whether this pattern of diminished FA is specific to SLE remains to be determined. Clinical and radiological work up of SLE patients should be extended to include both neuropsychological testing and DTI in both NPSLE and non-NPSLE patients, with the aim to specifically investigate neurocognitive dysfunction on the one hand and reductions in white matter integrity on the other hand. DTI with a scanning time between 8 and 12 min, together with advanced analysis methods, is generally suited to become part of the routine neuroradiological work up facilitating the investigation and assessment of white matter in SLE patients, possibly even providing at tool to support or discourage the diagnosis SLE and to monitor subtle changes in white matter integrity over time not assessable through conventional neuroimaging.
Acknowledgment
Tobias Schmidt-Wilcke was supported by a grant of the DFG (Deutsche Forschungsgemeinschaft, GZ: SchM 2665/1-1). Patricia Cagnoli was supported by MICHR Pilot Grant Program (UL1RR024986). Pia Sundgren has been supported by a Department of Radiology Seed Grant (U008039), by MICHR Pilot Grant (UL1TR000433) and by Alfred Österlund research grant (20111105/1), Sweden, and by the Scania University Hospital Foundation (#92504), Sweden.
Appendix A. Supplementary material
Supplementary material.
References
- American College of Rheumatology The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis and Rheumatism. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. 10211873 [DOI] [PubMed] [Google Scholar]
- Appenzeller S., Bonilha L., Rio P.A., Min Li L., Costallat L.T., Cendes F. Longitudinal analysis of gray and white matter loss in patients with systemic lupus erythematosus. NeuroImage. 2007;34(2):694–701. doi: 10.1016/j.neuroimage.2006.09.029. 17112740 [DOI] [PubMed] [Google Scholar]
- Appenzeller S., Li L.M., Costallat L.T., Cendes F. Evidence of reversible axonal dysfunction in systemic lupus erythematosus: a proton MRS study. Brain: A Journal of Neurology. 2005;128(12):2933–2940. doi: 10.1093/brain/awh646. 16195241 [DOI] [PubMed] [Google Scholar]
- Appenzeller S., Li L.M., Costallat L.T., Cendes F. Neurometabolic changes in normal white matter may predict appearance of hyperintense lesions in systemic lupus erythematosus. Lupus. 2007;16(12):963–971. doi: 10.1177/0961203307084723. 18042590 [DOI] [PubMed] [Google Scholar]
- Appenzeller S., Pike G.B., Clarke A.E. Magnetic resonance imaging in the evaluation of central nervous system manifestations in systemic lupus erythematosus. Clinical Reviews in Allergy & Immunology. 2008;34(3):361–366. doi: 10.1007/s12016-007-8060-z. 18084729 [DOI] [PubMed] [Google Scholar]
- Appenzeller S., Vasconcelos Faria A., Li L.M., Costallat L.T., Cendes F. Quantitative magnetic resonance imaging analyses and clinical significance of hyperintense white matter lesions in systemic lupus erythematosus patients. Annals of Neurology. 2008;64(6):635–643. doi: 10.1002/ana.21483. 19107986 [DOI] [PubMed] [Google Scholar]
- Bernatsky S., Joseph L., Pineau C.A., Tamblyn R., Feldman D.E., Clarke A.E. A population-based assessment of systemic lupus erythematosus incidence and prevalence — results and implications of using administrative data for epidemiological studies. Rheumatology. 2007;46(12):1814–1818. doi: 10.1093/rheumatology/kem233. [DOI] [PubMed] [Google Scholar]
- Bombardier C., Gladman D.D., Urowitz M.B., Caron D., Chang C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis and Rheumatism. 1992;35(6):630–640. doi: 10.1002/art.1780350606. 1599520 [DOI] [PubMed] [Google Scholar]
- Bosma G.P., Rood M.J., Zwinderman A.H., Huizinga T.W., van Buchem M.A. Evidence of central nervous system damage in patients with neuropsychiatric systemic lupus erythematosus, demonstrated by magnetization transfer imaging. Arthritis Rheum. 2000;43(1):48–54. doi: 10.1002/1529-0131(200001)43:1<48::AID-ANR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Brey R.L., Holliday S.L., Saklad A.R. Neuropsychiatric syndromes in lupus: Prevalence using standardized definitions. Neurology. 2002;58(8):1214–1220. doi: 10.1212/wnl.58.8.1214. 11971089 [DOI] [PubMed] [Google Scholar]
- Cagnoli P., Harris R.E., Frechtling D. Reduced insular glutamine and N-acetylaspartate in systemic lupus erythematosus: a single-voxel (1)H-MR spectroscopy study. Academic Radiology. 2013;20(10):1286–1296. doi: 10.1016/j.acra.2013.07.011. 24029061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnoli P.C., Sundgren P.C., Kairys A. Changes in regional brain morphology in neuropsychiatric systemic lupus erythematosus. Journal of Rheumatology. 2012;39(5):959–967. doi: 10.3899/jrheum.110833. 22467931 [DOI] [PubMed] [Google Scholar]
- Cotton F., Bouffard-Vercelli J., Hermier M. [MRI of central nervous system in a series of 58 systemic lupus erythematosus (SLE) patients with or without overt neuropsychiatric manifestations] La Revue de Médecine Interne / Fondée ... Par la Société Nationale Francaise de Médecine Interne. 2004;25(1):8–15. doi: 10.1016/s0248-8663(03)00265-0. 14736556 [DOI] [PubMed] [Google Scholar]
- DeGiorgio L.A., Konstantinov K.N., Lee S.C., Hardin J.A., Volpe B.T., Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nature Medicine. 2001;7(11):1189–1193. doi: 10.1038/nm1101-1189. 11689882 [DOI] [PubMed] [Google Scholar]
- Emmer B.J., Veer I.M., Steup-Beekman G.M., Huizinga T.W., van der Grond J., van Buchem M.A. Tract-based spatial statistics on diffusion tensor imaging in systemic lupus erythematosus reveals localized involvement of white matter tracts. Arthritis and Rheumatism. 2010;62(12):3716–3721. doi: 10.1002/art.27717. 20722009 [DOI] [PubMed] [Google Scholar]
- Fazekas F., Niederkorn K., Schmidt R. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke; a Journal of Cerebral Circulation. 1988;19(10):1285–1288. doi: 10.1161/01.str.19.10.1285. 3051534 [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. 1202204 [DOI] [PubMed] [Google Scholar]
- Gladman D., Ginzler E., Goldsmith C. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis and Rheumatism. 1996;39(3):363–369. doi: 10.1002/art.1780390303. 8607884 [DOI] [PubMed] [Google Scholar]
- Huerta P.T., Kowal C., DeGiorgio L.A., Volpe B.T., Diamond B. Immunity and behavior: antibodies alter emotion. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(3):678–683. doi: 10.1073/pnas.0510055103. 16407105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M., Sundgren P.C., Fan X. Diffusion tensor imaging in patients with acute onset of neuropsychiatric systemic lupus erythematosus: a prospective study of apparent diffusion coefficient, fractional anisotropy values, and eigenvalues in different regions of the brain. Acta Radiologica (Stockholm, Sweden: 1987) 2007;48(2):213–222. doi: 10.1080/02841850601105825. 17354144 [DOI] [PubMed] [Google Scholar]
- Jung R.E., Caprihan A., Chavez R.S. Diffusion tensor imaging in neuropsychiatric systemic lupus erythematosus. BMC Neurology. 2010;10:65. doi: 10.1186/1471-2377-10-65. 20667115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung R.E., Chavez R.S., Flores R.A., Qualls C., Sibbitt W.L., Jr., Roldan C.A. White matter correlates of neuropsychological dysfunction in systemic lupus erythematosus. PloS One. 2012;7(1):e28373. doi: 10.1371/journal.pone.0028373. 22291880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung R.E., Segall J.M., Grazioplene R.G., Qualls C., Sibbitt W.L., Roldan C.A. Cortical thickness and subcortical gray matter reductions in neuropsychiatric systemic lupus erythematosus. PloS One. 2010;5(3):e9302. doi: 10.1371/journal.pone.0009302. 20352085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyendijk J., Steens S.C., Ouwendijk W.J. Neuropsychiatric systemic lupus erythematosus: lessons learned from magnetic resonance imaging. Arthritis and Rheumatism. 2011;63(3):722–732. doi: 10.1002/art.30157. 21360502 [DOI] [PubMed] [Google Scholar]
- Matus S., Burgos P.V., Bravo-Zehnder M. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. Journal of Experimental Medicine. 2007;204(13):3221–3234. doi: 10.1084/jem.20071285. 18056288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastero R., Bettini P., Del Zotto E. Prevalence and pattern of cognitive impairment in systemic lupus erythematosus patients with and without overt neuropsychiatric manifestations. Journal of the Neurological Sciences. 2001;184(1):33–39. doi: 10.1016/s0022-510x(00)00492-5. 11231030 [DOI] [PubMed] [Google Scholar]
- Oda K., Matsushima E., Okubo Y. Abnormal regional cerebral blood flow in systemic lupus erythematosus patients with psychiatric symptoms. Journal of Clinical Psychiatry. 2005;66(7):907–913. doi: 10.4088/jcp.v66n0714. 16013907 [DOI] [PubMed] [Google Scholar]
- Otte A., Weiner S.M., Peter H.H. Brain glucose utilization in systemic lupus erythematosus with neuropsychiatric symptoms: a controlled positron emission tomography study. European Journal of Nuclear Medicine. 1997;24(7):787–791. doi: 10.1007/BF00879668. 9211766 [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Smith S.M., Nichols T.E. Adjusting the effect of nonstationarity in cluster-based and TFCE inference. NeuroImage. 2011;54(3):2006–2019. doi: 10.1016/j.neuroimage.2010.09.088. 20955803 [DOI] [PubMed] [Google Scholar]
- Schmidt R., Fazekas F., Kleinert G. Magnetic resonance imaging signal hyperintensities in the deep and subcortical white matter. A comparative study between stroke patients and normal volunteers. Archives of Neurology. 1992;49(8):825–827. doi: 10.1001/archneur.1992.00530320049011. 1524515 [DOI] [PubMed] [Google Scholar]
- Shen Y.Y., Kao C.H., Ho Y.J., Lee J.K. Regional cerebral blood flow in patients with systemic lupus erythematosus. Journal of Neuroimaging: Official Journal of the American Society of Neuroimaging. 1999;9(3):160–164. doi: 10.1111/jon199993160. 10436758 [DOI] [PubMed] [Google Scholar]
- Sibley J.T., Olszynski W.P., Decoteau W.E., Sundaram M.B. The incidence and prognosis of central nervous system disease in systemic lupus erythematosus. Journal of Rheumatology. 1992;19(1):47–52. 1556699 [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. 18501637 [DOI] [PubMed] [Google Scholar]
- Somers E.C., Marder W., Cagnoli P. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis & Rheumatology (Hoboken, N.J.) 2014;66(2):369–378. doi: 10.1002/art.38238. 24504809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steup-Beekman G.M., Zirkzee E.J., Cohen D. Neuropsychiatric manifestations in patients with systemic lupus erythematosus: epidemiology and radiology pointing to an immune-mediated cause. Annals of the Rheumatic Diseases. 2013;72(Suppl 2):ii76–ii79. doi: 10.1136/annrheumdis-2012-202369. 23253914 [DOI] [PubMed] [Google Scholar]
- Sundgren P.C., Jennings J., Attwood J.T. MRI and 2D-CSI MR spectroscopy of the brain in the evaluation of patients with acute onset of neuropsychiatric systemic lupus erythematosus. Neuroradiology. 2005;47(8):576–585. doi: 10.1007/s00234-005-1371-y. 16007461 [DOI] [PubMed] [Google Scholar]
- Tan E.M., Cohen A.S., Fries J.F. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. 7138600 [DOI] [PubMed] [Google Scholar]
- Wang P.I., Cagnoli P.C., McCune W.J. Perfusion-weighted MR imaging in cerebral lupus erythematosus. Academic Radiology. 2012;19(8):965–970. doi: 10.1016/j.acra.2012.03.023. 22608862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R.C., Rahbar H., Foerster B., Thurnher M., Sundgren P.C. Brain diffusivity in patients with neuropsychiatric systemic lupus erythematosus with new acute neurological symptoms. Journal of Magnetic Resonance Imaging: JMRI. 2007;26(3):541–551. doi: 10.1002/jmri.21036. 17729344 [DOI] [PubMed] [Google Scholar]
- Zivadinov R., Shucard J.L., Hussein S. Multimodal imaging in systemic lupus erythematosus patients with diffuse neuropsychiatric involvement. Lupus. 2013;22(7):675–683. doi: 10.1177/0961203313486193. 23640981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


