Abstract
We coupled capillary zone electrophoresis (CZE) with an ultrasensitive electrokinetically pumped nanospray ionization source for tandem mass spectrometry (MS/MS) analysis of complex proteomes. We first used the system for the parallel reaction monitoring (PRM) analysis of angiotensin II spiked in 0.45 mg/mL of bovine serum albumin (BSA) digest. A calibration curve was generated between the loading amount of angiotensin II and intensity of angiotensin II fragment ions. CZE-PRM generated a linear calibration curve across over 4.5 orders of magnitude dynamic range corresponding to angiotensin II loading amount from 2 amole to 150 fmole. The relative standard deviations (RSDs) of migration time were <4% and the RSDs of fragment ion intensity were ~20% or less except 150 fmole angiotensin II loading amount data (~36% RSD). We further applied the system for the first bottom up proteomic analysis of a human cell line using CZE-MS/MS. We generated 283 protein identifications from a 1 hour long, single-shot CZE MS/MS analysis of the MCF7 breast cancer cell line digest, corresponding to ~80 ng loading amount. The MCF7 digest was fractionated using a C18 solid phase extraction column; single-shot analysis of a single fraction resulted in 468 protein identifications, which is by far the largest number of protein identifications reported for a mammalian proteomic sample using CZE.
Keywords: CZE-ESI-MS/MS, CZE-PRM, ultrasensitive electrokinetically pumped sheath flow nanospray interface, shotgun proteomics, human cell lysate digest
1. Introduction
Capillary electrophoresis (CE)-electrospray ionization (ESI)-mass spectrometry (MS) has been used to characterize a wide range of analytes, including intact proteins, peptides, metabolites, etc. [1-4]. CE-ESI-MS applications have benefited from recent improvements in the electrospray interface [5]. As a result of these improvements, CEMS has attracted renewed interest as a tool for proteomics research [6-8].
There are several important recent developments in CE-MS interface designs. Moini reported a sheathless capillary electrophoresis - electrospray interface in 2007 [9], which employed a porous capillary tip as the nanospray emitter. The sheathless interface system was been used for analysis of peptides [10, 11], Arg-C-digested histones, a Pyrococcus furiosus tryptic digest [12-14], and intact proteins analysis [15, 16].
A second CE-nanospray interface was reported by our group in 2010 [17]. This sheath-flow interface employs a glass emitter with a ~5-μm orifice. Electro-osmosis at the glass surface drives the sheath fluid at very low rates. The interface has several advantages, including minimal sample dilution due to the very low sheath flow rate, elimination of mechanical pumps and nebulizing gas, use of a wide range of separation buffers, and stable operation in the nanospray regime. We have applied the electrokinetically pumped sheath flow nanospray interface CE-MS/MS system for shotgun proteomic analysis of the secretome of Mycobacterium marinum [18], a fraction of yeast lysate [19], the E.coli proteome [20-22], picogram amounts of RAW 264.7 cell lysate [23-25], and the PC12 cell lysate [26]. In addition, the system was also applied for top-down intact protein characterization [27], quantitative multiple reaction monitoring (MRM) of peptide abundance [28], and phosphopeptides characterization [29].
Very recently, we reported a simple modification to our interface that resulted in ultrasensitive performance. We etched a few millimeters of the outside of the separation capillary tip with hydrofluoric acid to reduce its outer diameter from ~150 μm to ~60 μm. This step allows the capillary tip to be placed within 200 μm of the emitter orifice, which results in a significant improvement in the system's sensitivity [30]. By employing 10 μm i.d. separation capillary, a Q-Exactive mass spectrometer, and the improved CE-MS interface, we obtained 1 zmole (1 zmol = 10−21 mol = 600 molecule) peptide detection limit (S/N = 3). Over 100 proteins were identified based on tandem mass spectra from 16 pg of E.coli digest and 154 peptides from 60 proteins were identified from 400 fg sample loading.
Besides the interfaces mentioned above, the flow-through microvial interface developed by Chen group is also widely used for metabolomic [31], glycan [32], and intact protein [33] analysis. In addition, Tang's group recently developed a novel sheathless CE-MS interface combining a large i.d. separation capillary and a detachable small i.d. porous ESI emitter [34]. This design combines a large sample loading volume with stable nanoESI operation, and the design has produced a 10 pM limit of quantification for standard peptides spiked in a BSA tryptic digest background.
CE-MS/MS has a brief history for analysis of complex protein digests. Yates’ group developed a solid-phase microextraction technique to prefractionate a yeast ribosome digest followed by CE-MS/MS analysis in 1999; 66 proteins were identified using an ion trap mass spectrometer from the eight fractions [35]. Recently, Yates’ group reported an improved on-line microextraction fractionation, transient isotachophoresis capillary electrophoresis−MS/MS system. They employed an etched porous capillary as the ESI sprayer to analyze a tryptic digest of Pyrococcus furiosus; 548 protein IDs were obtained in duplicate separations of eight factions [13]. Lindner's group employed a capillary zone electrophoresis (CZE)-MS/MS system with the porous capillary tip based sheathless interface for analysis of a rat testis linker histone protein sample digested by endoproteinase Arg-C, and eight non-histone H1 proteins were identified [12].
Our group used the electrokinetically pumped sheath flow interface for the capillary zone electrophoresis analysis of the secretome of M. marinum in 2012; 140 protein groups were identified [18]. We improved the peptide separation by using linear polyacrylamide coated capillary and stacking injection; ~ 300 protein groups were identified from 100 ng of E.coli digests by single shot analysis in less than 1 h [20]; the number of protein IDs was increased to 871 by analyzing seven E.coli digest fractions from offline C18-SPE fractionation [21]. We also employed a capillary isoelectric focusing MS/MS system with the electrokinetically pumped sheath flow interface for eight-plex iTRAQ based quantitative proteomic analysis of differentiating PC12 cells; 835 protein groups were identified [26]. To our knowledge, there are no publications employing CZE-MS/MS for analysis of a human cell line.
For target proteomics research, multiple/selected reaction monitoring (MRM/SRM) is typically employed with triple-quadrupole (QqQ) mass spectrometer [36, 37]. Briefly, the parent ion of a targeted peptide is isolated in the first quadrupole (Q1) and then fragmented in the second quadrupole (Q2). One or several fragment ions from the targeted peptide are further isolated by the third quadrupole (Q3) for detection [38, 39]. Recently, Coon's group introduced a new target proteomics technique, named parallel reaction monitoring (PRM), which was performed with a bench-top quadrupole-Orbitrap mass spectrometer [40]. For PRM, a target peptide was selected in the quadrupole and then fragmented in the collisional cell. The resulting fragment ions were analyzed in the Orbitrap to generate one full, high-resolution MS/MS spectrum. Because m/z ratios of fragment ions are not required during the method development step, the process is much easier than SRM/MRM. In addition, PRM has much better tolerance to the background matrix than SRM/MRM due to the high resolution of the Orbitrap analyzer [40].
In this work, we present the first example of CZE-PRM. We employ our improved electrokinetically pumped sheath flow nanospray interface [30] for peptide analysis. A standard peptide, angiotensin II, was spiked in a 0.45 mg/mL bovine serum albumin digest to evaluate the CZE-PRM system performance. We observed over four and a half orders of magnitude linear dynamic range for angiotensin II corresponding to loading amounts from 2 to 150,000 amole. We also presented the first example of CZEMS/MS for bottom-up analysis of a human cell line; nearly 300 proteins were identified from MCF7 whole cell lysate digest in a 1-hour single-shot CZE-MS/MS analysis with ~80 ng loading amount.
2. Experimental
2.1. Materials and reagents
Bovine pancreas TPCK-treated trypsin, bovine serum albumin (BSA), urea, ammonium bicarbonate (NH4HCO3), dithiothreitol (DTT), iodoacetamide (IAA), and angiotensin II (human, Asp-Arg-Val-Tyr-Ile-His-Pro-Phe) were purchased from Sigma−Aldrich (St. Louis, MO). Acetonitrile (ACN), formic acid (FA), and hydrofluoric acid (HF) were purchased from Fisher Scientific (Pittsburgh, PA). Methanol and water were purchased from Honeywell Burdick & Jackson (Wicklow, Ireland). Fused silica capillary (10 and 20 μm i.d./150μm o.d.) and linear polyacrylamide (LPA) coated capillary (50 μm i.d./150μm o.d.) were purchased from Polymicro Technologies (Phoenix, AZ).
Eagle's minimal essential medium (EMEM), fetal bovine serum (FBS), GlutaMAX™ (100×), insulin, and Antibiotic-Antimycotic (Anti-Anti, 100×) were purchased from Life Technologies Corporation (Grand Island, NY). Mammalian Cell-PE LB™ buffer for cell lysis was purchased from G-Biosciences (St. Louis, MO). Complete, mini protease inhibitor cocktail (provided in EASYpacks) was purchased from Roche (Indianapolis, IN).
2.2. Sample preparation
Bovine serum albumin (BSA, 0.5 mg/mL) in 100 mM NH4HCO3 (pH 8.0) containing 8 M urea was denatured at 37 °C for 30 min, followed by standard reduction and alkylation with DTT and IAA. After dilution with 100 mM NH4HCO3 (pH 8.0) to reduce the urea concentration below 2 M, protein digestion was performed for 12 h at 37 °C with trypsin at a trypsin/protein ratio of 1/30 (w/w). After acidification, the protein digest was desalted with a C18-SepPak column (Waters, Milford, MA), followed by lyophilization with a vacuum concentrator (Thermo Fisher Scientific, Marietta, OH). The dried sample was dissolved in 0.05% (v/v) FA to produce a 0.5 mg/mL solution and stored at −20 °C before use.
Angiotensin II solution was spiked into the BSA digest to generate five samples in 0.05% (v/v) FA containing 0.45 mg/mL BSA digest and different concentrations of angiotensin II (10 nM, 100 nM, 1 μM, 10 μM and 100 μM). Each sample was analyzed by CZE-PRM in triplicate.
The procedures for MCF7 cell culture, cell lysis, protein acetone precipitation, denaturation, reduction and alkylation were described before [41]. Briefly, after cell culture, MCF7 cells were lysed by sonication, followed by BCA protein concentration measurement, and acetone precipitation. Then, a 690 μg protein aliquot was dissolved in 8 M urea and 100 mM NH4HCO3 (pH ~8.0), denatured at 37 °C for 1 h, and reduced in ~40 mM DTT at 37 °C for 1.5 h, followed by alkylation with 100 mM IAA at room temperature for 30 min. After dilution with 100 mM NH4HCO3 (pH ~8.0) to reduce the urea concentration below 2 M, the proteins were digested by trypsin at a trypsin/protein ratio of 1/30 (w/w) overnight at 37 °C. The protein digest was acidified with FA (1% final concentration), and desalted with C18-SepPak column (Waters, Milford, MA), followed by lyophilization. The peptide mixture was dissolved in 0.04% (v/v) FA containing 20% (v/v) ACN to get a 3.5 mg/mL sample, followed by CZE-ESI-MS and MS/MS analysis. Around 300 μg of digest was lyophilized again, and redissolved in 0.1% (v/v) FA. The digest was loaded onto a C18-SepPak SPE column (Waters), and eluted by 1 mL of 12% (v/v) ACN containing 0.1% (v/v) FA. The eluate was lyophilized and further dissolved in 28 μL of 0.04% (v/v) FA and 30% (v/v) ACN for CZE-ESI-MS/MS analysis.
2.3. CZE-ESI-MS/MS analysis
The capillary electrophoresis system was assembled from components reported previously [17, 30]. Two Spellman CZE 1000R high-voltage power supplies provided voltages for separation and electrospray. An ultrasensitive electrokinetically pumped sheath flow interface [30] was used to couple CZE to a Q-Exactive mass spectrometer (Thermo Fisher Scientific). The electrospray emitter was a borosilicate glass capillary (1.0 mm o.d., 0.75 mm i.d.) pulled with a Sutter instrument P-1000 flaming/brown micropipet puller, and the size of the emitter orifice was 8-10 μm. Voltage programming was controlled by LabView software.
For the CZE-PRM experiment, a 32 cm long capillary (10 and 20 μm i.d./150 μm o.d.) with etched tip (~ 60 μm o.d., ~ 5 mm length) was used for the separation. The separation buffer was 0.5%(v/v) FA. The voltage at the injection end was 16.2 kV, and 1.2 kV was applied as spray voltage. For whole MCF7 cell lysate digest analysis, a 100 cm long capillary (20 μm i.d./150 μm o.d.) with ~ 5 mm length etched end (~ 60 μm o.d.) was employed, and the separation buffer was 0.5%(v/v) FA. For the MS1 only experiment, 29.2 kV was applied at the injection end; for MS/MS experiment in data dependent acquisition (DDA) mode, 21.2 kV was applied at the injection end. In both cases, 1.2 kV was applied for electrospray. For analysis of the 12%(v/v) ACN eluate of MCF7 cell lysate digest from C18-SPE column, an LPA-coated capillary (62 cm, 50 μm i.d./150 μm o.d.) with ~ 5 mm long etched end (~ 70 μm o.d.) was used, and the separation buffer was 0.12% (v/v) FA. The voltage applied at injection end was 16.2 kV and 1.2 kV was applied as spray voltage. For all the experiments, the sheath buffer was 10% (v/v) methanol and 0.1% (v/v) FA. The distance between the separation capillary end and spray emitter tip was around 200 μm. The sample was injected into the separation capillary by nitrogen pressure, and the injection volume was calculated based on Poiseuille's law. All the HF etching operations for separation capillaries were performed with the same protocol as reference [30]. The etched 10 and 20 μm i.d. separation capillary was successively flushed with sodium hydroxide (1 M), deionized water, hydrochloric acid (1 M), deionized water, and 0.5% (v/v) FA before use. The etched LPA-coated separation capillary (50 μm i.d.) was successively flushed with deionized water and 0.12% (v/v) FA before use.
For CZE-PRM experiment, the Q-Exactive was programmed in target-MS2 mode with inclusion list as on. In the inclusion list, 523.7734 and +2 were set as the target m/z and charge, respectively. The normalized collisional energy (NCE) was 25%. The other parameters were as follows: resolution for MS/MS as 17,500, AGC target as 5E5, maximum injection time as 500 ms, isolation window as 1 m/z, and microscans as 1. For analysis of the human cell line digest, both MS1-only and regular DDA mode experiments were performed. For the MS1-only experiment, MS spectra were acquired with 380-1800 m/z range, 70,000 resolution (at m/z 200), AGC target as 1E6, maximum injection time as 250 ms, and microscans as 1. For the DDA mode experiment, a top 12 method was employed. For MS1 full scan, the parameters were the same as the MS1-only experiment. For MS/MS, twelve most intense peaks from the MS spectrum were sequentially isolated in the quadrupole (isolation window as 2.0 m/z) and further fragmented in the higher energy collisional dissociation (HCD) cell (NCE as 28%), followed by Orbitrap analysis. The resolution as 35 000 (at m/z 200), AGC target as 1E6, maximum injection time as 120 ms and microscans as 1 were applied. The parent ions with charge states higher than +1 and intensity higher than 8.3E3 were chosen for fragmentation. Dynamic exclusion was set as 10 s for 20 μm i.d. capillary and 15 s for 50 μm i.d. capillary. Peptide match and exclude isotopes were turned on.
2.4. Data analysis
For CZE-PRM experiment, the data were manually analyzed with Thermo Xcalibur software. The peaks of fragment ions from angiotensin II were manually extracted with 10 ppm mass tolerance and Gaussian smoothing (5 points) was performed on the peaks.
For human cell lysate digest data, the raw files containing tandem spectra were analyzed with Proteome Discoverer 1.3 (Thermo Fisher Scientific). Mascot 2.2 was used for database searching against Swiss-Prot database with taxonomy as human (20,335 sequences). Trypsin was chosen as the digestion enzyme, and the maximum number of missed cleavages was set as 2. The mass tolerances for parent ions and fragment ions were set as 10 ppm and 0.05 Da, respectively. Dynamic modifications included oxidation (M), deamidation (NQ), and acetylation (K and protein N-terminus). Carbamidomethylation (C) was set as the fixed modification. Database searching against the corresponding reversed database was also performed in order to evaluate the false discovery rate (FDR) [42, 43].
Percolator software (version 1.17) integrated in the Proteome Discoverer 1.3 was used to evaluate the database searching results. For peptide level analysis, peptide confidence value as high was used to filter the peptide identification, corresponding to peptide-level FDR less than 1%. For peptides per protein settings, the following parameters were applied, including minimal number of peptides as 1, count only rank 1 peptides, and count peptide only in top scored proteins. In addition, protein grouping was enabled. The identified protein information is listed in supporting material I.
3. Results and discussions
3.1 CZE-PRM for peptide detection
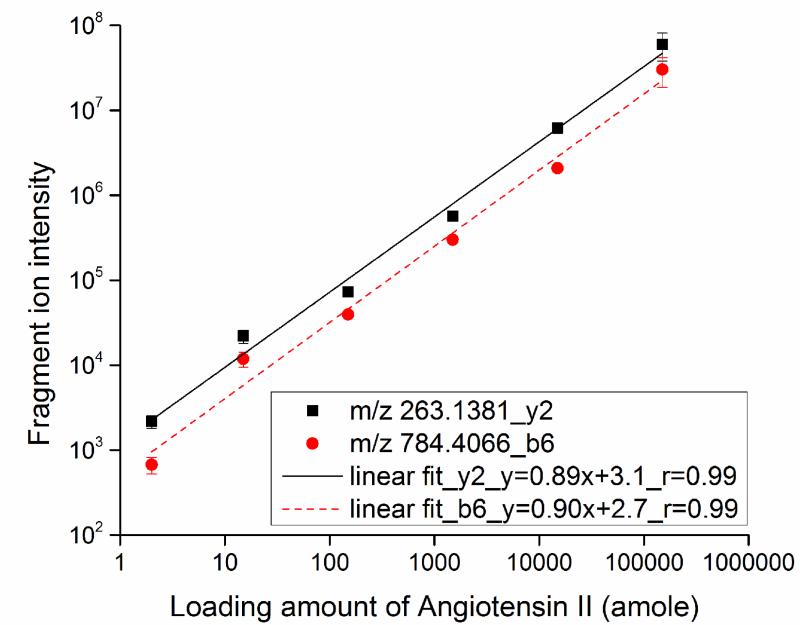
For CZE-PRM experiments, the Q-Exactive was programmed in target-MS2 mode. In the inclusion list, m/z 523.7734 (+2) corresponding to angiotensin II (human, Asp-Arg-Val-Tyr-Ile-His-Pro-Phe) was used. The angiotensin II standard peptide was spiked into 0.45 mg/mL BSA digest to generate a series of samples containing 10 nM, 100 nM, 1 μM, 10 μM, and 100 μM of angiotensin II, corresponding to 15 amole to 150 fmole of angiotensin II loaded onto the separation capillary (20 μm i.d.) in the presence of a constant background of BSA digest. We also employed a 10 μm i.d. separation capillary to analyze a sample made from 10 nM angiotensin II spiked in 0.45 mg/mL BSA digest; in this experiment, about 2 amole of angiotensin II was loaded onto the capillary. After triplicate CZE-PRM analyses, the two most intense fragment ions (b6+ and y2+) of angiotensin II were extracted from the acquired data with 10 ppm mass tolerance to construct a calibration curve, Fig. 1. We performed an unweighted weighting linear fit to the log-log data. The fragment ion signal increased linearly with angiotensin loading amount over 4.5 orders of magnitude (log-log b6+ slope = 0.90, r = 0.99; y2+ slope = 0.89, r = 0.99). We note that the two fragment ions of angiotensin II co-migrate in each CZE-PRM run, which confirms the detection of the standard peptide.
Fig. 1.
Wide dynamic range calibration curve for loading amounts and fragment ions (y2+ and b6+) intensity of angiotensin II after CZE-PRM analysis. The lines are the results of unweighted linear fit to the log-log data.
Use of 2 amole of angiotensin II in the presence of the 0.45 mg/mL (~7 μM) BSA digest background generated ~2.0E+03 signal for the most abundant fragment ion (y2+) of angiotensin II. This signal is ~20 times higher than that from our previous work that also injected ~2 amole of Leu-enkephalin, in the presence of 66 nM of BSA digest as background and using CZE-MRM analysis [28]. The dramatic improvement in signal amplitude has several causes. First, our first-generation electrokinetically pumped sheath flow interface [17] was employed in the earlier work [28], whereas the improved version of the interface [30] was used in this work. Because the distance between separation capillary tip and spray emitter tip in the improved interface [30] is five-times shorter than that in original version [17], diffusion of peptides in the spray emitter is decreased, peptide peaks are much sharper, and peptide signals are correspondingly more intense. Second, we used a separation capillary with much smaller inner diameter (10-20 μm vs. 50 μm) and separation buffer with much lower pH value (lower than 3 vs. 6-8), which reduces the flow rate in the separation capillary, generating better sensitivity. Third, online stacking was used in this work, which again sharpens peaks. The conductivity of sample matrix (0.05% (v/v) FA) is much lower than the separation buffer (0.5% FA), so that peptides are concentrated when high voltage is applied across the capillary. In addition, a much higher resolution mass analyzer (Orbitrap vs. quadrupole) was employed in this work, which generates vastly superior tolerance to the background matrix.
Recently, Marginean et al. employed narrow-bore chemically etched emitters for ESI at pL/min level flow rate, which significantly improved the ion utilization efficiency [44]. Cox et al. developed an electrospray emitter array based on chemically etched emitters with individualized sheath gas capillaries to improve the ESI efficiency at subambient pressures, and over an order of magnitude MS sensitivity improvement was obtained compared with standard atmospheric pressure single ESI emitter/heated capillary interface [45]. It will be valuable to couple the CZE separation with these techniques for analysis of trace amounts of biological samples, i.e. single cells.
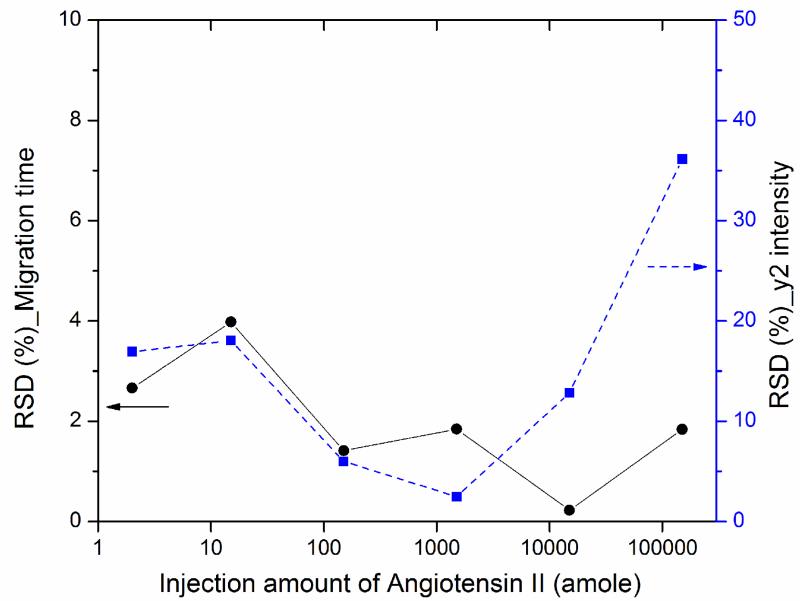
We also evaluated the reproducibility of the CZE-PRM system in terms of migration time and intensity of angiotensin II fragment ion (y2+), Fig. 2. The relative standard deviations (RSDs) of migration time for triplicate analysis were less than 4%. The RSDs of the fragment ion intensity for triplicate analysis were ~36% for 150 fmole loading amounts, around 10% or less for 150 amole to 15 fmole loading amounts and close to 20% for 2 and 15 amole loading amounts. The slightly higher RSDs for the 2 and 15 amole loading amount data are likely due to two reasons. First, the intensity of co-isolated BSA peptides will be much higher than angiotensin II, leading to higher detection variation for very low loading amounts. Second, the very sharp peaks of the fragment ions (full width at half height for the peaks are ≤2 s) for the 2 and 15 amole loading amount data (S-Fig.1 in supporting material II) leads to fewer data points across the peaks than the higher loading amounts data. For the 150-fmole loading amount data, the RSD is significant higher because the CE-MS system is slightly overloaded, which produces non-Gaussian peak shapes. In addition, the RSDs data of migration time and fragment ion intensity for angiotensin II fragment ion b6+ agree well with that for y2+.
Fig. 2.
Relative standard deviations (RSDs) of migration time and intensity of angiotensin II fragment ion (y2+) for different loading amounts (2 amole-150 fmole) from triplicate CZE-PRM analysis.
3.2 CZE-ESI-MS and MS/MS for human cell lysate digest analysis
Our initial report using the etched-tip electrokinetically pumped sheath flow nanospray interface based CZE-MS/MS system analyzed high-femtogram to midpicogram amounts of E. coli cell lysate digest; that study demonstrated that the improved system was highly efficient and quantitatively reproducible for separation and detection of a prokaryote proteome digest [30]. There have been a handful of other reports that use CZE-MS/MS for analysis of prokaryote proteomes. We have studied both the M. marinum secretome and the E. coli proteome [18, 20, 21]. The Yates group has reported study of the P. furiosus proteome [13]. To date, there has been no published bottom-up analysis of a human proteome using CZE-MS/MS.
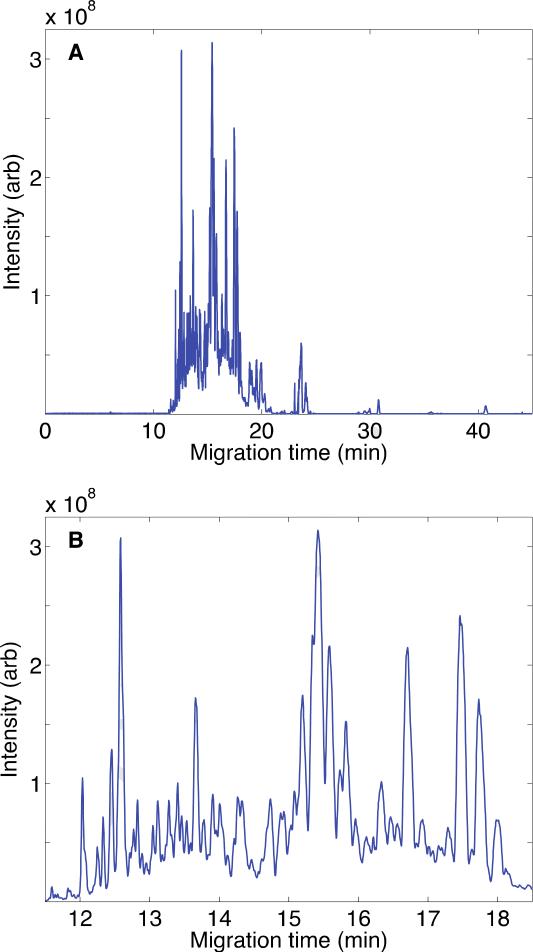
Here, we report the first application of CZE-MS/MS for the bottom-up analysis of a human proteome, the proteome of the MCF-7 human breast cancer cell line. We began by using the ultrasensitive interface for analysis of ~60 ng of a whole-cell digest, Fig. 3 and S-Fig. 2 in supporting material II. The separation was driven by a 280 V/cm electric field. Peptides began to migrate from the capillary at 11 min, and the separation was nearly complete by 26 min, Fig. 3A, although a few strong peaks were observed at later times, S-Fig. 2 in supporting material II. The separation is highly efficient and peaks are very sharp, Figure 3B. Roughly 10-20 peaks were resolved from the base peak electropherogram across the 1-min separation window from 13- to 14-min. We extracted one peptide (m/z 400.77161, z=+2) from the data with 2 ppm mass tolerance that generated 525,000 theoretical plates, S-Fig. 3 in supporting material II, which demonstrates the extraordinarily high separation efficient produced by this instrument.
Fig. 3.
Base peak electropherogram of MCF7 digest after CZE-ESI-MS analysis. (A) Entire separation; (B) Detail of the separation from 12 to 18 min. Data were treated with a Lowess filter with Gaussian kernel and span of 10 points. The sample loading amount was ~60 ng; the capillary was 20 μm i.d. and 100 cm long; the separation buffer was 0.5%(v/v) FA; and a field strength of 280 V/cm was used for the separation.
We next applied single-shot CZE-MS/MS for bottom-up analysis of the MCF7 whole cell lysate digest. Around 80 ng of peptides was loaded onto a 100-cm long, 20 μm i.d. separation capillary. The separation was performed at 200 V/cm electric field. After database searching of the tandem spectra, 1,159 peptides and 283 proteins were confidently identified, and 176 proteins were identified based on at least two peptides. The separation was complete in roughly 40 min.
To test how many peptides and proteins can be identified from the MCF7 proteome with single-shot CZE-MS/MS, we simplified the MCF7 cell lysate digest by fractionating the proteome. Peptides were first trapped on a C18 SPE column and then a fraction was eluted with 12% (v/v) ACN for analysis. An LPA-coated separation capillary was used to reduce electro-osmosis, which increases the separation capacity. To increase sample injection volume, we used a 50-μm ID capillary and injected about 130 nL of sample. After database searching, the single-shot analysis identified 1,199 peptides and 468 proteins. 219 proteins were identified based on at least two peptides. the separation was complete within 60 min. The combined datasets contain 2,005 peptide and 537 protein IDs.
Supplementary Material
Highlights.
Parallel reaction monitoring was used with capillary zone electrophoresis.
First use of CZE for bottom-up proteomic analysis of a human cell line.
468 proteins were identified in a single-shot analysis of the MCF-7 proteome.
Acknowledgements
We thank Dr. William Boggess in the Notre Dame Mass Spectrometry and Proteomics Facility for his help with this project. This project was supported by a grant from the National Institutes of Health (R01GM096767). Finally, we acknowledge Professor Peichang Lu for the inspiration for this work, and we congratulate him on his 90th birthday.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kraly J, Fazal MA, Schoenherr RM, Bonn R, Harwood MM, Turner E, Jones M, Dovichi NJ. Bioanalytical applications of capillary electrophoresis. Anal. Chem. 2006;78:4097–4110. doi: 10.1021/ac060704c. [DOI] [PubMed] [Google Scholar]
- 2.Haselberg R, de Jong GJ, Somsen GW. Capillary electrophoresis-mass spectrometry for the analysis of intact proteins 2007-2010. Electrophoresis. 2011;32:66–82. doi: 10.1002/elps.201000364. [DOI] [PubMed] [Google Scholar]
- 3.Good GM, et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol. Cell. Proteomics. 2010;9:2424–2437. doi: 10.1074/mcp.M110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramautar R, Somsen GW, de Jong GJ. CE-MS for metabolomics: developments and applications in the period 2010-2012. Electrophoresis. 2013;34:86–98. doi: 10.1002/elps.201200390. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell EJ, Chen DD. Twenty years of interface development for capillary electrophoresis-electrospray ionization-mass spectrometry. Anal. Chim. Acta. 2008;627:25–33. doi: 10.1016/j.aca.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Zhu G, Yan X, Champion MM, Dovichi NJ. Capillary zone electrophoresis for analysis of complex proteomes using an electrokinetically pumped sheath flow nanospray interface. Proteomics. 2014;14:622–628. doi: 10.1002/pmic.201300295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Zhu G, Yan X, Dovichi NJ. High sensitivity capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry for the rapid analysis of complex proteomes. Curr. Opin. Chem. Biol. 2013;17:795–800. doi: 10.1016/j.cbpa.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramautar R, Heemskerk AA, Hensbergen PJ, Deelder AM, Busnel JM, Mayboroda OA. CE-MS for proteomics: Advances in interface development and application. J. Proteomics. 2012;75:3814–3828. doi: 10.1016/j.jprot.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 9.Moini M. Simplifying CE-MS operation. 2. Interfacing low-flow separation techniques to mass spectrometry using a porous tip. Anal. Chem. 2007;79:4241–4246. doi: 10.1021/ac0704560. [DOI] [PubMed] [Google Scholar]
- 10.Busnel JM, Schoenmaker B, Ramautar R, Carrasco-Pancorbo A, Ratnayake C, Feitelson JS, Chapman JD, Deelder AM, Mayboroda OA. High capacity capillary electrophoresis-electrospray ionization mass spectrometry: coupling a porous sheathless interface with transient-isotachophoresis. Anal. Chem. 2010;82:9476–9483. doi: 10.1021/ac102159d. [DOI] [PubMed] [Google Scholar]
- 11.Medina-Casanellas S, Domínguez-Vega E, Benavente F, Sanz-Nebot V, Somsen GW, de Jong GJ. Low-picomolar analysis of peptides by on-line coupling of fritless solid-phase extraction to sheathless capillary electrophoresis-mass spectrometry. J. Chromatogr A. 2014;1328:1–6. doi: 10.1016/j.chroma.2013.12.080. [DOI] [PubMed] [Google Scholar]
- 12.Faserl K, Sarg B, Kremser L, Lindner H. Optimization and evaluation of a sheathless capillary electrophoresis-electrospray ionization mass spectrometry platform for peptide analysis: comparison to liquid chromatography-electrospray ionization mass spectrometry. Anal. Chem. 2011;83:7297–7305. doi: 10.1021/ac2010372. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Fonslow BR, Wong CCL, Nakorchevsky A, Yates JR., 3rd Improving the comprehensiveness and sensitivity of sheathless capillary electrophoresis-tandem mass spectrometry for proteomic analysis. Anal. Chem. 2012;84:8505–8513. doi: 10.1021/ac301091m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarg B, Faserl K, Kremser L, Halfinger B, Sebastiano R, Lindner HH. Comparing and combining capillary electrophoresis electrospray ionization mass spectrometry and nano-liquid chromatography electrospray ionization mass spectrometry for the characterization of post-translationally modified histones. Mol. Cell. Proteomics. 2013;12:2640–2656. doi: 10.1074/mcp.M112.024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haselberg R, de Jong GJ, Somsen GW. Low-flow sheathless capillary electrophoresis-mass spectrometry for sensitive glycoform profiling of intact pharmaceutical proteins. Anal. Chem. 2013;85:2289–2296. doi: 10.1021/ac303158f. [DOI] [PubMed] [Google Scholar]
- 16.Haselberg R, Ratnayake CK, de Jong GJ, Somsen GW. Performance of a sheathless porous tip sprayer for capillary electrophoresis-electrospray ionization-mass spectrometry of intact proteins. J. Chromatogr. A. 2010;1217:7605–7611. doi: 10.1016/j.chroma.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Wojcik R, Dada OO, Sadilek M, Dovichi NJ. Simplified capillary electrophoresis nanospray sheath-flow interface for high efficiency and sensitive peptide analysis. Rapid Commun. Mass Spectrom. 2010;24:2554–2560. doi: 10.1002/rcm.4672. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Champion MM, Sun L, Champion PA, Wojcik R, Dovichi NJ. Capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry as an alternative proteomics platform to ultraperformance liquid chromatography-electrospray ionization-tandem mass spectrometry for samples of intermediate complexity. Anal. Chem. 2012;84:1617–1622. doi: 10.1021/ac202899p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wojcik R, Li Y, Maccoss MJ, Dovichi NJ. Capillary electrophoresis with Orbitrap-Velos mass spectrometry detection. Talanta. 2012;88:324–329. doi: 10.1016/j.talanta.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu G, Sun L, Yan X, Dovichi NJ. Single-shot proteomics using capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry with production of more than 1250 Escherichia coli peptide identifications in a 50 min separation. Anal. Chem. 2013;85:2569–2573. doi: 10.1021/ac303750g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan X, Essaka DC, Sun L, Zhu G, Dovichi NJ. Bottom-up proteome analysis of E. coli using capillary zone electrophoresis-tandem mass spectrometry with an electrokinetic sheath-flow electrospray interface. Proteomics. 2013;13:2546–2551. doi: 10.1002/pmic.201300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu G, Sun L, Yan X, Dovichi NJ. Stable, reproducible, and automated capillary zone electrophoresis-tandem mass spectrometry system with an electrokinetically pumped sheath-flow nanospray interface. Anal. Chim. Acta. 2014;810:94–98. doi: 10.1016/j.aca.2013.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, Zhu G, Li Y, Wojcik R, Yang P, Dovichi NJ. CZE-ESI-MS/MS system for analysis of subnanogram amounts of tryptic digests of a cellular homogenate. Proteomics. 2012;12:3013–3019. doi: 10.1002/pmic.201200100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L, Zhu G, Dovichi NJ. Integrated capillary zone electrophoresis-electrospray ionization tandem mass spectrometry system with an immobilized trypsin microreactor for online digestion and analysis of picogram amounts of RAW 264.7 cell lysate. Anal. Chem. 2013;85:4187–4194. doi: 10.1021/ac400523x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, Li Y, Champion MM, Zhu G, Wojcik R, Dovichi NJ. Capillary zone electrophoresis-multiple reaction monitoring from 100 pg of RAW 264.7 cell lysate digest. Analyst. 2013;138:3181–3188. doi: 10.1039/c3an00287j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu G, Sun L, Keithley RB, Dovichi NJ. Capillary isoelectric focusing-tandem mass spectrometry and reversed-phase liquid chromatography-tandem mass spectrometry for quantitative proteomic analysis of differentiating PC12 cells by eight-plex isobaric tags for relative and absolute quantification. Anal. Chem. 2013;85:7221–7229. doi: 10.1021/ac4009868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L, Knierman MD, Zhu G, Dovichi NJ. Fast top-down intact protein characterization with capillary zone electrophoresis-electrospray ionization tandem mass spectrometry. Anal. Chem. 2013;85:5989–5995. doi: 10.1021/ac4008122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Wojcik R, Dovichi NJ, Champion MM. Quantitative multiple reaction monitoring of peptide abundance introduced via a capillary zone electrophoresis-electrospray interface. Anal. Chem. 2012;84:6116–6121. doi: 10.1021/ac300926h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mou S, Sun L, Dovichi NJ. Accurate determination of peptide phosphorylation stoichiometry via automated diagonal capillary electrophoresis coupled with mass spectrometry: proof of principle. Anal. Chem. 2013;85:10692–10696. doi: 10.1021/ac402858a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L, Zhu G, Zhao Y, Yan X, Mou S, Dovichi NJ. Ultrasensitive and fast bottom-up analysis of femtogram amounts of complex proteome digests. Angew. Chem. Int. Ed. Engl. 2013;52:13661–13664. doi: 10.1002/anie.201308139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindenburg PW, Ramautar R, Jayo RG, Chen DD, Hankemeier T. Capillary electrophoresis-mass spectrometry using a flow-through microvial interface for cationic metabolome analysis. Electrophoresis. 2014;35:1308–1314. doi: 10.1002/elps.201300357. [DOI] [PubMed] [Google Scholar]
- 32.Jayo RG, Thaysen-Andersen M, Lindenburg PW, Haselberg R, Hankemeier T, Ramautar R, Chen DD. Simple Capillary Electrophoresis-Mass Spectrometry Method for Complex Glycan Analysis Using a Flow-Through Microvial Interface. Anal. Chem. 2014 doi: 10.1021/ac5010212. DOI: 10.1021/ac5010212. [DOI] [PubMed] [Google Scholar]
- 33.Zhong X, Maxwell EJ, Ratnayake C, Mack S, Chen DD. Flow-through microvial facilitating interface of capillary isoelectric focusing and electrospray ionization mass spectrometry. Anal. Chem. 2011;83:8748–8755. doi: 10.1021/ac202130f. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Lee CS, Smith RD, Tang K. Capillary isotachophoresisnanoelectrospray ionization-selected reaction monitoring MS via a novel sheathless interface for high sensitivity sample quantification. Anal. Chem. 2013;85:7308–7315. doi: 10.1021/ac401202c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong W, Link A, Eng JK, Yates JR., 3rd Identification of proteins in complexes by solid-phase microextraction/multistep elution/capillary electrophoresis/tandem mass spectrometry. Anal. Chem. 1999;71:2270–2278. doi: 10.1021/ac9901182. [DOI] [PubMed] [Google Scholar]
- 36.Anderson L, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteomics. 2006;5:573–588. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Picotti P, Aebersold R. Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nat. Methods. 2012;9:555–566. doi: 10.1038/nmeth.2015. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn E, Wu J, Karl J, Liao H, Zolg W, Guild B. Quantification of C-reactive protein in the serum of patients with rheumatoid arthritis using multiple reaction monitoring mass spectrometry and 13C-labeled peptide standards. Proteomics. 2004;4:1175–1186. doi: 10.1002/pmic.200300670. [DOI] [PubMed] [Google Scholar]
- 39.Lange V, Picotti P, Domon B, Aebersold R. Selected reaction monitoring for quantitative proteomics: a tutorial. Mol. Syst. Biol. 2008;4:222. doi: 10.1038/msb.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson AC, Russell JD, Bailey DJ, Westphall MS, Coon JJ. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol. Cell. Proteomics. 2012;11:1475–1488. doi: 10.1074/mcp.O112.020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun L, Zhu G, Yan X, Mou S, Dovichi NJ. Uncovering immobilized trypsin digestion features from large-scale proteome data generated by high-resolution mass spectrometry. J. Chromatogr. A. 2014;1337:40–47. doi: 10.1016/j.chroma.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller A, Nesvizhskii AL, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 43.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 44.Marginean I, Tang K, Smith RD, Kelly RT. Picoelectrospray ionization mass spectrometry using narrow-bore chemically etched emitters. J. Am. Soc. Mass Spectrom. 2014;25:30–36. doi: 10.1007/s13361-013-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox JT, Marginean I, Kelly RT, Smith RD, Tang K. Improving the Sensitivity of Mass Spectrometry by Using a New Sheath Flow Electrospray Emitter Array at Subambient Pressures. J. Am. Soc. Mass Spectrom. 2014 doi: 10.1007/s13361-014-0856-5. DOI: 10.1007/s13361-014-0856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.