Abstract
Objective:
There have been few comparative studies of microsurgical excision vs conservative management of cerebral cavernous malformations (CCM) and none of them has reliably demonstrated a statistically and clinically significant difference.
Methods:
We conducted a prospective, population-based study to identify and independently validate definite CCM diagnoses first made in 1999–2003 in Scottish adult residents. We used multiple sources of prospective follow-up to assess adults' dependence and to identify and independently validate outcome events. We used univariate and multivariable survival analyses to test the influence of CCM excision on outcome, adjusted for prognostic factors and baseline imbalances.
Results:
Of 134 adults, 25 underwent CCM excision; these adults were younger (34 vs 43 years at diagnosis, p = 0.004) and more likely to present with symptomatic intracranial hemorrhage or focal neurologic deficit than adults managed conservatively (48% vs 26%; odds ratio 2.7, 95% confidence interval [CI] 1.1–6.5). During 5 years of follow-up, CCM excision was associated with a deterioration to an Oxford Handicap Scale score 2–6 sustained over at least 2 successive years (adjusted hazard ratio [HR] 2.2, 95% CI 1.1–4.3) and the occurrence of symptomatic intracranial hemorrhage or new focal neurologic deficit (adjusted HR 3.6, 95% CI 1.3–10.0).
Conclusions:
CCM excision was associated with worse outcomes over 5 years compared to conservative management. Long-term follow-up will determine whether this difference is sustained over patients' lifetimes. Meanwhile, a randomized controlled trial appears justified.
Classification of evidence:
This study provides Class III evidence that CCM excision worsens short-term disability scores and increases the risk of symptomatic intracranial hemorrhage and new focal neurologic deficits.
After cerebral cavernous malformation (CCM) diagnosis, 5-year risks are 2.4% for a first intracranial hemorrhage (ICH) and 29.5% for a recurrent ICH,1 which may be influenced by patient sex and CCM location.1,2 Because of these risks, microsurgical excision3–5 and stereotactic radiosurgery6 are sometimes used to treat CCM. However, ICH from CCM tends to be small (average volume ∼1.8 cm3),7 its functional outcome tends to be good,7 and the annual risk of recurrent ICH appears to subside within 5 years after the initial ICH,1,2,8 provoking uncertainty about whether to treat CCM.9
These uncertainties are partly attributable to the shortage of evidence supporting CCM treatment.10 A systematic review seeking studies of more than 20 adults, comparing CCM excision to conservative management in a concurrent or historical control group and reporting objective clinical outcomes, found only 11 eligible studies.11 Apart from one retrospective study of 31 patients, which found a beneficial effect of CCM excision on seizure freedom12 (that almost reached the threshold for a statistically significant and “dramatic” effect in an observational study13), the other 10 studies did not show clinically or statistically significant differences in death or functional outcome from CCM excision. Therefore, we compared clinical outcomes after CCM excision and conservative management in an observational study.
METHODS
Our primary research question was whether there was a difference in clinical outcome between adults undergoing CCM excision vs a concurrent control group undergoing conservative management nested within a prospective, population-based, observational inception cohort study (class III evidence). The Scottish Intracranial Vascular Malformation Study (SIVMS) uses anonymized data extracts from a National Health Service national clinical audit of adults who were aged ≥16 years and resident in Scotland when first diagnosed with any type of intracranial vascular malformation during 1999–2003 and 2006–2010 (The Scottish Audit of Intracranial Vascular Malformations [SAIVMs]; www.saivms.scot.nhs.uk). The SAIVMs and SIVMS identified patients through multiple overlapping sources of case ascertainment that included a Scotland-wide collaborative network of neurologists, neurosurgeons, radiologists, and pathologists and central registers of hospital discharges and death certificates.14
Standard protocol approvals, registrations, and patient consents.
The Multicentre Research Ethics Committee for Scotland (MREC/98/0/48) and the Fife and Forth Valley Research Ethics Committee (08/S0501/76) approved the conduct of observational studies (to which an opt-out consent policy applied) and postal questionnaire studies (which required opt-in consent). We performed all analyses of observational data on anonymized extracts of a National Health Service national audit dataset (www.isdscotland.org/Health-Topics/Quality-Improvement/SAIVM/). We published the audit protocol (www.saivms.scot.nhs.uk/pdf/2008_06_SAIVMs%20protocol_v2.pdf), which included a prespecified objective to “monitor the outcomes of patients who do and do not receive interventional treatment (and thereby monitor the beneficial and adverse effects of these interventions).” We registered the research protocol with the Directory of Clinical Databases (DoCDat; http://docdat.ic.nhs.uk).
Eligibility criteria.
We included every adult in SIVMS with a first-in-a-lifetime definite diagnosis of CCM in 1999–2003, on the basis of pathologic examination or brain MRI.15,16 We classified adults as being managed conservatively if they did not receive CCM treatment within 5 years of initial presentation or as being treated if they had undergone microsurgical excision or stereotactic radiosurgery for their CCM within this time period; the decision to treat was left to the discretion of patients' doctors.
Inception and follow-up.
The inception point for the conservatively managed group was an adult's “initial presentation,” which was the date of symptom onset or medical consultation (if asymptomatic) that led to CCM diagnosis. The inception point for the treated group was the date of the first CCM treatment within 5 years of initial presentation. We used uninterrupted annual surveillance of general (family) practitioner and hospital medical records, as well as annual postal questionnaires to general practitioners and consenting participants on each anniversary of CCM diagnosis, to identify outcome events and assess dependence prospectively during follow-up on the Oxford Handicap Scale (OHS), which is a derivative of the modified Rankin Scale, ranging from 0 (no symptoms) to 6 (death).17
Classification of baseline and outcome variables.
Two neuroradiologists used the diagnostic brain imaging that had been obtained in clinical practice to verify certainty of CCM diagnosis15,16 and collected data on CCM anatomical location and radiographic evidence of recent ICH.18 We reviewed medical records to establish demographics, medical histories, and the consequences of initial presentation on the OHS. We reviewed these medical records, brain imaging, and reports of pathologic examinations to classify the mode of initial presentation and clinical outcome events during follow-up. In attributing the mode and cause of death, we reviewed death certificates, autopsy reports if postmortem had been performed, and clinical records and brain imaging if death had occurred during hospital admission. Two investigators (C.P.W. or R.A.-S.S.) independently assessed outcome events using all the clinical, radiologic, and pathologic information available.
We used published criteria to distinguish ICH from focal neurologic deficit (FND) due to CCM.18 We regarded initial presentations as incidental if the adult had been asymptomatic or if we could not relate their symptoms (e.g., headache) to the underlying CCM. We attributed initial presentations to seizure if a seizure was neither symptomatic of a concomitant ICH nor more likely to be due to another cause. When assessing clinical events at initial presentation and during follow-up, we also classified whether they were definitely, possibly, or definitely not attributable to the CCM or a procedure complication. We classified events as possibly attributable to a CCM when the clinical features of an event were anatomically consistent with CCM location, but another cause (e.g., ischemic stroke) was possible and neuroradiologic investigation had identified neither CCM hemorrhage nor an alternative cause.
Statistical methods.
Baseline characteristics.
We categorized CCM location as brainstem (midbrain, pons, or medulla), cerebellar, deep (thalamus, basal ganglia, or choroidal), or lobar (cortex and subcortical areas of the cerebral hemispheres); we dichotomized location into brainstem vs other locations for analyses. If an adult had many CCM, we allocated a single “primary” location according to the location of the CCM that was treated or to the symptomatic CCM if managed conservatively; in asymptomatic adults, brainstem CCM location took precedence because it was a predictor of interest. If following initial presentation a clinical event occurred that led to CCM treatment, this event was the mode of presentation in the treated group to reflect the indication for treatment. We dichotomized initial presentation into ICH/FND vs others for analyses.
We used parametric statistics for between-group comparisons when continuous data obeyed a normal distribution and nonparametric statistics when they did not. We used odds ratios (95% confidence interval [CI]) for categorical variables. We used exact tests when cell counts were less than 5.
Follow-up.
The primary outcome was “poor outcome,” defined as at least 2 successive ratings of OHS ≥2 (signifying “some restrictions to lifestyle, but the patient can look after themselves,” or worse). We used only OHS ratings after initial presentation to time progression to this event at the midpoint between the last OHS score 0–1 and the first of the successive ratings of OHS ≥2 for the conservatively managed group. Because the study timed ratings of functional outcome in relation to the anniversaries of every adult's date of diagnosis rather than the date of CCM treatment, the first rating following treatment was the earliest within 6 to 18 months of treatment. The secondary outcome was a composite of symptomatic ICH or new FND, which was either definitely or possibly attributable to a CCM,18 or attributable to CCM treatment.
We quantified completeness of the follow-up data accrued as a proportion of all the potential follow-up time that could have been obtained prior to death or the end of 5-year follow-up.19 We used life tables and Kaplan-Meier estimates to analyze follow-up data accrued by February 2011. Survival analyses of time to first event started at inception and stopped at the date of the first outcome event or the date of censoring, whichever occurred sooner. We censored follow-up at the earliest occurrence of death unrelated to CCM, the last available follow-up, or 5 years after the start of follow-up. We performed univariate analyses using hazard ratios [HRs] and the log-rank test, quantified survival functions at 5 years, and performed Cox regression if proportional hazards assumptions were satisfied.20 In multivariable analyses with fixed entry of covariates, we adjusted HRs for prespecified covariates that differed between the groups at baseline (age at inception), that influence CCM outcome (sex and mode of initial presentation), or that influence the risk of CCM treatment (brainstem CCM location).
We did not prespecify our desired sample size, but instead we sought to identify every new definite CCM diagnosis over 5 years in 1 country (mid-2010 population estimate of adults aged ≥16 years was 4.31 million) and to accumulate sufficient numbers of primary and secondary outcomes to enable us to analyze our potential predictors in multivariable analyses. We used 2-tailed statistical tests (α = 0.05), IBM SPSS Statistics (version 19.0), Stata (version 11.2), StatsDirect (version 2.7.8), and Confidence Interval Analysis software.
RESULTS
During 1999–2003, 139 adult residents in Scotland were newly diagnosed with at least one definite CCM on brain MRI (n = 133), on pathologic examination following surgical excision (n = 1), or incidentally at autopsy (n = 5, who are not considered in this analysis).1 Most of the 134 adults initially presented in the fourth or fifth decade of life, with a solitary symptomatic supratentorial CCM, which had not limited their independence (table 1).
Table 1.
Baseline characteristics of adults with a definite diagnosis of CCM in Scotland (1999–2003), stratified by whether they underwent CCM excision or conservative management
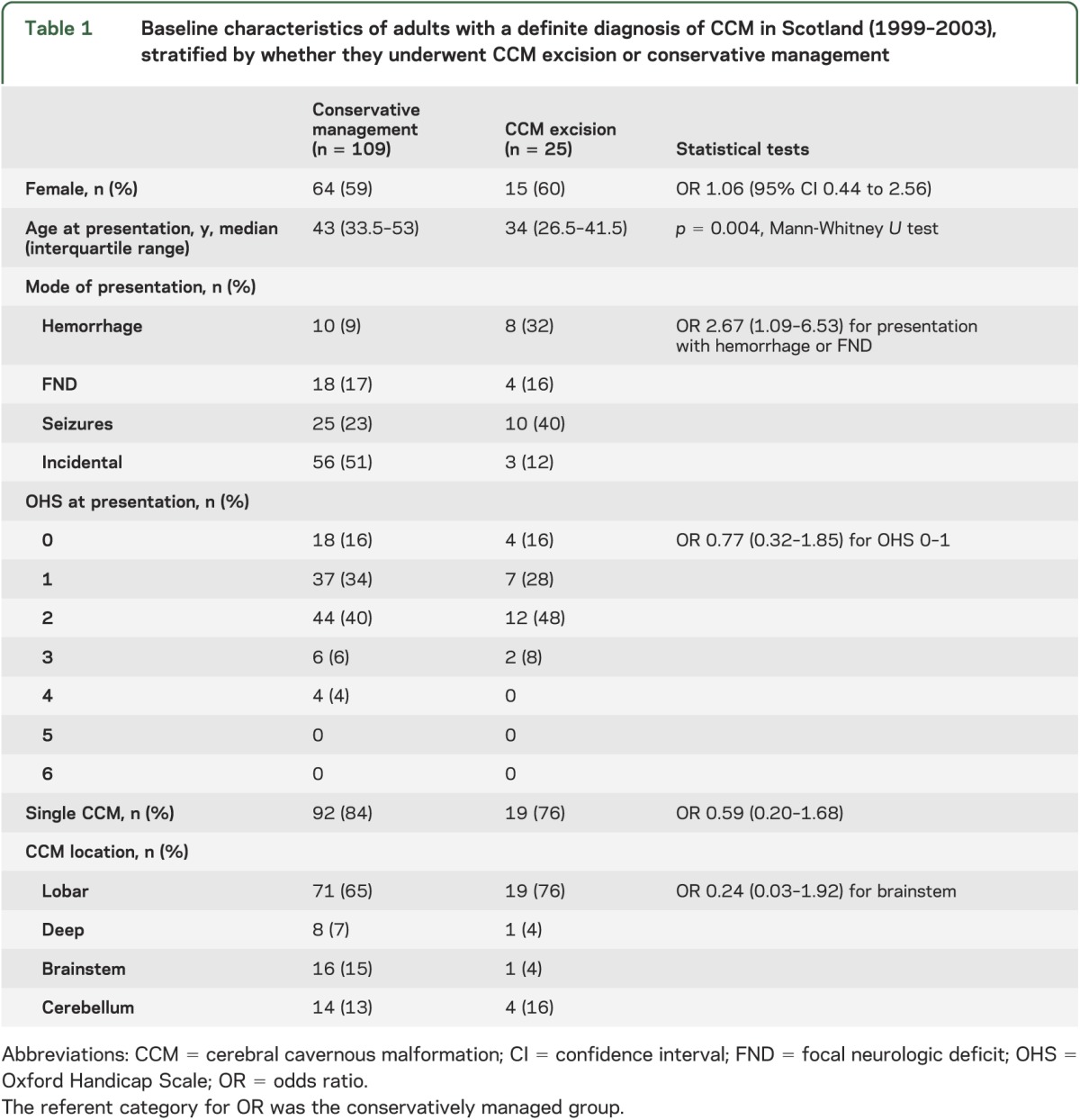
Twenty-five (19%) of these 134 adults underwent microsurgery for CCM excision after a median of 10 months (interquartile range 4.5–16) following initial presentation; none underwent stereotactic radiosurgery. At operation, excision was complete in 23 cases (2 patients required a second operation to achieve this), partial in one case, and abandoned in another case. Adults who underwent CCM treatment were younger by almost 1 decade and were more likely to have initially presented with ICH or FND than adults who were managed conservatively, but there were no detectable differences in other characteristics (table 1). Between initial presentation and CCM treatment (a period not included in our survival analyses), 5 of the 25 treated adults experienced 1 (n = 3), 2 (n = 1), or 3 (n = 1) ICH or FND that was definitely or possibly attributable to a CCM.
We followed up the 134 adults with CCM who were alive at initial presentation for 1,177 person-years (of 1,216 potential person-years, for an overall completeness of 97%).19
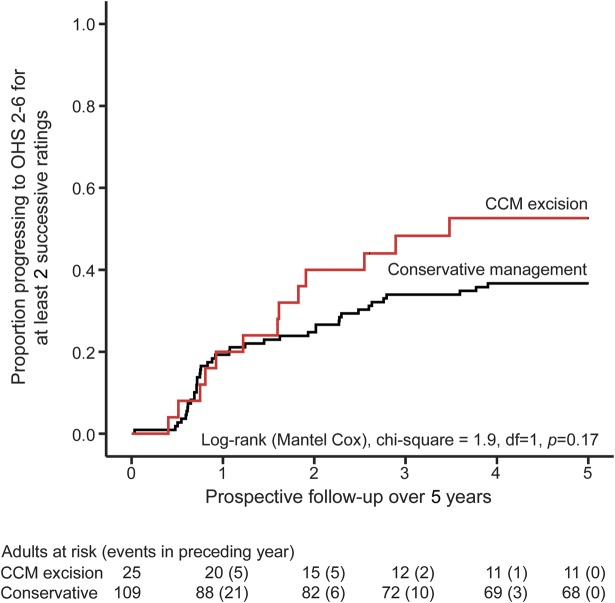
The primary outcome of sustained progression to OHS 2–6 for at least 2 successive annual ratings during 5 years of follow-up was reached by 13 (52%, 95% CI 34–70) of the 25 adults who underwent CCM excision and 40 (37%, 95% CI 28–46) of the 109 adults who were managed conservatively. This was not significant in univariate survival analysis (figure 1), but having confirmed that the proportional hazards assumption was fulfilled (figure e-1 on the Neurology® Web site at Neurology.org), our prespecified analysis of the primary outcome found that the risk of progressing to the primary outcome was doubled following CCM excision (adjusted HR 2.2, 95% CI 1.1–4.3; table 2). Seventeen patients died during follow-up (figure e-2). Three patients died between 0.5 and 2.5 years following CCM excision, 1 due to a seizure and 2 others due to metastatic malignancy. Fourteen patients died during conservative management: 1 death was due to an ICH attributed to the CCM, another death was due to pneumonia caused by a progressive neurologic deficit from a brainstem CCM, 2 deaths may have been due to the CCM (sudden death without autopsy, and pneumonia following neurologic deficit that may have been due to the CCM or a new ischemic stroke), and 10 deaths were unrelated to the CCM.
Figure 1. Progression to primary outcome, stratified by treatment group, during 5 years of prospective follow-up.
Kaplan-Meier estimate of progression to primary outcome (at least 2 successive ratings of 2 or more on the Oxford Handicap Scale [OHS]), stratified by treatment group (red line = cerebral cavernous malformation [CCM] excision, black line = conservative management), during 5 years of prospective follow-up.
Table 2.
Univariate and multivariable analyses of progression to the first occurrence of a primary or secondary outcome during 5 years of prospective follow-up
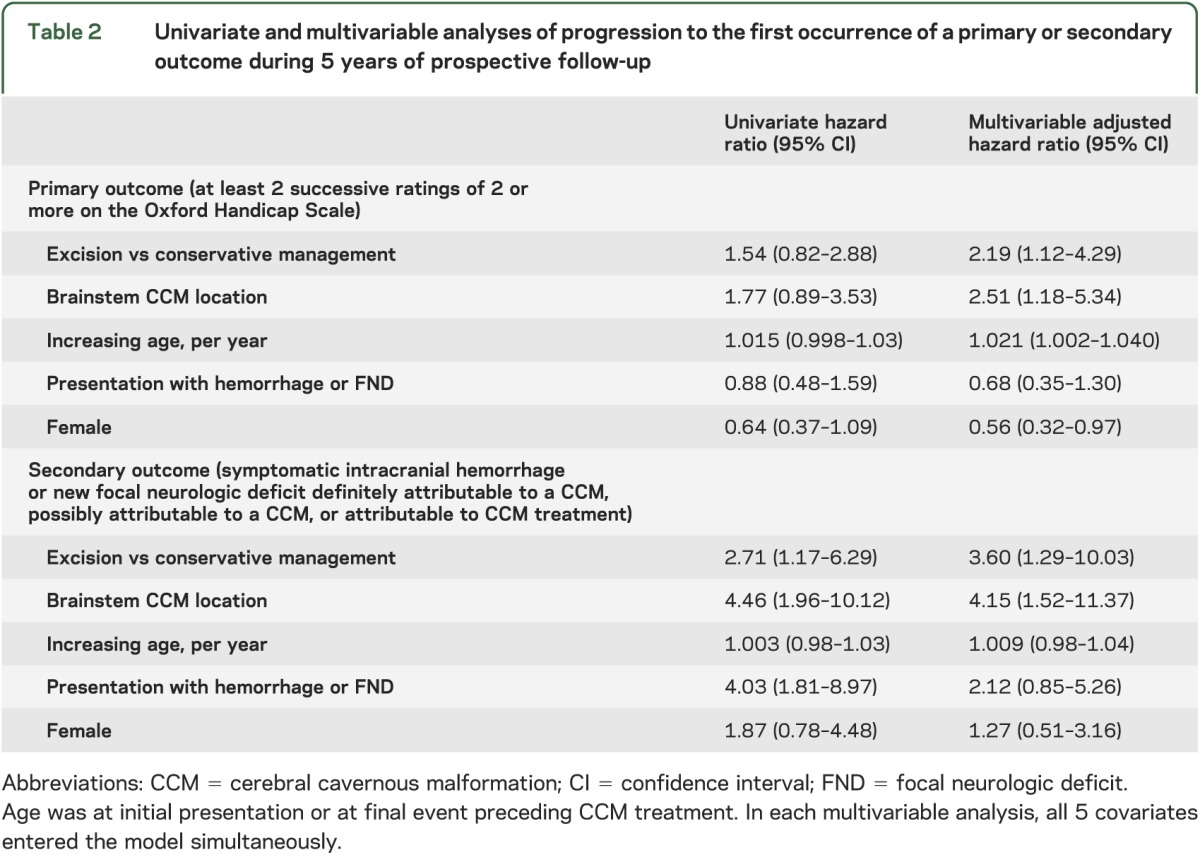
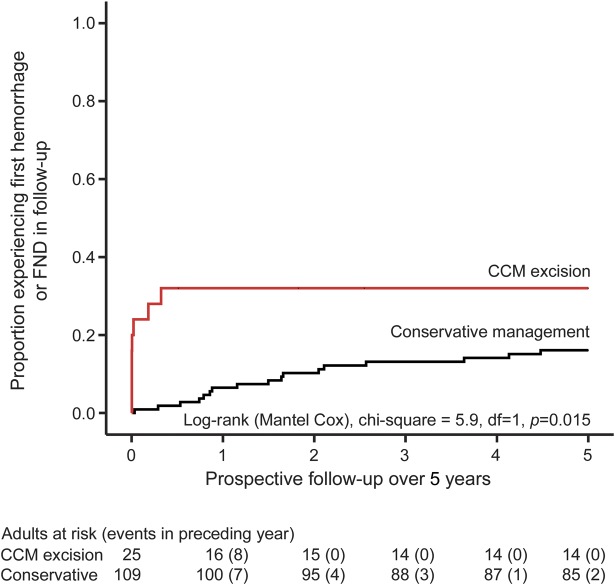
The secondary outcome of first ICH or new FND during 5 years of follow-up was reached by 17 (16%, 95% CI 10–24) of the 109 adults who were managed conservatively and 8 (32%, 95% CI 17–52) of the 25 adults who underwent CCM excision (6 [24%, 95% CI 7–41] of whom experienced a new FND [n = 5] or ICH [n = 1], but none died, within 30 days of CCM excision). CCM excision was associated with progression to the secondary outcome in univariate survival analysis (figure 2 and table 2). Having confirmed that the proportional hazards assumption was fulfilled (figure e-3), CCM excision more than trebled the risk of progression to the secondary outcome (adjusted HR 3.6, 95% CI 1.3–10.0; table 2). In sensitivity analyses of the secondary outcome excluding events possibly attributable to a CCM, there was no change in the significance, direction, or magnitude of the association. We also investigated whether the chance of attaining 2-year seizure freedom differed following CCM excision vs during conservative management for the 35 adults who had presented with seizures (table 1), and found no difference in a survival analysis (p = 0.26).
Figure 2. Progression to secondary outcome, stratified by treatment group, during 5 years of prospective follow-up.
Kaplan-Meier estimate of progression to secondary outcome (symptomatic intracranial hemorrhage or new focal neurologic deficit [FND] definitely attributable to a cerebral cavernous malformation [CCM], possibly attributable to a CCM, or attributable to CCM treatment), stratified by treatment group (red line = CCM excision, black line = conservative management), during 5 years of prospective follow-up.
DISCUSSION
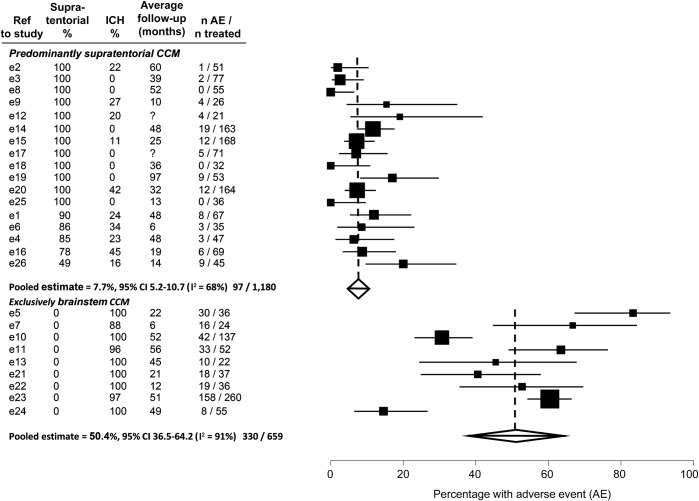
This prospective, population-based inception cohort study of adults with CCMs provides class III evidence that CCM microsurgical excision was associated with worse outcomes in comparison to a concurrent control group undergoing conservative management over 5 years, after adjusting for baseline imbalances and known predictors of outcome. The difference between the groups arose due to the recognized early complications of CCM excision (figure 2), which were as frequent as in some other series (figure 3).3–5 Although adults without early complications of CCM excision did not experience ICH or FND thereafter, their subsequent morbidity over 5 years reflects the impact of these complications (figure 1 and table 2). With further follow-up, this difference might diminish because of new neurologic events in the conservatively managed group, but this may not turn out to be the case because the risk of recurrent CCM hemorrhage seems to decline over time1,2,8,21,22 and CCM sometimes regrow after surgery and bleed again.
Figure 3. Postoperative adverse events in published studies of predominantly supratentorial or exclusively brainstem cerebral cavernous malformation.
Meta-analysis of proportions of patients with postoperative adverse events (death within 30 days, symptomatic intracranial hemorrhage (ICH), or new or worsened focal neurologic deficit) in studies of predominantly supratentorial or exclusively brainstem cerebral cavernous malformation (CCM). CI = confidence interval.
Our study's strengths were thorough case ascertainment in a population-based sampling frame (unlike all prior comparative studies11), a concurrent control group, almost complete follow-up (97%), independent imaging review and outcome assessment to minimize bias,15,16,18 estimates of the effect of CCM excision that we statistically adjusted for baseline imbalances (table 1), and a primary outcome that did not include OHS at presentation (which was not significantly imbalanced between the groups; table 1) and allowed for recovery from the known early complications after CCM excision.
The main limitation of this study was that it was not randomized. We adjusted for known predictors of poor outcome and baseline imbalances, which have also been identified by a recently published surgical grading scale,23 but the possibility of residual confounding remains. The sample size was modest, but it included all adults newly diagnosed with a CCM in an adult population of 4.3 million over 5 years, making it larger than any other comparative study,11 and it identified sufficient outcome events over 5 years of follow-up to power a multivariable analysis of the primary outcome (table 2). Only 19% of the cohort underwent CCM excision, thus the 24% (95% CI 7–41) risk of postoperative adverse events within 30 days in this study was an imprecise estimate. Only one of the excised CCMs was in the brainstem, which is a location that may confer higher risks after both CCM excision and conservative management.1,5 Although CCM excision did not affect the chance of seizure remission in this study, it may have missed a beneficial effect by being underpowered and not restricted to adults needing epilepsy surgery. None of the patients diagnosed with CCM in Scotland in 1999–2003 underwent stereotactic radiosurgery, so we and others should publish comparative studies of this technique in the future.
To put our findings in context, we performed 2 systematic reviews using electronic strategies for CCM (appendix e-1) to search for journal articles published prior to January 1, 2013, and indexed in OVID Medline or Embase. First, we updated our systematic review11 of studies involving more than 20 adults, which compared treatment with neurosurgery to conservative management in a concurrent or historical control group and reported objective clinical outcomes. We found 5 comparative studies involving 205 patients with brainstem CCM that had caused ICH/FND24–28 and 9 studies involving 278 patients with CCM that had caused seizures,13,29–36 which did not demonstrate dramatic beneficial or harmful effects on death or functional outcome,12 apart from one study that found a beneficial effect of CCM excision on seizure freedom, although we judged it to be at high risk of bias.13 Second, we quantified the risk of adverse events after CCM excision using the largest published case series from individual institutions, which included more than 20 adults who underwent excision of CCMs that were diagnosed by MRI or pathologic examination, and which described both patients' clinical presentation as well as objective clinical outcomes, stratified by CCM location. We included 26 case series (figure e-4, table e-1, table e-2, e-references) reporting the outcome after CCM excision from 1,839 adults over average postoperative follow-up ranging from 6 to 97 months.e1–e26 We used StatsDirect statistical software version 2.7.8 to perform a study-level meta-analysis of the proportion of patients undergoing CCM excision who had an adverse event (death within 30 days of surgery, symptomatic ICH, or a new or worsened FND); we used a random-effects model, and we used the I2 statistic to quantify inconsistency between studies. Postoperative adverse events were more frequent in studies reporting excision of exclusively brainstem CCM (50%, 95% CI 37–64) compared to studies reporting excision of predominantly supratentorial CCMs (8%, 95% CI 5–11; figure 3), although there was between-study heterogeneity (I2 91% and 68%, respectively).
Because comparative studies have been unable to show that CCM excision improves long-term outcome,11 clinical guidelines have been unable to recommend CCM excision.9 Our study casts further doubt on the superiority of CCM excision over conservative management. However, CCM excision continues to be undertaken for patients both with and without symptoms from their CCM (table 1),e1–e26,13,24–36 despite the low risk of the clinical course for some untreated CCMs.1,2 Therefore, future research should investigate the effects of CCM treatment (with excision or stereotactic radiosurgery) in comparison to conservative management, ideally in a randomized controlled trial of adults with CCM, presenting in any way, for whom there is uncertainty about CCM excision, taking into account known influences on outcome (table 2).1,23
Supplementary Material
ACKNOWLEDGMENT
The authors thank Rosemary Anderson, Aidan Hutchison, and all the adults in the SAIVMs; Brenda Thomas (Cochrane Stroke Review Group Trials Search Coordinator); and Dr. Adam Kobayashi, Dr. Elena Lebedeva, and Dr. Vida Demarin for translating foreign language publications for the systematic review.
GLOSSARY
- CCM
cerebral cavernous malformation
- CI
confidence interval
- FND
focal neurologic deficit
- HR
hazard ratio
- ICH
intracranial hemorrhage
- OHS
Oxford Handicap Scale
- SAIVMs
Scottish Audit of Intracranial Vascular Malformations
- SIVMS
Scottish Intracranial Vascular Malformation Study
Footnotes
Supplemental data at Neurology.org
Editorial, page 576
AUTHOR CONTRIBUTIONS
Dr. Moultrie contributed to study design, collected data, performed and critically appraised the literature search, drafted the paper, and reviewed the final version of the paper. M.A. Horne contributed to study design, collected data, drafted the paper, and reviewed the final version of the paper. Dr. Josephson collected data and reviewed the final version of the paper. Dr. Hall collected data, assessed brain imaging, and reviewed the final version of the paper. Dr. Counsell contributed to study design and reviewed the final version of the paper. Dr. Bhattacharya contributed to study design, assessed brain imaging, and reviewed the final version of the paper. Dr. Papanastassiou contributed to study design and reviewed the final version of the paper. R.J. Sellar contributed to study design, assessed brain imaging, and reviewed the final version of the paper. Dr. Warlow contributed to study design and reviewed the final version of the paper. Dr. Murray contributed to study design, supervised data analysis and interpretation, and reviewed the final version of the paper. Dr. Al-Shahi Salman designed the study; collected data; assessed brain imaging; checked, analyzed, and interpreted the data; performed, critically appraised, and meta-analyzed the literature search; drafted the paper; and reviewed the final version of the paper.
STUDY FUNDING
Supported by the Medical Research Council (clinical training fellowship G84/5176, clinician scientist fellowship G108/613, senior clinical fellowship G1002605, and the Edinburgh Hub for Trials Methodology Research G0800803); Scottish Government Chief Scientist Office (project grants K/MRS/50/C2704 and CZB/4/35); and Stroke Association (project grant TSA04/01).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Al-Shahi Salman R, Hall JM, Horne MA, et al. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol 2012;11:217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flemming KD, Link MJ, Christianson TJ, Brown RD., Jr Prospective hemorrhage risk of intracerebral cavernous malformations. Neurology 2012;78:632–636 [DOI] [PubMed] [Google Scholar]
- 3.Amin-Hanjani S, Ogilvy CS, Ojemann RG, Crowell RM. Risks of surgical management for cavernous malformations of the nervous system. Neurosurgery 1998;42:1220–1227 [DOI] [PubMed] [Google Scholar]
- 4.Ojemann RG, Ogilvy CS. Microsurgical treatment of supratentorial cavernous malformations. Neurosurg Clin N Am 1999;10:433–440 [PubMed] [Google Scholar]
- 5.Garrett M, Spetzler RF. Surgical treatment of brainstem cavernous malformations. Surg Neurol 2009;72(suppl 2):S3–S9 [DOI] [PubMed] [Google Scholar]
- 6.Kondziolka D, Flickinger JC, Lunsford LD. Radiosurgery for cavernous malformations. Prog Neurol Surg 2007;20:220–230 [DOI] [PubMed] [Google Scholar]
- 7.Cordonnier C, Al-Shahi Salman R, Bhattacharya JJ, et al. Differences between intracranial vascular malformation types in the characteristics of their presenting haemorrhages: prospective, population-based study. J Neurol Neurosurg Psychiatry 2008;79:47–51 [DOI] [PubMed] [Google Scholar]
- 8.Barker FG, II, Amin-Hanjani S, Butler WE, et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery 2001;49:15–24 [DOI] [PubMed] [Google Scholar]
- 9.Samarasekera N, Poorthuis M, Kontoh K, et al. Guidelines for the Management of Cerebral Cavernous Malformations in Adults. London: Genetic Alliance UK and Cavernoma Alliance UK; 2012 [Google Scholar]
- 10.Lippitz B. Treatment of cavernoma: an evidence-based dilemma? Acta Neurochir Suppl 2013;116:99–101 [DOI] [PubMed] [Google Scholar]
- 11.Poorthuis M, Samarasekera N, Kontoh K, et al. Comparative studies of the diagnosis and treatment of cerebral cavernous malformations in adults: systematic review. Acta Neurochir 2013;155:643–649 [DOI] [PubMed] [Google Scholar]
- 12.Glasziou P, Chalmers I, Rawlins M, McCulloch P. When are randomised trials unnecessary? Picking signal from noise. Br Med J 2007;334:349–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noto S, Fujii M, Akimura T, et al. Management of patients with cavernous angiomas presenting epileptic seizures. Surg Neurol 2005;64:495–498 [DOI] [PubMed] [Google Scholar]
- 14.Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Scottish Intracranial Vascular Malformation Study (SIVMS): evaluation of methods, ICD-10 coding, and potential sources of bias in a prospective, population-based cohort. Stroke 2003;34:1156–1162 [DOI] [PubMed] [Google Scholar]
- 15.Rigamonti D, Drayer BP, Johnson PC, Hadley MN, Zabramski J, Spetzler RF. The MRI appearance of cavernous malformations (angiomas). J Neurosurg 1987;67:518–524 [DOI] [PubMed] [Google Scholar]
- 16.Zabramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 1994;80:422–432 [DOI] [PubMed] [Google Scholar]
- 17.Bamford JM, Sandercock PA, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989;20:828. [DOI] [PubMed] [Google Scholar]
- 18.Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA; Angioma Alliance Scientific Advisory Board. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Stroke 2008;39:3222–3230 [DOI] [PubMed] [Google Scholar]
- 19.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet 2002;359:1309–1310 [DOI] [PubMed] [Google Scholar]
- 20.Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis part III: multivariate data analysis: choosing a model and assessing its adequacy and fit. Br J Cancer 2003;89:605–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathiesen T, Edner G, Kihlstrom L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg 2003;99:31–37 [DOI] [PubMed] [Google Scholar]
- 22.Wang C-C, Liu A, Zhang J-T, Sun B, Zhao Y-L. Surgical management of brain-stem cavernous malformations: report of 137 cases. Surg Neurol 2003;59:444–454 [DOI] [PubMed] [Google Scholar]
- 23.Kivelev J, Laakso A, Niemela M, Hernesniemi J. A proposed grading system of brain and spinal cavernomas. Neurosurgery 2011;69:807–813 [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Zhao Y, Zhou L, Zhu W, Pan Z, Mao Y. Surgical strategies in treating brainstem cavernous malformations. Neurosurgery 2011;68:609–620 [DOI] [PubMed] [Google Scholar]
- 25.Menon G, Gopalakrishnan CV, Rao BRM, Nair S, Sudhir J, Sharma M. A single institution series of cavernomas of the brainstem. J Clin Neurosci 2011;18:1210–1214 [DOI] [PubMed] [Google Scholar]
- 26.Esposito P, Coulbois S, Kehrli P, et al. Place of the surgery in the management of brainstem cavernomas: results of a multicentric study [in French]. Neurochirurgie 2003;49:5–12 [PubMed] [Google Scholar]
- 27.Huang AP, Chen JS, Yang CC, et al. Brain stem cavernous malformations. J Clin Neurosci 2010;17:74–79 [DOI] [PubMed] [Google Scholar]
- 28.Tarnaris A, Fernandes RP, Kitchen ND. Does conservative management for brain stem cavernomas have better long-term outcome? Br J Neurosurg 2008;22:748–757 [DOI] [PubMed] [Google Scholar]
- 29.Fernández S, Miró J, Falip M, et al. Surgical versus conservative treatment in patients with cerebral cavernomas and non refractory epilepsy. Seizure 2012;21:785–788 [DOI] [PubMed] [Google Scholar]
- 30.Banfi P, Guaschino E, Delodovici ML, Peron S, Tomei G, Bono G. Cerebral cavernous angiomas and epilepsy: clinical features and therapy in 20 cases. Bollettino Lega Italiana Contro L'Epilessia 2006;133–134 [Google Scholar]
- 31.Congia S, Saba S, Cannas A, et al. Epileptic seizures and cavernous angiomas. Bollettino Lega Italiana Contro L'Epilessia 2001;113–114:163–166 [Google Scholar]
- 32.Iakovlev G, Devaux B, Ghossoub M, Beuvon F, Brami F, Roux FX. Cerebral cavernomas, epilepsy and seizures: natural history and therapeutic strategy [in French]. Neurochirurgie 2005;51:3–14 [DOI] [PubMed] [Google Scholar]
- 33.Kivelev J, Niemela M, Kivisaari R, Dashti R, Laakso A, Hernesniemi J. Long-term outcome of patients with multiple cerebral cavernous malformations. Neurosurgery 2009;65:450–455 [DOI] [PubMed] [Google Scholar]
- 34.Rougier A, Castel JP, Cohadon F. Different modalities for the treatment of hemispheric cavernomas [in French]. Neurochirurgie 1989;35:115–119 [PubMed] [Google Scholar]
- 35.Secchi P, Calloni MV, Freschi R, et al. Epilepsy and cerebral vascular malformations: retrospective study of 71 cases. Bollettino Lega Italiana Contro L'Epilessia 1998;102–103:111–113 [Google Scholar]
- 36.Vespignani H, Bouilleret V, Taillandier L, et al. Cavernous angiomas revealed by epileptic seizures: 57 cases reports. Epilepsies 1994;6:97–111 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.