Abstract
Objective:
Determine whether United States Air Force (USAF) U-2 pilots (U2Ps) with occupational exposure to repeated hypobaria had lower neurocognitive performance compared to pilots without repeated hypobaric exposure and whether U2P neurocognitive performance correlated with white matter hyperintensity (WMH) burden.
Methods:
We collected Multidimensional Aptitude Battery–II (MAB-II) and MicroCog: Assessment of Cognitive Functioning (MicroCog) neurocognitive data on USAF U2Ps with a history of repeated occupational exposure to hypobaria and compared these with control data collected from USAF pilots (AFPs) without repeated hypobaric exposure (U2Ps/AFPs MAB-II 87/83; MicroCog 93/80). Additional comparisons were performed between U2Ps with high vs low WMH burden.
Results:
U2Ps with repeated hypobaric exposure had significantly lower scores than control pilots on reasoning/calculation (U2Ps/AFPs 99.4/106.5), memory (105.5/110.9), information processing accuracy (102.1/105.8), and general cognitive functioning (103.5/108.5). In addition, U2Ps with high whole-brain WMH count showed significantly lower scores on reasoning/calculation (high/low 96.8/104.1), memory (102.9/110.2), general cognitive functioning (101.5/107.2), and general cognitive proficiency (103.6/108.8) than U2Ps with low WMH burden (high/low WMH mean volume 0.213/0.003 cm3 and mean count 14.2/0.4).
Conclusion:
In these otherwise healthy, highly functioning individuals, pilots with occupational exposure to repeated hypobaria demonstrated lower neurocognitive performance, albeit demonstrable on only some tests, than pilots without repeated exposure. Furthermore, within the U2P population, higher WMH burden was associated with lower neurocognitive test performance. Hypobaric exposure may be a risk factor for subtle changes in neurocognition.
Neurologic decompression sickness (NDCS) is an occupational risk for high-altitude pilots1 characterized by a variety of neurologic symptoms including confusion, disorientation, concentration problems, memory deficits, and reduced neurocognitive processing,2 significantly affecting functional capacity.3 In response to an increased incidence of NDCS in the United States Air Force (USAF),4,5 research was performed that demonstrated pilots who experienced NDCS had increased volume and number of subcortical T2-weighted white matter hyperintensity (WMH) abnormalities.6 Follow-up research reported significant elevation of WMH in high-altitude pilots without clinical symptoms of NDCS.7 WMH are important markers of cerebral integrity in aging and brain disorders8 linked to executive functioning,9 processing speed, and general neurocognitive status,9–11 and are an important predictor of increased neurocognitive decline.12–15
We examined the relationship between WMH and neurocognitive performance in active duty USAF high-altitude pilots. This presents a unique opportunity to study the association between WMH burden caused by occupational factors rather than aging or illnesses in high-performing individuals with optimal health, free of the cardiovascular and metabolic risk factors frequently present in individuals with significant WMH burden.12–14,16,17 We studied the effects of occupational hypobaria on neurocognition by testing 2 hypotheses: (1) there will be a significant reduction in the neurocognitive test scores between USAF pilots (AFPs) repetitively exposed to hypobaria compared with AFPs not exposed to repetitive hypobaria, and (2) the volume and number of WMH will explain a significant proportion of the variability of the neurocognitive performance in AFPs who were occupationally exposed to repetitive hypobaria.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Air Force Research Laboratory Institutional Review Board. All participants were active duty members of the US military recruited with strict adherence to Department of Defense requirements regarding protection of human subjects in research. Participation in this study was voluntary, and commanding officers were not involved in subject participation. All participants acknowledged this was not an anonymous study and provided informed consent before testing.
Participants.
All participants were between the ages of 28 and 47 years, were healthy without any history of neurologic or psychiatric disease, and had undergone annual medical examinations within 12 months of participation. All participants at the time of testing met USAF Flying Class II neurologic standards, and all pilots were on active flying status.18 Briefly, exclusionary criteria for Flying Class II include a history of any of the following: head trauma with any loss of consciousness or amnesia; migraine headache; psychiatric or psychological disease requiring any medication or hospitalization; hypertension requiring more than a single angiotensin-converting enzyme inhibitor for control; hyperlipidemia requiring more than a single statin for control; diabetes or glucose intolerance; ischemic cardiac disease; any neurologic disease including infection, seizure, or stroke; or substance or drug abuse or dependence. Subjects were not evaluated for the presence of a patent foramen ovale because this is not a disqualifier for Flying Class II certification. Subjects were not compensated for participation, but their travel costs were reimbursed as permitted under Federal Government travel regulations.
All active duty USAF U-2 pilots (U2Ps) were invited to participate: 106 individuals agreed, exceeding a 90% participation rate. All U2Ps before flight and altitude exposure must undergo a physiologist-monitored 1-hour nitrogen degassing with 100% O2 and then remain on 100% O2 until returning to below 10,000-feet altitude. Hypobaric exposure during flight may be as long as 9 hours with cabin altitude of 28,000 to 30,000 feet. Exposure frequency is variable, but not more often than every third day. Sixteen (15%) reported symptoms of NDCS, with only 2 reporting more than a single episode. No episode of NDCS was associated with equipment failure or aircraft malfunction.
For structural MRI comparison, 132 active duty doctorate-degree volunteers (DOCs) matched for age and medical conditions were recruited as previously described.7 All DOCs were healthy at the time of study, without present or past history of any medical conditions associated with WMH or disqualifying for Flying Class II certification.
For neurocognitive comparison, testing results for 83 active duty AFPs matched for age at the time of the neurocognitive assessment and on active flying status meeting Flying Class II standards were obtained from the AFP neurocognitive testing dataset via record review.
All U2Ps and DOCs underwent high-resolution MRI as previously described.6,7 AFPs only participated in the neurocognitive assessment and did not undergo MRI. Neurocognitive assessment data were collected on U2Ps before imaging. Eighty-seven U2Ps completed the Multidimensional Aptitude Battery–II (MAB-II) and 93 completed the MicroCog: Assessment of Cognitive Functioning (MicroCog). From the USAF neurocognitive testing dataset, MAB-II assessment was available on 83, while MicroCog assessment was available on 80.
Cognitive assessment.
Computer-based MAB-II and MicroCog neurocognitive assessment tests are routinely used in aircrew by the USAF. The MAB-II is a broad-based evaluation of neurocognitive ability based on the Wechsler Adult Intelligence Scale–Revised (correlation 0.91).19,20 This is a computer-administered test that yields 3 summary scores (full-scale IQ, verbal IQ, and performance IQ) based on subtests of vocabulary, arithmetic, information, comprehension, similarities, digit symbol, picture arrangement, object assembly, picture completion, and spatial thinking. Similar to the Wechsler Adult Intelligence Scale–Revised, the MAB-II full-scale, verbal, and performance IQ scores are standardized to age with a mean of 100 and an SD of 15. Measures of reliability and construct validity for full-scale IQ have been demonstrated.19 Results on the MAB-II may inflate IQ estimates, but this systematic bias will be present in all subjects and therefore not affect group comparisons.
The MicroCog is a computer-based neurocognitive assessment test that consists of 18 subtests used to derive 9 index scores. Level 1 indexes include the 5 domains of reaction time, memory, attention and control, reasoning and calculation, and spatial processing.21 Level 2 indexes assess overall information processing speed and information processing accuracy, while level 3 indexes represent global neurocognitive functioning with general cognitive functioning weighing speed and accuracy equally and general cognitive proficiency weighing accuracy over speed.22–24 MicroCog was specifically designed to provide more accurate assessment of the reaction time and processing speed when compared with other neurocognitive assessment instruments.22 However, because MicroCog is a computer-based instrument, more comprehensive neuropsychological testing is required to draw conclusions about the general cognitive profile of subjects. Nonetheless, normative scores on the MicroCog have been established for age and education level,22 and overall, MicroCog-derived scores show good consistency with other neuropsychological instrument batteries.24
MRI assessment.
Structural MRI data for U2Ps were collected at the Research Imaging Institute, University of Texas Health Science Center, San Antonio, using a Siemens 3T Tim Trio scanner equipped with a 12-channel phase array coil (Siemens AG, Erlangen, Germany).6,7 Structural MRI data for DOCs were collected at Wilford Hall Ambulatory Surgery Clinic, Lackland Air Force Base, TX, using a Siemens 3T Verio scanner equipped with a 32-channel phase array coil. Both scanners are operated under quality control and assurance guidelines in accordance with recommendations by the American College of Radiology, and cross-calibration between scanners has been performed as previously described.7 Briefly, 3-dimensional imaging parameters were T1 magnetization-prepared rapid-acquisition gradient echo repetition time 2,200 milliseconds, echo time 2.85 milliseconds, and isotropic resolution 0.80 mm, and fluid-attenuated inversion recovery repetition time 4,500 milliseconds, echo time 311 milliseconds, and isotropic resolution 1.00 mm. Fluid-attenuated inversion recovery images were coregistered to a common Talairach atlas–based stereotactic frame permitting normalization of brain size and hence cross-individual comparison. An experienced neuroanatomist (intrarater reproducibility r = 0.95) blinded to group as previously described manually traced WMH,7 while a neuroradiologist provided oversight and clinical interpretation of MRIs. We manually counted the total number (count) of subcortical WMH and used in-house software to calculate the total volume (volume) of subcortical WMH in each lobe.
Stratification of data by WMH.
U2Ps were separated into low WMH volume/count and high WMH volume/count cohorts. We selected as the stratification point the median WMH volume/count of the 132 DOC MRIs, making the assumption that healthy young to middle-aged adults lacking comorbid risk factors for WMH can serve as a representative sample for the upper- and lower-half quartile of WMH burden. We hypothesized that in the absence of hypobaric exposure, there should be no significant differences in either the burden or distribution of WMH between DOCs and U2Ps. The DOC median WMH volume was 0.013 cm3 (mean DOCs/U2Ps 0.036/0.147 cm3; p < 0.001) and median WMH count was 2 (mean DOCs/U2Ps 2.8/9.7; p < 0.001). Within U2Ps, there were no significant differences between high and low WMH volume/count groups in either age (p = 0.921/0.342) or number of U-2 flight hours (p = 0.464/0.313).
Statistical analysis.
We used the 2-tailed Student t test with Sidak adjustment for multiple tests for comparison of MAB-II and MicroCog between U2Ps and AFPs. Similarly, we used the 2-tailed Student t test with Sidak adjustment for multiple tests for comparison of MAB-II and MicroCog within U2Ps separated into low and high WMH volume/count groups. We used the 2-tailed Student t test with Sidak adjustment for multiple tests for comparison of MicroCog within U2Ps separated into low-, mid-, and high-range quartile WMH volume/count, comparing each quartile individually with AFPs. Finally, we utilized Cohen d as a descriptive statistic to describe how substantial our findings are when utilizing the t test for comparing U2Ps and AFPs.
RESULTS
Demographics.
Mean age for U2Ps/AFPs was 36.8 ± 5.3/33.7 ± 5.1 years, with a male/female ratio for U2Ps of 95/2 and for AFPs of 78/5. No difference was present between U2Ps and AFPs on the Air Force Officer Qualifying Test performed before commissioning (p > 0.05) or baseline MAB-II performed at time of entry into undergraduate pilot training (table e-1 on the Neurology® Web site at Neurology.org). No difference in distribution of initial assigned aircraft after undergraduate pilot training or in subsequent aircraft flown was present between U2Ps and AFPs.
Current cognitive assessment group comparisons.
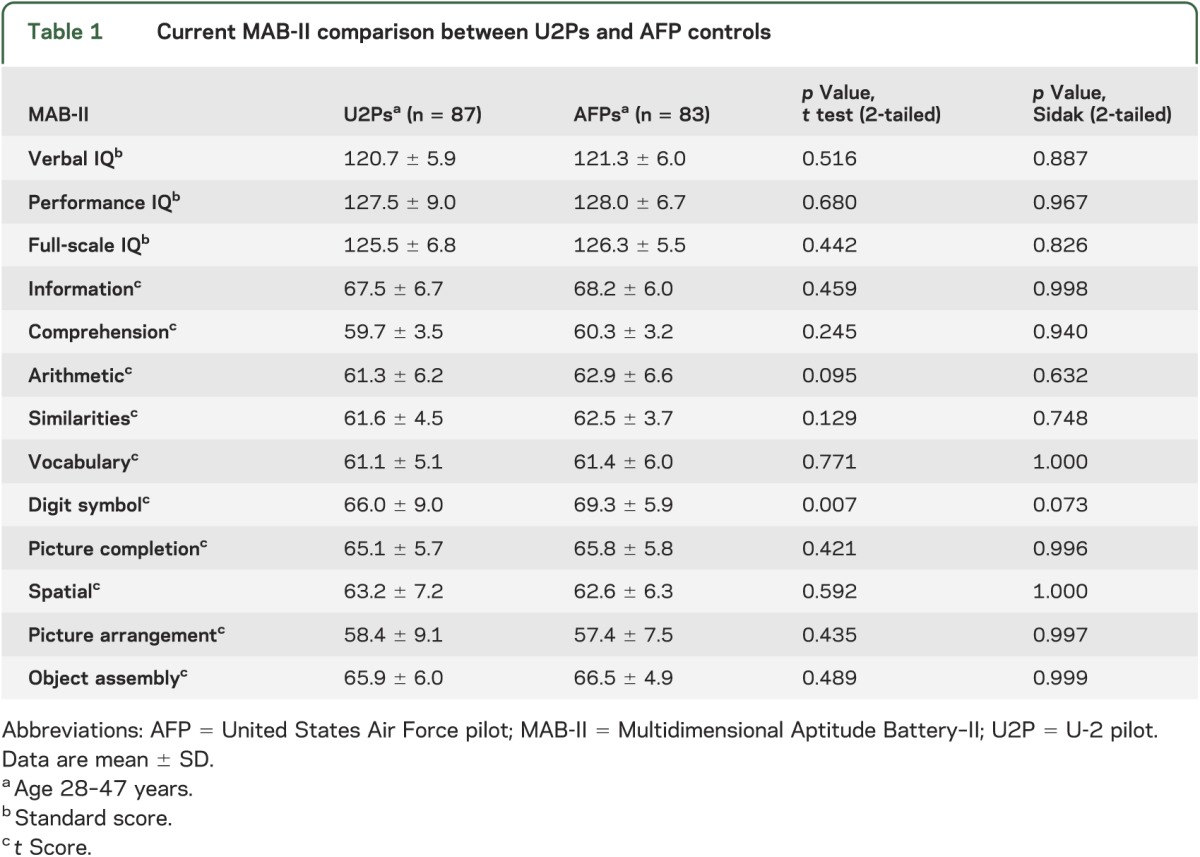
There were no significant differences between U2Ps and AFPs for any of the age-adjusted MAB-II measures after applying the Sidak adjustment (table 1). Performance for both groups was high when compared with population normative data, indicating that AFPs are highly functioning individuals.
Table 1.
Current MAB-II comparison between U2Ps and AFP controls
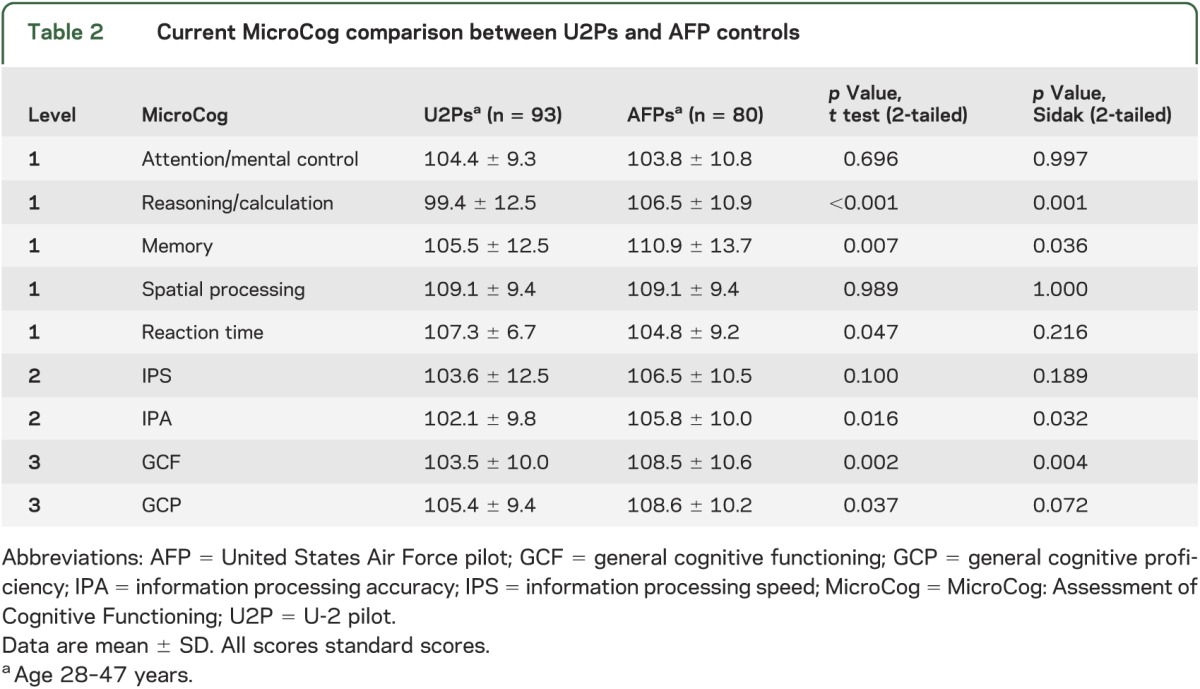
In contrast, age- and education-corrected MicroCog subtests demonstrated a significant difference between U2Ps and AFPs on reason/calculation, memory, information processing accuracy, and general cognitive functioning after applying the Sidak adjustment (table 2). Regardless, performance on all measures was in the average range of function relative to pilot peers.
Table 2.
Current MicroCog comparison between U2Ps and AFP controls
In the U2P cohort, the correlations between neurocognitive performance and the clinical occurrence of NDCS or the total hours or average frequency of hypobaric exposure were not significant (all p > 0.05).
WMH volume/count U2P comparison groups.
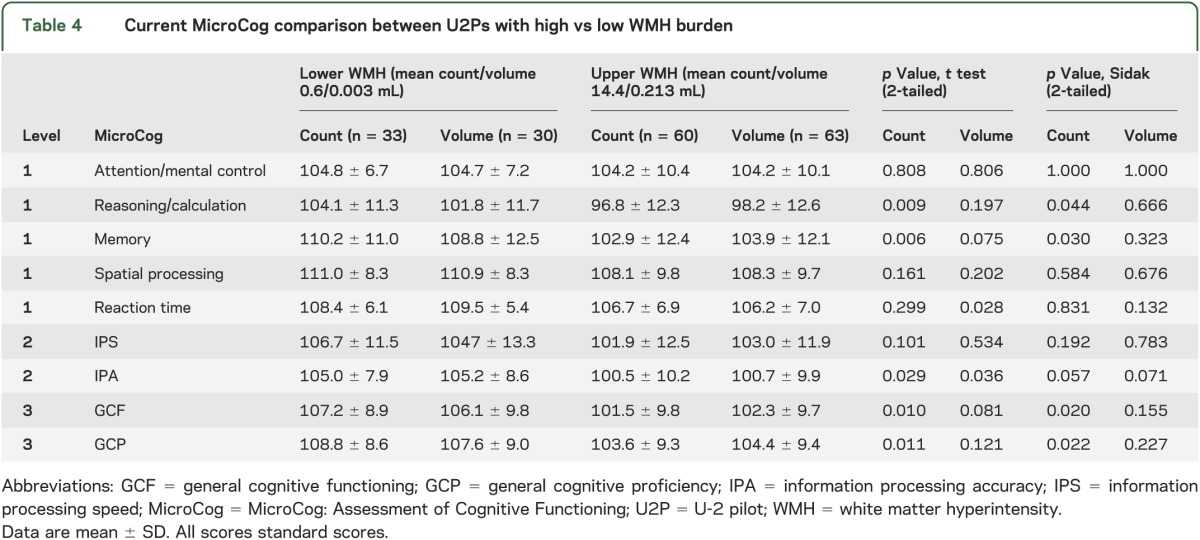
There were no significant differences in MAB-II current performance between the low and high WMH volume/count groups after applying the Sidak adjustment (table 3). However, MicroCog results demonstrated a significant difference in reasoning/calculation, memory, general cognitive functioning, and general cognitive proficiency (count) and a trend in information processing accuracy (count and volume) after applying the Sidak adjustment (table 4). Furthermore, there were no MicroCog differences between the low-quartile U2Ps and AFPs while there were significant differences between the mid- and high-quartile U2Ps and AFPs (tables e-2 and e-3).
Table 3.
Current MAB-II comparison between U-2 pilots with high vs low WMH burden
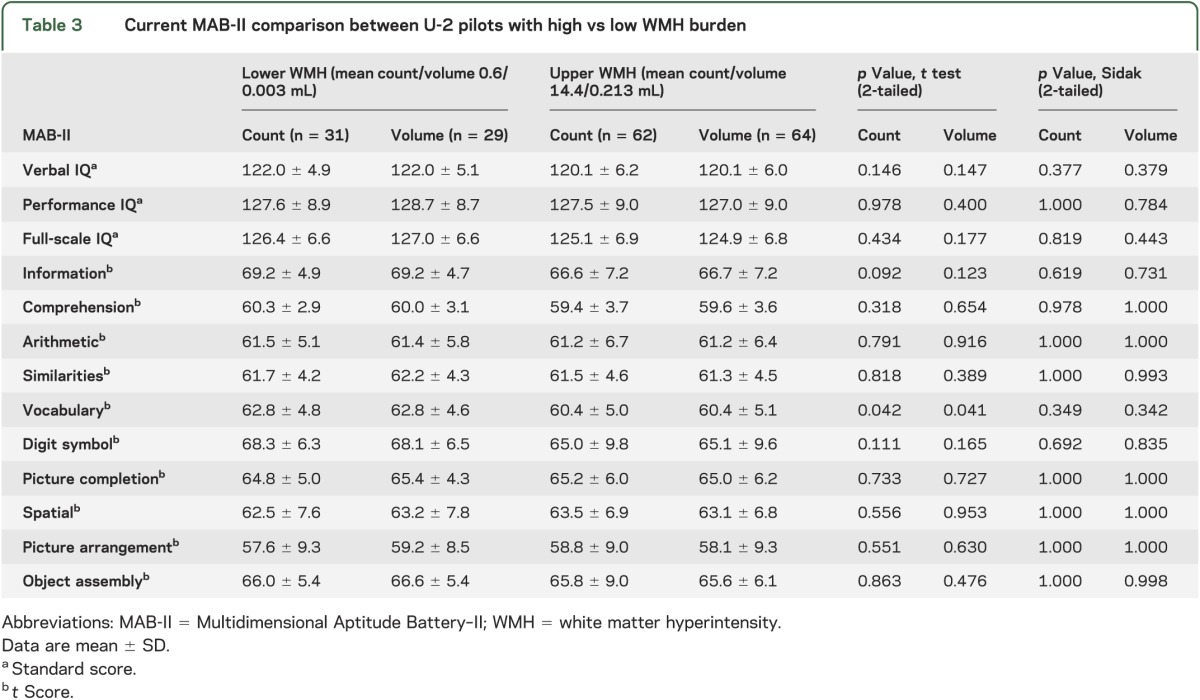
Table 4.
Current MicroCog comparison between U2Ps with high vs low WMH burden
Cohen d effect.
Cohen d values for MicroCog test results were substantial with moderate value for reasoning/calculation, mild to moderate values for memory and global cognitive functioning, and mild values for reaction time, information processing speed and accuracy, and global cognitive processing (table e-4).
DISCUSSION
Our study demonstrated that a subgroup of AFPs (U2Ps), occupationally exposed to repeated hypobaria but lacking any clinical deficits, had subtle changes on neurocognitive function, demonstrable as significantly reduced scores on several neurocognitive measures after Sidak adjustment for multiple tests, when compared to AFPs without repeated hypobaric exposure. Specifically, U2Ps had significantly lower scores on reasoning/calculation, memory, information processing accuracy, and general cognitive functioning with a nominal reduction in general cognitive proficiency. In addition, within the U2P population, higher WMH count was associated with significantly lower scores on reasoning/calculation, memory, general cognitive functioning, and general cognitive proficiency compared with lower WMH count. In addition, these subjects showed nominally significant reduction in information processing accuracy (count and volume).
One potential limitation of this work is the use of computer-based cognitive assessment instruments rather than human-administered evaluations. An advantage of computer-based cognitive assessment is it provides for more accurate and standardized assessment of processing speed and reaction time, 2 cognitive domains of importance for military pilots. However, stand-alone computer-based testing does limit conclusions on general cognitive profile. A second potential limitation is interpretation of significance when using multiple tests, even after applying the Sidak adjustment. While Sidak adjustment attempts to correct for the probability of false positives when conducting multiple tests, this also affects the critical value for rejecting the null hypothesis. Finally, while we observed that lower neurocognitive performance in U2Ps appears to be associated with higher WMH burden, the absence of MRI data in AFPs prevents a direct association between neurocognitive scores and WMH burden. Notably, regardless of the statistically significant differences in test performance, U2Ps continue to be on par with age- and cohort-specific normative data, indicating that the reduction in neurocognitive performance is not of immediate clinical significance.
The apparent lower neurocognitive performance in relationship to WMH burden in U2Ps is consistent with findings from cerebral aging research demonstrating an association between increased WMH burden and reduced performance on attention/processing speed,9,11,12 possibly reflecting decreased efficiency of the affected neural network and eventual loss of function.25 Interpretation of these associations between WMH abnormalities and neurocognition is complicated by both the nonspecific nature of sporadic WMH regions and the potential differences in the underlying pathophysiologic mechanisms of WMH. In aging studies, increased WMH burden is associated with many insidious and chronic conditions including cerebrovascular inflammation,16,26 small-vessel stenosis,27 diabetes, and hypertension.28 In addition, the associations between WMH abnormalities and neurocognitive performance are often drawn from observations in elderly and patient populations. Our study provides strong evidence that occupational hypobaria-associated WMH load may be an independent predictor of lower neurocognitive performance even among healthy and high-performing individuals, free of typical risk factors associated with elevated WMH.
Hypobaria is a known risk factor for decompression sickness that may include neurologic symptoms ranging from very mild (feeling of physical fatigue and complaints of slowed thought processes) to severe (anomia, confusion, and unresponsiveness),2 including permanent neurocognitive decline.3 At this time, the precise mechanisms of CNS damage due to hypobaria are uncertain. Previously, we hypothesized that CNS damage may be caused by microbubble occlusion of small arterioles, by platelet thrombi produced by accelerated coagulation in the presence of nitrogen microbubbles,29,30 or by direct tissue damage from microparticles and activation of proinflammatory leukocytes.7,31,32 In other words, exposure to hypobaria leads to bombardment of brain vasculature and thrombo-inflammatory damage in cerebral white matter that is visible as WMH. A significantly more uniform regional distribution of WMH across cerebral white matter in U2Ps compared with DOCs supports this hypothesis.7 Specifically, WMH were more uniformly distributed across the cerebral white matter in U2Ps, while the lesion burden was primarily observed in frontal areas in DOCs. How this pattern of injury would lead to lower neurocognitive performance is unknown.
We believe the significant difference in neurocognitive scores between U2Ps and AFPs is caused by repeated hypobaric exposure, although we cannot exclude other environmental contributors associated with high flight including radiation injury. U2Ps showed significantly reduced scores across a broad range of neurocognitive domains compared with AFPs. In addition, finding significantly lower neurocognitive scores in U2Ps with greater vs lesser WMH burden further suggests an association between WMH burden and reduced neurocognitive performance in U2Ps. Indeed, post hoc testing revealed U2Ps from the lower WMH group did not show significant difference from AFPs. In contrast, the neurocognitive scores from the U2Ps with mid-range or high WMH burden were significantly lower (tables e-2 and e-3). The lack of MRI data on AFPs, however, prevents the direct association of WMH burden and neurocognitive performance. Nonetheless, our finding supports the hypothesis that WMH changes may, by themselves, be associated with a decline in neurocognition.33 This is consistent with the current mechanist view of the brain as a collection of large-scale functional networks supporting higher neurocognitive functioning. However, this observation requires additional validation, and following this cohort as they age would be an important step in understanding whether or not these changes are progressively different than what is expected for a healthy, aging cohort.
Finally, despite the findings of statistical difference on neurocognitive performance, U2Ps remain very highly functioning individuals with neurocognitive scores above the average for the general public. They remain fully capable of performing the complex multitasking necessary of pilots in this challenging aircraft with no persistent behavioral abnormalities noted.34 Similar to reports in other populations,35 this may represent neurocognitive reserve present in these highly functioning individuals. A single U2P (excluded from all analysis) experienced neurocognitive impairment sufficient to preclude further pilot duties, but this was an unusual case not representative of the U-2 population.3 One pilot included in this study experienced repeated episodes of headache associated with high flight and was therefore restricted to pressurized aircraft with no subsequent clinical symptoms or impairments. In addition, although 2 pilots in this study did voluntarily withdraw from the U-2 program before testing, neither one demonstrated any clinical neurocognitive deficit and both remained on active flying status medically qualified for all USAF aircraft including the U-2 without restriction. However, our findings are a cause for concern and suggest that further investigation on the long-term significance of this difference is needed.
Analysis of other MRI parameters, including spectroscopy and volumetric parameters, is ongoing in an attempt to better understand the pathophysiologic process and impact on neurostructures and performance. In addition, a reliable laboratory model for neurologic injury secondary to hypobaric exposure is under development.
This study demonstrates that U2Ps with repeated occupational exposure to hypobaria have significantly lower neurocognitive test performance compared to AFPs without repeated hypobaric exposure. Moreover, higher WMH burden was significantly associated with reduced neurocognitive performance. In addition, our study suggests that other healthy, peak-of-function, young to middle-aged populations who are at high risk of increased WMH burden, such as concussion from athletic activities, may be at risk of significantly reduced neurocognitive performance.
Supplementary Material
ACKNOWLEDGMENT
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the United States Air Force, Department of Defense, or US Government. The authors thank Mr. Jared Haynes, US Air Force School of Aerospace Medicine, 711th Human Performance Wing, Wright–Patterson AFB, OH, who performed the statistical analysis. Approved for public release; distribution is unlimited. Case Number: SAF-2013-0695, 10 Jan 2014.
GLOSSARY
- AFP
United States Air Force pilot
- DOC
doctorate-degree volunteer
- MAB-II
Multidimensional Aptitude Battery–II
- MicroCog
MicroCog: Assessment of Cognitive Functioning
- NDCS
neurologic decompression sickness
- USAF
United States Air Force
- U2P
U-2 pilot
- WMH
white matter hyperintensity
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. McGuire: study concept, design, performance analysis, interpretation, and principal author of manuscript. Dr. Tate and Dr. Wood: study concept, design, performance, data analyses, and critical revision of the manuscript for important intellectual content. Dr. Sladky, Dr. McDonald, and Dr. Sherman: study concept, design, performance, analysis and interpretation. Ms. Kawano: critical scientific editorial assistance. Dr. Rowland, Ms. Patel, Ms. Wright, Dr. Hong, Dr. Rasmussen, and Dr. Willis: analysis and interpretation. Dr. Kochunov: study concept, design, performance, data analyses, and critical revision of the manuscript for important intellectual content.
STUDY FUNDING
Supported by United States Air Force Surgeon General grants (Log I-11-10 and I-11-44) to S.A.M.
DISCLOSURE
S. McGuire is supported by USAF Surgeon General grants Log I-11-10 and I-11-44. D. Tate is consultant to the Brigham and Women's Hospital, Boston, MA, for an NIH-funded grant of brain injury. J. Wood, J. Sladky, K. McDonald, P. Sherman, and E. Kawano report no disclosures. L. Rowland serves as an editorial board member of Schizophrenia Bulletin and is funded by NIH grants R01MH094520 and R01MH096263. B. Patel, S. Wright, E. Hong, J. Rasmussen, and A. Willis report no disclosures. P. Kochunov is funded by NIH grant R01EB015611. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Vann RD, Butler FK, Mitchell SJ, Moon RE. Decompression illness. Lancet 2011;377:153–164 [DOI] [PubMed] [Google Scholar]
- 2.Balldin UI, Pilmanis AA, Webb JT. Central nervous system decompression sickness and venous gas emboli in hypobaric conditions. Aviat Space Environ Med 2004;75:969–972 [PubMed] [Google Scholar]
- 3.Jersey SL, Baril RT, McCarty RD, Millhouse CM. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med 2010;81:64–68 [DOI] [PubMed] [Google Scholar]
- 4.Hundemer GL, Jersey SL, Stuart RP, Butler WP, Pilmanis AA. Altitude decompression sickness incidence among U-2 pilots: 1994–2010. Aviat Space Environ Med 2012;83:968–974 [DOI] [PubMed] [Google Scholar]
- 5.Jersey SL, Hundemer GL, Stuart RP, West KN, Michaelson RS, Pilmanis AA. Neurological altitude decompression sickness among U-2 pilots: 2002–2009. Aviat Space Environ Med 2011;82:673–682 [DOI] [PubMed] [Google Scholar]
- 6.McGuire SA, Sherman PM, Brown AC, et al. Hyperintense white matter lesions in 50 high-altitude pilots with neurologic decompression sickness. Aviat Space Environ Med 2012;83:1117–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuire S, Sherman P, Profenna L, et al. White matter hyperintensities on MRI in high-altitude U-2 pilots. Neurology 2013;81:729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochunov P, Thompson PM, Coyle TR, et al. Relationship among neuroimaging indices of cerebral health during normal aging. Hum Brain Mapp 2008;29:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochunov P, Robin DA, Royall DR, et al. Can structural MRI indices of cerebral integrity track cognitive trends in executive control function during normal maturation and adulthood? Hum Brain Mapp 2009;30:2581–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galluzzi S, Lanni C, Pantoni L, Filippi M, Frisoni GB. White matter lesions in the elderly: pathophysiological hypothesis on the effect on brain plasticity and reserve. J Neurol Sci 2008;273:3–9 [DOI] [PubMed] [Google Scholar]
- 11.Kochunov P, Coyle T, Lancaster J, et al. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage 2010;49:1190–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tate DF, Jefferson AL, Brickman AM, et al. Regional white matter signal abnormalities and cognitive correlates among geriatric patients with treated cardiovascular disease. Brain Imaging Behav 2008;2:200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoth KF, Poppas A, Moser DJ, Paul RH, Cohen RA. Cardiac dysfunction and cognition in older adults with heart failure. Cogn Behav Neurol 2008;21:65–72 [DOI] [PubMed] [Google Scholar]
- 14.Gunstad J, Keary TA, Spitznagel MB, et al. Blood pressure and cognitive function in older adults with cardiovascular disease. Int J Neurosci 2009;119:2228–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantarci K, Weigand SD, Przybelski SA, et al. MRI and MRS predictors of mild cognitive impairment in a population-based sample. Neurology 2013;81:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochunov P, Glahn D, Lancaster J, et al. Whole brain and regional hyperintense white matter volume and blood pressure: overlap of genetic loci produced by bivariate, whole-genome linkage analyses. Stroke 2010;41:2137–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kochunov P, Glahn DC, Lancaster J, et al. Blood pressure and cerebral white matter share common genetic factors in Mexican Americans. Hypertension 2011;57:330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Air Force. Medical Examinations and Standards. Washington, DC: Department of the Air Force; 2009. Air Force Instruction 48–123 [Google Scholar]
- 19.Kranzler JH. The construct validity of the multidimensional aptitude battery: a word of caution. J Clin Psychol 1991;47:691–697 [DOI] [PubMed] [Google Scholar]
- 20.Retzlaff PD, Callister JD, King RE. Clinical procedures for the neuropsychological evaluation of U.S. Air Force pilots. Mil Med 1999;164:514–519 [PubMed] [Google Scholar]
- 21.Helmes E, Miller M. A comparison of MicroCog and the Wechsler Memory Scale (3rd ed) in older adults. Appl Neuropsychol 2006;13:28–33 [DOI] [PubMed] [Google Scholar]
- 22.Powell DH, Kaplan EF, Whitla D, Weintraub S, Caitlin R, Funkenstein HH. MicroCog: Assessment of Cognitive Functioning Windows Edition (MicroCog for Windows) 2004, Manual/Installation Guide. San Antonio: Pearson; 2004 [Google Scholar]
- 23.Lopez SJ, Edwards LM, Floyd RK, Magyar-Moe J, Rehfeldt JD, Ryder JA. Note on comparability of MicroCog test forms. Percept Mot Skills 2001;93:825–828 [DOI] [PubMed] [Google Scholar]
- 24.Elwood RW. MicroCog: assessment of cognitive functioning. Neuropsychol Rev 2001;11:89–100 [DOI] [PubMed] [Google Scholar]
- 25.Frisoni GB, Galluzzi S, Pantoni L, Filippi M. The effect of white matter lesions on cognition in the elderly—small but detectable. Nat Clin Pract Neurol 2007;3:620–627 [DOI] [PubMed] [Google Scholar]
- 26.Kochunov P, Glahn DC, Hong LE, et al. P-selectin expression tracks cerebral atrophy in Mexican-Americans. Front Genet 2012;3:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kochunov P, Glahn D, Winkler A, et al. Analysis of genetic variability and whole genome linkage of whole-brain, subcortical, and ependymal hyperintense white matter volume. Stroke 2009;40:3685–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallenbeck JM, Bove AA, Moquin RB, Elliott DH. Accelerated coagulation of whole blood and cell-free plasma by bubbling in vitro. Aerosp Med 1973;44:712–714 [PubMed] [Google Scholar]
- 30.Tanoue K, Mano Y, Kuroiwa K, Suzuki H, Shibayama M, Yamazaki H. Consumption of platelets in decompression sickness of rabbits. J Appl Physiol 1987;62:1772–1779 [DOI] [PubMed] [Google Scholar]
- 31.Thom SR, Yang M, Bhopale VM, Huang S, Milovanova TN. Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J Appl Physiol 2011;110:340–351 [DOI] [PubMed] [Google Scholar]
- 32.Thom SR, Milovanova TN, Bogush M, et al. Microparticle production, neutrophil activation, and intravascular bubbles following open-water scuba diving. J Appl Physiol 2012;112:1268–1278 [DOI] [PubMed] [Google Scholar]
- 33.Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 2008;71:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013;81:1122–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farfel JM, Nitrini R, Suemoto CK, et al. Very low levels of education and cognitive reserve: a clinicopathological study. Neurology 2013;81:650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


