Abstract
Objective:
The objectives of the study were (1) to determine the prevalence and characteristics of pseudobulbar affect (PBA) in patients with primary lateral sclerosis (PLS) and amyotrophic lateral sclerosis (ALS) in an outpatient clinic population, and (2) to test the hypothesis that damage of inputs to the cerebellum, leading to cerebellar dysmodulation, is associated with PBA.
Methods:
Chart review of all patients with PLS and ALS seen between 2000 and 2013. The examining neurologist documented the presence or absence of PBA in 87 patients. Forty-seven patients also had diffusion tensor imaging (DTI) studies. Tract-based spatial statistics were used to compare DTI of patients with and without PBA to identify altered white matter tracts associated with PBA.
Results:
Thirty-one of 50 patients with PLS and 12 of 37 patients with ALS had PBA. Psychiatric/emotional assessment found congruence between mood and affect during episodes, but excessive magnitude of the response. DTI studies of 25 PLS and 22 ALS patient brains showed reduced fractional anisotropy of the corticospinal and callosal white matter tracts in all patients. Patients with PBA additionally had increased mean diffusivity of white matter tracts underlying the frontotemporal cortex, the transverse pontine fibers, and the middle cerebellar peduncle.
Conclusions:
PBA is common in PLS. Imaging findings showing disruption of corticopontocerebellar pathways support the hypothesis that PBA can be viewed as a “dysmetria” of emotional expression resulting from cerebellar dysmodulation.
Pseudobulbar affect (PBA) is a syndrome of involuntary emotional expression dissociated from one's true emotional experience.1 It occurs in several neurologic disorders, including amyotrophic lateral sclerosis (ALS).2,3 In one retrospective series, PBA occurred in 50% of patients with ALS.4 There have been case reports of PBA in patients with primary lateral sclerosis (PLS), a motor neuron disorder variant with relatively selective degeneration of upper motor neurons.5 However, the prevalence of PBA in PLS has not been evaluated in a large patient series.2,6 To assess the occurrence of PBA in PLS, we reviewed the charts of all patients seen in an upper motor neuron disorder clinic.
Two hypotheses have been proposed regarding the neural circuits that produce PBA. Wilson7 proposed that brainstem centers controlling laughter and crying were separately controlled by volitional and emotional pathways, and that lesions of the volitional pathway led to disinhibition of the involuntary emotional pathway. Parvizi et al.,8 who observed PBA in patients with cerebellar lesions, hypothesized that PBA results from loss of frontal cortex inputs that normally provide the cognitive, emotional, and social context that the cerebellum uses to modulate the duration and intensity of the facial and respiratory muscle activity producing emotional expression.9 The loss of such inputs would result in impaired cerebellar modulation, leading to a “dysmetria” of emotional expression. To distinguish between these hypotheses, we compared diffusion tensor imaging (DTI) between patients with and without PBA to identify white matter tracts with reduced integrity in patients with PBA.
METHODS
Standard protocol approvals, registrations, and patient consents.
All subjects gave written informed consent for protocols approved by the NIH CNS Institutional Review Board, in accord with the Declaration of Helsinki. Every patient was enrolled in a natural history study (NCT00015444); healthy controls and a subset of patients were enrolled in imaging or cognitive testing protocols (NCT00071435, NCT01517087).
Subjects.
This is a retrospective report. Ninety-eight patients evaluated in the National Institute of Neurological Disorders and Stroke upper motor neuron disorder clinic between 2000 and 2013 fulfilled a clinical diagnosis of PLS5 or ALS.10 Their charts were reviewed for clinical findings. Twenty-eight healthy controls underwent DTI scanning, neurologic examination (assessment of cranial nerves, motor strength, reflexes, tone, coordination, and sensation), and measures of finger-tapping and timed gait. All subjects were examined by a neurologist, and all healthy controls had normal neurologic examinations.
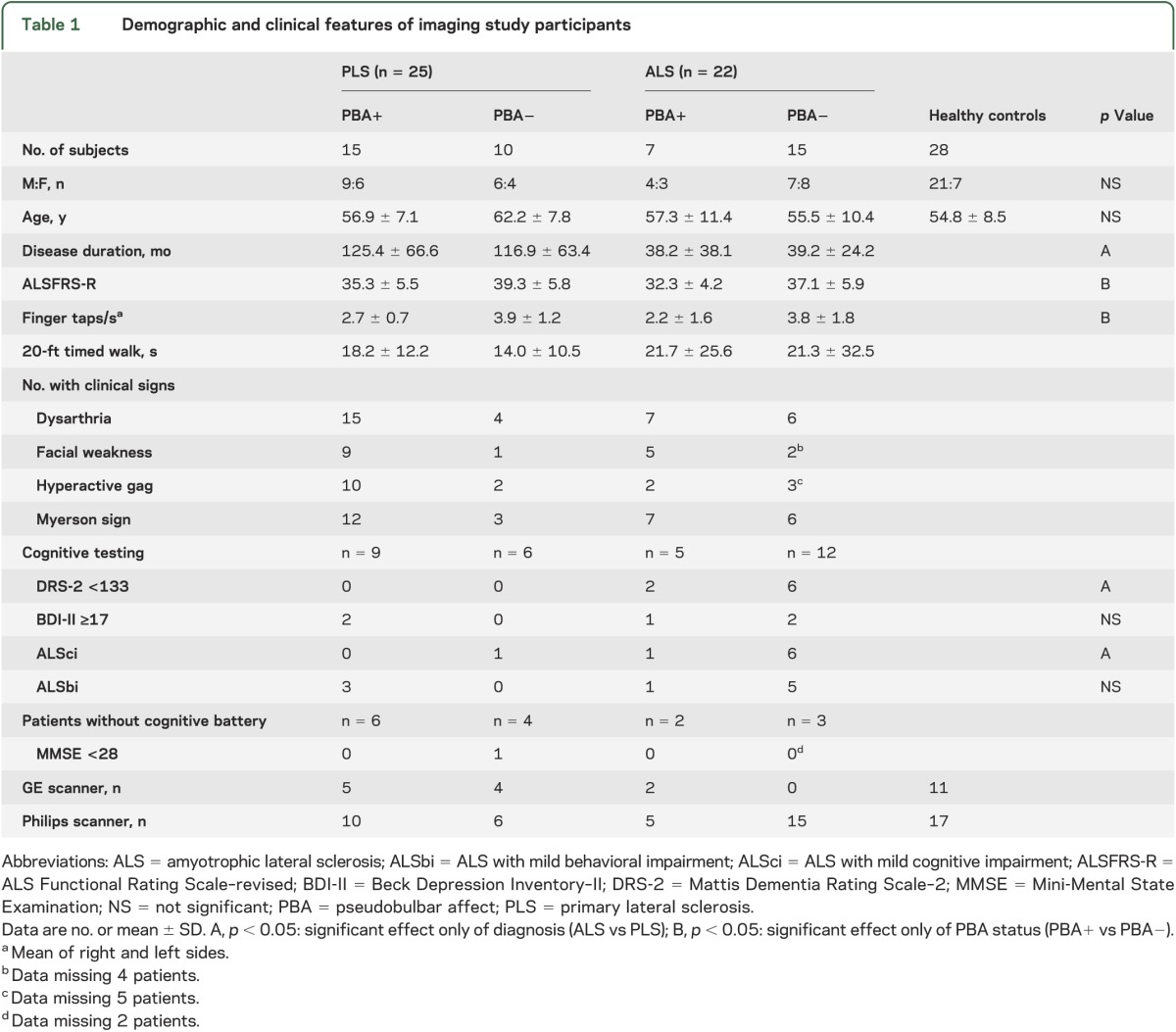
The examining neurologist had asked patients and caregivers whether symptoms of PBA were present in 87 patients (50 PLS, 37 ALS). The neurologist's classification of whether patients had PBA was used for the chart review and DTI analysis. Clinical findings of dysarthria, facial muscle weakness, brisk jaw jerk, hyperactive gag reflex, frontal release signs (suck, snout, or palmomental reflexes), Myerson sign (persistent blinking to glabellar taps), history of bladder urgency, and measures of finger-tapping rate and timed gait were tallied from the charts. Beginning in 2006, the ALS Functional Rating Scale–revised (ALSFRS-R) and Beck Depression Inventory–II (BDI-II) scores were routinely obtained in patients. DTI scans were performed in all patients without contraindications seen after 2006. Forty-seven patients (25 PLS, 22 ALS) and 28 healthy controls had DTI studies. The demographic features of the imaging subset of patients were similar to the full cohort (table 1).
Table 1.
Demographic and clinical features of imaging study participants
Between 2006 and 2010, 32 of the 47 patients who had DTI also had cognitive testing as part of a previously reported study.11 The testing battery included the Mattis Dementia Rating Scale–2 (DRS-2),12 the Delis-Kaplan Executive Function System,13 the Frontal Systems Behavioral Evaluation,14 and the University of California Los Angeles (UCLA) Neuropsychiatric Index.15 None of the patients met criteria for frontotemporal dementia.16 The Delis-Kaplan Executive Function System was used to classify patients as having ALS with mild cognitive impairment (ALSci), and the Frontal Systems Behavioral Evaluation and UCLA Neuropsychiatric Index were used to classify patients as having ALS with mild behavioral impairment (ALSbi) using consensus criteria.17 After 2010, patients were screened for cognitive deficits with the ALS Cognitive Behavioral Screen,18 the Montreal Cognitive Assessment (www.mocatest.org), or the Mini-Mental State Examination.
Although the neurologist's assessment of PBA was used for analyses, a substudy performed during this period provided additional confirmation. During 2006–2008, 30 patients had a full emotional/psychiatric evaluation that included administration of the Structured Clinical Interview for DSM Disorders (I/P)19 and prospective evaluation of symptoms of PBA by a geriatric psychiatrist with experience working with patients with motor neuron disease (E.D.H.). The psychiatric portion of the data from these evaluations has been previously published.20 Patients received the psychiatric/emotional evaluation based only on whether they were seen during this time period and were not selected on the basis of any patient characteristics. In the psychiatric interview, a diagnosis of PBA was defined as recurrent episodes of excessive or inappropriate emotional expression. All evaluations were corroborated with an informant. Congruence between mood and affect (i.e., crying triggered by sad stimuli and laughing by happy or funny stimuli), and relative magnitude of mood and affect changes were assessed (i.e., Are the changes in affect excessive compared with mood or are both mood and affect disproportionately increased?). The emotional/psychiatric evaluation of PBA was performed blinded to the results of the neurologist's evaluation.20
Imaging acquisition and processing.
MRI studies were performed on two 3T scanners (Philips Achieva, Best, the Netherlands; and GE Medical Systems, Milwaukee, WI) using a receive-only, 8-channel head coil. Multislice diffusion-weighted imaging was acquired using a single-shot echo-planar sequence with 55 to 64 contiguous axial slices (slice thickness = 2.5 mm, field of view = 240 × 240 mm). Diffusion weighting was performed along either 32 noncollinear directions with b = 0 and b = 1,000 (Philips) or 80 noncollinear directions with multiple b values: b = 0 s/mm2, b = 300 s/mm2, and b = 1,100 s/mm2 (GE). The diffusion sequence was repeated 4 times to increase the signal-to-noise ratio. The average scan time for each diffusion sequence was 4 to 6 minutes. Axial T2-weighted images were also acquired for echo-planar imaging distortion correction. Processing of diffusion-weighted images, including coregistration, correction for eddy currents and subject motion using a 12-mode affine transformation,21 skull stripping, and normalization to the ICBM-DTI-81 coordinates,22 was performed using TORTOISE (http://www.nitrc.org/projects/tortoise)23 and MRI Studio24 (www.MriStudio.org) software packages. The 6 elements of the diffusion tensor, the fractional anisotropy (FA), the eigenvectors, and the eigenvalues were calculated for each voxel. Voxel-wise statistical analysis of the FA skeletons was performed using tract-based spatial statistics (version 1.2; http://www.fmrib.ox.ac.uk/fsl/),25 with methods previously described.26
Imaging analysis.
A 2-step process was used to identify white matter tracts associated with PBA. First, each patient group was compared with healthy controls to identify motor neuron disease–related changes in white matter tracts. The Randomise tool in FSL (FMRIB's Software Library; version 2.1, 5,000 permutations), which conducts permutation-based inference on t-statistic maps,27 was used to identify clusters of voxels that differed between controls and each patient group in pairwise comparisons. The FSL TFCE (threshold-free cluster enhancement) with p < 0.05 with family-wise error correction for multiple comparisons was used. Age and scanner type (GE or Philips) were regressed as covariates. The second step of the analysis assessed changes associated with PBA, comparing PBA+ and PBA− patients only within white matter voxels that differed from controls. For this analysis, age, scanner type, ALSFRS-R, and disease diagnosis (ALS vs PLS) were analyzed as covariates, using p < 0.05 with TFCE as the threshold for significance. Voxel-wise comparisons of the mean diffusivity were conducted using the same approach. Certain computationally intensive processes, such as choosing the registration target and the permutation testing, were accelerated by the high-performance computational capabilities of the Biowulf Linux cluster (NIH, Bethesda, MD).
Statistical analysis of clinical data.
Continuous demographic and clinical data are presented as means ± SDs in the text and tables. In the chart review study, 2-sample t tests were used to test differences in continuous demographic data between patients with and without PBA. Variables were checked for normality and log transformation was applied to disease duration and an inverse square-root transformation was applied to measures of timed gait. Logistic regression was used to determine the association of clinical variables with PBA status. The first step consisted of selecting covariates to use in the regression model, using a low threshold of p < 0.1 in order to include variables with small or possible effects. The diagnosis (PLS vs ALS) was significant and was used as a covariate. Age and sex were not significant and were not included as covariates in the regression model. An exact p value was computed, and p < 0.01 was used to test the significance of association between PBA and clinical variables, adjusting for diagnosis. In the imaging substudy, Fisher exact test was used to determine the association between PBA (PBA+ vs PBA−) and clinical variables, DRS-2 (≥133 vs <133), the BDI-II (<17 vs ≥17), ALSci (present vs absent), and ALSbi (present vs absent), using p < 0.05 to determine significance. A 2-factor analysis of variance was used to assess the effect of disease diagnosis (ALS vs PLS) and PBA status on continuous clinical variables.
RESULTS
Chart review.
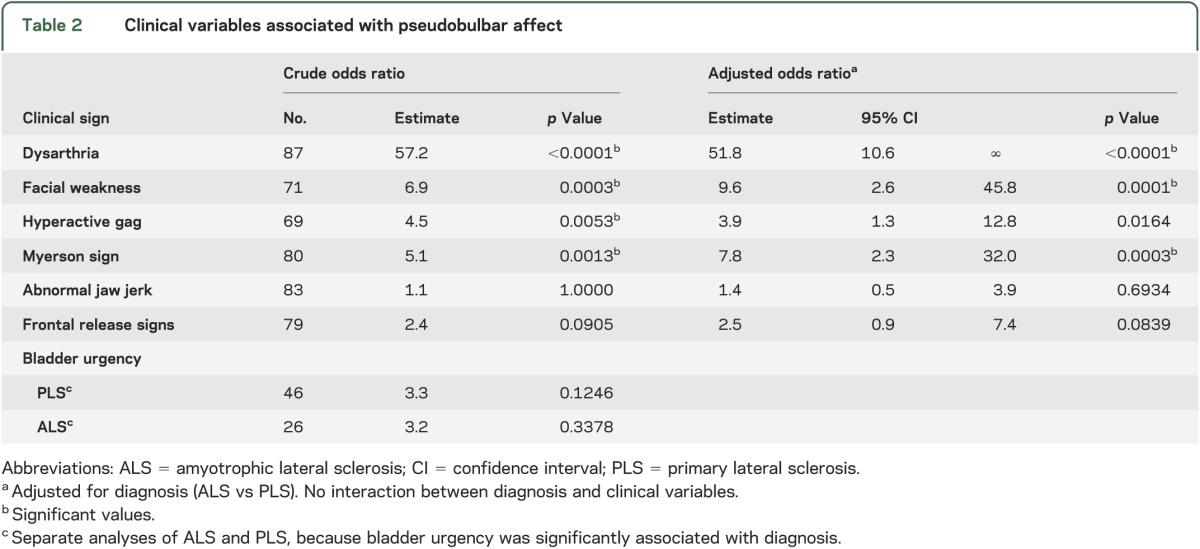
Among the 87 patients in the chart review, 43 had PBA (PBA+) and 44 did not have PBA (PBA−). Thirty-one of the 50 patients with PLS and 12 of 37 patients with ALS were PBA+. There were no significant differences in age (58.7 ± 8.7 vs 57.4 ± 9.8 years) or sex between PBA+ and PBA− groups. However, patients with PBA had slightly worse motor function as evidenced by slower finger-tapping rates (PBA+: 2.6 ± 1.1 taps/s; PBA−: 3.7 ± 1.6 taps/s; p < 0.001), although there was no difference in timed gait (PBA+: 27.8 ± 36.1 seconds; PBA−: 23.5 ± 33.1 seconds; p = 0.195). All PBA+ patients had upper motor neuron signs on clinical examination. Dysarthria was present in all PBA+ patients and half of the PBA− patients (figure 1). A logistic regression analysis, controlling for diagnosis of PLS or ALS, showed that facial muscle weakness, hyperactive gag reflex, and Myerson sign were associated with PBA+, but that brisk jaw jerks, frontal release signs, and bladder urgency were not associated with PBA+ (table 2).
Figure 1. Clinical findings associated with pseudobulbar affect in the chart review of 87 patients.
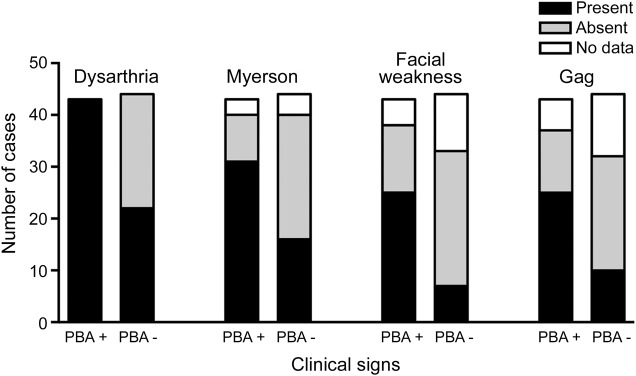
The clinical finding is noted above a pair of bars, one indicating patients with pseudobulbar affect (PBA+) and the other indicating patients without pseudobulbar affect (PBA−). Black bars indicate the number of patients in whom the clinical finding was present, gray bars indicate the number in whom the finding was absent, and open bars indicate patients for whom no documentation of the finding was recorded in the chart.
Table 2.
Clinical variables associated with pseudobulbar affect
In the prospective emotional/psychiatric evaluation, 12 of the 17 patients with PLS had PBA, and 2 of the 13 patients with ALS had PBA. The psychiatrist's determination of PBA agreed with the neurologist's assessment in all but 2 of the 30 subjects. In both discrepant cases, the patient met criteria for current major depressive disorder, suggesting that an active mood disorder may make the assessment of PBA more difficult. However, there was no significant difference in the proportion of subjects with PBA with active depressive symptoms (Fisher exact test, p = 0.2602), suggesting that, in this small sample, PBA did not affect the incidence of mood disorder diagnoses and vice versa. In all subjects with PBA, symptoms were mood-congruent. That is, excessive crying was triggered by sad stimuli and excessive laughing by happy or funny stimuli. In all but one subject with PBA, affect/emotional expression was increased out of proportion to mood changes. Several subjects mentioned the experience of laughing and crying excessively while internally noting that they no longer felt happy or sad.
Imaging study.
Clinical findings.
In the subset of 47 patients in the imaging study, there were no significant differences in age or sex between PBA+ and PBA− patients (table 1). A higher proportion of patients with PLS than patients with ALS had PBA, similar to the full cohort in the chart review. As expected, patients with PLS had longer disease durations than patients with ALS, but there was no significant interaction between diagnosis (ALS vs PLS) and PBA status (PBA+ vs PBA−). However, the ALSFRS-R score and finger-tapping rates were significantly lower for PBA+ patients compared with PBA− patients, suggesting more impaired motor function in PBA+. Of the 32 patients who had cognitive testing batteries, 8 of 17 patients with ALS scored below 133 on the DRS-2, but there was no difference in the proportions of ALS and PLS patients that were PBA+ and PBA− (Fisher exact test, p > 0.05). No patient with PLS in this subset had an abnormal DRS-2 score. The proportions of patients who met criteria for ALSci or ALSbi, or had BDI-II >17 did not differ between the PBA+ and PBA− patient groups. In the 15 patients who did not have full cognitive testing, the Mini-Mental State Examination did not differ.
Imaging findings.
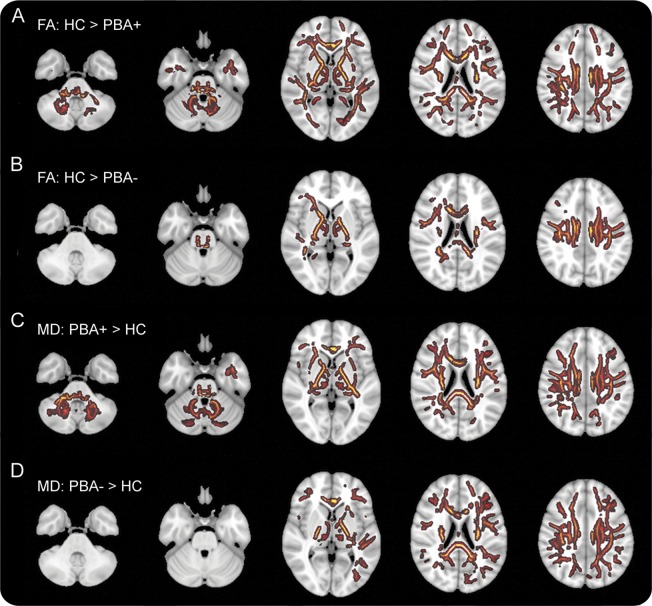
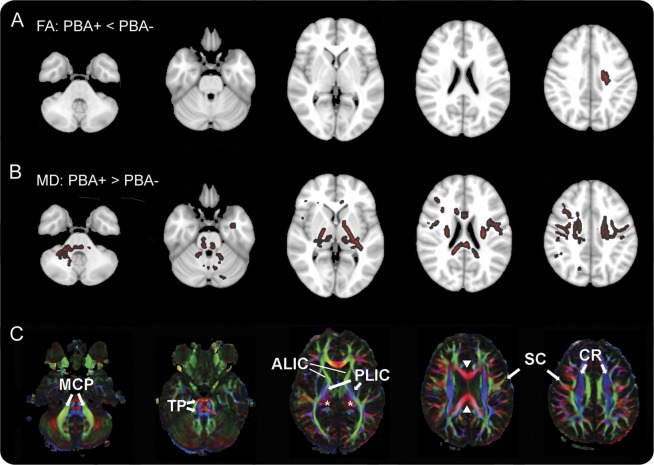
In the first step of the tract-based spatial statistics analysis, both PBA+ and PBA− patients were found to have reduced FA and increased mean diffusivity in multiple white matter areas compared with healthy controls (figure 2). The affected regions included the subcortical white matter, internal capsule, and the corpus callosum, consistent with other studies.26,28,29 In the second step, to identify white matter regions associated with PBA, white matter voxels that differed from controls were compared between PBA+ and PBA− patients, with ALSFRS-R and diagnosis (ALS vs PLS) as covariates. This analysis revealed a small region of white matter underlying the left motor cortex with reduced FA in PBA+ compared with PBA− patients (figure 3, row A). In addition, PBA+ patients had increased mean diffusivity in several white matter regions compared with PBA− patients (figure 3, row B). Notably, there was greater mean diffusivity in the right middle cerebellar peduncle, transverse pontine fibers, and white matter tracts underlying the frontotemporal cortex in PBA+ patients compared with PBA− patients.
Figure 2. Differences in diffusion tensor imaging between patients and healthy controls.
Tract-based spatial statistics showing differences in diffusion properties between white matter skeletons of healthy controls (HC) and patients with pseudobulbar affect (PBA+, rows A and C) and between healthy controls and patients without pseudobulbar affect (PBA−, rows B and D) projected on axial sections. White matter areas shown in color were significantly different between patients and healthy controls (threshold-free cluster enhancement with p < 0.05, correction using family-wise error rate). (A, B) Differences in fractional anisotropy (FA) maps. (C, D) Differences in mean diffusivity (MD) maps. Axial sections are shown in radiologic convention with right brain on the left side.
Figure 3. Differences in diffusion tensor imaging between patients with and without pseudobulbar affect.
(A, B) Color indicates white matter regions where diffusion properties differ between patients with pseudobulbar affect (PBA+) and patients without pseudobulbar affect (PBA−) (tract-based spatial statistics, threshold-free cluster enhancement, p < 0.05). (C) Directionally encoded color map providing labels for selected structures. (A) Fractional anisotropy (FA) was reduced in PBA+ patients only in a small region underlying the left motor cortex. (B) Mean diffusivity (MD) was greater in PBA+ patients in multiple white matter areas, including the middle cerebellar peduncles (MCP), transverse pontine fibers (TP), bilateral posterior limbs of the internal capsule (PLIC), left anterior limb of the internal capsule (ALIC), thalamus (asterisks), frontotemporal subcortical white matter, corona radiata (CR), white matter underlying the motor cortex (SC), and corpus callosum (solid arrowheads). Axial sections are shown in radiologic convention with right brain on the left side.
DISCUSSION
Two-thirds of the patients with PLS in our clinic had PBA, a frequency higher than published reports in ALS.4 All PBA+ patients had dysarthria and upper motor neuron signs. PBA was associated with other clinical findings, such as hyperactive gag and Myerson sign. Although all patients in the DTI study had abnormalities in the corticospinal tract and corpus callosum typical for motor neuron disease, patients with PBA also had increased mean diffusivity in white matter tracts underlying frontotemporal regions, the pons, and cerebellum. Increased mean diffusivity is thought to signify the loss of integrity of myelinated axons.30 The altered white matter tracts associated with PBA connect the frontal cortex to the cerebellum via the anterior limb of the internal capsule and through the pontine nuclei. Affected axons are likely to arise from neurons of cortical regions projecting to brainstem structures that have been implicated in emotional processing, including periaqueductal gray neurons projecting to medullary premotor interneurons,31 and from pontine nuclei, in regions where spectroscopy studies indicate neuronal loss.32 Functional imaging studies suggest that the network for emotional processing includes the prefrontal and orbitofrontal cortex, insula, anterior cingulate cortex, and regions of the cerebellum.1,33,34 Clinical observations of stroke patients who developed emotional facial paresis in the setting of preserved volitional expression also implicate the frontal cortex.7,35 The cerebellum has been shown to have a modulatory role in behavioral and cognitive control, receiving input from frontal and paralimbic cortex through pontine relay nuclei.34,36 In the hypothesis proposed by Parvizi et al.9 to explain PBA, inputs from the frontal and association cortex to the cerebellum are used to modulate the duration and intensity of facio-respiratory muscle activation in emotional expression. Our imaging data support this hypothesis, and suggest that PBA can be considered a “dysmetria” of emotional expression1,8 that results from loss of corticopontine inputs. However, it is important to note that diffusion changes were also seen in white matter tracts from the motor cortex. The combination of impaired volitional motor control and cerebellar dysmodulation may be necessary to produce PBA. Impairment of pathways for volitional expression of emotion, central to Wilson's7 proposal that disinhibition of pontine centers for emotional expression caused PBA, may be contributory.
PBA is one of a number of terms that have been used in the literature to describe disorders with paroxysmal outbursts of emotional expression. Emotional lability is the term applied to a subset of PBA in which episodes are congruent to mood but disproportionate to the triggering stimulus, and unable to be fully suppressed.1,9 The emotional/psychiatric interviews conducted in our study showed that affect was generally mood-congruent with a triggering stimulus, but out of proportion to mood. This fits with emotional lability, in agreement with previous studies of PBA in patients with ALS.37 The psychiatric/emotional data support the idea that the initiation of the emotional response arises from the areas normally involved in emotional processing and the motor expression of emotion.1,33,34 PBA in our subjects was also independent of mood disorders. As one subject with major depressive disorder and PBA said, “I can tell the difference between when I'm crying because I'm feeling depressed and when I'm crying inappropriately.” Clinical measures of cognitive status were not associated with PBA. Although previous studies found alterations in extramotor cortical areas associated with cognitive dysfunction in ALS or PLS,11,38 the primary structural correlates seem to be interhemispheric long association tracts. However, an association between oral naming and integrity of portions of cerebellar white matter was reported in one recent study39 that defined cognitive impairment in PLS less stringently than consensus criteria and did not assess for concurrent PBA.17 In our study, PBA was independent of cognitive impairment, and thus cognitive impairment seems unlikely to account for diffusivity changes observed in pontocerebellar tracts.
This retrospective study has several limitations. First, classification of patients as PBA+ or PBA− was based on a neurologist's clinical assessment, rather than using an instrument specific for measuring PBA.40 Nevertheless, there was good agreement of the neurologist's classification with the independent emotional/psychiatric evaluation. Another limitation is the likelihood of sample selection bias toward stable patients who are willing to travel to NIH to participate in research and are able to tolerate imaging studies. This may account for the relatively low proportion of patients with cognitive impairment.
The main finding of our chart review is that PBA is common among patients with PLS. PBA adversely affects the quality of life, leading to social isolation and housebound status in many different diseases, including ALS.6 Treatment can ameliorate symptoms of PBA and improve ratings of the quality of life in patients with ALS.3 Such treatment may be of benefit to patients with PLS as well. Our imaging study found white matter differences associated with PBA that implicate corticopontocerebellar circuits in its origin, supporting the view that PBA is a dysmetria of emotional expression.
GLOSSARY
- ALS
amyotrophic lateral sclerosis
- ALSbi
amyotrophic lateral sclerosis with mild behavioral impairment
- ALSci
amyotrophic lateral sclerosis with mild cognitive impairment
- ALSFRS-R
Amyotrophic Lateral Sclerosis Functional Rating Scale–revised
- BDI-II
Beck Depression Inventory–II
- DRS-2
Mattis Dementia Rating Scale–2
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FSL
FMRIB's Software Library
- PBA
pseudobulbar affect
- PLS
primary lateral sclerosis
- TFCE
threshold-free cluster enhancement
- UCLA
University of California Los Angeles
AUTHOR CONTRIBUTIONS
Dr. Floeter was responsible for study concept and design, acquisition of clinical data, overall analysis and interpretation, writing and revising the manuscript, and study supervision. Mr. Katipally was responsible for analysis and interpretation of imaging data. Ms. Kim was responsible for analysis and interpretation of chart review data. Ms. Schanz was responsible for acquisition of imaging data and initial data analysis and interpretation. Mr. Stephen was responsible for acquisition of clinical data and initial data analysis and interpretation. Ms. Danielian was responsible for acquisition of imaging data and analysis and interpretation. She trained the postbaccalaureate students in imaging analysis methods and performed quality checks of the imaging data analyses. She critically read the manuscript. Dr. Wu was responsible for statistical analysis and interpretation, and critical revision of the manuscript for important intellectual content. Dr. Huey was responsible for acquisition of psychiatric/emotional data and their analysis and interpretation, and for critical revision of the manuscript for important intellectual content. Dr. Meoded was responsible for designing MRI sequences for acquisition of imaging data, for analysis and interpretation of imaging data, critical revision of the manuscript for important intellectual content, and for study supervision.
STUDY FUNDING
Supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NINDS), NIH (Z01 NS002976). E.D. Huey was funded by NIH/NINDS grants R00NS060766 and R01NS076837 and Columbia University's CTSA grant UL1RR024156 from NCATS-NCRR/NIH.
DISCLOSURE
M. Floeter is an employee in the National Institute of Neurological Disorders and Stroke (NINDS) intramural program funded by Z01 NS002976. She is on the editorial board of Muscle and Nerve. R. Katipally and M. Kim were summer student interns in NINDS. O. Schanz is a postbaccalaureate trainee in NINDS. M. Stephen was a postbaccalaureate trainee in NINDS. L. Danielian is a biomedical engineer employed by NINDS. T. Wu is a statistician employed in the NINDS intramural program. E. Huey was a fellow in NINDS during the time of the study, and is currently an assistant professor of Psychiatry and Neurology at Columbia University, NY. He is funded by NIH/NINDS grants R00NS060766 and R01NS076837 and Columbia University's CTSA grant UL1RR024156 from NCATS-NCRR/NIH. A. Meoded is a radiologist who is currently a postdoctoral fellow in the NINDS intramural program. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lauterbach EC, Cummings JL, Kuppuswamy PS. Toward a more precise, clinically-informed pathophysiology of pathological laughing and crying. Neurosci Biobehav Rev 2013;37:1893–1916 [DOI] [PubMed] [Google Scholar]
- 2.Work SS, Colamonico JA, Bradley WG, Kaye RE. Pseudobulbar affect: an under-recognized and under-treated neurological disorder. Adv Ther 2011;28:586–601 [DOI] [PubMed] [Google Scholar]
- 3.Brooks BR, Thisted RA, Appel SH, et al. Treatment of pseudobulbar affect in ALS with dextromethorphan/quinidine: a randomized trial. Neurology 2004;63:1364–1370 [DOI] [PubMed] [Google Scholar]
- 4.Gallagher JP. Pathologic laughter and crying in ALS: a search for their origin. Acta Neurol Scand 1989;80:114–117 [DOI] [PubMed] [Google Scholar]
- 5.Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis: clinical features, neuropathology and diagnostic criteria. Brain 1992;115:495–520 [DOI] [PubMed] [Google Scholar]
- 6.Brooks BR, Crumpacker D, Fellus J, Kantor D, Kaye RE. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One 2013;8:e72232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson SAK. Some problems in neurology: II: pathological laughing and crying. J Neurol Psychopathol 1924;4:299–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parvizi J, Anderson SW, Martin CO, Damasio H, Damasio AR. Pathological laughter and crying: a link to the cerebellum. Brain 2001;124:1708–1719 [DOI] [PubMed] [Google Scholar]
- 9.Parvizi J, Coburn KL, Shillcutt SD, Coffey CE, Lauterbach EC, Mendez MF. Neuroanatomy of pathological laughing and crying: a report of the American Neuropsychiatric Association Committee on Research. J Neuropsychiatry Clin Neurosci 2009;21:75–87 [DOI] [PubMed] [Google Scholar]
- 10.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–299 [DOI] [PubMed] [Google Scholar]
- 11.Meoded A, Kwan JY, Peters TL, et al. Imaging findings associated with cognitive performance in primary lateral sclerosis and amyotrophic lateral sclerosis. Dement Geriatr Cogn Dis Extra 2013;3:233–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rascovsky K, Salmon DP, Hansen LA, Galasko D. Distinct cognitive profiles and rates of decline on the Mattis Dementia Rating Scale in autopsy-confirmed frontotemporal dementia and Alzheimer's disease. J Int Neuropsychol Soc 2008;14:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc 2004;10:301–303 [DOI] [PubMed] [Google Scholar]
- 14.Stout JC, Ready RE, Grace J, Malloy PF, Paulsen JS. Factor analysis of the Frontal Systems Behavior Scale (FrSBe). Assessment 2003;10:79–85 [DOI] [PubMed] [Google Scholar]
- 15.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314 [DOI] [PubMed] [Google Scholar]
- 16.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strong MJ, Grace GM, Freedman M, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2009;10:131–146 [DOI] [PubMed] [Google Scholar]
- 18.Woolley SC, York MK, Moore DH, et al. Detecting frontotemporal dysfunction in ALS: utility of the ALS Cognitive Behavioral Screen (ALS-CBS). Amyotroph Lateral Scler 2010;11:303–311 [DOI] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCID-I/P, version 2.0). New York: Biometrics Research, New York State Psychiatric Institute; 1995 [Google Scholar]
- 20.Huey ED, Koppel J, Armstrong N, Grafman J, Floeter MK. A pilot study of the prevalence of psychiatric disorders in PLS and ALS. Amyotroph Lateral Scler 2010;11:293–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang J, Hrabe J, Kangarlu A, et al. Correction of eddy-current distortions in diffusion tensor images using the known directions and strengths of diffusion gradients. J Magn Reson Imaging 2006;24:1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008;40:570–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierpaoli C, Walker L, Irfanoglu MO, et al. TORTOISE: an integrated software package for processing of diffusion MRI data. Presented at the 18th Annual Meeting of the International Society of Magnetic Resonance in Medicine; 2010; Stockholm; 1597
- 24.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 2006;81:106–116 [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–1505 [DOI] [PubMed] [Google Scholar]
- 26.Iwata NK, Kwan JY, Danielian LE, et al. White matter alterations differ in primary lateral sclerosis and amyotrophic lateral sclerosis. Brain 2011;134:2642–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002;15:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agosta F, Pagani E, Petrolini M, et al. Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor MR imaging tractography study. AJNR Am J Neuroradiol 2010;31:1457–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciccarelli O, Behrens TE, Johansen-Berg H, et al. Investigation of white matter pathology in ALS and PLS using tract-based spatial statistics. Hum Brain Mapp 2009;30:615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003;20:1714–1722 [DOI] [PubMed] [Google Scholar]
- 31.Holstege G, Bandler R, Saper CB. The emotional motor system. Prog Brain Res 1996;107:3–6 [DOI] [PubMed] [Google Scholar]
- 32.Cwik VA, Hanstock CC, Allen PS, Martin WR. Estimation of brainstem neuronal loss in amyotrophic lateral sclerosis with in vivo proton magnetic resonance spectroscopy. Neurology 1998;50:72–77 [DOI] [PubMed] [Google Scholar]
- 33.Lee GP, Meador KJ, Loring DW, et al. Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cogn Behav Neurol 2004;17:9–17 [DOI] [PubMed] [Google Scholar]
- 34.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 2010;46:831–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopf HC, Muller-Forell W, Hopf NJ. Localization of emotional and volitional facial paresis. Neurology 1992;42:1918–1923 [DOI] [PubMed] [Google Scholar]
- 36.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 2004;16:367–378 [DOI] [PubMed] [Google Scholar]
- 37.Olney NT, Goodkind MS, Lomen-Hoerth C, et al. Behaviour, physiology and experience of pathological laughing and crying in amyotrophic lateral sclerosis. Brain 2011;134:3458–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agosta F, Galantucci S, Riva N, et al. Intrahemispheric and interhemispheric structural network abnormalities in PLS and ALS. Hum Brain Mapp 2014;35:1710–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canu E, Agosta F, Galantucci S, et al. Extramotor damage is associated with cognition in primary lateral sclerosis patients. PLoS One 2013;8:e82017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore SR, Gresham LS, Bromberg MB, Kasarkis EJ, Smith RA. A self report measure of affective lability. J Neurol Neurosurg Psychiatry 1997;63:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]