Abstract
Objective:
To identify risk factors for intracranial hemorrhage in the natural history course of brain arteriovenous malformations (AVMs) using individual patient data meta-analysis of 4 existing cohorts.
Methods:
We harmonized data from Kaiser Permanente of Northern California (n = 856), University of California San Francisco (n = 787), Columbia University (n = 672), and the Scottish Intracranial Vascular Malformation Study (n = 210). We censored patients at first treatment, death, last visit, or 10-year follow-up, and performed stratified Cox regression analysis of time-to-hemorrhage after evaluating hemorrhagic presentation, sex, age at diagnosis, deep venous drainage, and AVM size as predictors. Multiple imputation was performed to assess impact of missing data.
Results:
A total of 141 hemorrhage events occurred during 6,074 patient-years of follow-up (annual rate of 2.3%, 95% confidence interval [CI] 2.0%–2.7%), higher for ruptured (4.8%, 3.9%–5.9%) than unruptured (1.3%, 1.0%–1.7%) AVMs at presentation. Hemorrhagic presentation (hazard ratio 3.86, 95% CI 2.42–6.14) and increasing age (1.34 per decade, 1.17–1.53) independently predicted hemorrhage and remained significant predictors in the imputed dataset. Female sex (1.49, 95% CI 0.96–2.30) and exclusively deep venous drainage (1.60, 0.95–2.68, p = 0.02 in imputed dataset) may be additional predictors. AVM size was not associated with intracerebral hemorrhage in multivariable models (p > 0.5).
Conclusion:
This large, individual patient data meta-analysis identified hemorrhagic presentation and increasing age as independent predictors of hemorrhage during follow-up. Additional AVM cohort data may further improve precision of estimates, identify new risk factors, and allow validation of prediction models.
Brain arteriovenous malformations (BAVMs) are the most common cause of intracranial hemorrhage in younger people, who are at risk of long-term morbidity and mortality. The most common presentation is hemorrhage (approximately 50%), followed by seizures and neurologic deficits.
Understanding hemorrhage risk in the natural history course of BAVM is crucial when making treatment decisions. However, obtaining accurate estimates is challenging because of short follow-up times from censoring by treatment, low event rates, and low prevalence of disease, which are major limitations of all BAVM longitudinal studies. Current BAVM annual hemorrhage rates range from 2%–4% overall1–9 to 1%–3% among unruptured patients,1,4,6,9 but vary widely depending on the number of overlapping risk factors.9 More importantly, the relative effects of risk factors, such as age, sex, anatomical location, and angioarchitectural attributes, also vary between studies. Sufficient sample size and events are generally not available within single centers to allow adequate assessment of subgroups.
These considerations form the rationale for the Multicenter AVM Research Study (MARS). We illustrate the feasibility of pooling longitudinal data from existing cohorts, and perform an individual patient data meta-analysis (IPDMA) to evaluate risk predictors.10,11 IPDMA is the PROGRESS-recommended12 approach for prognostic research and has a number of advantages over standard methods, allowing for meta-analysis of multivariable models and internal validation.
METHODS
Study cohorts.
Individual-level survival data were available from 4 cohorts participating in MARS: Kaiser Permanente of Northern California AVM Study (KPNC, n = 856),2 University of California San Francisco Brain AVM Study Project (UCSF, n = 787),4 the Columbia AVM Database Project (COL, n = 672),9 and the Scottish Intracranial Vascular Malformation Study (SIVMS, n = 210).13,14
COL and UCSF prospectively collected data from all consecutive BAVM cases seen at their institutions during 1989–2003 and 2000–2010, respectively.4,9 A multidisciplinary team of neurosurgeons, neurointerventional radiologists, and neurologists discuss all cases and confirm the diagnosis of BAVM on neuroimaging, and follow-up patients at clinic visits. At UCSF, the study coordinator obtains consent from all patients with BAVM, abstracts data, and performs annual phone follow-up in addition to seeing patients at clinic visits to obtain outcome data.
KPNC cases were identified from approximately 3 million health care members through 2006 using computerized search of all databases for ICD-9 code 747.81 and CPT-4 codes germane to BAVM.2 A trained medical record abstractor reviewed all charts to verify the diagnosis, and abstracted relevant clinical and outcome data. The definitive diagnosis of BAVM is based on 2 or more sources, including clinical history, neuroimaging (CT, MRI, or angiography as a single study or in combination), and pathology. Study neurologists adjudicated any suspect cases.
SIVMS is a prospective, population-based cohort study that used anonymized data extracts from multiple overlapping sources13 in a National Health Service clinical audit to identify all cases of intracranial vascular malformations in Scotland (www.saivms.scot.nhs.uk). For MARS, we included residents 16 years or older with a definite diagnosis of BAVM between 1999 and 2003, made on the basis of pathologic examination or neuroimaging and confirmed by 2 study neuroradiologists.14 Annual surveillance of general practitioner and hospital medical records, and annual postal questionnaires to consenting participants on each anniversary of BAVM diagnosis were used to identify events during follow-up.
Standard protocol approvals, registrations, and patient consents.
All studies were approved by institutional ethics committees. A waiver of consent was obtained for KPNC and COL because data were collected from medical records with no direct patient contact. Informed consent was obtained from patients with BAVM participating in the UCSF study and SIVMS to ascertain events from patients.
Data collection.
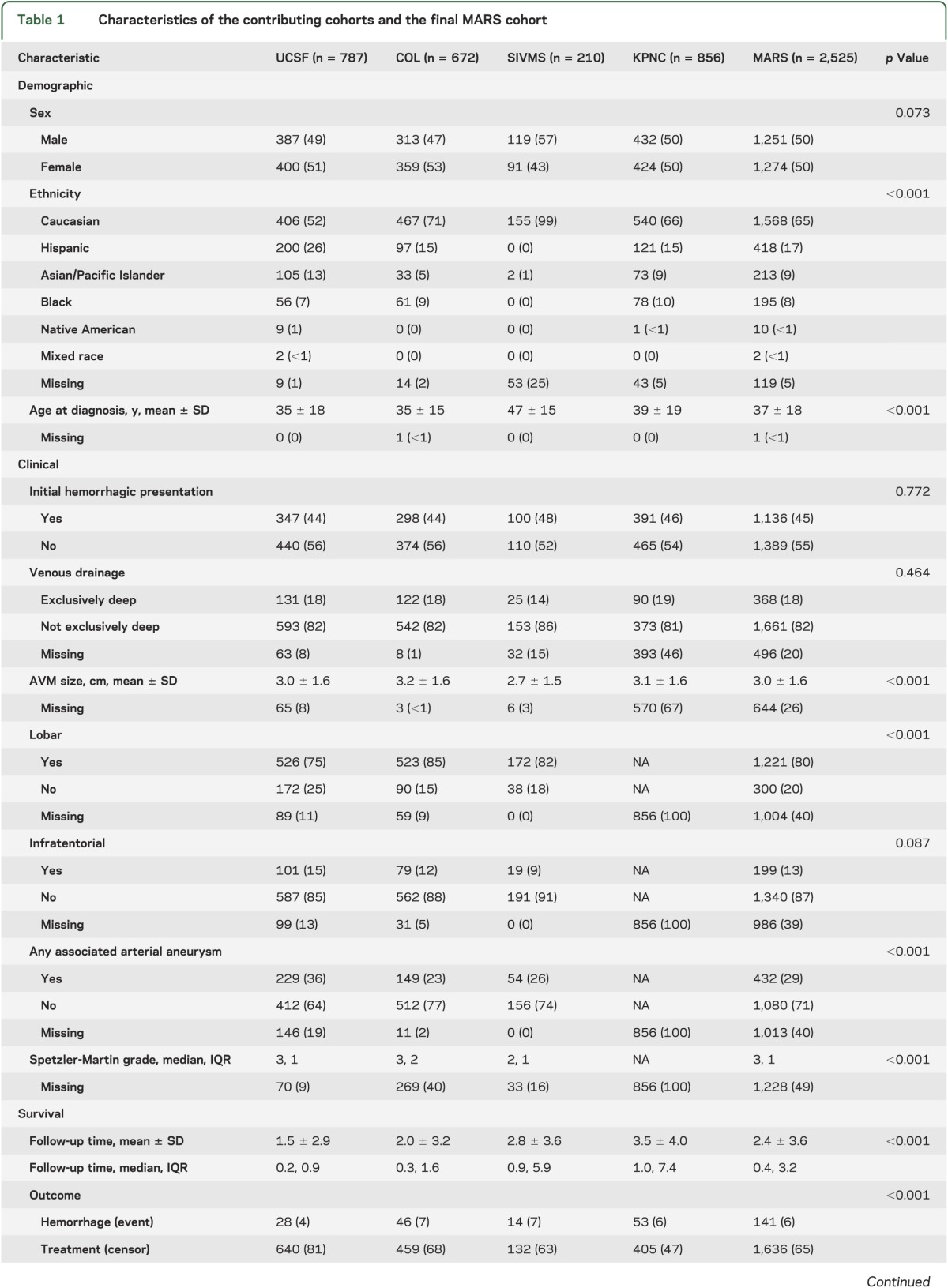
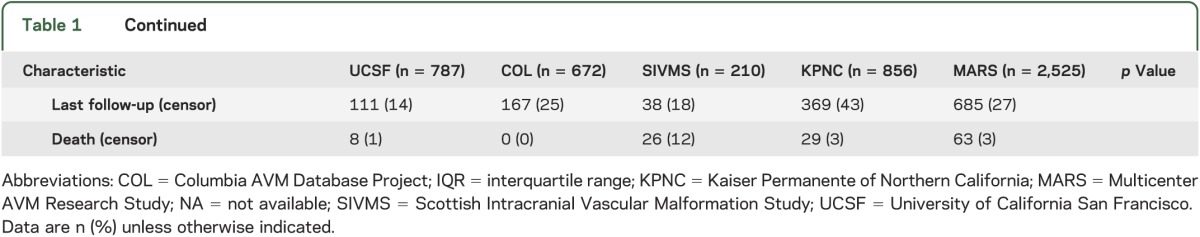
Data fields were harmonized following standardized guidelines for reporting AVM terminology15; the codebook is provided in table e-1 on the Neurology® Web site at Neurology.org. We focused on a limited subset of predictors in common among the 4 cohorts (age at presentation and diagnosis, initial presentation, and sex), and expanded to include angiographic data (maximum nidus diameter in centimeters [size], venous drainage pattern, lobar location, infratentorial location, and associated arterial aneurysm [AAA]), for which a variable degree of missing data was present (table 1). Completeness of angiographic variables was influenced by study design and the appropriateness of undertaking digital subtraction angiography for the patients in question. For analysis, we considered “age at diagnosis” to be the onset of symptoms or signs leading to diagnosis. Hemorrhagic presentation was defined as fresh bleeding into parenchyma or CSF spaces by CT or MRI with accompanying clinical signs or symptoms. “Associated arterial aneurysm” included any flow-related (feeding artery) aneurysm or intranidal aneurysm (excluding venous varices). Venous drainage pattern was dichotomized into exclusively deep vs not exclusively deep.
Table 1.
Characteristics of the contributing cohorts and the final MARS cohort
Statistical analysis.
Descriptive statistics were compared between cohorts using χ2 tests for categorical variables and analysis of variance for continuous variables. Kaplan–Meier survival curves and log-rank tests were used to describe hemorrhage-free survival rates. Patients were censored at earliest occurrence of first BAVM treatment, death, or last follow-up visit. Follow-up times were censored at 10 years to make the cohorts more comparable, resulting in different hemorrhage rates than previously published.
We performed IPDMA using a 1-step approach.10 Significant predictors (p < 0.05) in at least 1 of the 4 cohorts were included in combined multivariable Cox regression analysis of time-to-hemorrhage in the natural untreated course after diagnosis. Proportional hazards were tested using Schoenfeld residuals, and a time-varying covariate was included for predictors that violated the assumption. All pairwise interaction terms between cohort and predictors were examined by likelihood ratio test for inclusion in multivariable models; nonsignificant interaction terms (p > 0.10) were excluded from final models. To allow for differing hemorrhage rates across cohorts, we used stratified Cox models.
We addressed missing clinical data by comparing models of different complexity and by multiple imputation. Three multivariable Cox regression analyses were considered. Model A allowed for the largest sample size (n = 1,776), and included age at diagnosis (decades), female sex, initial hemorrhagic presentation, maximal BAVM size (cm), and exclusively deep venous drainage. Model B included all variables in model A plus 2 AVM location variables, AAA, and an interaction term of AAA with time because of nonproportional hazards (n = 1,412). As a sensitivity analysis, model C was restricted to model B patients (n = 1,412) but included the same variables as model A to evaluate whether model B results were attributable to additional angiographic variables or to the subset of patients included.
For multiple imputation, we generated 50 imputation datasets with a multivariate normal regression imputation algorithm,16 thereby allowing us to include all 2,525 patients in the IPDMA. Imputed values of age at diagnosis, AVM size, and venous drainage pattern were generated based on the following independent variables: cohort, sex, initial hemorrhagic presentation, hemorrhage event (yes or no), and nontruncated, log-transformed survival time.
We also used 10-fold cross-validation to assess overfitting and compared models on the basis of Harrell’s C statistic.17 All analyses were performed using Stata version 12.1 (StataCorp LP, College Station, TX); forest plots were generated using metan.18
RESULTS
Demographic, clinical, and survival analysis characteristics of the 4 cohorts are presented in table 1. Missing data for each variable are also listed by cohort where applicable.
Baseline characteristics.
There was no significant difference in sex (p = 0.07), initial hemorrhagic presentation (p = 0.77), exclusively deep venous drainage (p = 0.46), and infratentorial location (p = 0.09) between cohorts (table 1). The majority of patients with BAVM were of Caucasian race/ethnicity (65%), and this was the largest subgroup within each cohort. However, race-ethnic differences were observed reflecting the geographic catchment areas (p < 0.001). Patients with BAVM in the SIVMS cohort were also, on average, older (47 ± 15 years) and had smaller AVM size (2.7 ± 1.5 cm) compared with other cohorts. UCSF had a lower percentage of AVMs in lobar location (75%) but a greater percentage of AVMs with AAA (36%).
First and recurrent hemorrhage rates.
Among 2,525 patients, 141 hemorrhage events occurred during 6,074 person-years of follow-up, yielding an overall annual hemorrhage rate of 2.3% (95% confidence interval [CI] 2.0%–2.7%), which was higher in ruptured (4.8%, 95% CI 3.9%–5.9%) than unruptured (1.3%, 95% CI 1.0%–1.7%) AVMs at presentation (table 2). There was some evidence that hemorrhage rates varied by cohort (p = 0.051, figure 1); COL had the highest intracerebral hemorrhage rate (3.5%), followed by SIVMS (2.4%), UCSF (2.3%), and KPNC (1.8%) (table 2). Thus, to combine data into a single model for IPDMA, we allowed the baseline hazards for each cohort to vary in stratified Cox regression analysis. Sensitivity analysis of hemorrhage rates when truncating survival times to 1, 2, 5, and 10 years after diagnosis suggests that hemorrhage events were more likely to occur in a short window after presentation rather than at a later time. This is reflected by higher hemorrhage rates in the 1- and 2-year analyses and lower rates in the 5- and 10-year analyses (table e-2).
Table 2.
Hemorrhage rates (per 100 patient-years) overall and by mode of initial presentation
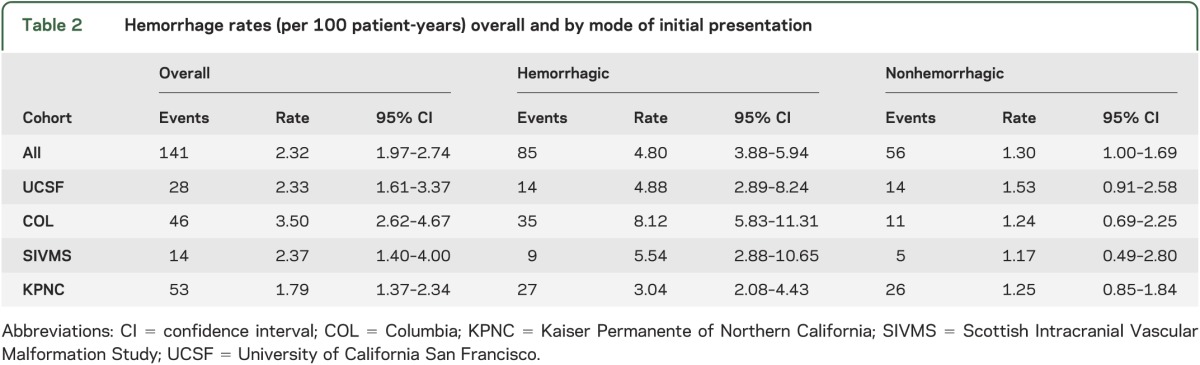
Figure 1. Survival curves of time-to-hemorrhage in patients with untreated brain AVM, by MARS cohort.
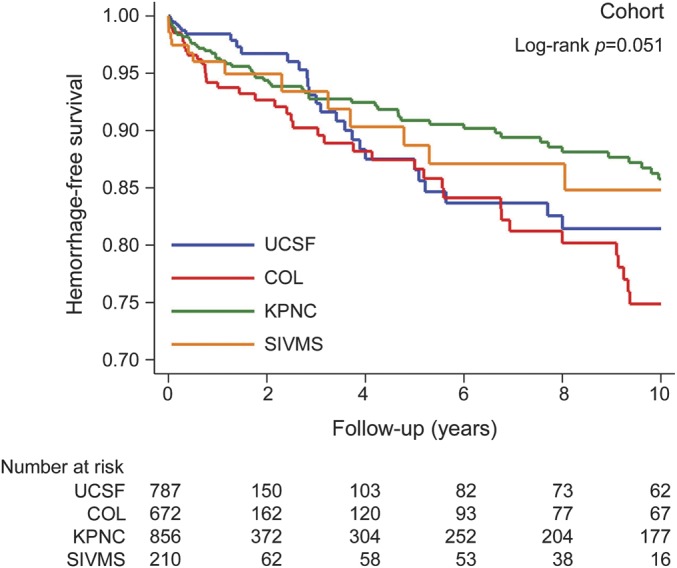
The y-axis shows the proportion of subjects who remain hemorrhage-free. The x-axis shows follow-up time after AVM diagnosis in years. The table below shows the number at risk at each follow-up time interval. AVM = arteriovenous malformation; COL = Columbia; KPNC = Kaiser Permanente of Northern California; MARS = Multicenter AVM Research Study; SIVMS = Scottish Intracranial Vascular Malformation Study; UCSF = University of California San Francisco.
Predictors of hemorrhage during follow-up.
Initial hemorrhagic presentation (p < 0.001), exclusively deep venous drainage (p = 0.001), and AAA (p = 0.026) were significant predictors of hemorrhage in univariable analysis (table 3). Kaplan–Meier survival curves for 4 predictors are shown in figure e-1.
Table 3.
Univariable and multivariable Cox regression analysis of time-to-hemorrhage in the natural history course
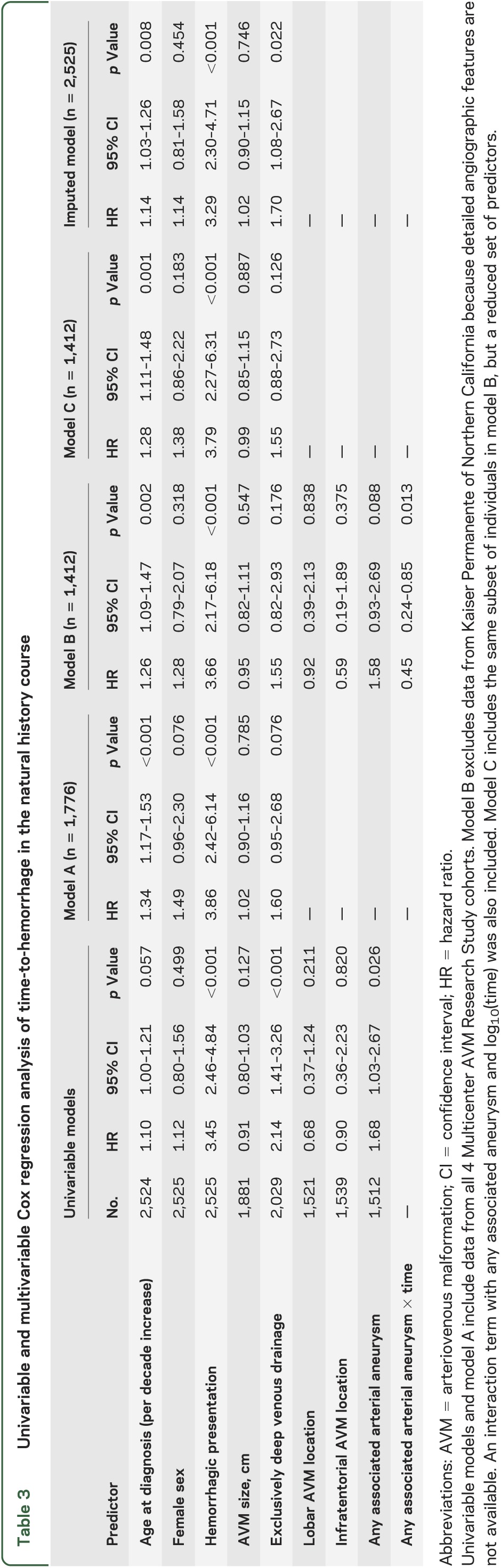
Results from multivariable Cox regression analyses are summarized in table 3. Initial hemorrhagic presentation was the strongest predictor of subsequent hemorrhage in both multivariable models (A and B), with patients presenting ruptured having an approximately 4-fold increased risk compared with those presenting unruptured (p < 0.001). Increasing age at diagnosis was a significant predictor of hemorrhage in both multivariable models A (p < 0.001) and B (p = 0.002), corresponding to an approximately 30% increased risk of hemorrhage for every decade increase in age. Model B also identified a time-dependent effect of AAA with subsequent hemorrhage (p = 0.013); the risk of hemorrhage 1 year after presentation was 58% higher in AVMs with AAA compared to those without aneurysms (hazard ratio [HR] = 1.58), but no long-term effect was observed (HR = 0.98 at 4 years). All other predictors in models A and B were not statistically significant, although the lower bound of the 95% CI for female sex and exclusively deep venous drainage just crossed the null value of 1.0.
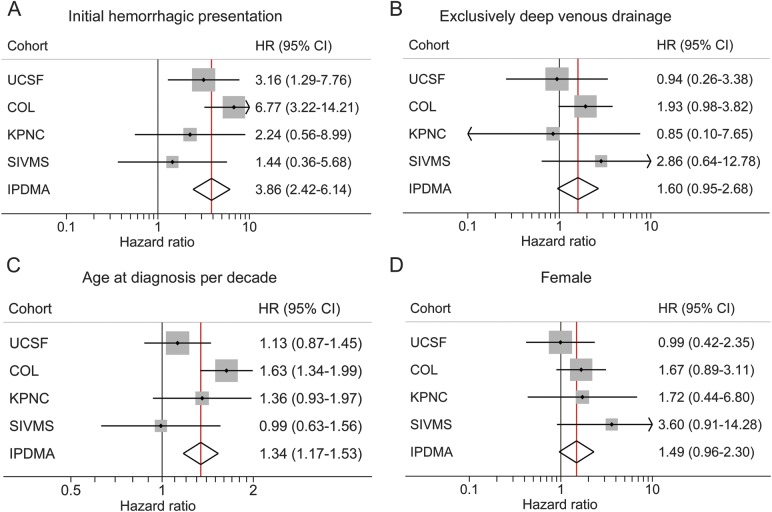
Figure 2 demonstrates increased precision of multivariable-adjusted estimates from model A. HRs for the 4 predictors shown were significant or borderline significant in IPDMA while generally not within any single cohort. The advantage of IPDMA is observed in the narrowed width of the 95% CI as compared to within each cohort.
Figure 2. Forest plots of multivariable-adjusted predictors by cohort and combined IPDMA.
(A) Initial hemorrhagic presentation. (B) Exclusively deep venous drainage. (C) Age at diagnosis. (D) Sex. The x-axis shows the HR with the vertical black line at 1.0 (no association) and the vertical red line indicating the overall estimate from IPDMA. CI = confidence interval; COL = Columbia; HR = hazard ratio; IPDMA = individual patient data meta-analysis; KPNC = Kaiser Permanente of Northern California; SIVMS = Scottish Intracranial Vascular Malformation Study; UCSF = University of California San Francisco.
All models performed reasonably well, with C statistics of 0.75 (model A), 0.76 (model B), and 0.74 (model C). Model C had a higher cross-validated C statistic (5 predictors, C = 0.73) compared with model B (9 predictors, C = 0.72), suggesting that the additional variables in model B did not improve prediction and fit over simpler models (models A and C).
Using model A estimates, we calculated the predicted probability of hemorrhage in the untreated course for an 80-year-old and a 40-year-old man with the following characteristics: unruptured, 3-cm AVM nidus with superficial venous drainage. This corresponds to a predicted probability of hemorrhage of 4.5% at 1 year, 17.3% at 5 years, and 27.9% at 10 years for the 80-year-old man and 1.4%, 5.6%, and 9.6%, respectively, for a 40-year-old man with similar characteristics (described in the text for figure e-2). Figure e-2 shows good separation of survival curves into low, intermediate, and high risk of hemorrhage. In this example, the 80-year-old man who has an unruptured BAVM has a similar hemorrhage risk to a 40-year-old man who presents with a ruptured BAVM.
To alleviate concern that results were dominated by older patients driving the age association, we assessed hemorrhage risk by age categories. Compared with children, the risk of hemorrhage was monotonically increasing across all successive age categories (table e-3), providing further justification for modeling age continuously.
Effect of missing data.
Model B excluded KPNC patients because data were not collected for lobar location, AAA, and infratentorial location. However, model C (n = 1,412) estimates were similar to those in model A (n = 1,776), suggesting that model B results were not biased due to the reduced subset of patients included (table 3). We also compared model A estimates when leaving the SIVMS cohort out because this cohort had patients with BAVM who were on average 10 years older. The age effect was similar in the reduced dataset (HR = 1.39, 95% CI 1.21–1.60).
Imputation of missing clinical data allowed us to include all 2,525 subjects in the multivariable analyses (table 3). Age at diagnosis (p = 0.008) and initial hemorrhagic presentation (p < 0.001) were again identified as significant independent predictors, although the HRs were reduced and more similar to observed univariable results (table 3). In addition, exclusively deep venous drainage remained a significant predictor in the imputed dataset (p = 0.022). These results suggest that there is some bias in the overall IPDMA because of excluding individuals with missing clinical data, but lend confidence to the robustness of findings for age at diagnosis and hemorrhagic presentation, which were significant across all multivariable models. Furthermore, imputation may have not only decreased the bias from missing data, but also increased precision of estimates (decreased standard errors) as reflected by the narrower 95% CI (table 3).
DISCUSSION
In this IPDMA of 4 BAVM cohorts, we identified hemorrhagic presentation and increasing age at diagnosis as significant independent predictors of hemorrhage in the natural history course. The finding that hemorrhagic presentation is the strongest risk factor for hemorrhage during follow-up is consistent with all previously reported longitudinal studies.1–9 However, the statistically and clinically significant influence of increasing age is an important result, given that it has only been reported in some9,19 but not all studies.1,5 We found that increasing age was associated with an approximately 30% increase in risk for every 10-year increase in age (95% CI 1.17–1.53). This corresponds to a 5-year predicted risk of hemorrhage of 17.3% for an 80-year-old man presenting with an unruptured, 3-cm AVM nidus with no deep venous drainage compared with only 5.6% for a 40-year-old man with similar characteristics. While older patients are also at higher risk of poor outcome after treatment,20 our data suggest that the risk-benefit of treatment in older patients bears reconsideration.
In the subset of patients with additional angiographic variables, we found that presence of AAA increased hemorrhage risk but this effect seemed to diminish over time. In the COL AVM study, an aneurysm effect was seen for initial hemorrhagic presentation (p < 0.001), but not on follow-up (HR 1.62, p = 0.17).9 The Toronto group also reported high hemorrhage rates in BAVM patients with associated aneurysms, but their follow-up time included events occurring during time of interventional treatment5; the effect of associated aneurysms did not remain statistically significant in their multivariable analysis (HR 1.59, p = 0.07). Our own findings cannot confirm a strong independent effect of associated aneurysms on hemorrhage risk on follow-up. However, this variable remains a potentially important clinical risk factor that has management implications.20
We report borderline associations with female sex and exclusively deep venous drainage. Others have also noted an increased risk of hemorrhage in females,6,19 although they used different statistical models and adjustment factors, making it difficult to directly compare. Exclusively deep venous drainage is typically identified as a significant univariable predictor of hemorrhage in all studies, but does not always remain significant in multivariable analysis. Deep venous drainage was borderline significant (p = 0.08) in our model A (n = 1,776), but remained a significant independent predictor when using imputed data (n = 2,525) with an HR of 1.6 (p = 0.02), suggesting some bias due to missing data. Including additional data may shed light on these suggestive risk factors.
While small AVM nidus size has been associated in prior studies,7,9 it was not a significant predictor of subsequent hemorrhage risk in any of our multivariable models (95% CI 0.90–1.16 in model A) or in several other studies.5,9,21 These results, however, are in contrast with those reported in the retrospective Finnish natural history study,1 where large AVMs (>50 mm) but not medium AVMs (25–50 mm) were associated with increased rupture risk compared with small AVMs (<25 mm) in various multivariable models. However, AVM size did not significantly affect the rupture rate during the first 5 years after admission (log-rank p = 0.807) or over the entire follow-up period (log-rank p = 0.22) in their cohort.1
Our study had several strengths and weaknesses. This analysis represents the largest study in terms of sample size, person-years of follow-up, and events to identify predictors of hemorrhage after BAVM diagnosis. We show that IPDMA of prognostic factors for intracerebral hemorrhage in the natural history course is feasible and yields improved precision of risk estimates, which were generally not significant within any single cohort. Furthermore, IPDMA allows for estimation of multivariable models, internal validation, and adjustment for missing data. Without individual-level data, we would not have been able to perform imputation to explore possible biases in the data. Our imputation results suggested some bias from excluding individuals with partially missing covariate data and increased precision of multivariable estimates by decreasing the standard error. Even a 10% decrease in standard error for a predictor has important implications for planning of future studies (roughly corresponds to 20% reduction in required sample size). As summarized in a recent meta-analysis of treatment outcomes, BAVMs remain a formidable management challenge.21 This premise derives not only from the challenge of safely undertaking invasive therapy, but also from the lack of accurate and reliable tools to rationally weigh risks of treatment against conservative management.
However, despite the large sample size, our study did not identify novel predictors of hemorrhage. While sex, exclusively deep venous drainage, and AAA failed to achieve statistical significance in the IPDMA, we cannot rule out these suggestive and clinically important risk factors given the magnitude of HRs and imputation results. This highlights the need for even larger datasets to overcome the limitation of short follow-up times in the natural history period due to censoring for treatment. Thus, these hemorrhage risk estimates cannot be extrapolated to the entire lifetime of a patient. Furthermore, we acknowledge potential selection bias in cases included given possible changes in referral patterns for unruptured BAVMs in recent years or aggressive treatment of BAVMs with AAA at referral centers.
The next steps for MARS will be a systematic literature review to identify new collaborators for this important effort with attention to methodologic quality of studies, including confounders, bias, and completeness of follow-up.22 Additional MARS cohorts should improve accuracy of risk estimates, identify novel hemorrhage predictors, and allow validation of risk prediction models that will be clinically useful and informative for design of future BAVM studies.
Supplementary Material
ACKNOWLEDGMENT
This article is dedicated in memoriam to our colleague and friend, William L. Young, in recognition of his major contribution to brain arteriovenous malformations research. The authors thank Tony Pourmohamad and Jeffrey Nelson for statistical assistance, and Voltaire Gungab for assistance with manuscript copyediting.
GLOSSARY
- AAA
associated arterial aneurysm
- AVM
arteriovenous malformation
- BAVM
brain arteriovenous malformation
- CI
confidence interval
- COL
Columbia
- CPT-4
Current Procedural Terminology, fourth edition
- HR
hazard ratio
- ICD-9
International Classification of Diseases, ninth revision
- IPDMA
individual patient data meta-analysis
- KPNC
Kaiser Permanente of Northern California
- MARS
Multicenter AVM Research Study
- SIVMS
Scottish Intracranial Vascular Malformation Study
- UCSF
University of California San Francisco
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
The MARS Writing Committee members wrote the manuscript and agreed to and had responsibility for the content and interpretation of this article. H.K. and C.E.M. contributed to data analysis. The MARS coinvestigators contributed to study recruitment and data collection for individual studies included in the meta-analysis.
STUDY FUNDING
UCSF, KPNC, and COL studies were supported by NIH grants R01 NS034949 (W.L. Young), P01 NS044155 (W.L. Young), and R01 NS040792 (J.P. Mohr). SIVMS was supported by the Chief Scientist Office of the Scottish Government Health Department and MRC clinical training, clinician scientist, and senior clinical fellowships (R. Al-Shahi Salman). H. Kim was supported in part by NIH K23 NS058357 and R01 NS034949.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hernesniemi JA, Dashti R, Juvela S, Vaart K, Niemela M, Laakso A. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery 2008;63:823–831 [DOI] [PubMed] [Google Scholar]
- 2.Halim AX, Johnston SC, Singh V, et al. Longitudinal risk of intracranial hemorrhage in patients with arteriovenous malformation of the brain within a defined population. Stroke 2004;35:1697–1702 [DOI] [PubMed] [Google Scholar]
- 3.Wedderburn CJ, van Beijnum J, Bhattacharya JJ, et al. Outcome after interventional or conservative management of unruptured brain arteriovenous malformations: a prospective, population-based cohort study. Lancet Neurol 2008;7:223–230 [DOI] [PubMed] [Google Scholar]
- 4.Kim H, Sidney S, McCulloch CE, et al. Racial/ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke 2007;38:2430–2437 [DOI] [PubMed] [Google Scholar]
- 5.da Costa L, Wallace MC, Ter Brugge KG, O'Kelly C, Willinsky RA, Tymianski M. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke 2009;40:100–105 [DOI] [PubMed] [Google Scholar]
- 6.Yamada S, Takagi Y, Nozaki K, Kikuta K, Hashimoto N. Risk factors for subsequent hemorrhage in patients with cerebral arteriovenous malformations. J Neurosurg 2007;107:965–972 [DOI] [PubMed] [Google Scholar]
- 7.Brown RD, Jr, Wiebers DO, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg 1988;68:352–357 [DOI] [PubMed] [Google Scholar]
- 8.Itoyama Y, Uemura S, Ushio Y, et al. Natural course of unoperated intracranial arteriovenous malformations: study of 50 cases. J Neurosurg 1989;71:805–809 [DOI] [PubMed] [Google Scholar]
- 9.Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 2006;66:1350–1355 [DOI] [PubMed] [Google Scholar]
- 10.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340:c221. [DOI] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000;283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 12.Riley RD, Hayden JA, Steyerberg EW, et al. Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLoS Med 2013;10:e1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Scottish Intracranial Vascular Malformation Study (SIVMS): evaluation of methods, ICD-10 coding, and potential sources of bias in a prospective, population-based cohort. Stroke 2003;34:1156–1162 [DOI] [PubMed] [Google Scholar]
- 14.Al-Shahi R, Bhattacharya JJ, Currie DG, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke 2003;34:1163–1169 [DOI] [PubMed] [Google Scholar]
- 15.Atkinson RP, Awad IA, Batjer HH, et al. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke 2001;32:1430–1442 [DOI] [PubMed] [Google Scholar]
- 16.Li KH. Imputation using Markov chains. J Stat Comput Simul 1988;30:57–79 [Google Scholar]
- 17.Harrell FE., Jr Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; 2001 [Google Scholar]
- 18.Bradburn MJ, Deeks JJ, Altman DG. Sbe 24: metan—an alternative meta-analysis command. Stata Tech Bull 1998;44:4–15 [Google Scholar]
- 19.Karlsson B, Lindquist C, Johansson A, Steiner L. Annual risk for the first hemorrhage from untreated cerebral arteriovenous malformations. Minim Invasive Neurosurg 1997;40:40–46 [DOI] [PubMed] [Google Scholar]
- 20.Almefty K, Spetzler RF. Arteriovenous malformations and associated aneurysms. World Neurosurg 2011;76:396–397 [DOI] [PubMed] [Google Scholar]
- 21.van Beijnum J, van der Worp HB, Buis DR, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA 2011;306:2011–2019 [DOI] [PubMed] [Google Scholar]
- 22.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet 2002;359:1309–1310 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.