Abstract
In recent years, the molecular etiology of parkinsonism has yielded to genetic analysis.1 Mutations in the gene leucine-rich repeat kinase 2 (LRRK2) have the highest genotypic and population attributable risk. Disparate penetrance estimates have been reported using a variety of statistical analyses, ethnic populations, and sample sizes.2 We compared the age-associated cumulative incidence (penetrance) of LRRK2 p.G2019S patients from Tunisia and Norway.
In recent years, the molecular etiology of parkinsonism has yielded to genetic analysis.1 Mutations in the gene leucine-rich repeat kinase 2 (LRRK2) have the highest genotypic and population attributable risk. Disparate penetrance estimates have been reported using a variety of statistical analyses, ethnic populations, and sample sizes.2 We compared the age-associated cumulative incidence (penetrance) of LRRK2 p.G2019S patients from Tunisia and Norway.
Methods.
Clinic-based population series.
The Tunisian Arab-Berber series includes 350 patients with idiopathic Parkinson disease (iPD) (mean onset 55.3 ± 14.3 years, range 12–83). An additional 220 are affected LRRK2 p.G2019S carriers (mean onset 57.1 ± 11.6 years, range 22–82); 12 unaffected LRRK2 carriers were also identified (mean age 56.7 ± 10.9 years). A total of 411 control subjects (spouses or unrelated individuals) were recruited through the Clinic and genotyped. Neither blood relatives of cases nor control subjects were included. The ethnic Norwegian series includes 443 with iPD (mean onset 58.8 ± 11.2 years, range 25–88). A total of 520 control subjects (mostly spouses or unrelated individuals) were recruited through the Clinic and genotyped. In Norway, an additional 27 are affected LRRK2 p.G2019S carriers (mean onset 63 ± 11.6 years, range 40–82) and 57 unaffected carriers were identified (mean age of 54.2 ± 15.2 years) as first- to third-degree relatives through field studies.3
All individuals, clinical data, and blood samples were obtained with local ethical approvals, independently reviewed by the University of British Columbia Research Ethics Board. Written informed consent was provided. Neurologists specializing in movement disorders (F.H. and J.O.A.) diagnosed patients using UK Brain Bank criteria4 for both the Tunisian and Norwegian populations. Age at onset was obtained from patient medical records or by self-report. DNA was extracted from blood; LRRK2 genotyping was verified by Sanger sequencing.5
Statistical analysis.
The penetrance was estimated for iPD and LRRK2 carriers using a Kaplan-Meier method (JMP software, SAS Institute Inc., Cary, NC) with age at onset as the time variable; asymptomatic carriers were right-censored at the age at last contact or age at death, using log-rank tests to assess significance.
Results.
In Tunisia and Norway samples the mean, distribution, and range of disease onset in iPD is similar and the cumulative incidence is comparable (figure, A; log-rank p = 0.67). By contrast, the penetrance of LRRK2 p.G2019S parkinsonism was significantly different between Tunisian Arab-Berbers and ethnic Norwegians (figure, B; log-rank p < 0.0001). In Tunisia, LRRK2 p.G2019S carriers have a median 10 years earlier age at onset compared to carriers in Norway. In Tunisia, 30%, 61%, and 86% of LRRK2 p.G2019S carriers had developed parkinsonism by 50, 60, and 70 years of age. In Norway, only 3%, 20%, and 43% had developed parkinsonism by 50, 60, and 70 years of age (table e-1 on the Neurology® Web site at Neurology.org).
Figure. Cumulative incidence of idiopathic Parkinson disease (A) and LRRK2 p.G2019S parkinsonism (B).
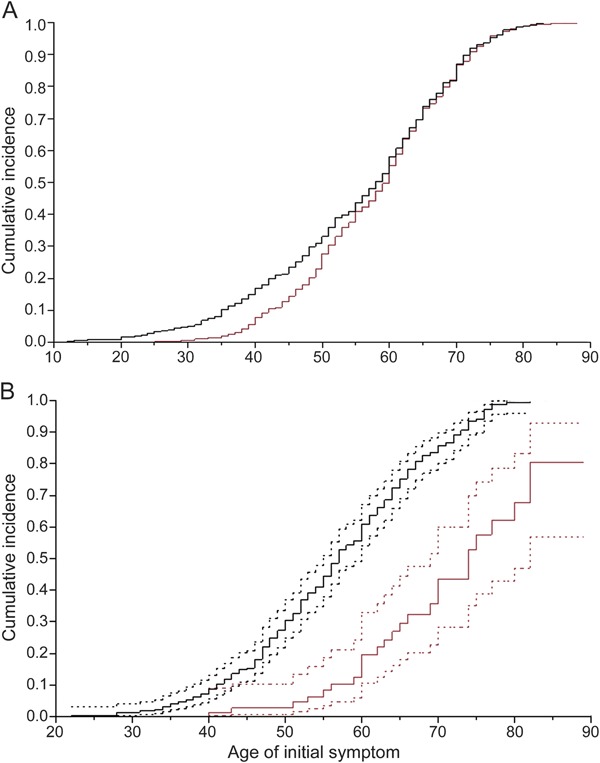
Kaplan-Meier analysis of parkinsonism with age at onset as the time variable. Black lines represent Tunisia and red lines represent Norway. Dotted lines represent confidence intervals. Unaffected LRRK2 p.G2019S carriers were right-censored.
Discussion.
LRRK2 p.G2019S phenoconversion from a motorically asymptomatic to an affected state probably reflects an age-associated failure to compensate for kinase dysfunction. However, the disparity in disease penetrance in LRRK2 p.G2019S carriers in Tunisia vs Norway is challenging to explain given that the cumulative incidence of iPD is almost identical. While the mean age at onset in Norway is higher than in Tunisia, the difference is not statistically different. However, in Tunisia a sex difference is observed in LRRK2 carriers, women having earlier-onset parkinsonism.6 The same trend appears in Norway but, perhaps due to a smaller sample size, is not significant.
Age at onset is a subjective measure but the difference between LRRK2 groups in these ethnicities is unlikely an artifact of analysis or study design. Kaplan-Meier analyses should be restricted to unrelated cases but these LRRK2 p.G2019S carriers have the same ancestral chromosome 12 haplotype7 and are derived from genetically homogeneous communities. In Norway, the LRRK2 p.G2019S genealogy dates to the 15th century3; in Tunisia, the origin of the mutation is more ancient. The use of convenient samples in lieu of prospective epidemiologic studies is clearly a limitation in making precise penetrance estimates. Nevertheless, available data suggest ancestral background/country of origin and associated genetic/environmental modifiers may account for differences in published penetrance estimates.2 Hence, ethnicity is an important caveat for genetic counseling.
Supplementary Material
Acknowledgments
Acknowledgment: The authors thank the individuals who participated in this study; Emil Gustavsson, Ilaria Guella, and Carles Vilarino-Guell for review comments; and Rachel Gibson, Lefkos Middleton, and the GlaxoSmithKline program team for prior collaborative efforts in Tunisia.
Footnotes
Supplemental data at Neurology.org
Author contributions: Dr. Hentati: study supervision, acquisition of data, analysis and interpretation. J. Trinh: analysis and first draft of the manuscript. C. Thompson: acquisition of data. Dr. Nosova: analysis and interpretation. Dr. Farrer: study concept, study supervision, analysis and interpretation, writing and revisions of the manuscript. Dr. Aasly: study supervision, acquisition of data, analysis and interpretation.
Study funding: Michael J. Fox Foundation (M.J.F., F.H., J.O.A.); the Canada Excellence Research Chairs program, the Canada Institutes of Health Research, and the Cundill Foundation (M.J.F.); Province of British Columbia, LifeLabs, and Genome BC for the Leading Edge Endowment Funds that support the Dr. Donald Rix BC Leadership Chair (M.J.F.) and Fellowship (J.T.).
Disclosure: F. Hentati receives limited sponsorship from H. Lundbeck A/S for studies related to LRRK2 p.G2019S. J. Trinh, C. Thompson, and E. Nosova report no disclosures relevant to the manuscript. M. Farrer has received royalties from H. Lundbeck A/S, licensing payments from Alnylam Pharmaceuticals, and consulting fees from Genzyme, Bristol Myers Squibb, and Sofinnova Ventures. J. Aasly is supported by the Norwegian Research Council and Reberg's Legacy. Mayo Foundation has received royalty payments from Athena Diagnostics for Drs. Aasly and Farrer for USA Patent 7,544,786 related to LRRK2 p.G2019S. Go to Neurology.org for full disclosures.
References
- 1.Trinh J, Farrer M. Advances in the genetics of Parkinson disease. Nat Rev Neurol 2013;9:445–454 [DOI] [PubMed] [Google Scholar]
- 2.Goldwurm S, Tunesi S, Tesei S, et al. Kin-cohort analysis of LRRK2-G2019S penetrance in Parkinson's disease. Mov Disord 2011;26:2144–2145 [DOI] [PubMed] [Google Scholar]
- 3.Johansen KK, Hasselberg K, White LR, Farrer MJ, Aasly JO. Genealogical studies in LRRK2-associated Parkinson's disease in central Norway. Parkinsonism Relat Disord 2010;16:527–530 [DOI] [PubMed] [Google Scholar]
- 4.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology 1992;42:1142–1146 [DOI] [PubMed] [Google Scholar]
- 5.Hulihan MM, Ishihara-Paul L, Kachergus J, et al. LRRK2 Gly2019Ser penetrance in Arab-Berber patients from Tunisia: a case-control genetic study. Lancet Neurol 2008;7:591–594 [DOI] [PubMed] [Google Scholar]
- 6.Trinh J, Amouri R, Duda JE, et al. A comparative study of Parkinson's disease and leucine-rich repeat kinase 2 p.G2019S parkinsonism. Neurobiol Aging 2014;35:1125–1131 [DOI] [PubMed] [Google Scholar]
- 7.Kachergus J, Mata IF, Hulihan M, et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am J Hum Genet 2005;76:672–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


