Abstract
Objective:
To relate neurophysiologic changes after mild/moderate traumatic brain injury to cognitive deficit in a longitudinal diffusion tensor imaging investigation.
Methods:
Fifty-three patients were scanned an average of 6 days postinjury (range = 1–14 days). Twenty-three patients were rescanned 1 year later. Thirty-three matched control subjects were recruited. At the time of scanning, participants completed cognitive testing. Tract-Based Spatial Statistics was used to conduct voxel-wise analysis on diffusion changes and to explore regressions between diffusion metrics and cognitive performance.
Results:
Acutely, increased axial diffusivity drove a fractional anisotropy (FA) increase, while decreased radial diffusivity drove a negative regression between FA and Verbal Letter Fluency across widespread white matter regions, but particularly in the ascending fibers of the corpus callosum. Raised FA is hypothesized to be caused by astrogliosis and compaction of axonal neurofilament, which would also affect cognitive functioning. Chronically, FA was decreased, suggesting myelin sheath disintegration, but still regressed negatively with Verbal Letter Fluency in the anterior forceps.
Conclusions:
Acute mild/moderate traumatic brain injury is characterized by increased tissue FA, which represents a clear neurobiological link between cognitive dysfunction and white matter injury after mild/moderate injury.
Diffuse axonal injury (DAI) resulting from traumatic brain injury (TBI) has been previously studied using diffusion tensor imaging (DTI). This research has led to a general consensus that after injury, white matter structural damage leads to increased water mobility (increased mean diffusivity [MD]1–4) and decreased directionality (decreased fractional anisotropy [FA]5–8). However, previous work predominantly examined the effect of severe and chronic injury, with disproportionately few studies focusing on patients with mild TBI, despite this patient demographic representing approximately 90% of TBI cases.9
Although studies focusing on acute, mild TBI often report similar results (e.g., see references 10–12), others have notably reported increased FA and decreased MD,13–15 contrary to these main observations in severe injury. Those observations suggest a different pathophysiology of tissue damage that might follow mild injury. Cytotoxic edema, the presence of which has previously been suggested to lower MD,16 is the primary hypothesized mechanism for these results,15 although a recent histopathologic investigation17 has indicated that astrogliosis may also cause acute FA increases in mild TBI. Greater understanding of the effects of mild injury on white matter integrity and its impact on clinical presentation requires longitudinal investigation coupled with assessment of cognitive function.
Herein, we present a comprehensive, longitudinal DTI study in a predominantly mild patient population. We hypothesized that poor cognitive performance would be directly related to white mater tract damage and therefore used Tract-Based Spatial Statistics18 (TBSS) to examine DTI metric changes between acute and chronic time points and to explore how these relate to acquired cognitive deficits after TBI.
METHODS
Participants.
Fifty-three patients with TBI were recruited from patients attending the Accident and Emergency and Neurosurgery departments of the Newcastle upon Tyne Hospitals Trust (age: 16–68 years, mean = 33.9, SD = 14.6; sex: 44 male, 9 female). Of these, 44 patients had a mild TBI (Glasgow Coma Scale [GCS] score 13–15) and 9 had a moderate TBI (GCS score 8–12). Thirty-three control subjects matched for age, sex, and level of education were recruited for comparison. Patients had no history of neurologic/psychological problems pre-TBI, no history of substance abuse, and no contraindication pertaining to MRI. Patients were studied as outpatients and underwent MRI a mean of 6 days postinjury (SD = 3.2, range = 1–14 days). Of the initial cohort, 23 patients (18 mild, 5 moderate) returned for a follow-up scan 1 year later (357–424 days after initial scan, mean = 383, SD = 22.5 days), with the remaining patients either declining to return (n = 4) or lost to follow-up (n = 26). Full participant demographic information is shown in table 1.
Table 1.
Participant demographic information
Standard protocol approvals, registrations, and patient consents.
The study was approved by the local research ethics committee and all subjects provided written informed consent.
Data acquisition.
Participants were scanned using a 3T Philips Achieva MRI scanner with an 8-channel SENSE head coil (Philips Medical Systems, Best, the Netherlands). A 3-dimensional T1-weighted sequence was used to obtain regional anatomy (magnetization-prepared rapid-acquisition gradient echo, repetition time [TR] = 8.1 milliseconds, echo time [TE] = 4.6 milliseconds, matrix size 240 × 216 × 180, isotropic 1-mm resolution). DTI scans used a single-shot echo-planar imaging diffusion sequence (TR/TE = 2,524/71 milliseconds; 24 slices; b = 0; 1,000 s·mm−2; 16 diffusion directions; 2 × 2 × 6 mm3 resolution). A magnetic field map sequence was acquired for correction of geometric distortion of the DTI scans (dual-echo gradient recalled echo, TR = 27 milliseconds, TE = 2.6/6.1 milliseconds, matrix 128 × 128 × 72, 2 mm resolution). The scan protocol also included measurements of cerebral blood flow, quantitative T1 and T2 mapping, and brain metabolism, which are not reported here. Participants were examined with a comprehensive neuropsychological test battery at each time of scanning, or within 7 days if the patient was unable to tolerate both in the same sitting (this was the case for 11 patients who were tested a mean of 2.6 days after their scan). A full list of tests included is available in e-Methods on the Neurology® Web site at Neurology.org; here, only Verbal Letter Fluency (VLF) and the National Adult Reading Test (NART)19 are considered in detail because these were the most informative under the analysis we conducted.
DTI data analysis.
DTI scans were processed using the FSL (FMRIB's Software Library) toolbox20 for analysis using TBSS18 (see e-Methods). TBSS was used to examine differences in acute DTI metrics (FA, MD, axial diffusivity [AD], and radial diffusivity [RD]) in a between-subjects control vs patient analysis. DTI metrics at follow-up were also compared between patients and control subjects. In all TBSS analyses, threshold-free cluster enhancement output images were used with p < 0.05 taken as significant.
Neuropsychological data analysis.
Acute and follow-up patient task performance was compared with controls using between-samples t-test analysis (Bonferroni-corrected). Multiple regression analysis was also conducted using group (patient/control) and NART (as a measure of premorbid IQ) as predictor variables for each task. TBSS was used to perform regression analysis of VLF test scores against FA, MD, AD, and RD at the acute and follow-up times and in controls and patients separately, controlling for IQ using NART. Seven patients were excluded acutely and 2 patients were excluded chronically from regression analysis because they were unable to complete both VLF and NART tasks for reasons relating to their injury. In addition, psychometric data for one control participant were absent for all tasks, reducing the control group to 32 for relevant testing.
RESULTS
Acute groupwise differences in diffusion metrics.
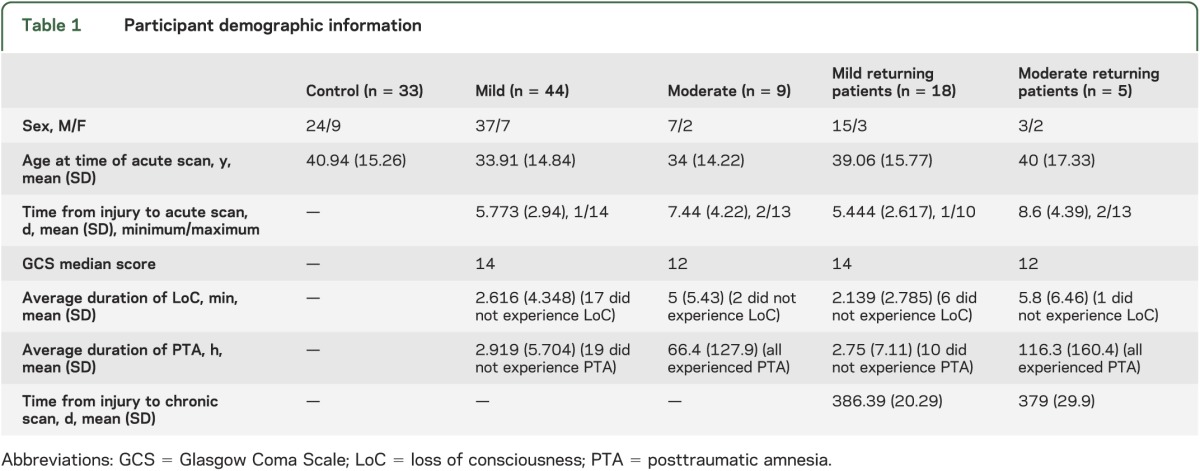
At the acute time point, groupwise patient vs control comparison revealed multiple, widespread locations of increased MD, FA, and AD in the patient group. There were no locations that showed a significant difference in RD (figure 1, table 2).
Figure 1. TBSS outputs demonstrating locations of metric changes after traumatic brain injury at the acute time point.
The analyzed white matter skeleton is shown in green with color overlay of significant differences. All changes are increases (colored red/yellow) in the patient group compared with controls. The z coordinates are based around the anterior commissure/posterior commissure line being z = 0. The RD row shows no differences but is included for comparison with figure 2. Color bar values are 1 − p value. AD = axial diffusivity; FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity; TBSS = Tract-Based Spatial Statistics.
Table 2.
Summary of results from groupwise and regression analysis
Chronic groupwise differences in diffusion metrics.
Comparison of follow-up patients (12 months postinjury) with control subjects again showed groupwise differences but with different imaging characteristics compared with those found at the acute time point. While MD remained significantly increased, patient FA was now decreased. In addition, patient AD showed no significant differences in any location, while RD was now increased (figure 2, table 2).
Figure 2. TBSS outputs demonstrating locations of metric changes after traumatic brain injury at the chronic time point.
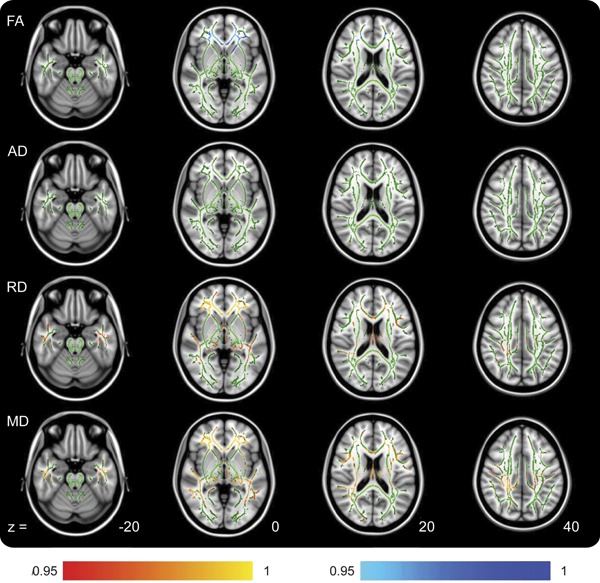
Increases in the patient group are shown in red, and decreases in the patient group are shown in blue. The AD row shows no differences but is included for comparison with figure 1. Color bar values are 1 − p value. AD = axial diffusivity; FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity; TBSS = Tract-Based Spatial Statistics.
Acute cognitive testing and TBSS analysis.
Full results of patient vs control task performance and regression analysis for the acute and chronic time points are available in table e-1. Despite recruiting control subjects matched to the patient population for duration of education, there was a significant difference in NART scores (control mean = 112.03, SD = 8.98, patient mean = 100, SD = 13.1, t77 = 4.88, p < 0.001). NART was therefore also included with group as predictor variables in multiple regression analysis. At the acute time point, patient VLF score (mean = 31.6, SD = 13.3) was significantly worse compared with controls (mean = 41.63, SD = 9.38, t78 = 3.95, p < 0.001). Multiple regression analysis indicated that group and NART explained 25.1% of the variance (R2 = 0.251, F2,75 = 12.54, p < 0.001). It was found that NART significantly predicted VLF performance (p = 0.001) while group was approaching significance (p = 0.075).
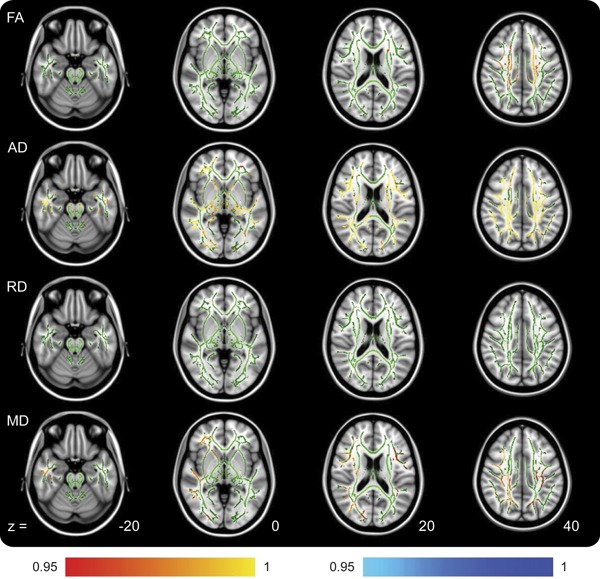
Despite this, TBSS analysis indicated that VLF scores (controlled for NART) negatively regressed with patient FA (worse performance, lower score, associated with higher FA) in widespread locations. Patient MD and RD were also found to hold positive regressions with VLF. Acute VLF score and patient AD did not demonstrate any locations of regression (figure 3, table 2). The control group did not show any significant regression between any diffusion metric and VLF performance, indicating that the variance in patient VLF score is attributable to their acquired injury.
Figure 3. TBSS outputs demonstrating locations of regression between acute patient diffusion tensor imaging metrics and Verbal Letter Fluency scores.
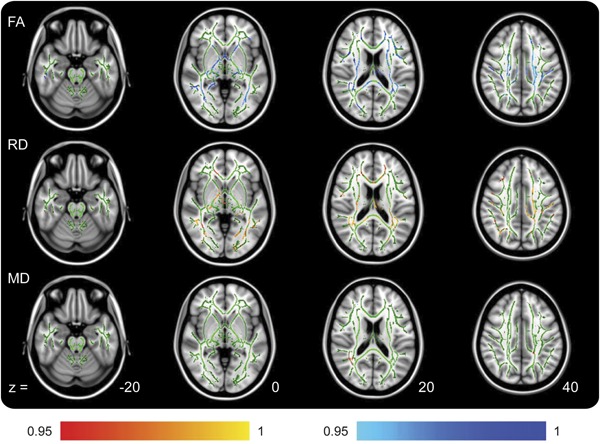
Positive regressions are shown in red and negative regressions are shown in blue. Axial diffusivity is not shown because no regressions were found for this. Color bar values are 1 − p value. FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity; TBSS = Tract-Based Spatial Statistics.
Chronic cognitive testing and TBSS analysis.
Follow-up patient VLF performance was not significantly different from control. Multiple regression analysis showed that group and NART now explained 14.4% of the variance (R2 = 0.144, F2,50 = 4.19, p = 0.021) in VLF performance. NART significantly predicted VLF performance (p = 0.01) while group did not. Despite this, when controlling for NART, negative regressions between FA/VLF were still found after TBSS analysis. AD was also found to negatively regress with VLF in one small location while RD/MD did not regress with VLF (figure e-1, table 2).
DISCUSSION
DTI has been used to study the white matter tracts in TBI.10,11 Injury and degeneration are generally considered to disrupt tissue structure at the cellular level, leading to increased MD and decreased FA. However, these findings come from a body of research weighted toward severe and chronic patients, while mild injury is by far the most common. Our study in a large cohort of patients with mild and moderate TBI has shown extensive increases in MD but these were accompanied by increased FA at the acute time point. These changes were strongly correlated with underperformance on cognitive testing and show a clear neurobiological basis for dysfunction postinjury.
Although elevated FA post-TBI is an unusual finding in the wider neuroimaging literature, similar observations have been reported in a minority of other small-scale imaging studies, particularly in patients with acute, mild TBI (e.g., see references 13–15, 21–23) and has been hypothesized to be due to the formation of edema.22 However, both in experimental models and clinical studies in TBI, the presence of cytotoxic edema is also associated with reduced MD (or its alternative metric: apparent diffusion coefficient24,25). In the current study, MD was either unchanged or increased. In addition, our groupwise increases in FA were associated principally with elevated diffusion along the axon (increased AD), while RD was unchanged. In previous studies reporting increased FA, RD was typically reduced where reported.15,21,22 Cytotoxic edema therefore does not explain our observations.
An alternative explanation may lie in the findings of other recent work that has suggested astrogliosis as a possible cause of acute FA increases. After injury, fibrous astrocytes often undergo reactive astrogliosis migrating to the site of injury, locally increasing the density of these cells.26 It was reported that the organization of these cells increased the AD within the affected tissue, while RD remained unchanged, thus increasing the measured FA.17 Although MD was not reported, this increase in AD (without any change in RD) would also increase the reported MD. Our observations are therefore more consistent with astrogliosis and with the known chronology of the immune response after CNS injury,27 and therefore we consider astrogliosis to be the most likely underlying cause of our finding of increased acute FA. Future research could expand to using the recent method of diffusional kurtosis in conjunction with DTI. Studies have shown that this combination brings increased sensitivity in detecting cognitively relevant physiologic change28 and astrogliosis29 compared with DTI alone.
Patients were found to underperform on the VLF task in the acute phase after injury with their performance strongly and negatively regressing with white matter FA and positively regressing with RD and MD. These regressions were located across widespread areas of the white matter tracts and particularly within the ascending fibers of the corpus callosum in the left hemisphere. This finding supports previous work that has shown the VLF network of healthy individuals to involve frontal, parietal, and temporal locations with an emphasis in the left hemisphere.30,31
While the groupwise increase in FA was mainly caused by increased AD, the relationship with cognitive performance was driven by a positive regression between RD and VLF, which implies the presence of a subtle reduction in RD. The contrasting observation that RD was not significantly different in the acute injury phase between the patient and control groups in groupwise analysis, but reduced RD was associated with differences in functional performance between patients, is likely attributable to differing statistical power between tests. The variance of RD values explained by regression within the acute patient group is large, whereas the difference in mean RD values between the 2 groups is small, making regression within groups more powerful than between-group comparisons.
Within the axon, undamaged neurofilament has been shown to be related to both larger axon diameter and more efficient conduction velocity,32 while DAI is associated with disruption of the neurofilament, involving loss of neurofilament sidearms via trauma-induced proteolysis.33 We therefore postulate that reduced axonal diameter may contribute to altering both diffusion properties (reducing RD and increasing FA) and to the deficit in VLF performance, which becomes more severe as RD decreases.
Diffusion imaging observations at 12-month follow-up supported the wider literature in chronic brain injury with patterns of an FA decrease driven by an underlying RD increase, while MD was increased extensively and AD was unchanged. Increased RD is evidence that the axonal membrane and myelin sheath have disintegrated.34 The reductions in FA were observed principally in the anterior forceps, suggesting that this region has experienced the most severe long-term damage after TBI. This finding is supported by the fact that anterior callosal fibers are known to be sensitive to DAI and damage to them relates to long-term prognosis.35 It should be noted that at both time points, locations of increased MD always overlapped with underlying increases of AD/RD. Therefore, while MD is one of the simplest diffusion metrics to measure and may therefore have a role in the general detection of tissue injury, our data demonstrate that interpretation of altered MD requires full assessment of the diffusion eigenvectors.
Chronically, patient performance on VLF was not significantly different from that of the controls, suggesting a degree of recovery regarding cognitive performance. However, negative regressions between FA and VLF were still found. These chronic regressions presented primarily in the body of the corpus callosum and the anterior forceps as opposed to the more diffuse pattern seen acutely. This shift of location to connecting fibers between the 2 hemispheres could be indicative of network reorganization to increase right hemisphere involvement as a compensatory mechanism, as has been demonstrated previously.36
Examining AD and RD did not provide evidence that either was specifically responsible for the chronic relationship between performance and FA, implying that relatively slight changes in each are contributing to this finding. The finding that decreased FA was associated with better VLF performance in the chronic phase is one that is challenging to explain because of its apparent contradiction to counterpart groupwise findings in the same tract location that indicate FA to decrease as a result of damage, and also its relative novelty regarding the wider literature. Many events are known to occur in the chronic phase of injury, such as a relative increase in the proportion of axons with smaller diameters,37 glial scarring,38 and network reorganization,36 all of which may contribute to an effect whereby the patients with the greatest VLF deficit have the highest FA, despite FA being generally reduced compared with controls. Further investigations are required to determine the exact mechanisms behind this finding, but it is highlighted as an avenue for future research.
Our study has several limitations. First, the loss of patients to follow-up reduced our statistical power to detect longitudinal change. Patients either declined to return for further testing (4/53) or were lost to further contact (26/53). We attribute these losses to the mild nature of our participants' injuries (possibly making them less inclined to devote further time to the study), and the relatively long (1-year) gap between assessments. It should be considered whether those patients returning at 12 months were either likely to have greater ongoing symptoms than those who did not return, or be influenced by socioeconomic or medical-legal factors (whereby a potential diagnosis of long-term damage or ongoing cognitive dysfunction may be seen as advantageous). This type of patient self-selection is difficult to quantify and cannot be ruled out in this cohort. We evaluated whether there was any difference in injury severity or cognitive deficits at baseline between those subjects who subsequently did or did not return for follow-up. Only verbal fluency performance was significant (p = 0.04), but, paradoxically, those who did not return had worse performance, contradicting the hypothesis that the returning patients were more severely affected.
A proportion of our patient group was also unable to complete psychometric testing for reasons pertaining to their injury (12% acutely and 9% at follow-up), reducing the sample size for the regression analyses. Because our hypothesis was that cognitive dysfunction is secondary to microstructural damage, those patients who were unable to complete the tests would be expected to have the greatest microstructural damage. Excluding such individuals would then bias against detecting an effect, suggesting that our observations are a lower estimate of the importance of white matter injury on cognitive performance after mild TBI.
Finally, our mixed mild and moderate cohort is a potential confound for understanding the effect of mild TBI alone. Comparison of the patient groups (mild vs moderate) did not show any significant differences in any diffusion metric. Furthermore, in comparison to healthy subjects and during regression against VLF performance, exclusion of the moderate patients did not change the direction or distribution of any findings (other than a reduction in statistical power expected with the smaller group size; data using the mild patients only are presented in figures e-2 to e-5). Finally, only 2 of 9 patients with moderate injury had GCS score less than 10, so this moderate group is toward the milder end of injury. The findings are therefore consistent with revealing the microstructural changes associated with mild injury severity.
This longitudinal study has produced a comprehensive picture of the diffusion imaging changes after mild/moderate TBI and how these can relate to cognition in a large cohort of patients. Detailed analysis of the complete set of diffusion metrics suggests that gliosis rather than cytotoxic edema is most consistent with changes in these metrics after acute, mild TBI. We have also further quantified acute findings by identifying a proportional relationship with verbal fluency, which we have shown persists into the chronic injury phase, at a more subtle but still detectable level. The importance of investigating the component parts of FA is also shown by groupwise/regression findings in the acute phase that would have proved conflicting had they not been shown to be separately driven by AD and RD, respectively. The full potential of DTI to detect different physiologic changes resulting from TBI, and to show which among these is affecting cognition, is highlighted while avenues for future research are also indicated.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the radiographers who assisted in this work: Tim Hodgson, Louise Ward, Carol Smith, and Tamsin Gaudie.
GLOSSARY
- AD
axial diffusivity
- DAI
diffuse axonal injury
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- GCS
Glasgow Coma Scale
- MD
mean diffusivity
- NART
National Adult Reading Test
- RD
radial diffusivity
- TBI
traumatic brain injury
- TBSS
Tract-Based Spatial Statistics
- TE
echo time
- TR
repetition time
- VLF
Verbal Letter Fluency
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
I.D. Croall and C.J.A. Cowie: design of study, analysis and interpretation of data, drafting and revising the manuscript. J. He: design of study, interpretation of data, revising the manuscript. A. Peel and J. Wood: analysis and interpretation of data, revising the manuscript. B.S. Aribisala: design of study, analysis of data, revising the manuscript. P. Mitchel and A.D. Mendelow: design and conceptualization of study, interpretation of data, revising the manuscript. F.E. Smith: interpretation of data, drafting and revising the manuscript. D. Millar and T. Kelly: design of study, analysis of data, revising the manuscript. A.M. Blamire: design and conceptualization of study, interpretation of data, drafting and revising the manuscript.
STUDY FUNDING
Funding for the study and a studentship to I.D.C. has been provided by Sir Jules Thorn Charitable Trust Biomedical Research Award.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Rugg-Gunn FJ, Symms MR, Barker GJ, Greenwood R, Duncan JS. Diffusion imaging shows abnormalities after blunt head trauma when conventional magnetic resonance imaging is normal. J Neurol Neurosurg Psychiatry 2001;70:530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goetz P, Blamire A, Rajagopalan B, Cadoux-Hudson T, Young D, Styles P. Increase in apparent diffusion coefficient in normal appearing white matter following human traumatic brain injury correlates with injury severity. J Neurotrauma 2004;21:645–654 [DOI] [PubMed] [Google Scholar]
- 3.Rutgers DR, Fillard P, Paradot G, Tadie M, Lasjaunias P, Ducreux D. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am J Neuroradiol 2008;29:1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newcombe V, Chatfield D, Outtrim J, et al. Mapping traumatic axonal injury using diffusion tensor imaging: correlations with functional outcome. PLoS One 2011;6:e19214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakayama N, Okumura A, Shinoda J, et al. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry 2006;77:850–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 2007;130:2508–2519 [DOI] [PubMed] [Google Scholar]
- 7.Palacios EM, Fernandez-Espejo D, Junque C, et al. Diffusion tensor imaging differences relate to memory deficits in diffuse traumatic brain injury. BMC Neurol 2011;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011;134:449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornhill S, Teasdale GM, Murray GD, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: prospective cohort study. BMJ 2000;320:1631–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg 2005;103:298–303 [DOI] [PubMed] [Google Scholar]
- 11.Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj 2008;22:115–122 [DOI] [PubMed] [Google Scholar]
- 12.Lipton ML, Gulko E, Zimmerman ME, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology 2009;252:816–824 [DOI] [PubMed] [Google Scholar]
- 13.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 2007;24:1447–1459 [DOI] [PubMed] [Google Scholar]
- 14.Wilde EA, McCauley SR, Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 2008;70:948–955 [DOI] [PubMed] [Google Scholar]
- 15.Chu Z, Wilde EA, Hunter JV, et al. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. AJNR Am J Neuroradiol 2010;31:340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barzo P, Marmarou A, Fatouros P, Hayasaki K, Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg 1997;87:900–907 [DOI] [PubMed] [Google Scholar]
- 17.Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain 2011;134:2248–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-Based Spatial Statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–1505 [DOI] [PubMed] [Google Scholar]
- 19.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York: Oxford University Press; 2006 [Google Scholar]
- 20.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–S219 [DOI] [PubMed] [Google Scholar]
- 21.Mayer AR, Ling J, Mannell MV, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 2010;74:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling JM, Pena A, Yeo RA, et al. Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain 2012;135:1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yallampalli R, Wilde EA, Bigler ED, et al. Acute white matter differences in the fornix following mild traumatic brain injury using diffusion tensor imaging. J Neuroimaging 2013;23:224–227 [DOI] [PubMed] [Google Scholar]
- 24.Marmarou A, Signoretti S, Fatouros PP, Portella G, Aygok GA, Bullock MR. Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J Neurosurg 2006;104:720–730 [DOI] [PubMed] [Google Scholar]
- 25.Ito J, Marmarou A, Barzo P, Fatouros P, Corwin F. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J Neurosurg 1996;84:97–103 [DOI] [PubMed] [Google Scholar]
- 26.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia 2005;50:427–434 [DOI] [PubMed] [Google Scholar]
- 27.Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci 2007;27:11869–11876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grossman EJ, Ge YL, Jensen JH, et al. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J Neurotrauma 2012;29:2318–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuo JC, Xu S, Proctor JL, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage 2012;59:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology 2000;54:180–185 [DOI] [PubMed] [Google Scholar]
- 31.Pihlajamaki M, Tanila H, Hanninen T, et al. Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann Neurol 2000;47:470–476 [PubMed] [Google Scholar]
- 32.Kriz J, Zhu QZ, Julien JP, Padjen AL. Electrophysiological properties of axons in mice lacking neurofilament subunit genes: disparity between conduction velocity and axon diameter in absence of NF-H. Brain Res 2000;885:32–44 [DOI] [PubMed] [Google Scholar]
- 33.Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma 1997;14:419–440 [DOI] [PubMed] [Google Scholar]
- 34.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002;17:1429–1436 [DOI] [PubMed] [Google Scholar]
- 35.Matsukawa H, Shinoda M, Fujii M, et al. Genu of corpus callosum as a prognostic factor in diffuse axonal injury. J Neurosurg 2011;115:1019–1024 [DOI] [PubMed] [Google Scholar]
- 36.Voets NL, Adcock JE, Flitney DE, et al. Distinct right frontal lobe activation in language processing following left hemisphere injury. Brain 2006;129:754–766 [DOI] [PubMed] [Google Scholar]
- 37.Jafari SS, Maxwell WL, Neilson M, Graham DI. Axonal cytoskeletal changes after non-disruptive axonal injury. J Neurocytol 1997;26:207–221 [DOI] [PubMed] [Google Scholar]
- 38.Stichel CC, Muller HW. The CNS lesion scar: new vistas on an old regeneration barrier. Cell Tissue Res 1998;294:1–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


