Abstract
Time perception is fundamental and heavily researched, but the field faces a number of obstacles to theoretical progress. In this advanced review, we focus on three pieces of ‘bad news’ for time perception research: temporal perception is highly labile across changes in experimental context and task; there are pronounced individual differences not just in overall performance but in the use of different timing strategies and the effect of key variables; and laboratory studies typically bear little relation to timing in the ‘real world’. We describe recent examples of these issues and in each case offer some ‘good news’ by showing how new research is addressing these challenges to provide rich insights into the neural and information-processing bases of timing and time perception.
INTRODUCTION
The perception of time is fundamental to our experience and central to virtually all of our activities. Correspondingly, time perception was one of the earliest topics of experimental psychology and has been extensively studied for well over a century.1,2 This research has brought many successes, such as the finding that, to a first approximation, timing across multiple species exhibits a scalar property such that the variability of temporal representations increases linearly with the timed duration for intervals ranging from perhaps hundreds of milliseconds to tens of minutes. In fact, timing by many species (including humans) is approximately scale invariant, meaning that the whole response distribution scales directly with the length of the interval being timed.3,4
However, it is also clear that, contrary to the hopes (or implicit assumptions) of early psychophysicists,5,6 the brain is not like the measuring devices of classical physics: there is no immutable mapping between external magnitudes and internal sensations that can be captured by simple mathematical functions.7 Indeed, time perception poses a number of unique challenges. For one thing, there is no time-sense organ or single pathway carrying temporal information from the periphery to the brain.8,9 Rather, all sensory channels support time perception, and it is unclear how far these representations are mediated by common structures and mechanisms. Moreover, timing occurs over massively varying scales, from microseconds to years,10 and at intermediate durations multiple mechanisms likely operate in parallel, complicating the search for simple information processing models and neural substrates.
Partly for these reasons, many ‘big questions’ about time perception remain incompletely answered. For example, researchers continue to debate the relationship between physical time and the growth of subjective duration, with some arguing for a linear relationship11 and others positing a non-linear mapping such as logarithmic compression.12 Similarly, there is disagreement about whether timing is based on local or centralized processes.13 Regarding the former, temporal representations may be abstracted from time-dependent changes in local cortical networks that are activated by specific stimuli.14 That is, in the same way that one can infer how long ago a rock was thrown into a pond by the pattern of ripples on the surface, so the brain may extract temporal information from the pattern of neural activity triggered by the presentation of a stimulus.15 Other accounts posit a dedicated ‘internal clock’, a centralized system for measuring time across stimuli and tasks.16 The representation of time by this clock might involve the accumulation of pulses emitted by a pacemaker, or the detection of coincident activity among a set of oscillators with different periods.17 Finally, the basis and generality of the scalar property of timing remain the subject of considerable debate, with scale invariance variously attributed to the pulse-rate of a dedicated pacemaker,12 the transfer of temporal representations into memory,18 or the emergent properties of low-level interactions between neurons.19,20 Indeed, although the scalar property is widely observed, studies of human duration discrimination suggest that the Weber fraction is not constant over the range of about 0.5–2 seconds, violating scalar timing and providing a further theoretical challenge.21,22
Rather than focusing on these specific theoretical issues, the current review discusses three general challenges in the study of interval timing: (1) the sensitivity of time perception to the specific circumstances in which the time interval is experienced; (2) the differences between individuals in both the mechanisms and substrates of interval timing; and (3) the fact that the ‘real world’ is much more complex and dynamic than the stimuli and tasks used in most interval timing experiments. In each case, we first outline the ‘bad news’ by describing examples from recent studies which illustrate how these issues complicate and potentially hinder progress in time perception research. We then offer an alternative assessment—the ‘good news’—by showing how new findings and theorizing are helping to meet these challenges, providing deeper insights into the representation and judgment of time.
We focus primarily on durations of a few hundred to a few thousand milliseconds, as this range of intervals is the most intensely studied in human participants; it also has the advantages of being long enough to engage ‘cognitive’ processes23 but short enough to permit repeated measurements from each participant. Toward the end of the paper, we consider whether results with these kinds of moderate durations generalize to the longer intervals judged in ‘real life’.
SENSITIVITY OF TIME PERCEPTION TO THE METHOD OF MEASUREMENT
The Bad News: Research Increasingly Demonstrates the Lability of Temporal Judgments
The quest for basic principles of time perception is blighted by the remarkable sensitivity of temporal judgment to ‘extraneous’ contextual factors. First, it is increasingly clear that the judgment of a given duration depends on both the non-temporal properties of the stimuli that define the interval and the task used to elicit a duration judgment. Second, even when these factors are held constant, the judgment of a given interval is influenced by both the overall ensemble of stimuli during the session (the ‘global’ context) and the most recently presented stimulus (the ‘local’ context). Finally, even when stimuli, task, and context are controlled, judgments change as a result of experience and practice, with recent work showing just how plastic temporal representations can be. Some of these effects have been long-documented; we focus on recent research that is revealing the wide- and far-ranging context-sensitivity of temporal judgments.
Stimulus and Task Sensitivity
One basic finding is that the same duration will be perceived differently depending on how it is delimited, giving rise to what have been called ‘temporal illusions’.24 The ever-expanding range of non-temporal factors which influence the perception of a given interval is truly impressive. Some of these factors, such as sensory modality,25,26 intensity,27 size,28 complexity,29 familiarity,30 and whether an interval is ‘filled’ or ‘empty’,31 have been recognized for some time; others, such as the biasing effects of symbolic magnitude,28 difference-from-background,27 emotionality,32 pitch,33 speed,34 and changes in speed35 have been identified in recent years, and are the focus of increasing attention. This plethora of effects poses a serious challenge for researchers who seek unifying principles of interval timing.16
In addition to these stimulus-based effects, it is increasingly clear that the results of timing experiments often depend upon the method used to measure the percept (see Grondin36,37for reviews of time perception methodology). For example, estimation tasks (e.g., ‘how long was that square visible?’) and production tasks (e.g., ‘press this button when the square has been on the screen for X seconds’) have traditionally been assumed to have a reciprocal relation—stimuli which ‘seem longer’ will elicit larger estimates but shorter productions38 (because, for example, the time needed to reach a given number of pacemaker pulses will be reduced). However, the two methodologies can produce divergent results. For example, one recent study found that novel images were judged to last longer than repeated ones (implying a faster internal clock, leading to longer subjective duration), but that they were also left on-screen for longer in a production task39 (implying a slower internal clock, leading to a shorter subjective duration); still greater methodological dependence has been reported in recent investigations of the effects of emotional arousal on subjective time, with angry faces judged longer than neutral ones in verbal estimation and temporal production tasks but not in temporal generalization or reproduction tasks.40 Many timing studies use a single judgment task, which may explain why researchers investigating ostensibly similar perceptual effects sometimes produce conflicting results. That is, apparent inconsistencies in the effects of a particular manipulation or factor may actually be due to overlooked differences in methodology. For example, Witherspoon and Allan found that familiar stimuli were judged longer than unstudied items30 whereas Ono and Kawahara found the reverse effects38; the former study used a category judgment task whereas the latter used temporal production, which might explain the contradictory results.
‘Global’ and ‘Local’ Context
Even when non-temporal properties and task requirements are held constant, time perception is sensitive to the set of durations used in the study—the ‘global’ context. For example, in temporal bisection tasks the participant classifies a set of intermediate durations according to whether they are closest to a ‘short’ or ‘long’ anchor duration. For rats and pigeons, the bisection point (the duration where ‘short’ and ‘long’ responses are equally likely) is typically near the geometric mean (GM) of the two standards,3 but in humans it has been shown to depend on the distribution of intermediate durations, being closer to the GM when their spacing is logarithmic, but closer to the arithmetic mean (AM) when it is linear.3,41,42 Likewise, temporal generalization gradients (which indicate the probability that a given duration will be judged equal to a particular standard) become steeper when the comparison intervals are clustered more closely around the standard,43 leading to deviations from scalar timing if task difficulty is not carefully controlled across different standards.3 This sensitivity to global context extends to the effects of non-temporal factors; for example, the effects on time judgments of changes in tone pitch and visual marker size depend on the other pitches/markers presented during the experiment33,44; correspondingly, identical manipulations can produce different results depending on whether they are effected within or between subjects.45
Temporal judgments also depend on the ‘local’ context—that is, on the microstructure of the experimental session (the stimulus presented on the previous trial, the choice of inter-stimulus interval, and so forth). As one example, researchers routinely seek to measure the precision of temporal representation by employing two-alternative forced-choice (2AFC) tasks (where a fixed standard S and a variable comparison C are presented one after the other and participants indicate which of the two was longer). It has been known since Fechner that the order of presentation affects the point of subjective equality (PSE, the comparison duration judged equal to the standard), with the second interval of a pair often being overestimated relative to the first—although the size and direction of this time order error is variable.46 Recent work has focussed on the finding that discrimination thresholds also depend on stimulus order, being lower when the standard precedes the comparison than when the comparison comes first. This ‘Type B’ effect47 challenges the long-standing and intuitive conception of discrimination as the computation of a difference between the two stimuli48; such a calculation clearly makes sense when the two intervals are regarded as discrete physical quantities, but it cannot accommodate the effect of stimulus order on discrimination accuracy, because subtraction is commutative.
Learning and Plasticity
Finally, even when non-temporal properties, task, judgment set, and microstructure are held constant, researchers are confronted with the plasticity of the observer. Researchers make repeated observations to reduce the noise in their data, but in the case of human perception this simply creates a moving target because neural plasticity and learning can produce dramatic changes in performance over relatively short periods of time. Such plasticity has recently been investigated in time perception research.49 In one study, the threshold for discriminating a 100-ms interval from longer intervals approximately halved over 10 days of training.50 Recent work by Matthews and Grondin51 found smaller but still pronounced reductions in the Weber Fraction for intervals ranging from 100 to 1500 ms; moreover, there was no indication that performance had plateaued even after 20 sessions (two per day) training on each interval. Such learning generalizes across non-temporal features of the interval markers such as tone frequency,50,52 but training at one standard duration has little effect on discrimination at other durations.50–52 Similarly, tactile temporal discrimination learning generalizes across skin locations but not durations,53 and visual temporal discrimination learning generalizes across hemispheres, implying that the plasticity is not at an early stage in processing.54
This lability is not simply a question of improvement over time: key patterns in the data can completely shift with repeated exposure. One example comes from a Herculean bout of self-experimentation by Alfred Kristofferson, who extensively practiced temporal discrimination using standards ranging from 100–1480 ms.55 Early in training the variability of temporal representations was linearly related to the base duration; after extensive practice, however, a striking ‘staircase’ pattern emerged, with regions of constant variability punctuated by approximate ‘doubling’ at intervals which themselves double as base duration increases (Figure 1). Such plasticity makes it impossible to treat the brain like a physical measurement device, but very few researchers routinely test whether practice effects might be modulating the manipulations they wish to study.
FIGURE 1.
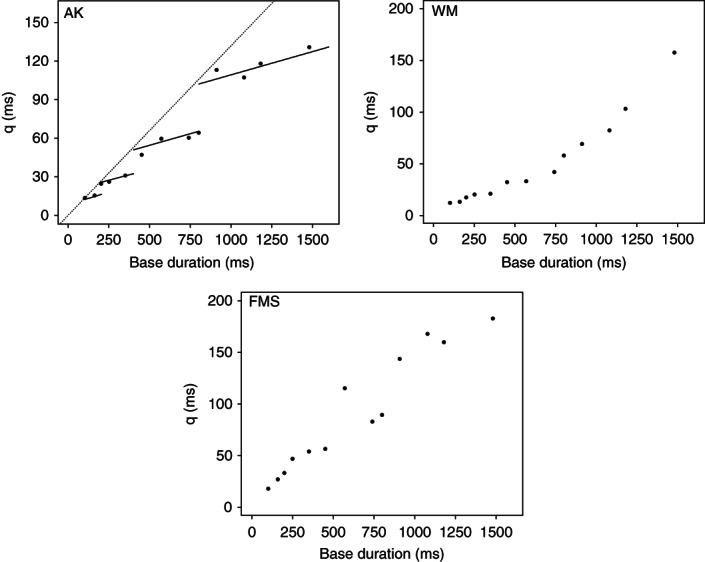
The effects of extended practice on the precision of temporal discrimination. Participants classified intervals as ‘short’ or ‘long’ according to whether the interval was shorter/longer than a given base duration. The top left panel shows data from one participant, Alfred Kristofferson, after extensive self-experimentation; it plots the variability in temporal representation (q, calculated according to a rather specific set of assumptions) as a function of base duration.55 The dashed line summarizes performance early in training; after prolonged practice, the data points ‘unfold’ from this line to give a staircase structure where both the height of the steps and the width of the treads double in magnitude with each successive step. Kristofferson interpreted this as evidence for a ‘time quantum’. The top right and bottom panels show similar data from two other participants (WM and FMS51). The eye of faith might discern some indication of a step pattern for these participants, but it is nowhere near as pronounced as for Kristofferson and the fit of Kristofferson's theoretically motivated step function is poor. (This function posits that the ‘treads’ of the staircase have the same near-horizontal slope but both their width and the step between them periodically doubles, corresponding to systematic doubling in the base of a triangular noise distribution; see Figure 4 of the paper by Matthews and Grondin51). Rather, for WM the variability in temporal representation is a quadratic function of base duration and for FMS the relation is linear. (Reprinted with permission from Ref 51)
The foregoing issues are not unique to time perception research, but they nonetheless pose enormous challenges for researchers who seek to use simple experimental and analytic tools to establish fundamental principles of timing and time perception.
The Good News: Recent Models Explain These Effects within Unifying Frameworks
Faced with this potentially overwhelming complexity, the good news is that systematic investigation of the effects of task, context, and microstructure can provide important insights into the representation and judgment of temporal information. We discuss just two recent examples.
Contextual Sensitivity Links Time Perception to General Principles of Cognition
The fact that the perception and judgment of a given item depends on the other items presented during the session is, of course, not unique to time perception.56,57 Correspondingly, careful investigation of these contextual dependencies can establish regularities that cut across multiple domains.
One example is provided by the application of range-frequency theory (RFT58) to temporal judgment. RFT posits that the judged value of a given stimulus is a compromise between (a) its position in the range defined by the smallest and largest items in the judgment set—the range principle—and (b) its relative rank position (i.e., the proportion of items in the set which are of lower magnitude)—the frequency principle. RFT emerged in the 1960s as a competitor to the idea that items are judged with respect to the mean of the stimulus set, and has been found to offer a superior account of context effects across multiple domains.59
Brown and colleagues41 have recently applied these ideas to time perception. They employed a temporal bisection task in which they varied the distribution of durations between the ‘short’ and ‘long’ anchor durations to be highly or moderately positively skewed (i.e., with lots of short durations and few long ones), arithmetically spaced, or moderately negatively skewed. The bisection points shifted progressively rightwards as the skew became more negative, and this effect was more pronounced when the ratio of long:short anchors was larger. The data were well-described by a temporal range-frequency theory (TFRT) account in which the subjective magnitude of each duration is given by its range-frequency value and the choice rule is based on the psychological distance between this magnitude and the subjective magnitude of the two standards (Figure 2). Moreover, the authors showed how this range-frequency model can account for the puzzling shifts in the bisection point noted in the literature,3 and for the effects of stimulus spacing on participants' ability to classify durations in an absolute identification task.41,60 More recently, this temporal range-frequency theory has been used to model the finding that spacing effects on duration judgments are larger for auditory stimuli than visual stimuli.61
FIGURE 2.
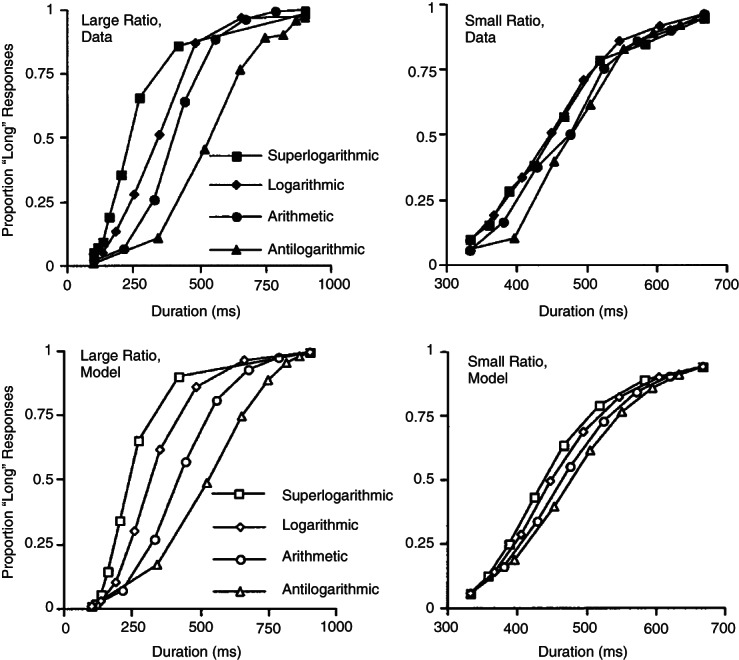
The effects of stimulus spacing on temporal bisection by humans. The top panels show the proportion of ‘long’ responses for super logarithmic (high positive skew), logarithmic (moderate positive skew), arithmetic (no skew), and antilogarithmic (moderate negative skew) spacing of the comparison durations when the two standard intervals are widely separated (left panel) or differ by only a small ratio (right panel). As the skew becomes more negative, the bisection point shifts to the right. The bottom panels show that these context effects are well-described by temporal range-frequency theory. (Reprinted with permission from Ref 41)
An alternative line of research has adopted a Bayesian framework to explain context effects within a wide-ranging and potentially unifying approach to perception and cognition.62 Jazayeri and Shadlen63 found that reproduced intervals gravitate toward the average of the set of durations in the experimental session. This effect was more pronounced for longer duration sets, where scalar variability implies greater uncertainty about the true time. Such central tendency of judgment is well-known and widespread,56 but Jazayeri and Shadlen offered a novel perspective by showing that the effect can be described by a Bayesian observer model in which a noisy measurement of the stimulus is combined with prior information about the distribution of stimuli to obtain a posterior probability distribution for the stimulus magnitude. Under this account, the central tendency of judgment is not simply a ‘nuisance’ bias; it reflects an optimizing strategy in which observers have implicit knowledge of the variability of their time representations and correspondingly adjust their use of prior probability information. More recent work has shown that, with some refinements to the prior distribution, this framework can be extended to explain the effects of modality and expertise.64 Specifically, the central tendency effect was seen for visual but not auditory stimuli; the representations of visually defined durations are typically less precise than auditory ones which, in the Bayesian framework, leads to greater reliance on the prior in the visual case. Moreover, highly trained drummers showed veridical reproductions in both modalities; their superior timing precision means they make little use of the prior even when the durations are defined by visual stimuli. The same Bayesian approach has also been used to model on- and off-medication timing performance by patients with Parkinson's disease, and other distortions in temporal memory tasks where the goal is to optimize timing performance under conditions of uncertainty.62
In short, the context effects that bedevil time-perception researchers actually link the field to a large body of empirical and theoretical work from other domains, offering the possibility of unified models of cognition and providing insights into both the functional basis for these effects and the otherwise puzzling instability of some empirical findings.
Context Effects Illuminate the Representation of Temporal Information
In addition to the descriptive accounts of the previous section, the context-sensitivity of time perception can also help clarify the ways in which temporal information is represented, combined, and evaluated. One recent example comes from the dynamic internal reference model (IRM48), developed to account for the finding that discrimination accuracy depends on the order of the standard and comparison stimuli (described above). Under this account, participants compare the second stimulus in each pair with an internal reference comprising a weighted combination of the first stimulus and the internal reference level from the previous trial. [Formally, In = gIn–1 + (1 – g)X1,n, where In is the reference level on the nth trial, X1,n is the internal representation of the first duration on the nth trial, and g is the weighting parameter.] If the difference between the second stimulus and this internal reference is positive, the second stimulus is judged to be longer than the first; if the difference is negative, the first stimulus is judged to be the longer.
The IRM predicts an effect of order on discrimination accuracy because the difference between the second stimulus and the internal reference is more variable when the first stimulus is the variable comparison duration (i.e., stimulus order <cs>) than when it is the fixed standard duration (order <sc>). Monte Carlo simulations show that the model predicts a similar effect irrespective of whether the stimulus orders are blocked or randomly intermixed, which matches the empirical pattern.48 Moreover, the model predicts sequential effects when stimulus order is fixed within blocks of trials: for order <cs>, a long comparison on the previous trial increases the internal reference level, making it less likely that the current standard stimulus will be judged the longer of the pair (and vice-versa when the previous comparison duration was short). By contrast, no sequential effects are predicted for the order <sc>, because the same standard duration is incorporated into the internal reference on every trial (in the model, only the first member of each pair contributes to the reference level). Again, these predictions nicely match the data.48
More recent work has shown that the effect of stimulus order is under attentional control. Informing participants which of the two intervals contains the variable comparison interval reduces the effect of stimulus order on the fidelity of discrimination, a modulation which can be modeled by reducing the weight given to previous trials. The IRM has also been used to account for order effects in equality judgments (‘are these two intervals the same or different?’65) and to the Vierordt effect in temporal productions.66
The IRM therefore builds on descriptive accounts of order effects on discrimination46 by providing an explicit mechanism for the formation of subjective standards which captures a wide range of findings. Similar progress has been made in developing quantitative, process models of both global context effects (i.e., the effects of stimulus range and the distribution of items within the set) and local context effects (i.e., the pattern of assimilation to the immediately preceding stimulus and contrast to stimuli earlier in the sequence) that arise when participants have to identify each of a set of durations (or other stimuli) with a unique label.41,57,60,67 Moreover, for relatively short durations (e.g., less than 1 second), researchers have modeled the effects of distractor signals and changes in inter-stimulus interval with state-dependent networks in which the time between two sensory events is represented by the changing state of local networks that respond to the marker signals.14,15
In short, the ‘instability’ of time perception can clarify the representation, combination, and evaluation of temporal information, allowing us to predict the microstructure of performance across an experimental session.
INDIVIDUAL DIFFERENCES
The Bad News: Recent Work Has Revealed Marked Individual Differences, Even for ‘Classic’ Effects
Although individual differences in perception and cognition have long been recognized as potentially important, most work in timing and time perception has ignored them and has focussed instead on establishing robust effects that emerge when data are averaged across participants. However, recent work is increasingly identifying cases where distinct sub-groups of participants, ostensibly from the same population, nonetheless show considerable heterogeneity—even for well-established effects. Such inter-participant differences are likely to be of considerable theoretical and practical importance.
One example comes from the widely reported finding that novel stimuli (‘oddballs’) have longer subjective duration than repeated items. This finding has been found in numerous experiments using diverse techniques,68 and is robust enough to be regarded as a standard ‘temporal illusion’.24 Data from one study of this effect (Experiment 2 of a study by Matthews39) are plotted in Figure 3. On each trial participants saw two photographs and judged whether the second (comparison) stimulus was presented for more or less time than the first (standard) stimulus (whose duration was always 506 ms). The second picture was either a repeat of the first or was novel. The top left panel shows the proportion of ‘longer’ responses, averaged across participants; the psychometric functions rise smoothly, and that for the ‘novel’ items is clearly shifted leftward, implying longer perceived duration for these items. A paired t-test conducted on the PSEs estimated from individual participant's data for each condition confirmed this as the overall pattern.
FIGURE 3.
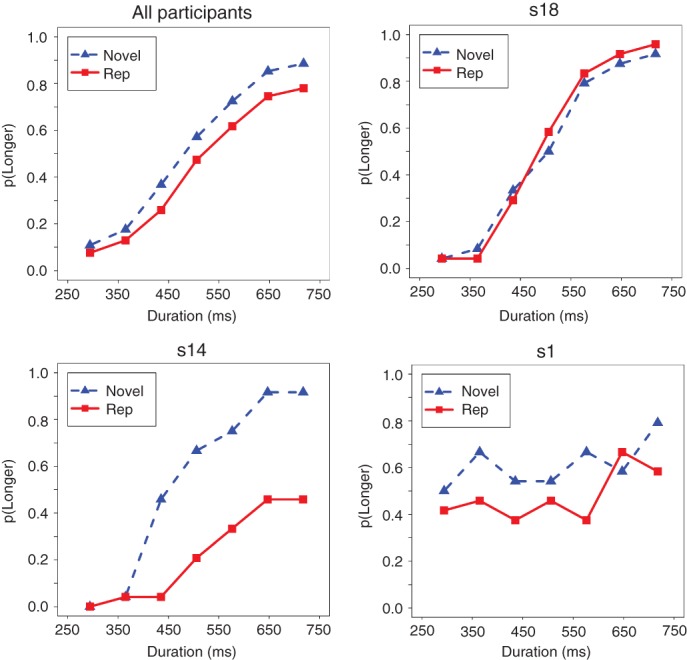
Individual differences in the effect of stimulus repetition on duration discrimination. Participants saw two images and indicated whether the second was longer or shorter than the first. The graphs show the probability of a ‘longer’ response as a function of the duration of the second (comparison) stimulus when the comparison image is a repeat of the standard image (Rep) and when it is different (Novel). The top left panel shows data averaged across participants; the top right shows a participant with good discrimination who was little affected by repetition; the bottom left shows a participant with reasonable overall discrimination but who judged novel images to be much longer than repeats of the same duration; the bottom right shows a participant with the same tendency but whose discrimination was very poor. Data are from a study reported by Matthews.39
However, there is marked variation among observers. The top right panel, for example, shows a participant with particularly good temporal discrimination who does not show the repetition effect; the bottom left shows a participant with reasonable discrimination who is nonetheless highly influenced by stimulus repetition; and the bottom right shows a participant whose temporal discrimination is poor, and who is effectively basing their judgments entirely on the novelty of the image. Thus, the overall size of the effect masks potentially important variation. A key point is that there may be systematic covariation between different aspects of performance and the effects of experimental manipulations such as the provision of feedback.69 In this study, for example, there was a substantial negative correlation between the size of the repetition effect and discrimination performance; people with poorer temporal discrimination were more affected by repetition.
This kind of heterogeneity arises in many studies of time perception, but is usually ignored or only briefly commented on. As another recent example, Figure 1 plots two additional participants who repeated the marathon discrimination training undertaken by Kristofferson55 (see above). Neither participant replicated Kristofferson's ‘step’ pattern; one shows a linear relation between variability and base duration while the other shows a quadratic pattern.51 Such large individual differences obviously make it harder to replicate a given effect, and ignoring them risks overlooking theoretically important relations between variables. Likewise, recent work by Hasuo and colleagues70 has shown pronounced individual differences in the ‘filled duration illusion’. This is the finding that a given interval seems longer when filled with continuous stimulation (e.g., a steady tone) than when it is ‘empty’ (e.g., a silent interval delimited by two brief clicks). This widely reported effect has informed both pacemaker-accumulator and alternative models of timing31,33,71; however, although the filled-duration illusion is robust in participant-averaged data, cluster analysis shows distinct sub-populations for whom the effect does and does not arise; indeed, at short durations a sub-group of people show the reverse of the usual effect.72
The Good News: Neuroimaging, Pharmacology, and Genetics Are Providing New Insights into Inter-Individual Variation
The good news is that emerging statistical techniques, including multilevel analysis73 and hierarchical Bayesian modeling,74 provide new ways to deconstruct performance in terms of underlying variables, and that neuroscience is increasingly providing insights into the origins of individual differences in timing performance. We focus here on three examples of the latter.
Individual Differences in Timing Strategy
One basic question concerns whether time perception is interval (duration) based or beat (rhythm) based.75 That is, how far does interval timing involve a stopwatch-like mechanism (e.g., the pacemaker-accumulator system of scalar timing theory16) and how far is it based on the synchronization of internal oscillatory activity with external stimulation (e.g., the entrainment model of Large and Jones76)? Behavioral and neural evidence suggests that both mechanisms are important and rest upon distinct (but perhaps connected) neural substrates.77
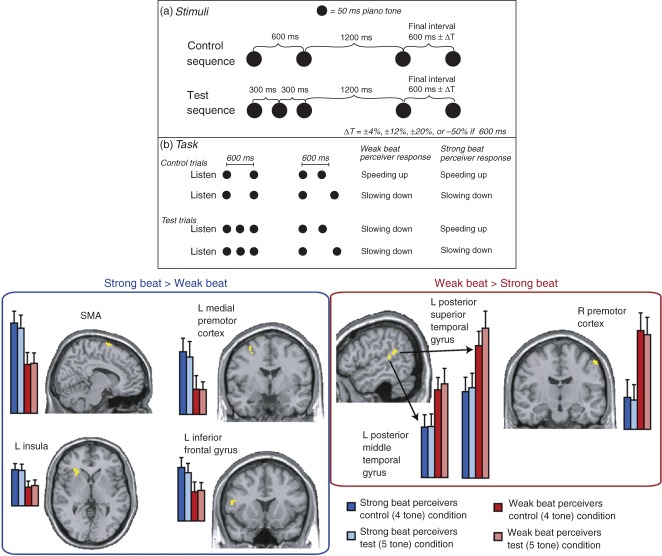
The issue is complicated by the fact that, as well as the ability of individual participants to employ both strategies, people differ in their ‘natural’ tendency to employ beat-based versus interval-based timing. Recent work has brought new clarity by establishing the neural correlates of individual differences in timing strategy. Grahn and McAuley78 had participants judge whether tone sequences were ‘speeding up’ or ‘slowing down’ (Figure 4). In the control condition the sequences comprised two pairs of tones, the first with an inter-tone interval (ITI) of 600 ms and the second with an ITI that varied around 600 ms. When the second interval was shorter than 600 ms, participants uniformly perceived the sequence as ‘speeding up’; when it was more than 600 ms, the sequence was perceived as ‘slowing down’. In the test condition, the first 2 tones were replaced by a sequence of 3 tones with an ITI of 300 ms, giving an implied periodic beat of 600 ms which was not explicitly emphasized. When judging these sequences, participants fell into two distinct groups: ‘strong’ beat perceivers, for whom final intervals less than 600 ms were systematically perceived as speeding up, and ‘weak’ beat perceivers, for whom final intervals less than 600 ms were perceived as slowing down. That is, ‘weak’ beat perceivers responded on the basis of the 300-ms separation between the first 3 tones, whereas ‘strong’ beat perceivers extracted the implied 600-ms interval. Importantly, the two groups did not differ in overall temporal sensitivity.
FIGURE 4.
Individual differences in beat perception. The top panel shows the stimulus sequences for the Control and Test trials, and the pattern of responses for weak and strong beat perceivers. In the control condition, listeners hear a pair of tones separated by 600 ms followed by another pair with a separation of 600 ms ±ΔT. Both strong and weak beat perceivers judge the sequence as ‘speeding up’ when the final separation is less than 600 ms and as ‘slowing down’ when it is more than 600 ms. On test trials, the first part of the sequence comprises a run of 3 tones separated by 300 ms so that the 600-ms periodicity is implicit. Strong beat-perceivers respond as for the control trials; weak beat-perceivers are insensitive to the 600-ms periodicity and judge all sequences as ‘slowing down’ because even the shortest final interval is longer than the 300 ms that separates the first 3 tones. The bottom panel shows the brain regions showing differential activity for the two groups of participants. Note that these neural differences arise even for the control sequences, where behavioral responses are identical. (Reprinted with permission from Ref 78)
Functional magnetic resonance imaging revealed that ‘strong’ beat perceivers showed greater activity than ‘weak’ beat perceivers in the supplementary motor area (SMA), left premotor cortex, and left insula; ‘weak’ beat perceivers showed greater activation in the right premotor cortex, the left posterior superior and left middle temporal gyri. Importantly, these differences arose even for the control sequences, where behavioral responding was the same for the two groups (Figure 4). Grahn and McAuley linked their data to the striatal beat-frequency model of interval timing,17,79 wherein neurons in the basal ganglia detect the coincident activity of oscillatory activity among cortical neurons, with particular patterns of coincidence representing particular durations. In this framework, ‘strong’ beat perceivers may more readily ‘tune in’ to the implied beat by entraining specific cortical oscillators. More recent work using electroencephalography suggests that differences in beat perception correspond to differential processing of the final tone in the sequence (with strong beat-perceivers showing greater build-up of contingent negative variation prior to the final tone and a larger parietal positivity after it) rather than differences in the sensory encoding of the initial sequence.80
These neuroimaging studies are complemented by the development of behavioral test batteries which can be used to identify both the precise nature of individual and group differences in timing and time perception and the inter-relations among different aspects of timing performance. The Battery for the Assessment of Auditory and Sensorimotor Timing Abilities (BAASTA) is one such tool; it includes duration discrimination, anisochrony detection, beat alignment, synchronization-continuation and tapping tasks, and has been used to compare musicians and non-musicians and to identify the specific timing impairments of patients with Parkinson's disease.81
Individual Differences in Response to Pharmacological Treatment
Lake and Meck82 had healthy young adults complete a peak-interval timing task (where the goal is to concentrate a sequence of spacebar presses around a trained duration) under the influence of d-amphetamine [an indirect dopamine (DA) receptor agonist], haloperidol (a direct DA receptor antagonist), and a placebo. Crucially, self-reported liking for the drug treatment moderated the effects of d-amphetamine on timing, with a positive correlation between liking and the shift in the timing of peak responding. Self-reported drug-liking also negatively correlated with a pre-measure of simple reaction time (SRT), an index of baseline attention which may reflect baseline DA levels. When the participants were divided according to this pre-measure, those high in attentional lapses (i.e., those with low drug liking) showed a leftward shift in the response curve when given d-amphetamine (relative to placebo) but a rightward shift following haloperidol. That is, for this group, increasing or decreasing DA activity produced the classic increase/decrease in ‘clock speed’ found in previous research.83 However, for participants low in baseline-lapses (i.e., high in drug liking), the response function following d-amphetamine was shifted to the right of the placebo, with haloperidol having little or no effect at the dose studied. The authors interpret this rightward shift as reflecting reduced attention to time among those people who particularly enjoyed the effects of d-amphetamine. That is, because reduced attention to ‘time in passing’ reduces perceived duration,84 so participants who experienced drug-induced euphoria following d-amphetamine administration focussed their attention on the hedonic effects of the drug rather than on their perception of time, and delayed their peak responding as a consequence.
The key point here is that the psychological effects of pharmacological challenge are modulated by individual differences in subjective experience, which in turn map on to measurable differences in pre-test performance and, perhaps, to underlying differences in neurotransmitter levels.85
Individual Differences in Genotype
Perhaps the most fundamental way to link individual differences in timing and time perception to neurochemistry is by studying genetic variation. A comprehensive example was recently provided by Sysoeva and colleagues.86 These authors recruited right-handed Russian male participants for whom DNA samples had previously been taken, and selected a sample comprising a mix of rare and common genotypes of genes involved in regulating dopaminergic and serotonergic activity. The participants completed a duration discrimination task in which they judged whether the first or second of two visual stimuli was shown for the shorter time.
As in many other studies employing this kind of 2AFC task, most participants showed a negative time order error, such that the second interval was over-estimated relative to the first (the two intervals were equally likely to be judged ‘shorter’ when the first was in fact longer than the second), which the authors interpreted in terms of leakage from a neural accumulator representing the first interval. The key finding was that the magnitude of this effect (indexed by both the PSE and by formal estimation of the leakage parameter) was related to differences in genotypes. The PSE was more negative (overestimation of the second interval was greater, implying greater leakage from the accumulator) for carriers of genotypes associated with greater serotonin (5-HT) transmission. Specifically, the PSE was more negative for (1) carriers of the SS genotype of the 5-HTT gene (associated with lower 5-HT reuptake) than for carriers of the SL and LL variants, (2) carriers of the low-expression variant of monoamine oxidase A (MAOA, associated with lower 5-HT breakdown) than for carriers of the high-expression variant, and (3) carriers of the TT genotype of the 5-HT2a receptor (associated with higher receptor density) than for CC-carriers. In contrast, differences in time perception were not related to differences in the genes encoding components of the DA system, although more recent genetic studies have shown DA receptors, transporter, and enzyme systems to be important for interval timing.87–89
In summary, new examinations of the specific neural, pharmacological, and genetic bases for individual differences in time perception are helping to account for heterogeneous performance and shed light on long-standing theoretical questions such as the relative importance of beat-based versus interval-based timing, the role of attention in time perception, and the basis for the time-order error. Moreover, these insights are supplemented by recent work linking differences in timing variability to cortical anatomy.90
TIME PERCEPTION IN THE ‘REAL WORLD’
The Bad News: Most Time Perception Research Uses Simple Stimuli and Artificial Tasks
In most studies of temporal judgment, researchers present a limited set of items many times, eliciting repeated judgments of the same stimulus and with the participant fully aware that a time judgment will be required for each item (i.e., their judgments are ‘prospective’). These tasks bear little relation to ‘normal life’, where time estimates are usually one-off judgments of single (often complex, dynamic, temporally structured) events. There have, of course, been studies of ‘retrospective’ time judgments (where the participant only learns that a time judgment is required after the event), and comparisons of these with ‘prospective’ judgments.91 However, the one-off nature of most retrospective judgments presents a practical barrier to large-scale data collection, and the number of studies comparing prospective and retrospective paradigms with other task dimensions held constant is small,92 with only a handful that have used intervals longer than a few minutes.93 There is a similar paucity of studies exploring whether principles established for brief stimuli presented multiple times (e.g. modality differences, intensity effects) also apply to naturalistic one-off judgments of longer intervals defined by complex event sequences.
A related issue concerns the kinds of stimuli used in most previous research. Studies of time perception typically use homogenous stimuli such as pure tones, steady lights, or static images, and although there is a large literature on rhythm perception, the effect of temporal structure on the perceived duration of the overall sequence has received relatively little attention (with some exceptions94). However, most naturally occurring stimuli are dynamic and temporally structured; there is increasing appreciation of the importance of temporal information to such ‘real world’ tasks as face processing,95 scene memory,96–98 and emotion recognition,99 which renders pressing the need for models of timing that can accommodate dynamic event sequences.
The bad news, then, is that most of our research has traditionally concerned stimuli and tasks which bear little relation to ‘real life’, making it hard to know whether we are missing theoretically important effects and interactions (by focusing on rather simplistic stimuli) and whether the principles in the laboratory will generalize to real-life situations.
The Good News: Recent Data Show that Lab Results Generalize and Comprehensive Models Are within Reach
On a positive note, researchers are increasingly supplementing the traditional approaches outlined above with (1) more naturalistic judgment tasks and (2) temporally structured, dynamic stimuli; this work gives reason for optimism about the generality of effects previously studied in the laboratory, and about the potential for formal models of timing that can accommodate the judgment of complex stimuli.
Naturalistic Tasks
One example of this kind of ecologically valid study was recently provided by Grondin and colleagues.93 These authors recruited 116 video Gamers at gaming centers in Quebec—players who had arrived at the centers to play a game of their choice as a normal leisure activity, rather than being explicitly recruited for an experiment. After 12, 35, or 58 min of their chosen game the players were asked to estimate how long they had been playing. Each player estimated a single duration; players in the prospective condition had been notified in advance that a time judgment would be requested whereas those in the retrospective condition had not. The ratio of judged-to-actual duration was greater than 1.0 in all conditions (that is, players overestimated their game time), and this tendency was significantly more pronounced in the 12-min duration than the 35- and 59-min conditions (which did not differ). Moreover, judged durations were longer in the prospective condition than in the retrospective condition (irrespective of physical duration). In other words, this very naturalistic study produced data that show several features found in more traditional research (namely, a general overestimation of time that relatively ‘flattens off’ at longer durations and that is more pronounced when the time estimate is anticipated; there was also some indication of greater absolute variability in prospective than retrospective judgments, but this effect missed significance).
It is reassuring that traditional experimental effects extend to the ‘real world’, but we must sound two notes of caution. First, not all naturalistic studies produce such replications. Boltz,100 for example, reported no difference between prospective and retrospective judgments of naturalistic events (video clips of people eating, playing basketball, etc.), and Darlow and colleagues101 recently found no effect of changes in tempo on one-off judgments of music, reading, or perceptuo-motor tasks, despite such tempo-changes having very pronounced effects in psychophysical studies.33 Second, when effects do generalize, this can prompt questions about the traditional explanations of these effects. For example, prospective judgments are usually argued to be longer because participants are paying ‘greater attention to time’ (such that, for example, a larger number of pacemaker pulses accumulates during a given interval than when processing is directed to non-temporal information). Does this idea hold water when, in the aforementioned video-game study, the participant is estimating a duration of nearly an hour while engaged in a highly absorbing leisure activity? Was mentioning at the start of the gaming session the fact that they would be asked for a time estimate really enough to produce a systematic shift in the amount of ‘attention to time’ during their gameplay? Other explanations are certainly possible. For example, forewarning about the judgment may have prompted participants to produce a preliminary estimate, perhaps based on their estimate of how long they planned to stay (e.g., ‘I'll be here for about four hours’) or for the length of time they were ‘likely to be asked about’ (e.g., ‘I expect she'll ask me after about an hour’). Such a pre-estimate might serve as an ‘anchor’ when the time came to make the actual judgment (such anchoring effects are very common102). These caveats notwithstanding, the increasing use of naturalistic studies provide important steps toward unified, general models of timing; similar work has been described in other recent studies.103–106
Dynamic Stimuli
A second line of attack involves using traditional laboratory tasks but employing dynamic, temporally structured stimuli rather than the homogenous lights and tones of conventional research. It has long been known that the division of an interval into sub-intervals tends to increase its apparent duration,71 and that the relative lengths of these sub-intervals also affects temporal judgment.107 Similarly, moving stimuli typically seem to last longer than static ones,34 with perceived duration a positive function of speed.34,108 More recent work has begun to extend these core observations in several ways.
First, careful psychophysical investigations have sought to establish the precise basis for these effects, for example by teasing apart the contributions of speed and temporal frequency.109,110
Second, researchers have moved beyond studying the effect of speed/tempo and begun to explore the influence of higher-order dynamics. In the first study of this type, Matthews35 had participants judge the duration of rotating and translating shapes which either moved with constant, gradually accelerating, or gradually decelerating speed (with mean speed held constant across conditions). Contrary to many theoretical accounts, perceived duration was longest for the constant-speed stimuli and shortest for the accelerating items. The finding that decelerating stimuli seem to last longer than accelerating ones has since been replicated by Sasaki and colleagues111 with sine-wave gratings; indeed, a discrete change in speed halfway through stimulus presentation was sufficient to elicit the effect. More recent work has investigated the effects of changes in tempo on the perceived duration of tone sequences.33 Again, constant-rate stimuli had the longest perceived duration, but the effects of temporal structure were modulated by such factors as overall physical duration, number of tones in the sequence, rate of acceleration/deceleration, and non-temporal aspects of the stimuli (specifically, the pitch-relations between successive sequence elements). In particular, accelerating sequences were judged longer than decelerating ones at 600 ms, but this pattern reversed at 1200 ms (Figure 5, left panel). These complex results provide strong empirical constraints on theoretical accounts of time perception.
FIGURE 5.
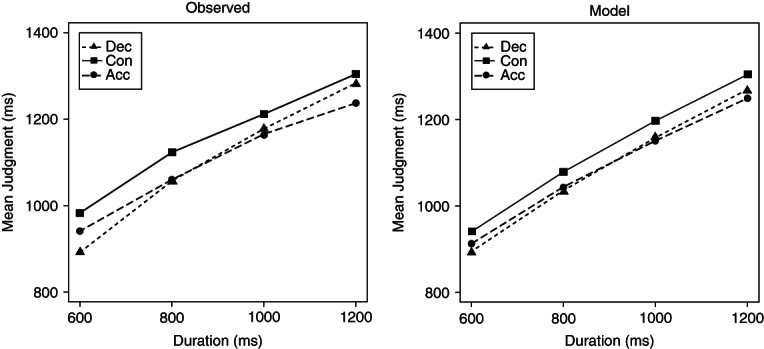
The effects of sequence structure on the judged duration of tone sequences. The left panel shows judgments of decelerating (Dec), Accelerating (Acc) and Constant-tempo (Con) sequences of 5 tones as a function of physical duration. At short durations, accelerating sequences are judged longer than decelerating ones; at longer durations, this pattern reverses. Constant-tempo stimuli are consistently judged to be the longest of all. The right-hand panel shows the predictions of a weighted sum of segments model, which successfully captures this complex pattern (see text for details). (Reprinted with permission from Ref 33)
Finally, researchers have begun to formally model the effects of motion and structure on perceived time. Thus, Beckman and Young112 fit different versions of scalar timing theory18,62,113 to a temporal bisection task that used stimuli rotating at different speeds. In one version of the model, stimulus speed affected the rate of the internal pacemaker; in the other version, speed affected the magnitude of the to-be-judged interval, on the assumption that organisms discriminate amounts of change rather than durations per se. The latter, change-based account provided a clearly superior fit, predicting shifts in the bisection point without the corresponding changes in discrimination precision that characterized the pacemaker account. In contrast, Matthews35 has argued against a change-based explanation for the effects of second-order dynamics, and has modeled the differences between accelerating, decelerating, and constant-speed stimuli by assuming that pacemaker-rate is a function of speed and that the output of the pacemaker is weighted differently over the course of the interval. More recently, a comprehensive weighted sum of segments model has been proposed to account for the effects of sequence structure when intervals are sub-divided into discrete sub-intervals.33 This model assumes (1) that the subjective duration of each segment is a negatively accelerated (e.g., logarithmic) function of its physical extent, (2) that the judgment of the overall interval is obtained by summing the subjective durations of each sub-interval, and (3) that during this summation, more recent segments are weighted more heavily (e.g., because the older representations are subject to exponential decay). For the specific case of tone sequences, the model further assumes a positive relation between pitch and perceived time. This model captures the differences between accelerating, decelerating, and constant-tempo sequences, and the dependency of these effects on the physical duration of the sequence (Figure 5, right panel). It also captures the complex interactions among these factors and the number of sub-intervals, the rate of acceleration/deceleration, and the direction of pitch change (ascending vs. descending).
The good news, then, is that researchers are beginning to study timing and time perception using the kinds of behavioral tasks and situations that apply outside the laboratory, to explore the effects of complex stimulus dynamics on perceived duration, and to develop quantitative models of these effects. Of course, the complexity of the natural world means that the context dependencies described earlier are likely to be even more pronounced and unpredictable than in the laboratory, but there is reason for optimism about our ability to model and explain such effects.
CONCLUSION
We hope that this brief survey is sufficient to encourage researchers that the complexity and lability of time perception is not all ‘bad news’. A number of directions stand out for future research, including: (1) the development of progressively more unified information-processing models, which connect time perception to other aspects of cognition such as working memory, (2) the simultaneous application of genetic, pharmacological, neuroimaging, and behavioral techniques to establish comprehensive accounts of the biological basis of interval timing, and (3) the extension of existing paradigms and principles to more naturalistic situations and tasks, particularly those with important practical consequences.
REFERENCES
- 1.Roeckelein JE. History of conceptions and accounts of time and early time perception research. In: Grondin S, editor. Psychology of Time. Bingley, UK: Emerald Press; 2008. pp. 1–50. [Google Scholar]
- 2.James W. The Principles of Psychology. Vol. 1. New York: Holt; 1890. The perception of time. [Google Scholar]
- 3.Wearden JH, Lejeune H. Scalar properties in human timing: conformity and violations. Q J Exp Psychol. 2008;61:569–587. doi: 10.1080/17470210701282576. [DOI] [PubMed] [Google Scholar]
- 4.Lejeune H, Wearden JH. Scalar properties in animal timing: conformity and violations. Q J Exp Psychol. 2006;59:1875–1908. doi: 10.1080/17470210600784649. [DOI] [PubMed] [Google Scholar]
- 5.Fechner GT. Elemente der psychophysik. Vol. 1. Leipzig: Breitkopf und Härtel; 1860. [Google Scholar]
- 6.Stevens SS. On the psychophysical law. Psychol Rev. 1957;64:153–181. doi: 10.1037/h0046162. [DOI] [PubMed] [Google Scholar]
- 7.Laming D. The measurement of sensation. Oxford, UK: Oxford University Press; 1997. [Google Scholar]
- 8.Gibson JJ. Events are perceivable but time is not. In: Fraser JT, Lawrence N, editors. The Study of Time II. New York: Springer; 1975. pp. 295–301. [Google Scholar]
- 9.Vroomen J, Keetels M. Perception and intersensory synchrony: a tutorial review. Atten Percept Psychophys. 2010;72:871–884. doi: 10.3758/APP.72.4.871. [DOI] [PubMed] [Google Scholar]
- 10.Buonomano DV. The biology of time across different scales. Nat Chem Biol. 2007;3:594–597. doi: 10.1038/nchembio1007-594. [DOI] [PubMed] [Google Scholar]
- 11.Wearden JH, Jones LA. Is the growth of subjective time in humans a linear or nonlinear function of real time? Q J Exp Psychol. 2007;60:1289–1302. doi: 10.1080/17470210600971576. [DOI] [PubMed] [Google Scholar]
- 12.Taatgen NA, van Rijn H, Anderson J. An integrated theory of prospective time interval estimation: the role of cognition, attention, and learning. Psychol Rev. 2007;114:577–598. doi: 10.1037/0033-295X.114.3.577. [DOI] [PubMed] [Google Scholar]
- 13.Merchant H, Harrington DL, Meck WH. Neural basis of the perception and estimation of time. Annu Rev Neurosci. 2013;36:313–336. doi: 10.1146/annurev-neuro-062012-170349. [DOI] [PubMed] [Google Scholar]
- 14.Karmarkar UR, Buonomano DV. Timing in the absence of clocks: encoding time in neural network states. Neuron. 2007;53:427–438. doi: 10.1016/j.neuron.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buonomano DV, Bramen J, Khodadadifar M. Influence of the interstimulus interval on temporal processing and learning: testing the state-dependent network model. Philos Trans R Soc B Biol Sci. 2009;364:1865–1873. doi: 10.1098/rstb.2009.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allman MJ, Teki S, Griffin TD, Meck WH. Properties of the internal clock: first- and second-order principles of subjective time. Annu Rev Psychol. 2014;65:743–771. doi: 10.1146/annurev-psych-010213-115117. [DOI] [PubMed] [Google Scholar]
- 17.Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cogn Brain Res. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Gibbon J, Church RM, Meck WH. Scalar timing in memory. Ann NY Acad Sci. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- 19.Simen P, Balci F, deSouza L, Cohen JD, Holmes P. A model of interval timing by neural integration. J Neurosci. 2011;31:9238–9253. doi: 10.1523/JNEUROSCI.3121-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oprisan AA, Buhusi CV. What is all the noise about in interval timing? Philos Trans R Soc B Biol Sci. 2014;369:1–12. doi: 10.1098/rstb.2012.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grondin S. Violation of the scalar property for time perception between 1 and 2 seconds: evidence from interval discrimination, reproduction, and categorization. J Exp Psychol Hum Percept Perform. 2012;38:880–890. doi: 10.1037/a0027188. [DOI] [PubMed] [Google Scholar]
- 22.Grondin S. From physical time to the first and second moments of psychological time. Psychol Bull. 2001;127:22–44. doi: 10.1037/0033-2909.127.1.22. [DOI] [PubMed] [Google Scholar]
- 23.Rammsayer TH, Lima SD. Duration discrimination of filled and empty auditory intervals: cognitive and perceptual factors. Percept Psychophys. 1991;50:565–574. doi: 10.3758/bf03207541. [DOI] [PubMed] [Google Scholar]
- 24.Eagleman DM. Human time perception and its illusions. Curr Opin Neurobiol. 2008;18:131–136. doi: 10.1016/j.conb.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grondin S, Rousseau R. Judging the relative duration of multimodal short empty time intervals. Percept Psychophys. 1991;49:245–256. doi: 10.3758/bf03214309. [DOI] [PubMed] [Google Scholar]
- 26.Penney TB, Gibbon J, Meck WH. Differential effects of auditory and visual signals on clock speed and temporal memory. J Exp Psychol Hum Percept Perform. 2000;26:1770–1787. doi: 10.1037//0096-1523.26.6.1770. [DOI] [PubMed] [Google Scholar]
- 27.Matthews WJ, Stewart N, Wearden JH. Stimulus intensity and the perception of duration. J Exp Psychol Hum Percept Perform. 2011;37:303–313. doi: 10.1037/a0019961. [DOI] [PubMed] [Google Scholar]
- 28.Xuan B, Zhang D, He S, Chen XC. Larger stimuli are judged to last longer. J Vis. 2007;7:1–5. doi: 10.1167/7.10.2. [DOI] [PubMed] [Google Scholar]
- 29.Aubry F, Guillaume N, Mogicato G, Bergeret L, Celsis P. Stimulus complexity and prospective timing: clues for a parallel process model of time perception. Acta Psychol (Amst) 2008;128:63–74. doi: 10.1016/j.actpsy.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Witherspoon D, Allan LG. The effect of a prior presentation on temporal judgments in a perceptual identification task. Mem Cognit. 1985;13:101–111. doi: 10.3758/bf03197003. [DOI] [PubMed] [Google Scholar]
- 31.Wearden JH, Norton R, Martin S, Montford-Bebb O. Internal clock processes and the filled-duration illusion. J Exp Psychol Hum Percept Perform. 2007;33:716–729. doi: 10.1037/0096-1523.33.3.716. [DOI] [PubMed] [Google Scholar]
- 32.Droit-Volet S, Fayolle S, Lamotte M, Gil S. Time, emotion and the embodiment of timing. Timing Time Percep. 2013;1:99–126. [Google Scholar]
- 33.Matthews WJ. How does sequence structure affect the judgment of time? Exploring a weighted sum of segments model. Cogn Psychol. 2013;66:259–282. doi: 10.1016/j.cogpsych.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Brown SW. Time, change, and motion: the effects of stimulus movement on temporal perception. Percept Psychophys. 1995;57:105–116. doi: 10.3758/bf03211853. [DOI] [PubMed] [Google Scholar]
- 35.Matthews WJ. How do changes in speed affect the perception of duration? J Exp Psychol Hum Percept Perform. 2011;37:1617–1627. doi: 10.1037/a0022193. [DOI] [PubMed] [Google Scholar]
- 36.Grondin S. Methods for studying psychological time. In: Grondin S, editor. Psychology of Time. Bingley, UK: Emerald Press; 2008. pp. 51–74. [Google Scholar]
- 37.Grondin S. Timing and time perception: a review of recent behavioral and neuroscience findings and theoretical directions. Atten Percept Psychophys. 2010;72:561–582. doi: 10.3758/APP.72.3.561. [DOI] [PubMed] [Google Scholar]
- 38.Ono F, Kawahara J. The effect of unconscious priming on temporal production. Conscious Cogn. 2005;14:474–482. doi: 10.1016/j.concog.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Matthews WJ. Stimulus repetition and the perception of time: the effects of prior exposure on temporal discrimination, judgment, and production. PLoS One. 2011;6:e19815. doi: 10.1371/journal.pone.0019815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gil S, Droit-Volet S. “Time flies in the presence of angry faces” … depending on the temporal task used! Acta Psychol (Amst) 2011;136:354–362. doi: 10.1016/j.actpsy.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Brown GDA, McCormack T, Smith M, Stewart N. Identification and bisection of temporal durations and tone frequencies: Common models for temporal and nontemporal stimuli. J Exp Psychol Hum Percept Perform. 2005;31:919–938. doi: 10.1037/0096-1523.31.5.919. [DOI] [PubMed] [Google Scholar]
- 42.Wearden JH, Ferrara A. Stimulus spacing effects in temporal bisection by humans. Q J Exp Psychol. 1995;48B:289–310. [PubMed] [Google Scholar]
- 43.Ferrara A, Lejeune H, Wearden JH. Changing sensitivity to duration in human scalar timing: An experiment, a review, and some possible explanations. Q J Exp Psychol. 1997;50B:217–237. [Google Scholar]
- 44.Matthews WJ. Can we use verbal estimation to dissect the internal clock? Differentiating the effects of pacemaker rate, switch latencies, and judgment processes. Behav Processes. 2011;86:68–74. doi: 10.1016/j.beproc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Gomez LM, Robertson LC. The filled-duration illusion: the function of temporal and non-temporal set. Percept Psychophys. 1979;25:432–438. doi: 10.3758/bf03199853. [DOI] [PubMed] [Google Scholar]
- 46.Hellström Å. The time-order error and its relatives—mirrors of cognitive processes in comparing. Psychol Bull. 1985;97:35–61. [Google Scholar]
- 47.Ulrich R, Vorberg D. Estimating the difference limen in 2AFC tasks: Pitfalls and improved estimators. Atten Percept Psychophys. 2009;71:1219–1227. doi: 10.3758/APP.71.6.1219. [DOI] [PubMed] [Google Scholar]
- 48.Dyjas O, Bausenhart KM, Ulrich R. Trial-by-trial updating of an internal reference in discrimination tasks: Evidence from effects of stimulus order and trial sequence. Atten Percept Psychophys. 2012;74:1819–1841. doi: 10.3758/s13414-012-0362-4. [DOI] [PubMed] [Google Scholar]
- 49.Bueti D, Buonomano DV. Temporal perceptual learning. Timing Time Percep. In press. [Google Scholar]
- 50.Wright BA, Buonomano DV, Mahncke HW, Merzenich MM. Learning and generalization of auditory temporal-interval discrimination in humans. J Neurosci. 1997;17:3956–3963. doi: 10.1523/JNEUROSCI.17-10-03956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthews WJ, Grondin S. On the replication of Kristofferson's (1980) quantal timing for duration discrimination: some learning but no quanta and not much of a Weber constant. Atten Percept Psychophys. 2012;74:1056–1072. doi: 10.3758/s13414-012-0282-3. [DOI] [PubMed] [Google Scholar]
- 52.Karmarkar UR, Buonomano DV. Temporal specificity of perceptual learning in an auditory discrimination task. Learn Mem. 2003;10:141–147. doi: 10.1101/lm.55503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagarajan SS, Blake DT, Wright BA, Byl N, Merzenich MM. Practice-related improvements in somatosensory interval discrimination are temporally specific but generalize across skin location, hemisphere, and modality. J Neurosci. 1998;18:1559–1570. doi: 10.1523/JNEUROSCI.18-04-01559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westheimer G. Discrimination of short time intervals by the human observer. Exp Brain Res. 1999;129:121–126. doi: 10.1007/s002210050942. [DOI] [PubMed] [Google Scholar]
- 55.Kristofferson AB. A quantal step function in duration discrimination. Percept Psychophys. 1980;27:300–306. doi: 10.3758/bf03206118. [DOI] [PubMed] [Google Scholar]
- 56.Matthews WJ, Stewart N. Psychophysics and the judgment of price: judging complex objects on a non-physical dimension elicits sequential effects like those in perceptual tasks. Judgment Decision Making. 2009;4:64–81. [Google Scholar]
- 57.Matthews WJ, Stewart N. The effect of interstimulus interval on sequential effects in absolute identification. Q J Exp Psychol. 2009;62:2014–2029. doi: 10.1080/17470210802649285. [DOI] [PubMed] [Google Scholar]
- 58.Parducci A. Category judgment: a range-frequency model. Psychol Rev. 1965;72:407–418. doi: 10.1037/h0022602. [DOI] [PubMed] [Google Scholar]
- 59.Parducci A. Happiness, pleasure, and judgment: the contextual theory and its applications. Mahwah, NJ: Lawrence Erlbaum Associates; 1995. [Google Scholar]
- 60.Stewart N, Brown GDA, Chater N. Absolute identification by relative judgment. Psychol Rev. 2005;112:881–911. doi: 10.1037/0033-295X.112.4.881. [DOI] [PubMed] [Google Scholar]
- 61.Penney TB, Brown GDA, Wong JKL. Stimulus spacing effects in duration perception are larger for auditory stimuli: data and a model. Acta Psychol (Amst) 2014;147:97–104. doi: 10.1016/j.actpsy.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 62.Shi Z, Church RM, Meck WH. Bayesian optimization of time perception. Trends Cogn Sci. 2013;17:556–564. doi: 10.1016/j.tics.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Jazayeri M, Shadlen MN. Temporal context calibrates interval timing. Nat Neurosci. 2010;13:1020–1028. doi: 10.1038/nn.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cicchini GM, Arrighi R, Cecchetti L, Giusti M, Burr DC. Optimal encoding of interval timing in expert percussionists. J Neurosci. 2012;32:1056–1060. doi: 10.1523/JNEUROSCI.3411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dyjas O, Ulrich R. Effects of stimulus order on discrimination processes in comparative and equality judgments: data and models. Q J Exp Psychol. 2014;67:1121–1150. doi: 10.1080/17470218.2013.847968. [DOI] [PubMed] [Google Scholar]
- 66.Bausenhart KM, Dyjas O, Ulrich R. Temporal reproductions are influenced by an internal reference: Explaining the Vierordt effect. Acta Psychol (Amst) 2014;147:60–67. doi: 10.1016/j.actpsy.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Brown SD, Marley AAJ, Donkin C, Heathcote A. An integrated model of choices and response times in absolute identification. Psychol Rev. 2008;115:396–425. doi: 10.1037/0033-295X.115.2.396. [DOI] [PubMed] [Google Scholar]
- 68.Tse PU, Intriligator J, Rivest J, Cavanagh P. Attention and the subjective expansion of time. Percept Psychophys. 2004;66:1171–1189. doi: 10.3758/bf03196844. [DOI] [PubMed] [Google Scholar]
- 69.Gu B-M, Meck WH. Multidisciplinary Aspects of Time and Time Perception. Vol. 6789. Berlin/Heidelberg: Springer; 2011. New perspectives on Vierordt's law: memory-mixing in ordinal temporal comparison tasks; pp. 67–78. Lecture Notes in Computer Science. [Google Scholar]
- 70.Hasuo E, Nakajima Y, Tomimatsu E, Grondin S, Ueda K. The occurrence of the filled duration illusion: a comparison of the method of adjustment with the method of magnitude estimation. Acta Psychol (Amst) 2014;147:111–121. doi: 10.1016/j.actpsy.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Thomas EAC, Brown I. Time perception and the filled-duration illusion. Percept Psychophys. 1974;16:449–458. [Google Scholar]
- 72.Hasuo E, Nakajima Y, Ueda K. Does filled duration illusion occur for very short time intervals? Acoust Sci Technol. 2011;32:82–85. [Google Scholar]
- 73.Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59:390–412. [Google Scholar]
- 74.Shiffrin R, Lee M, Kim W, Wagenmakers EJ. A survey of model evaluation approaches with a tutorial on hierarchical Bayesian methods. Cognit Sci. 2008;32:1248–1284. doi: 10.1080/03640210802414826. [DOI] [PubMed] [Google Scholar]
- 75.McAuley JD, Jones MR. Modeling effects of rhythmic context on perceived duration: a comparison of interval and entrainment approaches to short-interval timing. J Exp Psychol Hum Percept Perform. 2003;29:1102–1125. doi: 10.1037/0096-1523.29.6.1102. [DOI] [PubMed] [Google Scholar]
- 76.Large EW, Jones MR. The dynamics of attending: how people track time-varying events. Psychol Rev. 1999;106:119–159. [Google Scholar]
- 77.Teki S, Grube M, Griffiths TD. A unified model of time perception accounts for duration-based and beat-based timing mechanisms. Front Integr Neurosci. 2012;5:Article 90. doi: 10.3389/fnint.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grahn JA, McAuley JD. Neural bases of individual differences in beat perception. Neuroimage. 2009;47:1894–1903. doi: 10.1016/j.neuroimage.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 79.Allman MJ, Meck WH. Pathophysiological distortions in time perception and timed performance. Brain. 2012;135:656–677. doi: 10.1093/brain/awr210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Snyder JS, Pasinski AC, McAuley JD. Listening strategy for auditory rhythms modulates neural correlates of expectancy and cognitive processing. Psychophysiology. 2011;48:198–207. doi: 10.1111/j.1469-8986.2010.01053.x. [DOI] [PubMed] [Google Scholar]
- 81.Farrugia N, Benoit C-E, Harding E, Kotz SA, Dalla Bella S. 2012. BAASTA: battery for the assessment of auditory sensorimotor and timing abilities. In: Proceedings of the 12th International Conference on Music Perception and Cognition and the 8th Triennial Conference of the European Society for the Cognitive Sciences of Music. Tessaloniki, Greece.
- 82.Lake JI, Meck WH. Differential effects of amphetamine and haloperidol on temporal reproduction: dopaminergic regulation of attention and clock speed. Neuropsychologia. 2013;51:284–292. doi: 10.1016/j.neuropsychologia.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown SW. Time and attention: review of the literature. In: Grondin S, editor. Psychology of Time. Bingley, UK: Emerald Press; 2008. pp. 111–138. [Google Scholar]
- 85.Coull JT, Hwang HJ, Leyton M, Dagher A. Dopaminergic modulation of motor timing in healthy volunteers differs as a function of baseline DA precursor activity. Timing Time Percep. 2013;1:77–98. [Google Scholar]
- 86.Sysoeva OV, Tonevitsky AG, Wackermann J. Genetic determinants of time perception mediated by the serotonergic system. PLoS One. 2010;5:e12650. doi: 10.1371/journal.pone.0012650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balci F, Wiener M, Cavdaroglu B, Coslett HB. Epistasis effects of dopamine genes on interval timing and reward magnitude in humans. Neuropsychologia. 2013;51:293–308. doi: 10.1016/j.neuropsychologia.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 88.Meck WH, Cheng RK, MacDonald CJ, Gainetdinov RR, Caron MG, Cevik MO. Gene-dose dependent effects of methamphetamine on interval timing in dopamine-transporter knockout mice. Neuropharmacology. 2012;62:1221–1229. doi: 10.1016/j.neuropharm.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 89.Wiener M, Lohoff FW, Coslett HB. Double dissociation of dopamine genes and timing in humans. J Cogn Neurosci. 2011;23:2811–2821. doi: 10.1162/jocn.2011.21626. [DOI] [PubMed] [Google Scholar]
- 90.Gilaie-Dotan S, Kanai R, Rees G. Anatomy of human sensory cortices reflects inter-individual variability in time estimation. Front Integrat Neurosci. 2011;5:Article 76. doi: 10.3389/fnint.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Block RA, Zakay D. Prospective and retrospective duration judgments: a meta-analytic review. Psychon Bull Rev. 1997;4:184–197. doi: 10.3758/BF03209393. [DOI] [PubMed] [Google Scholar]
- 92.Block RA, Hancock PA, Zakay D. How cognitive load affects duration judgments: a meta-analytic review. Acta Psychol (Amst) 2010;134:330–343. doi: 10.1016/j.actpsy.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 93.Tobin S, Bisson N, Grondin S. An ecological approach to prospective and retrospective timing of long durations: a study involving gamers. PLoS One. 2010;5:e9271. doi: 10.1371/journal.pone.0009271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones MR, Boltz M. Dynamic attending and responses to time. Psychol Rev. 1989;96:459–491. doi: 10.1037/0033-295x.96.3.459. [DOI] [PubMed] [Google Scholar]
- 95.Lander K, Bruce V. The role of motion in learning new faces. Vis Cogn. 2003;10:897–912. [Google Scholar]
- 96.Matthews WJ, Benjamin C, Osborne C. Memory for moving and static images. Psychon Bull Rev. 2007;14:989–993. doi: 10.3758/bf03194133. [DOI] [PubMed] [Google Scholar]
- 97.Buratto LG, Matthews WJ, Lamberts K. When are moving images remembered better? Study-test congruence and the dynamic superiority effect. Q J Exp Psychol. 2009;62:1896–1903. doi: 10.1080/17470210902883263. [DOI] [PubMed] [Google Scholar]
- 98.Matthews WJ, Buratto LG, Lamberts K. Exploring the memory advantage for moving scenes. Vis Cogn. 2010;18:1393–1419. [Google Scholar]
- 99.Krumhaber EG, Kappas A, Manstead ASR. Effects of dynamic aspects of facial expression: a review. Emotion Rev. 2013;5:41–46. [Google Scholar]
- 100.Boltz MG. Duration judgments of naturalistic events in the auditory and visual modalities. Percept Psychophys. 2005;67:1362–1375. doi: 10.3758/bf03193641. [DOI] [PubMed] [Google Scholar]
- 101.Darlow HM, Dylman AS, Gheorghiu AI, Matthews WJ. Do changes in the pace of events affect one-off judgments of duration? PLoS One. 2013;8:e59847. doi: 10.1371/journal.pone.0059847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matthews WJ. What might judgment and decision making research be like if we took a Bayesian approach to hypothesis testing? Judgment Decision Making. 2011;6:843–856. [Google Scholar]
- 103.Tobin S, Grondin S. Time perception is enhanced by task duration knowledge: evidence from experienced swimmers. Mem Cognit. 2012;40:1339–1351. doi: 10.3758/s13421-012-0231-3. [DOI] [PubMed] [Google Scholar]
- 104.Tobin S, Grondin S. Video games and the perception of very long durations by adolescents. Comput Hum Behav. 2009;25:554–559. [Google Scholar]
- 105.Bisson N, Tobin S, Grondin S. Prospective and retrospective time estimates of children: a comparison based on ecological tasks. PLoS One. 2012;7:e33049. doi: 10.1371/journal.pone.0033049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bisson N, Grondin S. Time estimates of internet surfing and video gaming. Timing Time Percep. 2013;1:39–64. [Google Scholar]
- 107.Adams RD. Intervening stimulus effects on category judgments of duration. Percept Psychophys. 1977;21:527–534. [Google Scholar]
- 108.Tomassini A, Gori M, Burr D, Sandini G, Morrone MC. Perceived duration of visual and tactile stimuli depends on perceived speed. Front Integrat Neurosci. 2011;5:Article 51. doi: 10.3389/fnint.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kanai R, Paffen CLE, Hogendoorn H, Verstraten FAJ. Time dilation in dynamic visual display. J Vis. 2006;6:1421–1430. doi: 10.1167/6.12.8. [DOI] [PubMed] [Google Scholar]
- 110.Kaneko S, Murakami I. Perceived duration of visual motion increases with speed. J Vis. 2009;9:11–12. doi: 10.1167/9.7.14. [DOI] [PubMed] [Google Scholar]
- 111.Sasaki K, Yamamoto K, Miura K. The difference in speed sequence influences perceived duration. Perception. 2013;42:198–207. doi: 10.1068/p7241. [DOI] [PubMed] [Google Scholar]
- 112.Beckmann JS, Young ME. Stimulus dynamics and temporal discrimination: implications for pacemakers. J Exp Psychol Anim Behav Process. 2009;35:525–537. doi: 10.1037/a0015891. [DOI] [PubMed] [Google Scholar]
- 113.van Rijn H, Gu B-M, Meck WH. Dedicated clock/timing-circuit theories of interval timing. In: Merchant H, Lafuente Vd, editors. Neurobiology of Interval Timing. New York: Springer; In press. [Google Scholar]
FURTHER READING/RESOURCES
- Agostino PV, Golombek DA, Meck WH. Unwinding the molecular basis of interval and circadian timing. Front Integr Neurosci. 2011;5:64. doi: 10.3389/fnint.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- (These two p psychology and neurobiology of timing and time perception spanning a range from msec to 24 hrs in order to capture the interaction between circadian and interval timing mechanisms.)
- Meck WH, Doyère V, Gruart A. Interval timing and time-based decision making. Front Integr Neurosci. 2012;6:13. doi: 10.3389/fnint.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (Introduces mprising 58 research, opinion, and review articles covering multiple aspects of interval timing.)
- Merchant H, de Lafuente V. Neurobiology of Interval Timing. New York: Springer; in press. [DOI] [PubMed] [Google Scholar]
- (Provides advanced reviews of work on the psychophysics, neural correlates, and formal modelling of interval timing.)
RELATED ARTICLES
- Berniker M, Kording K. Bayesian approaches to sensory integration for motor control. WIREs Cognitive Sci. 2011;2:419–428. doi: 10.1002/wcs.125. doi: 10.1002/wcs.125. [DOI] [PubMed] [Google Scholar]
- Braunlich K, Seger C. The basal ganglia. WIREs Cognitive Sci. 2013;4:135–148. doi: 10.1002/wcs.1217. doi: 10.1002/wcs.1217. [DOI] [PubMed] [Google Scholar]
- Goel V. Neural basis of thinking: laboratory problems versus real-world problems. WIREs Cognitive Sci. 2010;1:613–621. doi: 10.1002/wcs.71. doi: 10.1002/wcs.71. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Náñez JE, Watanabe T. Recent progress in perceptual learning research. WIREs Cognitive Sci. 2012;3:293–299. doi: 10.1002/wcs.1175. doi: 10.1002/wcs.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]