Abstract
Two major types of intercellular communication are found in the central nervous system (CNS), namely wiring transmission (WT; point-to-point communication via private channels, e.g. synaptic transmission) and volume transmission (VT; communication in the extracellular fluid and in the cerebrospinal fluid). Volume and synaptic transmission become integrated because their chemical signals activate different types of interacting receptors in heteroreceptor complexes located synaptically and extrasynaptically in the plasma membrane. In VT, we focus on the role of the extracellular-vesicle type of VT, and in WT, on the potential role of the tunnelling-nanotube (TNT) type of WT. The so-called exosomes appear to be the major vesicular carrier for intercellular communication but the larger microvesicles also participate. Extracellular vesicles are released from cultured cortical neurons and different types of glial cells and modulate the signalling of the neuronal–glial networks of the CNS. This type of VT has pathological relevance, and epigenetic mechanisms may participate in the modulation of extracellular-vesicle-mediated VT. Gerdes and co-workers proposed the existence of a novel type of WT based on TNTs, which are straight transcellular channels leading to the formation in vitro of syncytial cellular networks found also in neuronal and glial cultures.
Keywords: wiring transmission, volume transmission, tunnelling nanotubes, extracellular vesicles, exosomes, microvesicles
1. Introduction
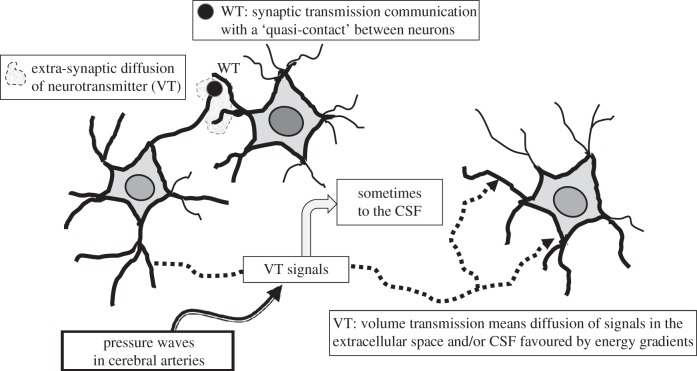
Two major complementary modes of intercellular communication exist in the central nervous system (CNS), namely wiring transmission (WT) and volume transmission (VT) [1–11]. WT is a point-to-point communication in the CNS via private channels involving synapses between nerve cells or gap junctions (GJs) directly connecting the cytoplasm of two cells, mainly found between astroglial cells (table 1 and figure 1). VT makes communication possible between cells of the brain and the spinal cord via diffusion and flow of neurotransmitters, neuromodulators, ions, trophic factors, etc., in the extracellular fluid (ECF) and cerebrospinal fluid (CSF) mainly targeting high-affinity receptors (table 1 and figure 1). Here, they act as VT signals enabling information handling and trophic communication between cells, including neuronal–glial, glial–glial and neuronal–glial–endothelial interactions via extrasynaptic and long-distance VT mainly along paravascular and paraaxonal channels and also involving CSF (table 1 and figure 1). Criteria for VT features and experimental evidence for its existence and its functional implications have been provided [3,12–15]. The prototype of WT is synaptic transmission where the channels are private, represented by axons and nerve terminals. However, in VT, the channels are open extracellular communication pathways formed within the matrix of the extracellular space in the CNS parenchyma and also using the CSF.
Table 1.
Classical dichotomy classification of communication modes in the central nervous system. ‘Private channel’ in WT means that the communication channel is a physically delimited pathway represented by, for example, the axon and its terminals forming synapses. ‘Widespread (diffuse)’ in VT means that the available extracellular and cerebrospinal fluid between the source and target can be used to transfer the signal.
| channel | main features |
|---|---|
| wiring transmission (WT): point-to-point communication via private channels | |
| synaptic contacts | private and highly localized transmission of transmitter signals in the synaptic cleft between neurons |
| gap junctions | private and highly localized transmission of signals mediated by an intercellular network of protein channels that facilitates the cell-to-cell passage of molecules, e.g. ions and neurotransmitters, mainly between astrocytes |
| volume transmission (VT): widespread (diffuse) communication in the extracellular space via extracellular channel plexa and in the CSF | |
| local extracellular channel plexa mainly in the µm range (extrasynaptic VT) | broadcasted transmission linked to synaptic transmission; through extrasynaptic release and synapse spillover involving a role of local field potentials, the ions and transmitters diffuse at the local circuit level to influence nerve cells, glial cells and endothelial cells, mainly via targeting local receptors |
| extracellular fluid pathways mainly along paravascular and paraaxonal extracellular channels; long-distance migration, also above 1 mm | broadcasted transmission; long-distance diffusion and flow of signals in the ECF involving mainly peptides and proteins targeting distant receptors; flow is generated by temperature and pressure gradients |
| cerebrospinal fluid pathways; long-distance migration, also above 1 mm |
broadcasted transmission; long-distance transmission of signals in the CSF, facilitated by flow generated by the arterial pulse |
Figure 1.
Schematic of wiring transmission and volume transmission in neural networks. Wiring transmission is illustrated by means of a synaptic contact. Volume transmission is the diffusion and flow of signals in the extracellular space (ECS) of the brain and in the cerebrospinal fluid (CSF) along the energy gradients. Thus, VT is illustrated as: the local extrasynaptic diffusion of neurotransmitters in the ECS; the long-distance diffusion and flow of some classical neurotransmitters and neuropeptides in the ECS; the flow of VT chemical signals via the CSF. Finally, the effects of the pressure waves in cerebral arteries on the diffusion and flow of the VT signals in the ECS and CSF are indicated (see [6,7,12,13]).
VT becomes mainly integrated with synaptic transmission via receptor–receptor interactions which are based on the existence of heteroreceptor complexes located synaptically and extrasynaptically [16–19]. Of special interest is that they can be built up of ion channel receptors and G-protein-coupled receptors (GPCRs) [20] highly suited to integrate the signals of synaptic transmission and VT at the plasma membrane level. In addition, the impact of GPCR–receptor tyrosine kinase (RTK) interactions in heteroreceptor complexes as mediators of integrative VT signalling on the plasma membrane in neuronal plasticity and trophism was recently introduced [4,21–23].
This review deals with novel types of VT and WT in the CNS. In VT, we focus on the role of extracellular-vesicle-mediated VT, also called the roamer type of VT (table 2) [13,26]. This communication is based on original work in the 1980s on receptor externalization and shedding in reticulocytes [27,28] and in the 1990s on secretion of antigen-presenting vesicles [29,30]. Of special interest is the paper by Simons & Raposo [31] giving an increased understanding of the mechanisms underlying the function of exosomes as vesicular carriers of intercellular communication in the body.
Table 2.
Classification of the tunnelling-nanotube (TNT)- and extracellular-vesicle (EV)-mediated intercellular communication in the central nervous system into wiring transmission and volume transmission, respectively. See Schneider and Simons [24] and van der Pol et al. [25] for further information on extracellular-vesicle-mediated intercellular communication.
| intercellular communication mode | channel | cargo transfer | mechanism of transport | origin |
|---|---|---|---|---|
| TNT type of WT (cultures of neuronal and glial cells) | TNT (15–60 µm long; 50–200 nm diameter) transient, membrane continuity between cells | organelles (especially vesicles), but also mtDNA, proteins, inside or along the TNT plasma membrane surface | actin-driven | no in vivo data in the CNS |
| EV type of VT (CNS: neurons, glial cells, brain endothelial cells) | extracellular space and CSF | exosomes (40–100 nm) containing proteins, lipids, mRNA, miRNA, mtDNA | diffusion and flow along energy gradients in the extracellular space | most cell types: plasma membrane; endosomes |
| shedding vesicles: microvesicles (100–1000 nm) insufficiently known composition |
most cell types: plasma membrane | |||
| apoptotic bodies (1–4 µm) histones, DNA | all cell types: plasma membrane |
In WT, the focus is on the potential role of the tunnelling-nanotube type of WT in the brain (table 2) [3,13] based on the original work of Gerdes and co-workers [32–34].
2. Different forms of volume transmission and integration with synaptic transmission
There are different forms of VT in the CNS. There is extrasynaptic VT operating at the local circuit level through diffusion over short distances (µm range) upon synaptic spillover or extrasynaptic release to activate especially high-affinity GPCRs [12,35]. An ephaptic VT has also been discussed based on flow of ions between neurons in the ECF (electrical VT) [13], first proposed by Golgi in 1906 [36].
(a). Long-distance and cerebrospinal fluid volume transmission
Peptide neurons likely operate via long-distance VT with distances over 1 mm also involving flow in the CSF [3]. One of the best examples is CSF-delivered beta-endorphin (2500 pmol in 5 μl) which could accumulate in nerve cell body populations and their dendrites all over the paraventricular hypothalamus as seen 15 min after CSF injection [37]. Striatally microinjected beta-endorphin can also reach the CSF as an intact peptide as shown with mass spectrometry [38]. The results taken together give strong indications that beta-endorphin can migrate for long distances in the ECF and CSF after its release from beta-endorphin immunoreactivity nerve terminal networks.
These results are also of relevance for understanding cotransmission in monoamine neurons often containing neuropeptides. The release of neuropeptides may allow the monoamine neurons to send VT signals to cellular networks further away from the monoamine terminals. Peptides and proteins may have a high stability and/or act via active peptide fragments which can make long-distance VT possible also involving CSF VT [3]. A temporal code of VT related to dynamic changes in release of transmitters is likely to exist especially over short distances. Thus, a phasic control of, for example, local circuits can occur via extrasynaptic VT. However, with long distances of diffusion/flow (mainly peptides/proteins), especially along extracellular channels of myelinated fibre bundles and paravascular extracellular channels, the dynamic changes in VT modulating the wired networks may be less pronounced but of high relevance for a tonic control of cell network function [3]. This was illustrated in the demonstration of the critical role of long-distance oxytocin VT in social attachment [15,39]. In this review, we summarize the interesting role of extracellular-vesicle-mediated VT in the CNS [13,31].
3. Different forms of wiring transmission
The specific feature of this mode of intercellular communication is the existence of a virtual wire connecting the cell source of the signal (message) with the cell target of the signal.
(a). Synaptic transmission
The most important and well known is certainly the synaptic transmission. Classic synaptic wiring, which depends on the transmission of action potentials, is the most important example of WT and the primary mechanism through which neurons transmit information and control behaviour. It represents the prototype of WT because it is characterized by a virtually continuous wire connecting the source of the message with its targets [3,13].
(b). Gap junctions
Connexins, a large family of homologous membrane proteins in vertebrates, form GJ channels that provide a direct pathway for electrical and metabolic signalling between cells, especially astrocytes [40–42]. Usually, different astrocytes are coupled through GJs to form large intercellular networks [43,44]. The function of these GJs is to minimize the differences not only between individual processes of the same astrocyte, but also between astrocytes by mediating the sharing of substrates such as glucose.
Furthermore, they play a role in dissipating extracellular K+ or glutamate, whose extracellular accumulation can be detrimental for proper neuronal function. The communication network generated by GJs in astrocytes is of significant support to the main processing network formed by the synaptic wiring among neurons. A potential new type of wiring communication in the CNS can be represented by the tunnelling nanotubes (TNTs) [32]. We summarize the exciting role of this type of WT later.
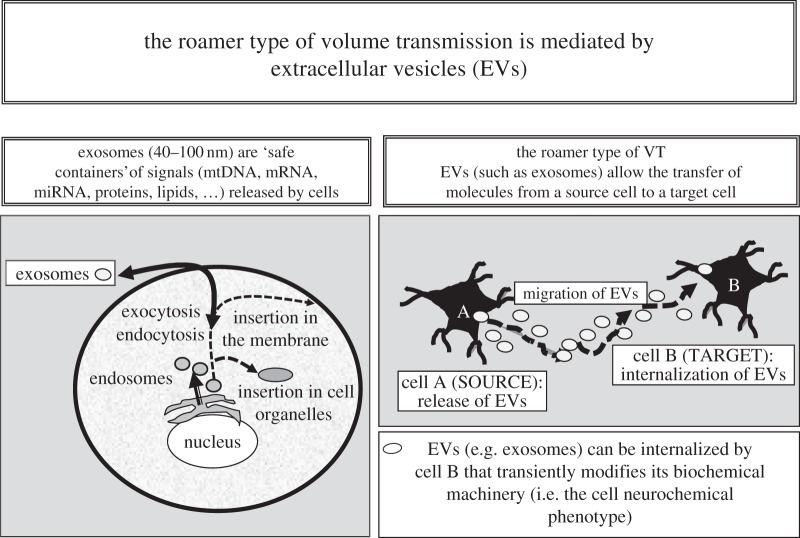
4. Extracellular-vesicle-mediated volume transmission, the roamer type of volume transmission
Extracellular vesicles were first shown to be released from reticulocytes with an origin in multivesicular bodies (MVBs) [27,28]. Today, it is believed that almost all cells can release vesicles into the ECF and send a set of messages to surrounding and distant cells [31]. A large number of possibly different extracellular vesicles were described, but it proved difficult to subdivide them into well-defined classes [24,25]. The so-called exosomes (endosome-derived), however, appear to be the major vesicular carrier for intercellular communication (table 2) [31,45] but microvesicles (shedding vesicles from the plasma membrane) also play a significant role (table 2) [46–49].
Exosomes are endosome-derived vesicles (diameter 40–100 nm) produced during the formation of MVBs. Occasionally, the MVBs fuse with the plasma membrane releasing their intraluminal vesicles into the extracellular media, which are then known as exosomes (figure 2 and table 2).
Figure 2.
Schematic of some main aspects of the roamer type of VT and of its possible implications for integrative actions in the CNS. It was shown inter alia that (i) exosomes can mediate oligodendrocyte–neuron communication [77]; (ii) exosomes can play a role in interconnections between brain and peripheral organs since, for example, cardiac myocytes release exosomes [84] and exosomes can cross the blood–brain barrier [85]; and (iii) exosomes can cause transient cell phenotype changes. Thus, it was shown that exosomes allow intercellular transfer of GPCRs [53].
As there are no exosome-specific markers, proteins that are enriched in exosomes from different cellular origins are commonly used for exosome detection. These are proteins such as tetraspanins (e.g. CD9, CD63 and CD81), cytoskeleton-associated protein (e.g. ezrin) and proteins involved in multivesicular biogenesis (e.g. Tsg101 and Alix). However, there are also cell-specific proteins found in exosomes such as A33 (intestinal epithelial cells), CD3 (T cells) and MHC class II (antigen-presenting cells). Thus, depending on from what cell type the exosomes are released, the markers for detection vary.
Detection of proteins enriched in exosomes, such as CD63, Tsg101 and Alix, and the concomitant absence of proteins such as the endoplasmic reticulum protein calnexin, is an indication that the exosome-enriched pellet is indeed of exosomes and not contaminating vesicles from other compartments of the cell [50].
A major question was how proteins and lipids in multivesicular endosomes can be sorted into different types of intraluminal vesicles, one type destined for degradation in lysosomes and one destined to become exosomes with release into the extracellular space. A major breakthrough came with the paper of Simons and co-workers [51] demonstrating that the sphingolipid ceramide induces budding of exosomes into multivesicular endosomes. This process was independent of the action of the endosomal sorting complex required for transport but promoted by neutral sphingomyelinases, the inhibition of which reduces the release of exosomes from cells [45]. Thus, ceramide may have a key role in exosome secretion and thus in accomplishing the pathway leading to vesicular intercellular communication [31]. As mentioned above, the MVBs can fuse with the plasma membrane releasing their intraluminal vesicles (exosomes) into the extracellular media [29]. However, they can also be formed from endosome-like domains of the plasma membrane [52].
Exosomes appear as round vesicles under electron microscopy and it was shown that they can transfer lipids and proteins, including receptors, Rab GTPases, tetraspanins, cholesterol, sphingolipids and ceramide. Within them, we can also find subsets of mRNAs and non-coding regulatory microRNAs [31,53,54]. They probably have a major role in moving the target cells into a novel but transient phenotypic state. Circulating miRNAs are also used as biomarkers for, for example, cardiovascular disease and are proposed to be novel types of signalling molecules in exosomal communication [55]. It should be noted that higher-order oligomerization targets plasma membrane proteins to exosomal sorting and release [56]. Thus, it seems possible that high-order hetero- and homoreceptor complexes [14] also are present in some exosomes.
Microvesicles, however, are usually regarded as extracellular vesicles formed in the plasma membrane through shedding (also called shedding vesicles). They have a variable size from 100 to 1000 nm (table 2). The microvesicles are produced directly through the outward budding and fission of membrane vesicles from the plasma membrane. They show surface markers largely dependent on the composition of the membrane of origin. Size, density, profile of several markers and indications of origin are used to classify them and separate them from exosomes.
(a). Epigenetic mechanisms in the modulation of extracellular-vesicle-mediated volume transmission
A set of histones H2A, H2B, H3 and H4 are known to package DNA. Their N-terminal tails play a major role because their covalent modification by acetylation, methylation, phosphorylation and ubiquination markedly changes the chromatin architecture and leads to changes in gene expression [57]. It is therefore of interest that histone methylation can influence the release of extracellular vesicles from adipocytes carrying specific RNAs and glycosylphosphatidylinositol-anchored proteins [58]. Inhibition of histone H3 lysine9 methyltransferase G9a or inhibition of histone H3 lysine4 demethylase LSD1 diminished the release of extracellular vesicles from large but not small adipocytes in response to a hydrogen peroxide challenge. It was associated with an upregulation of lipid synthesis and a downregulation of lipolysis. There were no effects on release of extracellular vesicles with combined inhibition of the two enzymes. Therefore, it seems as if H3 methylation at lysine 4 and 9 influences the release of extracellular vesicles in an interdependent way and is an epigenetic mechanism in control of extracellular vesicle release [58]. Usually, methylation of lysine9 in histone H3 correlates with transcriptional silencing, whereas methylation of lysine4 usually correlates with transcriptional activation [59,60]. It is of interest that histone H3 lysine4 demethylation is a target for non-selective antidepressant medications [61]. Thus, tranylcypromine is a potent inhibitor of histone demethylation, an action which is 10 times greater than its ability to inhibit monoamine oxidases. It opens up the possibility that demethylation mechanisms are overactive in depression and can be a target for novel antidepressant drugs. It may be speculated that transcriptional activation via inhibition of demethylation at histone H3 lysine4 leading to possible increases inter alia in extracellular-vesicle-mediated VT could be one mechanism involved in such a scenario.
(b). On the function of the extracellular vesicles
The major function of the exosomes and microvesicles appears to be in cell–cell communication [31] although they also have a role in the cellular clearance of biomolecular waste and in cell migration by extracellular delivery of chemoattractants [62]. Of special relevance for cell–cell communication is their ability through their expression of, for example, different patterns of cell adhesion molecules to specifically target cells in their environment. Cell types in the immune system can, for example, catch exosomes via their expression of intercellular adhesion molecule 1 [63]. Phosphatidylserine receptors on target cells can also capture exosomes via phosphatidylserine located on their membrane surface [64].
Functional complexes of different types exist in the exosomes which can regulate the cell function in multiple ways. However, we have only begun to understand the mechanisms by which exosomes regulate these functions [31]. One mechanism is internalization of the exosome into the endosomes from which it can deliver its contents into the cytoplasm after fusion with the limiting endosomal membrane. Protein and lipid ligands on the surface of the exosomes can also directly activate cell surface receptors, dissociate from them and migrate towards other cells leading to potential activation of multiple cells. Still another type of interaction involves a merger (fusion) with the plasma membrane with transfer of exosome membrane receptors and other membrane-linked molecules into the plasma membrane of the recipient cell and delivery of the exosome contents into the cytoplasm. Multiple types of exosomes exist with different molecular composition, and therefore each exosomal subtype may have its own preference with regard to its mechanism of interaction with the recipient cell.
(c). Central nervous system
In 2006, it was possible to demonstrate that ‘exosomes and microvesicles’, used as an umbrella term (EMV) [24], can be released from cultured cortical neurons [65,66]. The AMPA GluR2/3 subunits (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; a non-NMDA-type ionotropic transmembrane receptor for glutamate), the specific cell adhesion molecule L1 and the GPI-anchored prion protein were found in the EMV fractions of these cultures and the EMV release was enhanced by potassium-induced depolarization. It was proposed that EMV can have a regulatory function at synapses [65]. It is possible that that the presence of the GluR2/3 subunits of AMPA receptors in MEV, known to be enriched in the somatodendritic compartment, can lead to an increased transient formation of heterotetrameric AMPA receptors built up of dimeric GluR2 and dimeric GluR3 subunits in the cortical neurons. Thus, AMPA receptor-mediated glutamate transmission may be speculated to have become altered by EMV-mediated VT between neurons [67].
Because GPCRs are key molecules in decoding neurotransmitter information and previous studies have demonstrated that extracellular vesicles carry some G proteins, we investigated whether extracellular vesicles can transport functionally competent GPCRs from a donor to a target cell. Thus, the ability was investigated whether target cells can recognize and decode signals by means of receptors that they did not previously express [54]. The findings demonstrated that adenosine A2A receptors capable of recognizing and decoding extracellular signals can be safely transferred via extracellular vesicles from source to target cells in cellular models. The evidence indicated that GPCRs could be transported via extracellular vesicles to recipient cells likely mainly in the form of mRNA but also as proteins, which adds a further level of plasticity. In fact, a recipient cell, after receiving the extracellular vesicle cargo, can induce the translation process of the extracellular vesicle-derived mRNA, and the receptor formed can acquire the ability to respond to its neurotransmitter ligand [54]. This mechanism can increase the plasticity of the cells. However, it is also possible that an unspecific delivery of GPCRs via this mechanism can induce a misresponse to the neurotransmitter in the acceptor cells, which can lead to malfunction.
In addition, other cells in the CNS such as astrocytes [68,69], microglial cells [70] and oligodendrocytes [71] release EMVs (table 3). Astrocytes can release EMVs by an ATP-induced stimulation of P2X7 receptors followed by an action of acid sphingomyelinase [72]. The astrocytic EMVs move into the extracellular space and carry a large number of transfer compounds such as mitochondria, mitochondrial DNA (mtDNA), ATP, Hsp70, functional glutamate transporters, FGF-2, miRNA, etc. (table 3) [68,73–78]. The acceptor cells are both astrocytes and neurons and dependent on the cargo in the EMVs they may produce, for example, neuroprotection or degeneration [68,79,80]. It is of substantial interest that astrocytic EMVs can contain excitatory functional amino acid transporters (table 3) which are targeted to EMVs by protein kinase C and the activity of cells [75]. Thus, these types of EMVs from astrocytes may have a special role in VT by scavenging glutamate in the ECF, reducing excitation and neurodegeneration.
Table 3.
Characterization of the extracellular-vesicle-mediated volume transmission from the various cell types of the CNS.
| cell type | type of EVs | transfer compounds, acceptor cells, function |
|---|---|---|
| neurons | exosomes and microvesicles | Hsp, Flottilin, miRNA, GluR2; nerve cells; synaptic and extrasynaptic exocytosis leading to neuronal plasticity |
| astrocytes | exosomes and microvesicles | mitochondria, ATP, Hsp70, synapsin1, functional glutamate transporters, FGF-2, VEGF, matrix metallo-proteinases, microRNA-29; astrocytes and neurons; potential role in repair and neurodegeneration |
| oligodendrocytes | exosomes | myelin proteins (e.g. PLP, CNP), mRNA miRNA, glycolytic enzymes, tetraspanins, Hsp; neurons, especially axons, oligodendroglia (autocrine role), MHC class II microglia (degradation); trophic support of axons via internalization of oligodendrocyte exosomes |
| microglia | exosomes and microvesicles | externalized phosphatidylserine (high levels), interleukin-1beta, caspase-1, P2X7 receptor, aminopeptidase N, MHC class II, monocarboxylate transporter 1; recipient microglia, neurons; enhanced inflammation and neurotransmission |
| endothelial cells | endothelial microparticles | shedding from plasma membrane (less than 1 µm in diameter); phosphatidyl overexpression, adhesion molecules specific for mature endothelial cells; in circulation, interacting especially with monocytes; role in stroke and CNS inflammation |
The microglia are regarded as dynamic sensors of CNS disease, trauma and degeneration [81]. EMVs containing P2X7 receptors (belonging to the family of ionotropic purinoceptors for ATP), interleukin-1beta (IL-1beta) and its processing enzyme caspase-1 are released from reactive microglia by ATP activation (table 3) [82]. Furthermore, activation of the P2X7 receptors in the microglial EMVs may lead to IL-1beta processing and release of IL-1beta into the ECF from the EMVs and development and spread of inflammation in the CNS [48]. This involves the transmission of the microglial EMVs to recipient microglia (table 3) with upregulation of the expression of genes enhancing inflammation (IL-1beta, IL-6, cyclooxygenase-2, etc.) [47]. Microglial/macrophage EMV-mediated VT appears to play a relevant role in neuroinflammation, for example, in multiple sclerosis and represents a target for treatment of CNS inflammation. The possible differential roles in VT of diffusion and flow of soluble peptides and proteins in the ECF versus their transport in EMVs which diffuse and flow in ECF remain to be determined.
Microglial EMVs via VT also communicate with neurons and enhance excitatory transmission [46,76,77]. These EMVs can also express a number of proteins found also in EMVs from B cells and dendritic cells [70,83]. Microglial EMVs also express MHC class II molecules upregulated by interferon-gamma [70]. Their relevance for antigen presentation and CNS immunity is, however, unknown [76]. In inflammation, T cells can penetrate the blood–brain barrier and reach antigens presented by microglia which produces a number of effector functions, including cytotoxic functions [83].
It is of interest that microglial EMVs inter alia contain aminopeptidase CD13 which inactivates leucine- and methionine-enkephalins [70]. Thus, it seems possible that this may result in an extracellular-vesicle-mediated increase in enkephalin VT.
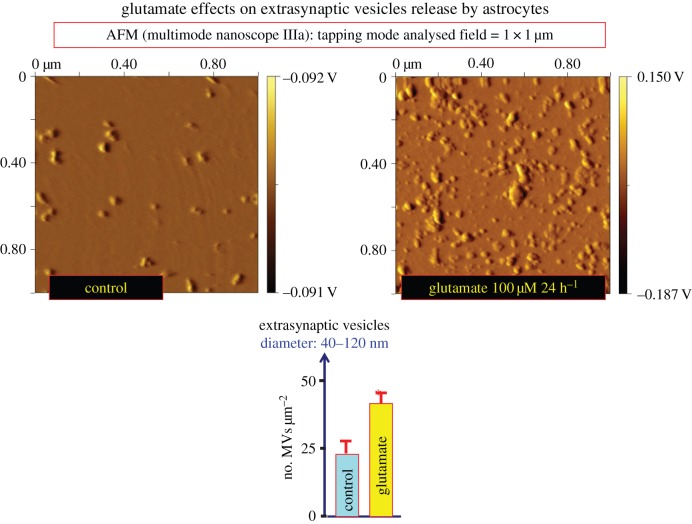
The oligodendrocyte exosomes contain myelin proteins such as PLP and CNP, glycolytic enzymes, stress protective proteins, mRNA and miRNA [71] (table 3). Axonally released glutamate activates exosome release from oligodendroglia mediated mainly by glial NMDA receptors [77]. It is linked to the electrical activity of neurons which in turn can internalize the oligodendroglial exosomes. This process also takes place in vivo and occurs especially at the axonal but also at the somatodendritic level leading to maintenance of axonal integrity and protection of neurons from oxidative stress. It demonstrates that glutamate-triggered exosome transfer mediates oligodendrocyte–neuron communication maintaining the health of axons [77]. In our group, glutamate (100 μM, 24 h) was also found to increase extracellular vesicle release from glioblastoma cells as demonstrated with atomic force microscopy (figure 3).
Figure 3.
Effects of glutamate (100 μM) for 24 h on the release of extracellular vesicles from glioblastoma cultures (U87MG). Conditioned medium (33 ml) was collected and processed for exosome isolation. Extracellular vesicles were purified by differential centrifugation at 4°C, starting with a centrifugation at 300g (10 min) and followed by centrifugations at 12 000g for (20 min), and 100 000g (120 min). The resulting extracellular vesicle pellets were washed with phosphate-buffered saline (PBS) and then collected again by ultracentrifugation at 100 000g (120 min) and resuspended in 500 µl PBS. Purified exosomes were further diluted up to 1 : 150 ratio with PBS and evaluated for number and size by atomic force microscopy (AFM) analysis. In detail, 10 μl of the obtained suspension was adsorbed to freshly cleaved mica sheets for 15 min at room temperature, rinsed with deionized water, and air dried. A nanoscope IIIa multimode AFM (Veeco) in tapping mode with silicon probes (K ≈ 50 N m−1) was used. Constant force was maintained for imaging all samples. Topographic (height) and amplitude images were recorded simultaneously at 512 × 512 pixels at a scan rate of 2.03 Hz. The height and amplitude (equivalent to a map of the slope of the sample) images are representative of the exosome morphological characteristics. Height image processing was performed using Gwyddion 2.5 software. Data are presented as number of extracellular vesicles µm−2 ± s.e. (n = 9–12). The colour scale on the right expresses the height of the surface features in intrinsic units (voltage), representing the voltage generated by the piezoelectric cantilever when the tip is moved at each given z-coordinate. This voltage is linearly related to the height in natural coordinates (nm). Thus, the colour bar has as a minimum value of elevation, the brown colour, and as a maximum value of elevation, the white colour (L. F. Agnati, D. Guidolin, G. Maura, C.Tortorella, M. Marcoli, G. Leo, C. Carone, S. Genedani, D.O. Borroto-Escuela and K. Fuxe 2013, unpublished data). (Online version in colour.)
A possible fundamental role could be played by extracellular vesicles called endothelial microparticles (EMPs) released from the brain endothelial cells lining capillaries and forming part of the blood–brain barrier for the bidirectional brain–body connections [83]. Brain endothelial cell extracellular vesicles can externalize brain-specific biomarkers into the blood stream and, via transcytosis, allow the migration of blood-borne molecules into the brain [86,87]. They are present in the circulation and are increased in infectious and thrombotic states indicating an involvement in such states [87,88]. Their blood levels are positively correlated to stroke severity [83]. In brain inflammation, cytokines such as IFN-gamma increase the release of EMPs from the brain endothelial cells. EMPs appear to preferentially interact with monocytes and EMP–monocyte conjugates are elevated in multiple sclerosis [89]. Such complexes also demonstrate an increased ability to migrate through human brain microvascular endothelial cell monolayers. These observations open up new perspectives for diagnosis and therapeutic interventions in brain diseases. Thus, endothelial cells can release EMPs to be used as diagnostic/prognostic blood-accessible biomarkers for some brain pathologies. Furthermore, the administration of suitable extracellular vesicles can allow drug delivery via transcytosis across the blood–brain barrier [85]. Thus, exosomes derived from dendritic cells can be used as targeted vehicles of drug delivery to the brain. Another example is the use of T-cell-derived exosomes to deliver anti-inflammatory drugs to the mouse brain via their injection into the nasal region [90]. It is of high interest that intravenous administration in rats of exosomes released from mesenchymal stromal cells promotes functional recovery and neurovascular plasticity after stroke [91]. In addition, it is possible that extracellular vesicles from brain endothelial cells can target and modulate the signalling of the trophic/neurovascular units of the CNS [92–94] via the roamer type of VT. Thus, the VT signalling of these units between the neuronal–glial networks and the endothelial cells may not only involve the ECF diffusion of soluble signalling molecules such as transmitters and trophic factors but also the roamer type of VT.
The extracellular vesicles in the CNS use the extracellular space for migration to target cells by means of pressure, temperature and concentration gradients. This extracellular vesicle-mediated communication therefore represents a special type of VT [13,26]. It has been called the roamer type of VT, because the extracellular vesicles, like the roamer's bag, are filled with important material. This type of VT may have the unique feature of building up a new molecular network of receptors in the target cells by intercellular transfer via the extracellular vesicles of competent GPCRs not otherwise expressed by the target cell [53].
(d). Pathological relevance in the central nervous system
Exosomes appear to be vehicles for transfer of toxic proteins such as amyloid-beta-derived peptides and alpha-synuclein in the brain increasing the spread of neurodegeneration in Alzheimer's disease and Parkinson's disease, respectively [95,96]. Furthermore, there is the transfer of the chemokine receptor CCR5 between cells by extracellular vesicles which is a mechanism for the spread of cellular human immunodeficiency virus 1 infection (HIV1) [97]. CCR5 is the principal coreceptor for HIV1.
Extracellular vesicles are also vehicles for cancer genes [98]. They, for example, transfer the oncogenic receptor epidermal growth factor receptor EGFRvII from glioma cells to neighbouring glial and endothelial cells inducing proliferation, and vascular endothelial growth factor, which leads to increased vascularization [99]. Furthermore, epigenetic reprogramming takes place via mRNA and/or miRNA transfer importantly contributing to the change in the phenotypic state of the recipient cells [53]. Glioblastoma cells can also transfer EGFR via extracellular vesicles [100] underlining that RTK transfer via this type of VT can play a major role in tumour growth. Novel therapeutic avenues will emerge by targeting different types of mechanisms in the extracellular vesicles [31].
It is of substantial interest that the extracellular-vesicle-mediated VT appears to modulate the plasticity of the neuronal–glial networks of the CNS via transfer especially of ion channel receptors, GPCRs and/or RTKs through receptor mRNA and/or protein which become functional in the target cells. Receptor homomers and heteromers may also be transferred. However, experimental support for this view is needed.
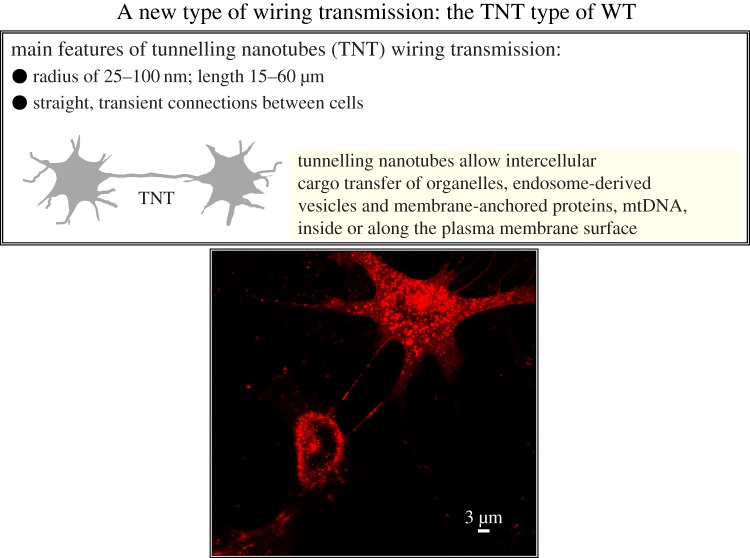
5. Tunnelling-nanotube type of wiring transmission
In a breakthrough paper, Gerdes and co-workers proposed the existence of a novel type of intercellular communication based on nanotubular highways for intercellular organelle transport and transport of membrane vesicles [32]. They were identified in a variety of cultured cell systems, including cells of the immune system, kidney cells, PC12 cells and cells from human glioblastoma (figure 4 and table 2) [13,32,33,101,102]. TNTs have a diameter of 50–200 nm and a length up to several cell diameters. These straight transcellular channels could lead to the formation of syncytial cellular networks [32,33,103,104]. They are transient structures having variable lifetimes ranging from less than 60 min (T cells, neuronal cells) up to several hours (PC12 cells) [101]. There are two processes that can lead to the formation of TNTs [104]. We have actin-driven protrusions from one cell which can connect with another cell via open-ended or closed TNTs. The possibility that protrusions from two cells can meet should be considered, but has so far not been demonstrated. The other process can involve the coming together of two cells which then move away from each other. It is a time-dependent process taking several minutes.
Figure 4.
Upper panel: schematic of some main aspects of the tunnelling nanotubes (TNTs) WT [32–34,107]. Lower panel: morphological evidence that TNTs can connect rat primary neuronal cells. The experimental procedure was as follows: TNT fluorescence labelling was performed by a fluorescent wheat germ agglutinin conjugate (WGA; Alexa Fluor 594 conjugate, 1 mg ml−1; Molecular Probes, Invitrogen, Eugene, OR, USA) directly added to the culture medium (1 : 400) at room temperature for 10 min. Subsequently, cells were washed with serum-free medium, and fresh medium was added. Live cell images were taken with a Leica DMIRE2 confocal microscope equipped with a 63× oil objective. (Online version in colour.)
(a). Neuronal cells
In neuronal cells (figure 4), there is an actin-driven protrusion of the TNT which may connect with a close-by cell. F-actin is the cytoskeletal component and membrane continuity exists [32]. Cargo transfer of endosome-derived vesicles and membrane-anchored proteins takes place. The mechanism involves a unidirectional vesicle transfer which is based on actin action [101]. It seems possible that membrane receptors can move via TNTs from the plasma membrane of one cell to the plasma membrane of another cell [53]. Through such a mechanism, one cell can acquire for a transient time period the capability to recognize and transduce signals which otherwise would not be recognized.
Thus, TNTs likely represent an interesting and significant type of WT in the CNS. Strong support exists for the view that this WT mode of communication is indeed used by neurons and/or by the other cell types in the CNS [3,13]. In fact, blockade of TNTs by cytochalasin B, which inhibits both the rate of actin polymerization and the interaction of actin filaments in solution, inhibits intercellular transfer between neuronal cells [105]. However, studies are required to better characterize not only the cell types that in vivo can use this possible mode of WT but also the triggering mechanisms for the formation of TNTs from the initiating cells and the signals allowing the TNT connection with the proper target cell. Significant in vivo data, however, do exist. The first in vivo experimental evidence of TNTs was found in bone-marrow-derived MHC class II+ cells in the corneal stroma of green fluorescent protein (GFP) chimeric mice [106]. In corneal whole mounts from wild-type, transgenic and enhanced GFP chimeric mice, long fine cellular processes were found originating from MH class II+ dendritic cells in the corneal stroma. Some of these structures formed distinct intercellular bridges between MHC class II+ dendritic cells. Furthermore, a number of in vivo studies demonstrate TNT-like structures during developmental processes in embryogenic tissues of vertebrates and invertebrates [107].
(b). Astrocytes
In some types of neuronal cells such as PC12 cells, TNT formation is independent of p53 expression [108]. However, in astrocytes, it was found that the TNT formation is dependent on p53 activation, a tumour suppressor protein giving cellular and genomic stability [74]. When p53 function is deleted by either dominant negative constructs or siRNAs, TNT development is inhibited. It was also shown that among the genes activated by p53, the epidermal growth factor receptor was important for TNT development. Akt, phosphoinositide 3-kinase and mTOR also play a role forming a signalling cascade activated by p53. It is of substantial interest that the mammalian protein M-Sec in immune cells and in astrocytes appears to have a key role in membrane nanotube formation by interacting with Ral, a small GTPase, and its downstream effector, the exocyst complex [109]. The M–Sec–Ral–exocyst pathway seems activated by both p53 and the p53-induced Akt–PI3K–mTOR cascade. Furthermore, hydrogen peroxide changed membrane and cytoskeleton properties and increased TNT connections in astrocytes [110] and induced TNT in hippocampal astrocytes and neurons [74]. Taken together, TNT development in cells appears to take place particularly under stress and may be one way to transfer cellular substances from stressed cells to healthy cells which may increase their stress resistance. However, TNT existence is not always dependent on stress and is also linked to cell type [33,34].
(c). Astrocyte–neuron crosstalk in development
Developing hippocampal neurons were demonstrated to form transient nanotubes with distant astrocytes [107,111]. TNTs were found to mediate both transient calcium signals and depolarization from distant astrocytes to neurons within a limited maturation period. This was associated with high expression of neuronal connexin 43. The TNTs may offer an interesting mechanism for how astrocytes may give instructions for neuronal migration. It is of interest that calcium signalling in the developing neurons was absent when they were not electrically coupled with astrocytes. A model of calcium diffusion alone through TNTs was therefore not supported. Instead, the findings indicated a local influx of calcium ions into neurons through activated low-voltage calcium channels which open at low depolarization thresholds [111]. It may be that VT via ATP and glutamate released from astrocytes in development is not as effective in producing depolarization and increasing neuronal calcium signalling via purinoceptors and NMDA receptors, respectively.
(d). Direction determination in neurons and astrocytes
It was recently demonstrated that the small calcium binding protein S100A4 has a role in guiding TNTs as well as its potential receptor (receptor for advanced glycation end products) [112]. For TNTs between astrocytes, S100A4 was sufficient. However, for TNTs between neurons and astroglia, neuronal activation was also required for targeting. This may be a mechanism for TNTs to recognize healthy nerve cells [112]. The mechanism for the S100A4 action appears to be that in TNT-initiating cells, p53 activates caspase-3, which leads to S100A4 cleavage and its subsequent decrease in cellular concentration. The decrease in cellular S100A4 induces the formation of a gradient of S100A4, from a low concentration in initiating cells towards a high concentration in target cells.
(e). Pathological relevance of tunnelling nanotubes in the central nervous system
It was reported that prions (PrPSc) hijack TNTs for intercellular spread [113]. In neuronal CATHa-differentiated (CAD) cells (CNS catecholaminergic cell line) PrPSc spread between infected and naive cells via TNTs. Transfer also occurred via TNTs between bone-marrow-derived dendritic cells and primary neurons. Thus, it is likely that PrPSc can spread among CNS neurons via TNTs. It is also of substantial interest that Brundin et al. [114] found a prion-like transmission of protein aggregates in neurodegenerative diseases. It is therefore possible that TNTs can participate in the spread of pathological proteins and their aggregates in conformational protein disorders. Support for this view was recently obtained by the demonstration of transfer of polyglutamine aggregates in neuronal cells through TNTs [115]. It should also be noted that mutated but not wild-type Huntington fragments increased the number of TNTs, which increases the capacity for transfer. Secreted aggregates did not become transferred to naive cells. In rotenone-poisoned glioblastoma cells, mitochondria were transferred via TNTs to healthy cells, and amyloid precursor protein was found in TNTs from glioblastoma cell cultures [13].
New concepts are being developed on WT in the CNS based on the discovery of TNTs in neuronal and glial cultures. This exciting field of research will, in the future, give the answer as to their existence in vivo in the brain and to their functional and pathological significance.
6. Future developments
For CNS communication, it will become of paramount importance to validate and understand the role of extracellular-vesicle-mediated VT and tunnelling-nanotube-mediated WT in the neuronal–glial–endothelial networks of the CNS. What functions can these types of VT and WT transmissions perform that cannot be executed by other classical forms of VT (extrasynaptic and long-distance communication in ECF and CSF) and WT (synaptic and GJ communication)? The specificity and distance of extracellular vesicle communication in the CNS is still unclear, but it certainly offers an exciting new way to change the phenotype of CNS cell populations. A substantial number of observations by many groups give strong support to the concept that these novel forms of VT and WT can strongly contribute to the spread of prions and of prion-like neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease as well as of human immunodeficiency virus type 1.
Acknowledgements
We thank our collaborators Prof. Genedani, Dr Carone, Dr Leo, Dr Guidolin and Dr Borroto-Escuela for substantial contributions. The results presented in figure 3 are part of larger projects that are presently in progress in our laboratory.
Funding statement
This work was supported by the Swedish Research Council (04X-715).
References
- 1.Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F. 1986. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol. Scand. 128, 201–207. ( 10.1111/j.1748-1716.1986.tb07967.x) [DOI] [PubMed] [Google Scholar]
- 2.Agnati LF, Guidolin D, Baluska F, Leo G, Barlow PW, Carone C, Genedani S. 2010. A new hypothesis of pathogenesis based on the divorce between mitochondria and their host cells: possible relevance for Alzheimer's disease. Curr. Alzheimer Res. 7, 307–322. ( 10.2174/156720510791162395) [DOI] [PubMed] [Google Scholar]
- 3.Fuxe K, Dahlstrom AB, Jonsson G, Marcellino D, Guescini M, Dam M, Manger P, Agnati L. 2010. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog. Neurobiol. 90, 82–100. ( 10.1016/j.pneurobio.2009.10.012) [DOI] [PubMed] [Google Scholar]
- 4.Fuxe K, et al. 2007. From the Golgi–Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res. Rev. 55, 17–54. ( 10.1016/j.brainresrev.2007.02.009) [DOI] [PubMed] [Google Scholar]
- 5.Fuxe K, Agnati LF, Zoli M, Cintra A, Harfstrand A, von Euler G, Grimaldi R, Kalia M, Eneroth P. 1988. The opioid peptide systems: their organization and role in volume transmission and neuroendocrine regulation. In Regulatory roles of opioid peptides (eds Illes P, Farsang C.), pp. 33–68. Weinheim, Germany: VCH. [Google Scholar]
- 6.Fuxe K, Agnati LF. 1991. Volume transmission in the brain, novel mechanisms for neural transmission. New York, NY: Raven Press. [Google Scholar]
- 7.Agnati LF, Fuxe K, Nicholson C, Sykova E. 2000. Volume transmission revisited. Amsterdam, The Netherlands: Elsevier Science BV. [Google Scholar]
- 8.Descarries L, Mechawar N. 2000. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog. Brain Res. 125, 27–47. ( 10.1016/S0079-6123(00)25005-X) [DOI] [PubMed] [Google Scholar]
- 9.Nicholson C, Sykova E. 1998. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21, 207–215. ( 10.1016/S0166-2236(98)01261-2) [DOI] [PubMed] [Google Scholar]
- 10.De-Miguel FF, Trueta C. 2005. Synaptic and extrasynaptic secretion of serotonin. Cell. Mol. Neurobiol. 25, 297–312. ( 10.1007/s10571-005-3061-z) [DOI] [PubMed] [Google Scholar]
- 11.Sykova E, Vargova L. 2008. Extrasynaptic transmission and the diffusion parameters of the extracellular space. Neurochem. Int. 52, 5–13. ( 10.1016/j.neuint.2007.04.007) [DOI] [PubMed] [Google Scholar]
- 12.Fuxe K, et al. 2012. Extrasynaptic neurotransmission in the modulation of brain function. Focus on the striatal neuronal–glial networks. Front. Physiol. 3, 136 ( 10.3389/fphys.2012.00136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. 2010. Understanding wiring and volume transmission. Brain Res. Rev. 64, 137–159. ( 10.1016/j.brainresrev.2010.03.003) [DOI] [PubMed] [Google Scholar]
- 14.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Zhang WB, Agnati LF. 2013. Volume transmission and its different forms in the central nervous system. Chin. J. Integr. Med. 19, 323–329. ( 10.1007/s11655-013-1455-1) [DOI] [PubMed] [Google Scholar]
- 15.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Ciruela F, Manger P, Leo G, Diaz-Cabiale Z, Agnati LF. 2012. On the role of volume transmission and receptor-receptor interactions in social behaviour: focus on central catecholamine and oxytocin neurons. Brain Res. 1476, 119–131. ( 10.1016/j.brainres.2012.01.062) [DOI] [PubMed] [Google Scholar]
- 16.Agnati LF, Ferre S, Lluis C, Franco R, Fuxe K. 2003. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol. Rev. 55, 509–550. ( 10.1124/pr.55.3.2) [DOI] [PubMed] [Google Scholar]
- 17.Fuxe K, et al. 2007. Intramembrane receptor-receptor interactions: a novel principle in molecular medicine. J. Neural Transm. 114, 49–75. ( 10.1007/s00702-006-0589-0) [DOI] [PubMed] [Google Scholar]
- 18.Borroto-Escuela DO, Romero-Fernandez W, Rivera A, Van Craenenbroeck K, Tarakanov AO, Agnati LF, Fuxe K. 2013. On the G-protein-coupled receptor heteromers and their allosteric receptor-receptor interactions in the central nervous system: focus on their role in pain modulation. eCAM 2013, 563716 ( 10.1155/2013/563716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borroto-Escuela DO, Romero-Fernandez W, Garriga P, Ciruela F, Narvaez M, Tarakanov AO, Palkovits M, Agnati LF, Fuxe K. 2013. G protein-coupled receptor heterodimerization in the brain. Methods Enzymol. 521, 281–294. ( 10.1016/B978-0-12-391862-8.00015-6) [DOI] [PubMed] [Google Scholar]
- 20.Wang M, Wong AH, Liu F. 2012. Interactions between NMDA and dopamine receptors: a potential therapeutic target. Brain Res. 1476, 154–163. ( 10.1016/j.brainres.2012.03.029) [DOI] [PubMed] [Google Scholar]
- 21.Borroto-Escuela DO, et al. 2012. Fibroblast growth factor receptor 1–5-hydroxytryptamine 1A heteroreceptor complexes and their enhancement of hippocampal plasticity. Biol. Psychiatry 71, 84–91. ( 10.1016/j.biopsych.2011.09.012) [DOI] [PubMed] [Google Scholar]
- 22.Borroto-Escuela DO, Corrales F, Narvaez M, Oflijan J, Agnati LF, Palkovits M, Fuxe K. 2013. Dynamic modulation of FGFR1–5-HT1A heteroreceptor complexes. Agonist treatment enhances participation of FGFR1 and 5-HT1A homodimers and recruitment of beta-arrestin2. Biochem. Biophys. Res. Commun. 441, 387–392. ( 10.1016/j.bbrc.2013.10.067) [DOI] [PubMed] [Google Scholar]
- 23.Borroto-Escuela DO, Flajolet M, Agnati LF, Greengard P, Fuxe K. 2013. Bioluminescence resonance energy transfer methods to study G protein-coupled receptor-receptor tyrosine kinase heteroreceptor complexes. Methods Cell Biol. 117, 141–164. ( 10.1016/B978-0-12-408143-7.00008-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider A, Simons M. 2013. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 352, 33–47. ( 10.1007/s00441-012-1428-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. 2012. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 64, 676–705. ( 10.1124/pr.112.005983) [DOI] [PubMed] [Google Scholar]
- 26.Trueta C, De-Miguel FF. 2012. Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front. Physiol. 3, 319 ( 10.3389/fphys.2012.00319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan BT, Johnstone RM. 1983. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33, 967–978. ( 10.1016/0092-8674(83)90040-5) [DOI] [PubMed] [Google Scholar]
- 28.Harding C, Heuser J, Stahl P. 1984. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur. J. Cell Biol. 35, 256–263. [PubMed] [Google Scholar]
- 29.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. 1996. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172. ( 10.1084/jem.183.3.1161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. 2002. The biogenesis and functions of exosomes. Traffic 3, 321–330. ( 10.1034/j.1600-0854.2002.30502.x) [DOI] [PubMed] [Google Scholar]
- 31.Simons M, Raposo G. 2009. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581. ( 10.1016/j.ceb.2009.03.007) [DOI] [PubMed] [Google Scholar]
- 32.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. 2004. Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010. ( 10.1126/science.1093133) [DOI] [PubMed] [Google Scholar]
- 33.Gerdes HH, Carvalho RN. 2008. Intercellular transfer mediated by tunneling nanotubes. Curr. Opin. Cell Biol. 20, 470–475. ( 10.1016/j.ceb.2008.03.005) [DOI] [PubMed] [Google Scholar]
- 34.Gerdes HH, Bukoreshtliev NV, Barroso JF. 2007. Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 581, 2194–2201. ( 10.1016/j.febslet.2007.03.071) [DOI] [PubMed] [Google Scholar]
- 35.Okubo Y, Sekiya H, Namiki S, Sakamoto H, Iinuma S, Yamasaki M, Watanabe M, Hirose K, Iino M. 2010. Imaging extrasynaptic glutamate dynamics in the brain. Proc. Natl Acad. Sci. USA 107, 6526–6531. ( 10.1073/pnas.0913154107). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golgi C. 1906. The neuron doctrine-theory and facts. Stockholm, Sweden: See www.nobelprize.org. [Google Scholar]
- 37.Agnati LF, Bjelke B, Fuxe K. 1992. Volume transmission in the brain. Am. Sci. 80, 362–373. [Google Scholar]
- 38.Hoistad M, Samskog J, Jacobsen KX, Olsson A, Hansson HA, Brodin E, Fuxe K. 2005. Detection of beta-endorphin in the cerebrospinal fluid after intrastriatal microinjection into the rat brain. Brain Res. 1041, 167–180. ( 10.1016/j.brainres.2005.02.014) [DOI] [PubMed] [Google Scholar]
- 39.Insel TR, Young LJ. 2001. The neurobiology of attachment. Nat. Rev. Neurosci. 2, 129–136. ( 10.1038/35053579) [DOI] [PubMed] [Google Scholar]
- 40.LeBeau FE, Traub RD, Monyer H, Whittington MA, Buhl EH. 2003. The role of electrical signaling via gap junctions in the generation of fast network oscillations. Brain Res. Bull. 62, 3–13. ( 10.1016/j.brainresbull.2003.07.004) [DOI] [PubMed] [Google Scholar]
- 41.Herve JC, Bourmeyster N, Sarrouilhe D, Duffy HS. 2007. Gap junctional complexes: from partners to functions. Prog. Biophys. Mol. Biol. 94, 29–65. ( 10.1016/j.pbiomolbio.2007.03.010) [DOI] [PubMed] [Google Scholar]
- 42.Yeager M, Harris AL. 2007. Gap junction channel structure in the early 21st century: facts and fantasies. Curr. Opin. Cell Biol. 19, 521–528. ( 10.1016/j.ceb.2007.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theis M, Sohl G, Eiberger J, Willecke K. 2005. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 28, 188–195. ( 10.1016/j.tins.2005.02.006) [DOI] [PubMed] [Google Scholar]
- 44.Volterra A, Meldolesi J. 2005. Astrocytes, from brain glue to communication elements: the revolution continues. Nat. Rev. Neurosci. 6, 626–640. ( 10.1038/nrn1722) [DOI] [PubMed] [Google Scholar]
- 45.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. 2006. Exosomes: a common pathway for a specialized function. J. Biochem. 140, 13–21. ( 10.1093/jb/mvj128) [DOI] [PubMed] [Google Scholar]
- 46.Antonucci F, et al. 2012. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 31, 1231–1240. ( 10.1038/emboj.2011.489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verderio C, et al. 2012. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 72, 610–624. ( 10.1002/ana.23627) [DOI] [PubMed] [Google Scholar]
- 48.Prada I, Furlan R, Matteoli M, Verderio C. 2013. Classical and unconventional pathways of vesicular release in microglia. Glia 61, 1003–1017. ( 10.1002/glia.22497) [DOI] [PubMed] [Google Scholar]
- 49.Joshi P, et al. 2013. Microglia convert aggregated amyloid-beta into neurotoxic forms through the shedding of microvesicles. Cell Death Differ. 21, 582–593. ( 10.1038/cdd.2013.180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lasser C, Eldh M, Lotvall J. 2012. Isolation and characterization of RNA-containing exosomes. J. Visual. Exp. 59, e3037 ( 10.3791/3037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247. ( 10.1126/science.1153124) [DOI] [PubMed] [Google Scholar]
- 52.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. 2006. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J. Cell Biol. 172, 923–935. ( 10.1083/jcb.200508014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659. ( 10.1038/ncb1596) [DOI] [PubMed] [Google Scholar]
- 54.Guescini M, et al. 2012. Microvesicle and tunneling nanotube mediated intercellular transfer of G-protein coupled receptors in cell cultures. Exp. Cell Res. 318, 603–613. ( 10.1016/j.yexcr.2012.01.005) [DOI] [PubMed] [Google Scholar]
- 55.Gupta SK, Bang C, Thum T. 2010. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ. Cardiovasc. Genet. 3, 484–488. ( 10.1161/CIRCGENETICS.110.958363) [DOI] [PubMed] [Google Scholar]
- 56.Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. 2007. Higher-order oligomerization targets plasma membrane proteins and HIV Gag to exosomes. PLoS Biol. 5, e158 ( 10.1371/journal.pbio.0050158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strahl BD, Allis CD. 2000. The language of covalent histone modifications. Nature 403, 41–45. ( 10.1038/47412) [DOI] [PubMed] [Google Scholar]
- 58.Mueller G, Schneider M, Gassenhuber J, Wied S. 2012. Release of exosomes and microvesicles harbouring specific RNAs and glycosylphosphatidylinositol-anchored protein from rat and human adipocytes is controlled by histone methylation. Am. J. Mol. Biol. 2, 187–209. ( 10.4236/ajmb.2012.23020) [DOI] [Google Scholar]
- 59.Kouzarides T. 2002. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 12, 198–209. ( 10.1016/S0959-437X(02)00287-3) [DOI] [PubMed] [Google Scholar]
- 60.Martin C, Zhang Y. 2005. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 6, 838–849. ( 10.1038/nrm1761) [DOI] [PubMed] [Google Scholar]
- 61.Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R. 2006. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem. Biol. 13, 563–567. ( 10.1016/j.chembiol.2006.05.004) [DOI] [PubMed] [Google Scholar]
- 62.Kriebel PW, Barr VA, Rericha EC, Zhang G, Parent CA. 2008. Collective cell migration requires vesicular trafficking for chemoattractant delivery at the trailing edge. J. Cell Biol. 183, 949–961. ( 10.1083/jcb.200808105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, Amigorena S, Thery C. 2005. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 106, 216–223. ( 10.1182/blood-2005-01-0220) [DOI] [PubMed] [Google Scholar]
- 64.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. 2007. Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439. ( 10.1038/nature06307) [DOI] [PubMed] [Google Scholar]
- 65.Faure J, et al. 2006. Exosomes are released by cultured cortical neurones. Mol. Cell. Neurosci. 31, 642–648. ( 10.1016/j.mcn.2005.12.003) [DOI] [PubMed] [Google Scholar]
- 66.Chivet M, Javalet C, Hemming F, Pernet-Gallay K, Laulagnier K, Fraboulet S, Sadoul R. 2013. Exosomes as a novel way of interneuronal communication. Biochem. Soc. Trans. 41, 241–244. ( 10.1042/BST20120266) [DOI] [PubMed] [Google Scholar]
- 67.Lachenal G, et al. 2011. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 46, 409–418. ( 10.1016/j.mcn.2010.11.004) [DOI] [PubMed] [Google Scholar]
- 68.Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE. 2007. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev. Neurobiol. 67, 1815–1829. ( 10.1002/dneu.20559) [DOI] [PubMed] [Google Scholar]
- 69.Guescini M, Genedani S, Stocchi V, Agnati LF. 2010. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 117, 1–4. ( 10.1007/s00702-009-0288-8) [DOI] [PubMed] [Google Scholar]
- 70.Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, Santambrogio L. 2005. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J. Immunol. 175, 2237–2243. ( 10.4049/jimmunol.175.4.2237) [DOI] [PubMed] [Google Scholar]
- 71.Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C, Mobius W, Berger H, Nave KA, Schild H, Trotter J. 2007. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics Clin. Appl. 1, 1446–1461. ( 10.1002/prca.200700522) [DOI] [PubMed] [Google Scholar]
- 72.Bianco F, et al. 2009. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 28, 1043–1054. ( 10.1038/emboj.2009.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Falchi AM, Sogos V, Saba F, Piras M, Congiu T, Piludu M. 2013. Astrocytes shed large membrane vesicles that contain mitochondria, lipid droplets and ATP. Histochem. Cell Biol. 139, 221–231. ( 10.1007/s00418-012-1045-x) [DOI] [PubMed] [Google Scholar]
- 74.Wang Y, Cui J, Sun X, Zhang Y. 2011. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 18, 732–742. ( 10.1038/cdd.2010.147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gosselin RD, Meylan P, Decosterd I. 2013. Extracellular microvesicles from astrocytes contain functional glutamate transporters: regulation by protein kinase C and cell activation. Front. Cell. Neurosci. 7, 251 ( 10.3389/fncel.2013.00251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fruhbeis C, Frohlich D, Kuo WP, Kramer-Albers EM. 2013. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell. Neurosci. 7, 182 ( 10.3389/fncel.2013.00182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fruhbeis C, et al. 2013. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 11, e1001604 ( 10.1371/journal.pbio.1001604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sbai O, et al. 2010. Differential vesicular distribution and trafficking of MMP-2, MMP-9, and their inhibitors in astrocytes. Glia 58, 344–366. ( 10.1002/glia.20927) [DOI] [PubMed] [Google Scholar]
- 79.Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, Mayer-Proschel M, Bieberich E. 2012. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD). J. Biol. Chem. 287, 21 384–21 395. ( 10.1074/jbc.M112.340513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu G, Yao H, Chaudhuri AD, Duan M, Yelamanchili SV, Wen H, Cheney PD, Fox HS, Buch S. 2012. Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell Death Dis. 3, e381 ( 10.1038/cddis.2012.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kettenmann H. 2007. Neuroscience: the brain's garbage men. Nature 446, 987–989. ( 10.1038/nature05713) [DOI] [PubMed] [Google Scholar]
- 82.Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. 2005. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J. Immunol. 174, 7268–7277. ( 10.4049/jimmunol.174.11.7268) [DOI] [PubMed] [Google Scholar]
- 83.Cossetti C, Smith JA, Iraci N, Leonardi T, Alfaro-Cervello C, Pluchino S. 2012. Extracellular membrane vesicles and immune regulation in the brain. Front. Physiol. 3, 117 ( 10.3389/fphys.2012.00117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cosme J, Liu PP, Gramolini AO. 2013. The cardiovascular exosome: current perspectives and potential. Proteomics 13, 1654–1659. ( 10.1002/pmic.201200441) [DOI] [PubMed] [Google Scholar]
- 85.Lakhal S, Wood MJ. 2011. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays 33, 737–741. ( 10.1002/bies.201100076) [DOI] [PubMed] [Google Scholar]
- 86.Haqqani AS, Delaney CE, Tremblay TL, Sodja C, Sandhu JK, Stanimirovic DB. 2013. Method for isolation and molecular characterization of extracellular microvesicles released from brain endothelial cells. Fluids Barriers CNS 10, 4 ( 10.1186/2045-8118-10-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morel O, Morel N, Jesel L, Freyssinet JM, Toti F. 2011. Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis. Semin. Immunopathol. 33, 469–486. ( 10.1007/s00281-010-0239-3) [DOI] [PubMed] [Google Scholar]
- 88.Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. 2009. Endothelial microparticles in diseases. Cell Tissue Res. 335, 143–151. ( 10.1007/s00441-008-0710-9) [DOI] [PubMed] [Google Scholar]
- 89.Jy W, Minagar A, Jimenez JJ, Sheremata WA, Mauro LM, Horstman LL, Bidot C, Ahn YS. 2004. Endothelial microparticles (EMP) bind and activate monocytes: elevated EMP-monocyte conjugates in multiple sclerosis. Front. Biosci. 9, 3137–3144. ( 10.2741/1466) [DOI] [PubMed] [Google Scholar]
- 90.Zhuang X, et al. 2011. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 19, 1769–1779. ( 10.1038/mt.2011.164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. 2013. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 33, 1711–1715. ( 10.1038/jcbfm.2013.152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agnati LF, Cortelli P, Pettersson R, Fuxe K. 1995. The concept of trophic units in the central nervous system. Prog. Neurobiol. 46, 561–574. ( 10.1016/0301-0082(95)00017-P) [DOI] [PubMed] [Google Scholar]
- 93.Park JA, Choi KS, Kim SY, Kim KW. 2003. Coordinated interaction of the vascular and nervous systems: from molecule- to cell-based approaches. Biochem. Biophys. Res. Commun. 311, 247–253. ( 10.1016/j.bbrc.2003.09.129) [DOI] [PubMed] [Google Scholar]
- 94.Abbott NJ, Ronnback L, Hansson E. 2006. Astrocyte-endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 7, 41–53. ( 10.1038/nrn1824). [DOI] [PubMed] [Google Scholar]
- 95.Bellingham SA, Guo BB, Coleman BM, Hill AF. 2012. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front. Physiol. 3, 124 ( 10.3389/fphys.2012.00124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. 2010. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30, 6838–6851. ( 10.1523/JNEUROSCI.5699-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mack M, et al. 2000. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 6, 769–775. ( 10.1038/77498) [DOI] [PubMed] [Google Scholar]
- 98.Rak J, Guha A. 2012. Extracellular vesicles--vehicles that spread cancer genes. Bioessays 34, 489–497. ( 10.1002/bies.201100169) [DOI] [PubMed] [Google Scholar]
- 99.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. 2008. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619–624. ( 10.1038/ncb1725) [DOI] [PubMed] [Google Scholar]
- 100.Skog J, et al. 2008. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476. ( 10.1038/ncb1800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gurke S, Barroso JF, Gerdes HH. 2008. The art of cellular communication: tunneling nanotubes bridge the divide. Histochem. Cell Biol. 129, 539–550. ( 10.1007/s00418-008-0412-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marzo L, Gousset K, Zurzolo C. 2012. Multifaceted roles of tunneling nanotubes in intercellular communication. Front. Physiol. 3, 72 ( 10.3389/fphys.2012.00072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Onfelt B, et al. 2006. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J. Immunol. 177, 8476–8483. ( 10.4049/jimmunol.177.12.8476) [DOI] [PubMed] [Google Scholar]
- 104.Davis DM, Sowinski S. 2008. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat. Rev. Mol. Cell Biol. 9, 431–436. ( 10.1038/nrm2399) [DOI] [PubMed] [Google Scholar]
- 105.Bukoreshtliev NV, Wang X, Hodneland E, Gurke S, Barroso JF, Gerdes HH. 2009. Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett. 583, 1481–1488. ( 10.1016/j.febslet.2009.03.065) [DOI] [PubMed] [Google Scholar]
- 106.Chinnery HR, Pearlman E, McMenamin PG. 2008. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J. Immunol. 180, 5779–5783. ( 10.4049/jimmunol.180.9.5779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gerdes HH, Rustom A, Wang X. 2013. Tunneling nanotubes, an emerging intercellular communication route in development. Mech. Dev. 130, 381–387. ( 10.1016/j.mod.2012.11.006) [DOI] [PubMed] [Google Scholar]
- 108.Andresen V, Wang X, Ghimire S, Omsland M, Gjertsen BT, Gerdes HH. 2013. Tunneling nanotube (TNT) formation is independent of p53 expression. Cell Death Differ. 20, 1124 ( 10.1038/cdd.2013.61) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hase K, et al. 2009. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 11, 1427–1432. ( 10.1038/ncb1990) [DOI] [PubMed] [Google Scholar]
- 110.Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. 2005. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J. Cell Sci. 118, 3695–3703. ( 10.1242/jcs.02507) [DOI] [PubMed] [Google Scholar]
- 111.Wang X, Bukoreshtliev NV, Gerdes HH. 2012. Developing neurons form transient nanotubes facilitating electrical coupling and calcium signaling with distant astrocytes. PLoS ONE 7, e47429 ( 10.1371/journal.pone.0047429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun X, Wang Y, Zhang J, Tu J, Wang XJ, Su XD, Wang L, Zhang Y. 2012. Tunneling-nanotube direction determination in neurons and astrocytes. Cell Death Dis. 3, e438 ( 10.1038/cddis.2012.177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gousset K, et al. 2009. Prions hijack tunnelling nanotubes for intercellular spread. Nat. Cell Biol. 11, 328–336. ( 10.1038/ncb1841) [DOI] [PubMed] [Google Scholar]
- 114.Brundin P, Melki R, Kopito R. 2010. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 11, 301–307. ( 10.1038/nrm2873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Costanzo M, Abounit S, Marzo L, Danckaert A, Chamoun Z, Roux P, Zurzolo C. 2013. Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J. Cell Sci. 126, 3678–3685. ( 10.1242/jcs.126086) [DOI] [PubMed] [Google Scholar]