Abstract
Exosomes are small membranous vesicles of endocytic origin that are released by almost every cell type. They exert versatile functions in intercellular communication important for many physiological and pathological processes. Recently, exosomes attracted interest with regard to their role in cell–cell communication in the nervous system. We have shown that exosomes released from oligodendrocytes upon stimulation with the neurotransmitter glutamate are internalized by neurons and enhance the neuronal stress tolerance. Here, we demonstrate that oligodendroglial exosomes also promote neuronal survival during oxygen–glucose deprivation, a model of cerebral ischaemia. We show the transfer from oligodendrocytes to neurons of superoxide dismutase and catalase, enzymes which are known to help cells to resist oxidative stress. Additionally, we identify various effects of oligodendroglial exosomes on neuronal physiology. Electrophysiological analysis using in vitro multi-electrode arrays revealed an increased firing rate of neurons exposed to oligodendroglial exosomes. Moreover, gene expression analysis and phosphorylation arrays uncovered differentially expressed genes and altered signal transduction pathways in neurons after exosome treatment. Our study thus provides new insight into the broad spectrum of action of oligodendroglial exosomes and their effects on neuronal physiology. The exchange of extracellular vesicles between neural cells may exhibit remarkable potential to impact brain performance.
Keywords: neuron–glia interaction, extracellular vesicles, exosomes, oligodendrocytes, signal transduction, gene regulation
1. Introduction
In the central nervous system (CNS), oligodendrocytes wrap axons with a multi-layered myelin sheath forming a functional axon–glia unit. Generation and maintenance of this unit requires intense communication between neurons and surrounding glia cells. Classically, intercellular communication is mediated through direct cell–cell contact via gap junctions and adhesion molecules, or secretion of paracrine factors. Recently, additional non-conventional routes of cell–cell communication have been uncovered. Extracellular vesicles (EVs) such as shedding microvesicles (MVs) and exosomes shuttle sets of proteins and RNAs to target cells (for review, see [1]). While MVs directly pinch-off from the plasma membrane, exosomes originate from the endosomal system and are significantly smaller in size (40–100 nm in diameter). They are released into the extracellular space by fusion of multivesicular bodies (MVBs) with the plasma membrane. Cells can use exosomes to dispose of obsolete proteins, but importantly, exosomes can also influence the target cell phenotype in various ways [2]. They can protect recipient cells, affect cellular signal transduction, promote malignant transformation and facilitate tumour growth [3]. Furthermore, exosomes contribute to immune responses and spread viruses and pathogenic proteins [4,5]. Exosomal delivery of genetic material, especially mRNA and miRNA, to target cells has gained particular interest. After transfer, mRNAs are translated resulting in newly synthesized proteins in the recipient cells [6]. On the other hand, miRNAs can mediate gene silencing in target cells [7–9].
Oligodendrocytes and all major cell types of the nervous system, including neurons, astrocytes and microglia, release EVs (for review, see [10]). Upon glutamatergic stimulation, cortical neurons secrete exosomes containing the neuronal cell adhesion molecule L1 as well as the AMPA receptor subunits GluR2/3, suggesting a role in synaptic plasticity [11–13]. Exosome release from oligodendrocytes is triggered in a similar way by the neurotransmitter glutamate, which is released by electrically active neurons and acts on oligodendroglial ionotropic glutamate receptors [14]. These exosomes carry a multitude of proteins (e.g. Alix, Tsg101, tetraspanins, heat shock proteins (HSPs) and the myelin proteins PLP and CNP) as well as RNA and are endocytosed by neurons followed by the functional recovery of their cargo. Neurons supplied with exosomes exhibit increased stress tolerance resulting in an improved survival under conditions of oxidative stress or starvation [14]. In addition, oligodendroglial exosomes can act in an autoinhibitory fashion inhibiting myelin formation [15] or, alternatively, become cleared from the extracellular space by microglia in an immunologically silent manner [16]. In the peripheral nervous system, Schwann cells secrete exosomes that are internalized by axons providing local axonal support and enhancing axonal regeneration after nerve damage [17].
In this study, we analyse the versatile impact of oligodendroglial exosomes on neuron physiology using different experimental approaches. We show that exposure of neurons to exosomes increases their action potential firing rate, alters cellular signal transduction pathways, and changes their transcriptome. Moreover, in an in vitro model of ischaemia, oligodendroglial exosomes exert beneficial effects on neurons, potentially via the transfer of protective proteins such as catalase and superoxide dismutase (SOD).
2. Material and methods
(a). Animals and cell culture
Primary cells were prepared from the C57Bl/6-N mouse strain. Embryonic day 14 mice of either sex were used for preparation of primary oligodendrocyte (pOL) and primary cortical neuron (pCN) cultures. pOL and the cell line Oli-neu were prepared and cultured in Sato 1% horse serum (HS) as described before [18]. pCN were cultured in Neurobasal feeding medium (Neurobasal (Gibco); 20 ml l−1 B27 (Invitrogen); 0.5 mM l-glutamine; 10 ml l−1 100× Pen-Strep (63.2 μg ml−1 Penicillin G K-salt; 135 μg ml−1 Streptomycin sulfate)) as described before [19]. O4-positive pOL were prepared from postnatal day 7 mice by using the Neural Tissue Dissociation Kit, gentleMACS dissociator, MACS Separator and anti-O4 MicroBeads (all obtained from Miltenyi Biotec) according to manufacturer's instructions.
(b). Antibodies and plasmids
The following antibodies were used: rat PLP (clone aa3), rabbit Catalase (Rockland), rabbit βIII-Tubulin (Tuj1; Covance), mouse α-Tub (Sigma), guinea pig doublecortin (DCX; Chemicon), rabbit GFP (green fluorescent protein; Abcam), rabbit phospho-Akt (Cell Signalling), rabbit Akt (Cell Signalling), mouse phospho-Erk1/2 (Cell Signalling), rabbit Erk1/2 (Cell Signalling). The following expression plasmids were used: pEGFP/N1, pEGFP/N1-hSOD1-wt (kind gift of Dr. Albrecht Clement, Mainz, Germany).
(c). Isolation of exosomes
Exosomes were isolated by differential centrifugation of tissue culture supernatants collected from pOL or Oli-neu cells according to standard procedures [20,21]. All steps were carried out at 4°C. First, dead cells and cell debris were removed by centrifugation for 10 min at 130g and for 20 min at 10 000g, respectively. Subsequently, the supernatant was centrifuged for 1 h at 100 000g to pellet the exosomes, which were resuspended in 100 µl phosphate-buffered saline (PBS) or recovered from a sucrose cushion (1.8 M sucrose in Tris-buffered saline (TBS)). Nanoparticle tracking analysis of isolated exosomes using the Nanosight LM10 system revealed an average number of 279 ± 12.3 (s.e.m.) × 106 particles of a size between 50 and 120 nm per 1 × 106 pOL (over a collection period of 24 h).
(d). Boyden chamber co-culture
Boyden chamber co-culture was performed as described previously [14]. Briefly, 7 days old pOL (1.25 × 106 per insert) or Oli-neu cells (2 × 105) were co-cultured with pCN (0.7 × 106 per well) in Boyden chambers, which allow contact-free co-culture. Six-well companion plates (353502) and six-well cell culture inserts (353102) were obtained from BD Falcon. To follow exosome transfer, either Oli-neu cells were transfected with 10 µg PLP-EGFP and 10 µg Sirt2-EYFP plasmids, or pOL were stained with the lipophilic dye PKH67 (Sigma) according to the manufacturer's protocol. Briefly, adherent pOL (4 days in vitro (DIV)) were incubated with 4 µl PKH67 diluted in 1 ml Diluent C for 4 min. Subsequently, reaction was stopped by adding 1 ml PBS + 10% HS for 1 min and cells were extensively washed (five times) with complete medium to remove residual dye. One day later, medium was exchanged and co-culture was started (pOL DIV 7). Immunocytochemical staining of PKH67-labelled oligodendrocytes confirmed enrichment of the dye in the endosomal system. After 1–2 days of co-culture, pCN were either immunocytochemically stained, lysed and analysed by Western blotting, or alternatively, neuronal RNA was prepared and analysed using microarrays and quantitative reverse transcription polymerase chain reaction (qRT-PCR).
(e). Cell lysates and Western blotting
Cells were scraped off the culture dish in 10 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 and protease inhibitor cocktail (Roche complete). Nuclei were pelleted by centrifugation for 10 min at 300g. Cell lysates and exosome samples were subjected to sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting (10 or 12% gels, Bio-Rad Mini PROTEAN electrophoresis system). Alternatively, NuPAGE pre-cast gradient (4–12%) gels (Invitrogen) with either MOPS or MES buffer were used according to the manufacturer's protocol. Western blotting onto a PVDF membrane was performed using Bio-Rad Mini Trans-Blot Electrophoretic Transfer Cell system for 3 h at 300 mA. The membrane was blocked with 4% milk powder/0.1% Tween in PBS. Proteins were detected by sequential incubation of the membrane with primary and horseradish peroxidase-coupled secondary antibodies and developed with enhanced chemiluminescence reagents (Millipore).
(f). Immunocytochemistry
Coverslips were washed with PBS and fixed for 15 min in 4% paraformaldehyde at room temperature. After washing with PBS (3×), cells were permeabilized with 0.1% Triton X100 in PBS for 2 min and washed again with PBS. Cells were then incubated in PBS + 10% HS for at least 15 min before applying the primary antibody diluted in PBS + 10% HS for 1 h. After washing with PBS, the secondary antibody was added diluted in PBS + 10% HS for 30 min. Nuclei were stained with 4′6-diamidino-2-phenylindole (DAPI) for 2 min. After final washing, the coverslips were briefly submerged in ddH2O before mounting them in Moviol on object slides. Fluorescence images were acquired using a DM6000 fluorescence microscope (Leica).
(g). Microarray and qRT-PCR
pOL (1.25 × 106 per insert) and pCN (0.7 × 106 per well) were prepared and co-cultured in Boyden chambers for 2 days as described previously [14]. Four individually prepared cultures of pCN were either co-cultured with pOL or with empty inserts containing exosome-depleted conditioned pOL medium as control. After co-culture, total RNA was prepared from pCN using the Qiagen miRNeasy mini Kit according to the manufacturer's protocol including on-column DNA digestion. Sample preparation for the microarrays was done using the Illumina TotalPrep RNA Amplification Kit including oligo dT primers for cDNA synthesis. For measuring gene expression, the BeadArray Mouse v2 microarray platform from Illumina was used. The microarray data were analysed using R and Bioconductor in particular the ‘limma’ package [22]. For validation of the microarray results, 0.7 × 106 pCN (7 DIV) were either co-cultured with 1.25 × 106 pOL (7 DIV) for 2 days or treated for 24 h with exosomes released by 8–10 × 106 pOL over a time period of 48 h. Subsequently, total RNA was prepared from pCN using the Qiagen miRNeasy mini Kit and qRT-PCR was performed with the StepOne Real-Time PCR System (Applied Biosystems) using TaqMan assays recognizing Ier3, Ccnd1, Cort, Metrn, Ldb2, Vgf, Acta2, Eif4ebp1, Chl1, Cdk1, Bdnf, Plp, Cnp, Mbp and Pgk1 (Applied Biosystems).
(h). Multi-electrode arrays
pCN were prepared from embryonic day 14 C57Bl/6 mouse brains and plated on 6-well multi-electrode arrays (MEA chips; Multi Channel Systems, Reutlingen, Germany; 2 × 105 cells per well). Each well contained 3 × 3 recording electrodes with 200 μm spacing. Exosomes released from 6 to 10 × 106 pOL (7 DIV) over a time period of 48 h were prepared, resuspended in 25 µl Neurobasal feeding medium, and applied to one well of pCN (DIV 14). As a control, 25 µl Neurobasal feeding medium alone was applied. The extracellular activity was recorded by using a MEA 1060-INV-BC interface. Recording of the spike activity was started 1 h before exosome treatment and lasted at least 12 h. Only experiments in which neuronal activity was recorded at three or more electrodes were further analysed for spontaneous firing rate, relative burst index and spike amplitude. For further details, see [23,24].
(i). Oxygen–glucose deprivation and analysis of neuronal viability
In Boyden chamber co-culture assays, pCN (10 DIV; 0.7 × 106/6-well) were cultured with O4-positive pOL (1 × 106/insert) for 48 h in Neurobasal/B27 or with blank inserts containing oligodendrocyte-conditioned medium. Before oxygen–glucose deprivation (OGD) exposure, the culture media was replaced by glucose-free Neurobasal and cells were transferred to an anaerobic chamber saturated with 95% N2 and 5% CO2 at 37°C. The co-cultures remained in an oxygen-free atmosphere for 2 h in order to mimic an ischaemic condition. Simultaneously, control cultures were grown under normoxic conditions in glucose-containing Neurobasal/B27. To allow subsequent reoxygenation (recovery), cells exposed to the OGD were returned to glucose-containing Neurobasal/B27 and were kept under normoxic conditions for 1 h. Media exchange was equally performed in the normoxic and OGD group. Neuronal viability was determined by the MTT assay. Briefly, 0.75 mg ml−1 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) was added to culture medium for 2 h. Formazan crystals formed were solubilized in a buffer containing 40% (v/v) dimethyl-formamide (Sigma), 10% (w/v) SDS and 2% (v/v) acetic acid overnight. The absorbance was measured at 562 nm using a plate reader (Tecan Infinite 2000).
(j). Phosphorylation arrays
Exosomes released by 1.5 × 106 O4-positive pOL (5 DIV) over a time period of 48 h were collected by differential centrifugation, resuspended in PBS, and applied to 0.7 × 106 pCN (7 DIV) on a 3 cm culture dish for certain time periods (0 min, 15 min, 30 min, 1 h, 2 h, 15 h and 24 h) before lysis in buffer supplemented with Halt Phosphatase Inhibitor Cocktail (Thermo Scientific). Control neurons (0 min) were treated with PBS. When exosome treatment of pCN was combined with oxidative stress or nutrient deprivation (ND), pCN were co-cultured with pOL in Boyden chambers for 48 h in neurobasal/B27 and compared to control cultures treated with pOL-conditioned, exosome-depleted medium as described previously [14]. Briefly, oxidative stress was induced by exposing neurons to 25 µM H2O2 for 1 h after 48 h of co-culture with pOL and before lysis. For ND, the pCN–pOL co-culture was grown in neurobasal without B27 supplement for 48 h before neurons were lysed. For further analysis, pCN lysates were subjected to SDS-PAGE and Western blotting. To examine the influence of exosome treatment on kinase activation in stressed neurons, 1.4 × 106 pCN (7 DIV) were treated with purified oligodendroglial exosomes obtained from 3 × 106 O4-positive pOL for 15 h and subsequently challenged with 25 µM H2O2 for 1 h before lysis. Control cells were treated with PBS before the exposure to H2O2. pCN lysates were subjected to protein determination using the BCA Protein Assay Kit (Novagen). One hundred micrograms of total protein was applied onto array membranes of the Proteome Profiler Human Phospho-MAPK Array kit (R&D Systems) according to the manufacturer's instructions.
3. Results
(a). Oligodendroglial exosomes influence neuronal activity
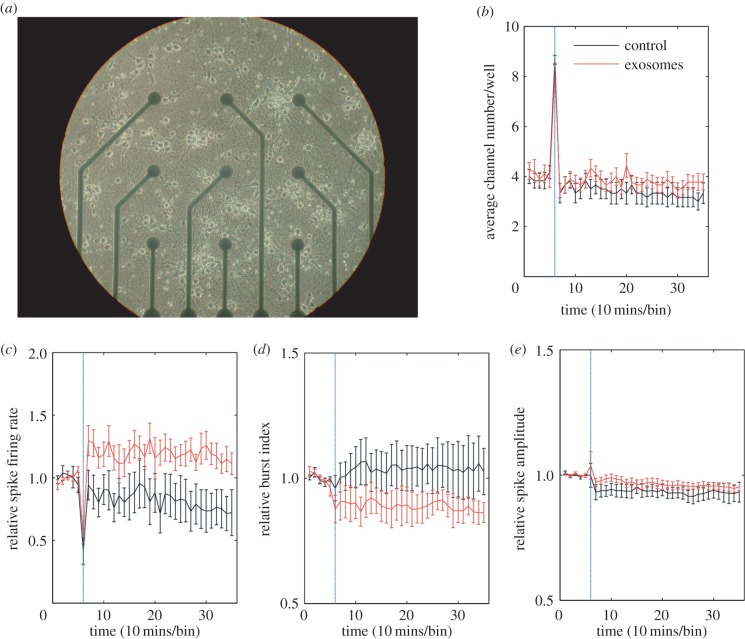
One of the most basic physiological functions of neurons is to generate action potentials in order to transmit information and communicate with other neurons. Previously, it has been shown that microglia-derived MVs can influence neuronal electrical activity [25]. To test whether oligodendroglial exosomes also have the potential to alter neuronal activity, in vitro MEA recordings were performed. pCNs were cultured on 6-well MEA chips (nine electrodes per well) for 14 days (figure 1a) to allow establishment of neuronal circuits and acquisition of spontaneous electrical activity. Recordings of neuronal spike activity over 6 h are depicted in figure 1b–e. Exosomes derived from cultured pOLs were applied 1 h after recording was started. Subsequently, the relative firing rate (figure 1c), burst index (figure 1d), spike amplitude (figure 1e) and the average channel number per well (figure 1b) were calculated. The results were only taken into account if the average number of active channels (electrodes) was higher than three during the recording. While the relative spike amplitude was not changed after application of oligodendroglial exosomes, the relative firing rate increased significantly and immediately (figure 1c). In contrast to the firing rate, the relative burst index decreased slightly after exosome treatment (figure 1d). The relative burst index is a measurement for the synchronicity of the neuronal network activity. A lower burst index indicates that neurons fire in a less synchronized fashion [23,24]. In summary, administration of oligodendroglial exosomes resulted in an increased number of action potentials with unaltered spike amplitude. Concomitantly, exosome-treated neurons fired less synchronously.
Figure 1.
Impact of oligodendroglial exosomes on neuronal electrical activity. (a) Image of a 14 DIV pCN culture grown in a 9-channel MEA. Electrode spacing is 200 μm. (b–e) Recordings were performed at DIV 14. The blue vertical line indicates the application of pOL exosomes (red graph, n = 9) or sham control (black graph, n = 6) after 1 h of recording. Average channel number/well (b), relative spike firing rate (c), relative burst index (d) and relative spike amplitude (e) are depicted over 6 h of recording. Error bars ± s.d. (*p < 0.05; Students t-test).
(b). Stress protective function of oligodendroglial exosomes
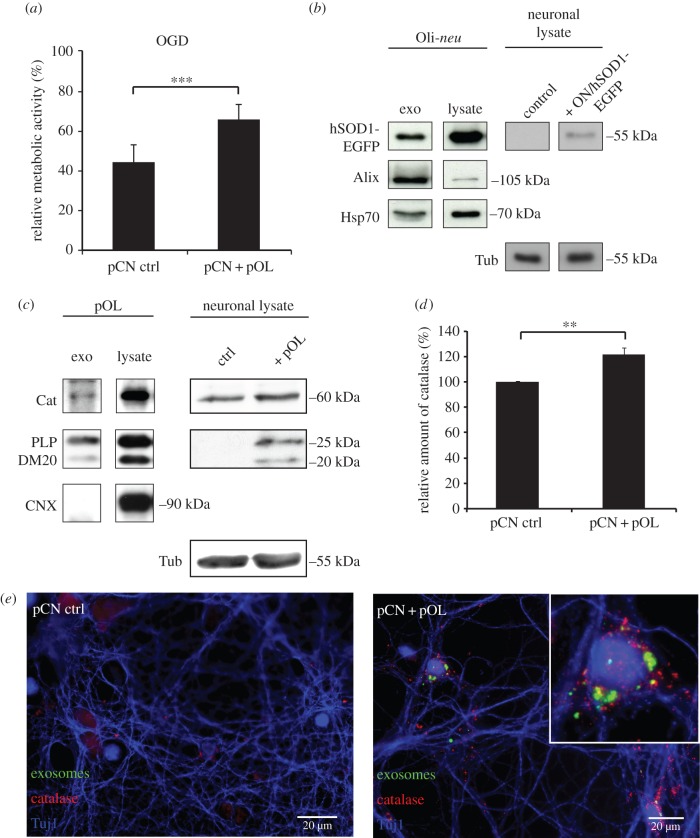
Our previous work revealed that oligodendroglial exosomes have the potential to protect recipient neurons from oxidative stress and ND [14]. To investigate the supportive effects of oligodendroglial exosomes in a diseased background, we used OGD, which is a widely accepted in vitro stroke model. OGD mimics ischaemia resulting in apoptotic and necrotic cell death [26]. pOLs and pCNs were co-cultured in Boyden chambers, allowing a constant supply of the neurons with oligodendroglial exosomes. Control neurons were cultured without oligodendrocytes, but in the presence of oligodendrocyte-conditioned medium depleted of exosomes, to exclude effects mediated by soluble factors. Co-cultures were subjected to OGD followed by a recovery phase and neuronal viability was assessed in relation to normoxic cultures by MTT assay (figure 2a). Intriguingly, neurons grown under ischaemic conditions but supplied with oligodendroglial exosomes revealed a significantly higher metabolic activity compared with control cells (increase of 21.5 ± 7.6%). This indicates that exosomes protect neurons under ischaemic conditions.
Figure 2.
Neuroprotection mediated by exosomes. (a) pCN exposed to pOL exosomes in Boyden chamber co-cultures and subjected to OGD compared to control conditions (pCN ctrl, pOL-conditioned medium depleted of exosomes). After OGD followed by reoxygenation, neuronal metabolic activity was determined by MTT assay. The relative metabolic activity correlates OGD co-cultures to respective cultures grown under normoxic conditions. Error bars, s.e.m. (***p < 0.001; n = 5, Students t-test). (b–d) Western blot analysis of pCN lysates after co-culture with Oli-neu cells (ON) or pOL. (b) Presence of hSOD1-EGFP in isolated exosomes (left) and transfer of hSOD1-EGFP from transfected ON cells to pCN (right). Control pCN were co-cultured with untransfected ON. Alix and Hsp70 identify exosomes. (c) Western blotting of isolated pOL-derived exosomes demonstrates presence of catalase (Cat, left). PLP/DM20 serves as an oligodendrocyte-specific exosome marker and calnexin (CNX) as a contamination marker. Catalase levels determined after co-culture of pCN with pOL (right). Detection of PLP/DM20 in pCN indicates exosome transfer. (d) Quantification of catalase normalized to tubulin (Tub) as derived from Western blot signals in (c) (right). Error bars, s.e.m. (**p < 0.01; Wilcoxon test, n = 10). (e) Images of pCN co-cultured with pOL (right panel), which were stained with PKH67 to label pOL-derived exosomes (green). Control pCN were cultured in absence of pOL (left panel). pCN were immuno-stained for catalase (red) and the neuronal marker Tuj1 (blue). Scale bar, 20 µm.
A possible mechanism of protection may be that exosome-mediated transfer of enzymes assists the relief of cell stress. Since oxygen radicals form especially during the reperfusion phase [27] after ischaemia, transferred enzymes such as catalase and SOD may reduce oxidative stress. To this end, we investigated the exosome-mediated transfer of these two enzymes to neurons. hSOD1-EGFP expressed in the oligodendroglial cell line Oli-neu is present in exosomes derived from these cells and can be detected in lysates of pCNs co-cultured in Boyden chambers (figure 2b), indicating delivery of hSOD1-EGFP to neurons via oligodendroglial exosomes. Furthermore, catalase can be detected in exosomes isolated from pOLs and catalase protein levels in co-cultured neurons were elevated by 22 ± 5% in coincidence with PLP/DM20 appearing in neurons as a result of exosome transfer (figure 2c,d). By immunocytochemical staining of neurons co-cultured with oligodendrocytes that were labelled with the dye PKH67, we observed that the signal for catalase was increased, in particular in those neurons that have internalized PKH67-labelled exosomes, suggesting that an exosome-mediated transfer of catalase from oligodendrocytes to neurons may have occurred (figure 2e). However, it is also possible that higher catalase levels in target neurons are due to upregulation of its expression. Taken together, these results suggest that exosomes deliver antioxidant enzymes such as catalase and SOD1, conferring enhanced stress resistance to exosome-recipient neurons.
(c). Activation of signalling pathways by oligodendroglial exosomes
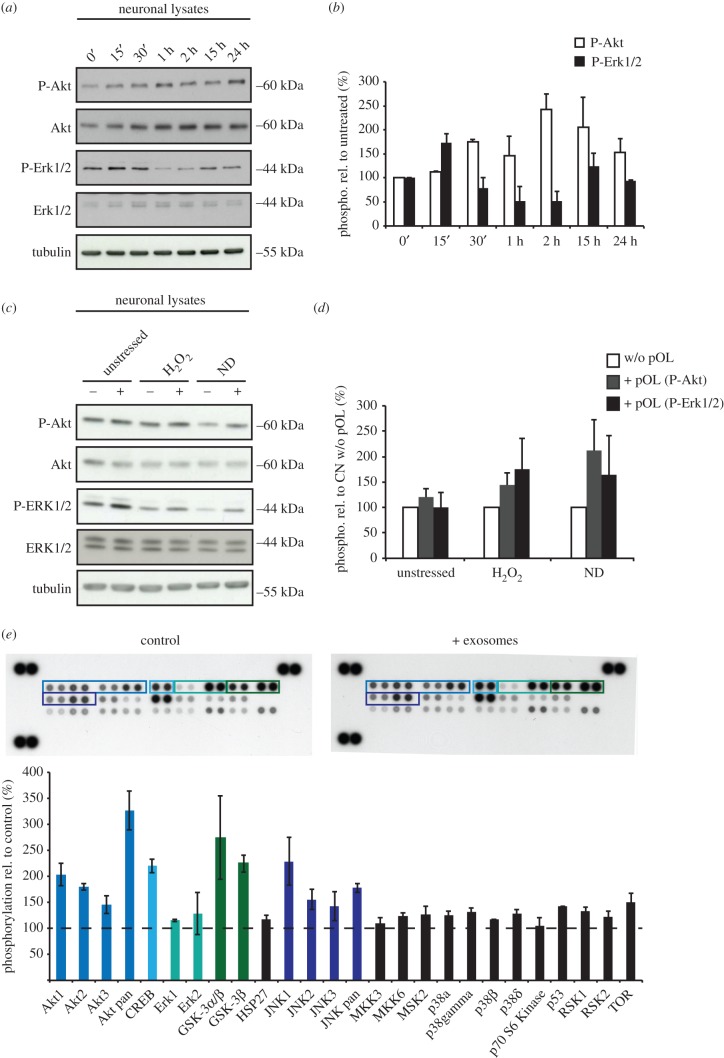
To further elucidate the mechanisms underlying exosome-mediated neuroprotection, we investigated the activation of pro-survival signalling pathways such as MEK/Erk and PI3K/Akt [28–31] in neurons in response to oligodendroglial exosomes under normal and stress conditions. We first aimed to elucidate the kinetics of kinase activation after exosome addition. Hence, pCNs were treated with purified oligodendroglial exosomes for different time periods (figure 3a,b). Subsequent analysis of neuronal lysates by Western blotting revealed the activation of Erk1/2 after 15 min and Akt after 30 min, reaching the maximal phosphorylation level at 15 min and 2 h, respectively (figure 3b). Whether activation of the signalling pathways occurs immediately after binding of exosomes to the cell surface or later during the internalization process will critically influence the activation kinetics.
Figure 3.
Analysis of exosome-dependent signalling pathways in neurons. (a,b) Purified oligodendroglial exosomes were applied to pCN for 0 min, 15 min, 30 min, 1 h, 2 h, 15 h and 24 h. P-Akt and P-Erk1/2 levels determined by Western blotting were normalized to total Akt or Erk and expressed in relation to untreated pCN at timepoint 0 min (n = 2). (c,d) Analysis of exosome signalling in recipient neurons under stress conditions. pCN co-cultured in Boyden chambers with (+) or without (−) pOL for 48 h were subjected to oxidative stress (25 µM H2O2 for 1 h before lysis) or ND (culture in absence of B27 supplement during co-culture) followed by Western blot analysis compared to unstressed cells. (d) Relative P-Akt and P-Erk1/2 levels in pCN are expressed in relation to control cells (−) of each condition to selectively visualize exosome-dependent kinase activation (n = 3). (e) Phospho-MAPK Array for phosphorylated proteins detected in neuronal lysates. pCN were either treated with PBS (control) or purified oligodendroglial exosomes and subjected to oxidative stress (n = 4). Abundance of phosphorylated proteins was quantified by densitometry and compared to PBS-treated pCN (dashed line). Proteins showing a higher phosphorylation level compared to control are highlighted by coloured boxes. Error bars, s.e.m.
To test the activation of both pathways under stress, we analysed phosphorylation levels in neurons co-cultured with pOLs in Boyden chambers, which were exposed to oxidative stress or ND (figure 3c,d). Control neurons were cultured without oligodendrocytes, but in oligodendrocyte-conditioned culture supernatant depleted of exosomes. Neurons challenged with either stress exhibit activation of Akt and Erk1/2 when co-cultured with oligodendrocytes (figure 3d). These results suggest that stimulation of Akt and Erk signalling pathways may play a role in exosome-dependent neuroprotection.
To identify additional kinases phosphorylated in neurons due to exosome treatment, we used the Phospho-MAPK (Mitogen Activated Protein Kinases) Array. pCNs were treated with purified oligodendroglial exosomes or vehicle for 15 h and subsequently subjected to oxidative stress for 1 h. The assay confirmed Akt activation and revealed an exosome-mediated increase in the phosphorylation state of CREB, GSK-3α/β, GSK-3β and JNK, while ERK did not exhibit robust activation under these conditions (figure 3e; highlighted in colours). Thus, oligodendroglial exosomes promote phosphorylation of distinct proteins involved in signalling.
(d). Influence of exosomes on neuronal gene expression
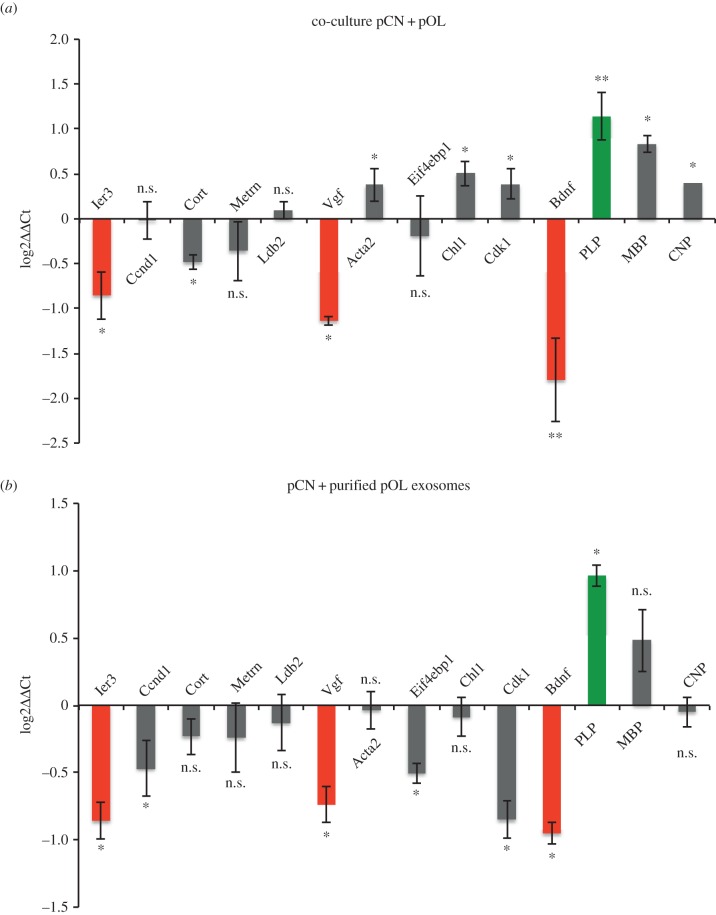
Having shown that exosomes modulate distinct signalling pathways in recipient cells, we analysed potential downstream effects on gene expression. One effect of signalling may be the activation or repression of certain genes, for instance by phosphorylation of the transcription factor cAMP response element-binding protein (CREB; figure 3e) [32,33]. Additionally, exosomes contain distinct mRNAs and miRNAs, which may exhibit pronounced effects on neuronal gene expression [6,7]. To test whether neuronal gene expression is altered by exposure of neurons to oligodendroglial exosomes, we performed a microarray analysis. pCNs were co-cultured in Boyden chambers with pOLs for 2 days. As a control, neurons were cultured in the absence of oligodendrocytes in oligodendrocyte-conditioned, exosome-depleted medium providing all soluble factors released by oligodendrocytes excluding exosomes. Subsequently, neuronal mRNA was prepared and differentially expressed genes were evaluated by microarray analysis. The quality of the raw microarray data was primarily assessed by looking at the spatial distribution of raw intensities on the array surface and by comparing the distribution of bead intensities between the individual arrays (data not shown). The expression data were normalized between the individual samples using the quantile normalization, and the data were analysed for differential gene expression using the approach of the R-package limma [34]. A complete list of the most significant differentially regulated genes is displayed in the electronic supplementary material, table S1 (False-Discovery Rate (FDR) ≤ 20%).
A number of selected candidates (table 1) that appeared promising in the context of CNS function were further validated by qRT-PCR in independent biological replicates (figure 4a). Validation revealed significantly altered expression of the following genes: immediate early response 3 (Ier3), cortistatin (Cort), VGF nerve growth factor inducible (Vgf), actin alpha 2 (Acta2), cell adhesion molecule with homology to L1CAM (Chl1), cyclin-dependent kinase 1 (Cdk1) and brain-derived neurotrophic factor (Bdnf). Moreover, the oligodendrocyte-specific transcripts of PLP, CNP and MBP were detectable in pCNs after co-culture with pOLs. Since the mRNAs of PLP and MBP are present in oligodendroglial exosomes (data not shown), these transcripts may be transferred to neurons.
Table 1.
Neuronal genes regulated in exosome-dependent fashion. Selection of differentially regulated genes detected by microarray analysis (down to a false-discovery rate (FDR) of 20%; n = 4), which were further subjected to qRT-PCR validation. The logFC value is the logarithmic representation of the fold change (FC) value. For a complete list of regulated genes, see the electronic supplementary material, table S1.
| ID | symbol | description | logFC | FDR (p-value) |
|---|---|---|---|---|
| ILMN_2737200 | Mbp | myelin basic protein | 0,87 | 1.79 × 10−7 |
| ILMN_1216764 | Ier3 | immediate early response 3 | −0,52 | 1.48 × 10−4 |
| ILMN_1221503 | Ccnd1 | cyclin D1 | −0,52 | 1.48 × 10−4 |
| ILMN_2601471 | Ccnd1 | cyclin D1 | −0,47 | 3.10 × 10−3 |
| ILMN_2955725 | Cort | cortistatin | −0,44 | 3.10 × 10−3 |
| ILMN_1226607 | Cort | cortistatin | −0,42 | 3.10 × 10−3 |
| ILMN_2739843 | Metrn | meteorin, glial cell differentiation regulator | −0,42 | 3.10 × 10−3 |
| ILMN_2754435 | Ldb2 | LIM domain binding 2 | 0,38 | 1.44 × 10−2 |
| ILMN_2925923 | Vgf | VGF nerve growth factor inducible | −0,36 | 2.52 × 10−2 |
| ILMN_2693895 | Acta2 | actin, alpha 2, smooth muscle, aorta | 0,60 | 2.92 × 10−2 |
| ILMN_2670398 | Eif4ebp1 | eukaryotic translation initiation factor 4E binding protein 1 | −0,35 | 4.64 × 10−2 |
| ILMN_2644008 | Chl1 | cell adhesion molecule with homology to L1CAM | 0,34 | 4.72 × 10−2 |
| ILMN_2657844 | Cdk1 | cyclin-dependent kinase 1 | −0,32 | 7.45 × 10−2 |
| ILMN_3105417 | Bdnf | brain-derived neurotrophic factor | −0,33 | 1.28 × 10−1 |
Figure 4.
Validation of exosome-dependent neuronal gene regulation. qRT-PCR analysis of candidate genes selected from microarray analysis. (a) pCN co-cultured in Boyden chambers with pOL compared to control pCN (n = 6; BDNF and PLP, n = 14). (b) pCN treated with isolated pOL-derived exosomes compared to untreated pCN (n = 7; BDNF, PLP, MBP and CNP, n = 5). Pgk1 was used as normalization standard. Log2 values of the ΔΔCt values are displayed. Genes validated in both paradigms as downregulated or upregulated are indicated by red bars (dark grey in print) and green bars (light grey in print), respectively. Error bars, s.e.m. (n.s., not significant; *p < 0.05; **p < 0.01; Wilcoxon test). (Online version in colour.)
To confirm that the observed effects can be attributed to exosomes, isolated oligodendroglial exosomes were applied to pCNs and the expression of candidate genes was determined by qRT-PCR (figure 4b). Comparison of the qRT-PCR results derived from the co-cultured and exosome-treated neurons revealed a proportion of likewise regulated genes with statistical significance. Ier3, Vgf and Bdnf are significantly downregulated, while Plp is significantly upregulated in both experimental settings, indicating a robust, exosome-mediated effect. Taken together, these results indicate that oligodendroglial exosomes alter neuronal gene expression and furthermore identify Ier3, Vgf and Bdnf as candidate target genes silenced in response to horizontal exosome transfer from oligodendrocytes to neurons.
(e). Transfer of oligodendroglial exosomes decreases neuronal doublecortin expression
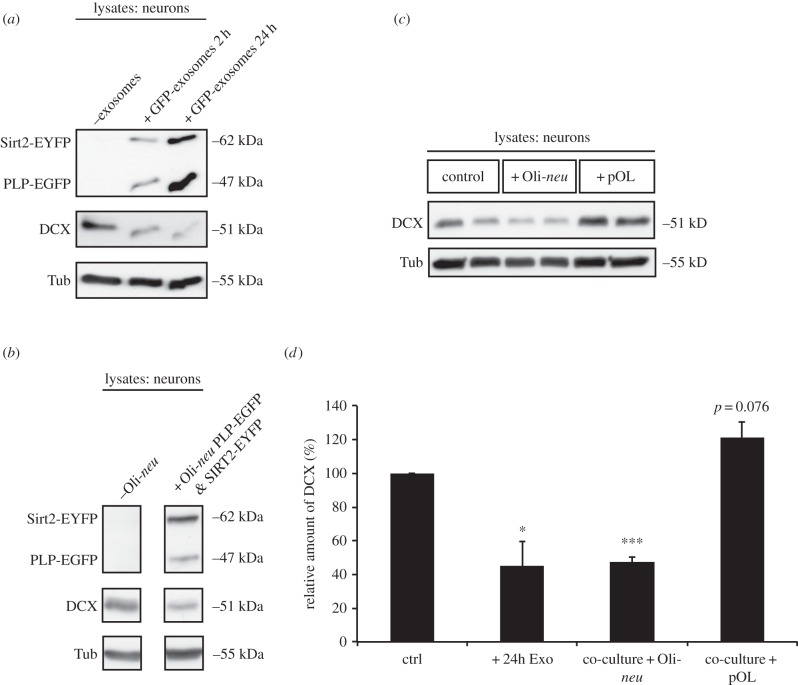
Oligodendroglial exosomes may influence neuronal gene expression by delivery of miRNAs mediating gene silencing. Thus, we investigated the presence of specific miRNAs in oligodendroglial exosomes that had been previously reported in oligodendrocytes [35]. By qRT-PCR, we detected the miRNAs miR-9 and miR-19a in exosomes derived from the oligodendroglial cell line Oli-neu (data not shown). Both miRNAs were predicted to bind DCX (algorithm from www.microRNA.org). DCX is a microtubule-stabilizing protein [36], which is downregulated during neuronal differentiation. To test whether exosome-mediated transfer of these miRNAs influences neuronal DCX expression, we applied oligodendroglial exosomes to cultured pCNs and analysed neuronal DCX levels by Western blotting. Application of exosomes derived from the oligodendrocyte precursor cell line Oli-neu to pCNs (figure 5a,d) or Boyden chamber co-culture of pCNs with Oli-neu cells (figure 5b–d), likewise resulted in a decreased DCX expression in recipient neurons. To label the glial exosomes, Oli-neu cells ectopically expressed the exosomal proteins Sirt2-EYFP and PLP-EGFP, which were also detectable in neuronal lysates and thus served as indicators for exosome transfer (figure 5a,b). To the contrary, co-culture with mature pOLs had the tendency to increase neuronal DCX expression (figure 5c,d). Taken together, exosomes derived from an oligodendroglial precursor cell line but not from mature oligodendrocytes resulted in decreased neuronal DCX expression in vitro.
Figure 5.
Downregulation of DCX in neurons in response to exosomes derived from oligodendrocyte precursor cells. (a–c) Western blot analysis of neuronal lysates. (a) pCN were treated with exosomes isolated from Oli-neu cells ectopically expressing Sirt2-EYFP and PLP-EGFP for 2 and 24 h, respectively (n = 6). (b,c) Boyden chamber co-culture of pCN either with Oli-neu cells ectopically expressing Sirt-EYFP and PLP-EGFP (b, n = 4) or with pOL and Oli-neu (c, n = 6) for 2 days. (d) Doublecortin (DCX) expression levels normalized to tubulin (Tub) as quantified from Western blots. Control pCN (untreated) are referred to as 100%. Error bars, s.e.m. (*p < 0.05; **p < 0.01; ***p < 0.001; Students t-test).
4. Discussion
This study provides evidence that oligodendroglial exosomes exert a variety of effects on recipient neurons and influence a broad spectrum of neuronal physiology. While this is not an in-depth analysis of individual exosome functions, the study indicates that exosomes are multifunctional entities in the brain and identifies putative molecular pathways of their action in neurons. Exosomes carry a complex assortment of different biomolecules with distinct activities that become integrated by target cells, most probably producing distinct functional outputs. We show that oligodendroglial exosomes modify neuronal electrical activity, activate certain signalling pathways, influence neuronal gene expression and deliver supportive molecules increasing neuronal stress tolerance under distinct stress conditions such as OGD. These effects may vary depending on the brain region where the transfer takes place and, moreover, on the status of the cells. Exosomes released by oligodendrocytes under conditions of cell stress may differ in composition from exosomes released by unstressed oligodendrocytes. It has been shown before that exosomes secreted from mast cells exposed to oxidative stress vary in their mRNA content to those from unstressed cells and increase resistance of recipient mast cells to oxidative stress [37]. Similarly, exosomes released from astrocytes subjected to heat stress are altered in their composition and are specifically enriched in protective HSP 70 [38]. On the other hand, the status of the recipient cells may further influence the effect of transferred exosomes. Neuronal subpopulations may differ in their internalization of exosomes and vary in their usage of exosomal cargo. Moreover, during disease, pathogens may influence the composition and effect of transferred exosomes. Exosomes can be hijacked by pathogens in the CNS and facilitate their spreading. Exosomes can carry and transfer α-synuclein, PrPSc (the scrapie and disease-specific form of the prion protein), amyloid precursor protein (APP) and several APP cleavage products including the Aβ peptide, phosphorylated tau and SOD, which are involved in Parkinson's disease, prion disease, Alzheimer's disease and amyotrophic lateral sclerosis (ALS), respectively [4,39].
(a). Oligodendroglial exosomes influence neuronal activity
Here, we demonstrate that treatment of cultured neurons with oligodendroglial exosomes enhances spontaneous neuronal electrical activity. Cultured neurons with already established synaptic connections exposed to exosomes generate more action potentials but at the same time fire in a less synchronized fashion. In general, these findings are consistent with the idea that oligodendrocyte-derived exosomes support the energy metabolism of neurons enabling an increased firing rate. However, the impact of exosomes on neuronal excitability in vivo remains to be proven. An excitatory effect has been reported for microglia-derived MVs that increase spontaneous and evoked excitatory transmission in hippocampal neurons [25]. Microglia-derived MVs act at the presynaptic site of the excitatory synapse by increasing the release probability of synaptic vesicles through induction of sphingolipid metabolism. A similar mechanism could be responsible for the increase of neuronal activity by oligodendrocyte-derived exosomes.
(b). Stress protective function of oligodendroglial exosomes
We have demonstrated previously that oligodendroglial exosomes ship protective cargo to neurons increasing their resistance against oxidative stress and starvation [14]. Here, we add further evidence that exosomes are also protective during OGD, which is a well-established in vitro model of cerebral ischaemia [40]. During brain ischaemia, the extracellular glutamate concentration increases, which can lead to neuronal cell death [40,41]. Exosome release from mature oligodendrocytes is actually triggered by glutamate [14], which in fact may help to prevent neuronal cell death during stroke, confining the ischaemic insult. Putative candidates exhibiting supportive function when transferred via exosomes may include HSPs as well as proteins involved in the oxidative stress response, like catalase and SOD. The role of HSPs in neuroprotection is well established and it has been previously shown that they can be supplemented from adjacent glial cells [42–45]. Our recent findings indicate that Hsc/Hsp70 [14], catalase and SOD1 are present in oligodendroglial exosomes and transferred to neurons. Since catalase mediates the breakdown of H2O2 to water and oxygen, this enzyme supplied to neurons by adjacent oligodendrocytes may protect neurons from H2O2-induced oxidative stress. Catalase is a peroxisome-resident protein and it may be interesting to note that intact peroxisomes in oligodendrocytes are required to maintain axon integrity in vivo [46]. SOD is another important enzyme involved in the antioxidant defence acting upstream of catalase by catalysing the dismutation of superoxide into oxygen and H2O2. It has been demonstrated previously that wild-type human SOD1 as well as their mutant forms are released in association with exosomes [47,48], which may be implicated in ALS pathogenesis.
(c). Activation of signalling pathways by oligodendroglial exosomes
Oligodendroglial exosomes contribute to neuronal survival under distinct stress conditions. However, the molecular determinants of enhanced survival are still unknown. Here, we identify several kinases that are activated in the target neurons upon treatment with oligodendroglial exosomes under normal as well as stress conditions. Cellular phosphorylation levels of Akt and Erk1/2 kinases are enhanced within minutes in response to oligodendroglial exosomes. Erk exhibited faster activation kinetics compared with Akt, indicating that it may act more upstream in the signalling cascade. Both kinases have been implicated in promoting survival [28–31]. In addition to Akt and Erk1/2, we identified CREB, GSK-3α/β, GSK-3β and JNK as phosphorylated in response to oligodendroglial exosomes. CREB is present in all cells of the CNS and functions as a transcription factor promoting cell survival and, furthermore, has a well-documented role in neuronal plasticity and the transcriptional control of memory formation [49–51]. Intriguingly, the Akt pathway activates CREB and downstream targets of CREB include the transcripts of BDNF and VGF [52–55], which we identified to be significantly regulated in neurons exposed to oligodendroglial exosomes. Additionally, phosphorylation of GSK-3α/β and GSK-3β, further downstream targets of Akt [56], may contribute to the neuroprotective function of oligodendroglial exosomes [57–59]. JNKs (c-Jun N-terminal kinases) are stress-induced protein kinases with various functions [60,61]. Recently, it has been reported that activated JNK is required for axon formation [62]. The transcription factor ATF-2, a member of the ATF/CREB family, is a target of JNK and was suggested to play a role in neuronal survival [62–64]. Taken together, these results reveal a variety of pathways potentially involved in mediating the neuroprotective effect of oligodendroglial exosomes. It is open at present whether signalling pathway activation requires exosome endocytosis or whether cell surface binding of exosomes is sufficient to elicit signalling.
(d). Influence of exosomes on neuronal gene expression
Initiation of signalling pathways in recipient neurons may result in differentially expressed genes. In addition, exosomal mRNAs and miRNAs or transcription factors are likely to modify the neuronal transcriptome. However, the ability to modify neuronal gene expression by the release of exosomes enables oligodendrocytes to broadly modulate the phenotype of surrounding neurons.
The most significantly regulated genes are Plp, Ier3, Vgf and Bdnf. Since PLP mRNA can be found in oligodendroglial exosomes, the upregulation of Plp may be a consequence of exosomal transfer of Plp mRNA. It has been reported previously that mRNA is transferred between mast cells by the means of exosomes and translated into functional proteins within the target cells [6]. Knockdown of Ier3, Vgf and Bdnf may be a result of transferred miRNAs. The transfer of miRNAs and the subsequent knockdown of their target genes have been shown before [7]. Ier3 belongs to the immediate early genes that are induced by a variety of stimuli such as growth factors, cytokines, ionizing radiation, viral infection and other kinds of cellular stress [65]. IER3 plays a complex and contradictory role in apoptosis and cell cycle control depending on the cellular context and growth conditions [65]. Both VGF and BDNF belong to the class of growth factors and are essential mediators of neuronal development, survival, axonal outgrowth and synaptic plasticity. BDNF plays a role in hippocampal-dependent associative memory [66], neurogenesis of hippocampal neurons [67] and during the pathology of depression [68]. BDNF induces the expression of Vgf via the transcription factor CREB [69]. Thus, reduced Vgf levels may be a consequence of decreased Bdnf expression. VGF is further processed into distinct polypeptides, which are subsequently released and exhibit a variety of functions, for instance the regulation of hippocampal synaptic plasticity and depressive behaviour in rodents [70,71]. However, the results indicate that oligodendroglial exosomes have the potential to influence neuronal physiology on a broad spectrum by regulating neuronal gene expression either via induction of distinct signalling pathways or by the selective transfer of mRNAs and miRNAs.
(e). Transfer of exosomes decreases neuronal doublecortin expression
Uptake of exosomes derived from the oligodendroglial precursor cell line Oli-neu but not from mature oligodendrocytes leads to a decreased DCX expression in recipient neurons, possibly by the transfer of glial miRNAs. We found the miRNAs miR-9 and miR-19a with predicted binding to DCX in oligodendroglial exosomes. It has been shown before that expression of both miRNAs is downregulated during oligodendrocyte maturation [35]. This is consistent with the finding that transfer of exosomes derived from the oligodendroglial precursor cell line Oli-neu but not from mature oligodendrocytes decreases neuronal DCX expression. DCX is a microtubule-stabilizing protein, which is mainly expressed by neuronal precursor cells and immature neurons [36]. Decreased DCX expression is associated with neuronal maturation and differentiation. The exosome-mediated downregulation of DCX may be important during development, where oligodendroglial exosomes could provide a differentiation signal to neurons, either initiating differentiation of neuronal precursor cells or keeping mature neurons in a differentiated state. Alternatively, neuronal precursors that migrate from the subventricular zone to the olfactory bulb along the rostral migratory stream could start to differentiate once they have come into contact with exosomes. And finally, oligodendroglial precursors accumulate at CNS injuries [72] and may be involved in on-going repair processes of the adult brain by stimulating axonal regeneration via the secretion of exosomes.
5. Conclusion
In this study, we provide evidence that oligodendroglial exosomes exhibit versatile effects on neuronal physiology. We identify exosomes as signalling entity in the brain able to influence neurons in multiple ways. However, we are only beginning to understand these effects, and future studies are needed to unravel the detailed mechanisms. Elucidating these mechanisms will enhance our understanding of brain performance in health and disease.
Supplementary Material
Acknowledgements
We thank Lilja Niedens for technical help, Bruno Kyewski for support and Albrecht Clement for reagents.
Funding statement
This study was supported by the DFG (grant KR 3668/1–1 to E.M.K.A.). C.F. received internal research funding for early career researchers from JGU Mainz. D.F. and W.P.K. received stipends from the Stipendien Stiftung Rheinland Pfalz, the Kalkhof-Rose Foundation and the Focus Program Translational Neuroscience JGU Mainz, respectively.
References
- 1.Raposo G, Stoorvogel W. 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383. ( 10.1083/jcb.201211138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnstone RM, Mathew A, Mason AB, Teng K. 1991. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J. Cell Physiol. 147, 27–36. ( 10.1002/jcp.1041470105) [DOI] [PubMed] [Google Scholar]
- 3.Ludwig AK, Giebel B. 2012. Exosomes: small vesicles participating in intercellular communication. Int. J. Biochem. Cell Biol. 44, 11–15. ( 10.1016/j.biocel.2011.10.005) [DOI] [PubMed] [Google Scholar]
- 4.Bellingham SA, Guo BB, Coleman BM, Hill AF. 2012. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front. Physiol. 3, 124 ( 10.3389/fphys.2012.00124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thery C, Ostrowski M, Segura E. 2009. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593. ( 10.1038/nri2567) [DOI] [PubMed] [Google Scholar]
- 6.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659. ( 10.1038/ncb1596) [DOI] [PubMed] [Google Scholar]
- 7.Pegtel DM, et al. 2010. Functional delivery of viral miRNAs via exosomes. Proc. Natl Acad. Sci. USA 107, 6328–6333. ( 10.1073/pnas.0914843107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. 2008. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476. ( 10.1038/ncb1800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, et al. 2010. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell 39, 133–144. ( 10.1016/j.molcel.2010.06.010) [DOI] [PubMed] [Google Scholar]
- 10.Frühbeis C, Fröhlich D, Kuo WP, Krämer-Albers E-M. 2013. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell Neurosci. 7, 182 ( 10.3389/fncel.2013.00182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lachenal G, et al. 2011. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell Neurosci. 46, 409–418. ( 10.1016/j.mcn.2010.11.004) [DOI] [PubMed] [Google Scholar]
- 12.Chivet M, Hemming F, Pernet-Gallay K, Fraboulet S, Sadoul R. 2012. Emerging role of neuronal exosomes in the central nervous system. Front. Physiol. 3, 145 ( 10.3389/fphys.2012.00145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faure J, et al. 2006. Exosomes are released by cultured cortical neurones. Mol. Cell Neurosci. 31, 642–648. ( 10.1016/j.mcn.2005.12.003) [DOI] [PubMed] [Google Scholar]
- 14.Frühbeis C, et al. 2013. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 11, e1001604 ( 10.1371/journal.pbio.1001604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakhti M, Winter C, Simons M. 2011. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 286, 787–796. ( 10.1074/jbc.M110.190009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch U-K, Simons M. 2011. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 124, 447–458. ( 10.1242/jcs.074088) [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Verrilli MA, Picou F, Court FA. 2013. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 61, 1795–1806. ( 10.1002/glia.22558) [DOI] [PubMed] [Google Scholar]
- 18.Krämer EM, et al. 1997. Oligodendrocytes direct glycosyl phosphatidylinositol-anchored proteins to the myelin sheath in glycosphingolipid-rich complexes. J. Biol. Chem. 272, 8937–8945. ( 10.1074/jbc.272.16.10558) [DOI] [PubMed] [Google Scholar]
- 19.Feldmann A, et al. 2011. Transport of the major myelin proteolipid protein is directed by VAMP3 and VAMP7. J. Neurosci. 31, 5659–5672. ( 10.1523/JNEUROSCI.6638-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould SJ, Raposo G. 2013. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2, 20389 ( 10.3402/jev.v2i0.20389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witwer KW, et al. 2013. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2, 20360 ( 10.3402/jev.v2i0.20360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentleman RC, et al. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 ( 10.1186/gb-2004-5-10-r80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nimmervoll B, White R, Yang J-W, An S, Henn C, Sun J-J, Luhmann HJ. 2013. LPS-induced microglial secretion of TNFalpha increases activity-dependent neuronal apoptosis in the neonatal cerebral cortex. Cereb. Cortex 23, 1742–1755. ( 10.1093/cercor/bhs156) [DOI] [PubMed] [Google Scholar]
- 24.Sun JJ, Kilb W, Luhmann HJ. 2010. Self-organization of repetitive spike patterns in developing neuronal networks in vitro. Eur. J. Neurosci. 32, 1289–1299. ( 10.1111/j.1460-9568.2010.07383.x) [DOI] [PubMed] [Google Scholar]
- 25.Antonucci F, et al. 2012. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 31, 1231–1240. ( 10.1038/emboj.2011.489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newcomb-Fernandez JK, Zhao X, Pike BR, Wang KKW, Kampfl A, Beer R, DeFord SM, Hayes RL. 2001. Concurrent assessment of calpain and caspase-3 activation after oxygen–glucose deprivation in primary septo-hippocampal cultures. J. Cereb. Blood Flow Metab. 21, 1281–1294. ( 10.1097/00004647-200111000-00004) [DOI] [PubMed] [Google Scholar]
- 27.Abramov AY, Scorziello A, Duchen MR. 2007. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci. 27, 1129–1138. ( 10.1523/JNEUROSCI.4468-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. 1997. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275, 661–665. ( 10.1126/science.275.5300.661) [DOI] [PubMed] [Google Scholar]
- 29.Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. 1997. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 11, 701–713. ( 10.1101/gad.11.6.701) [DOI] [PubMed] [Google Scholar]
- 30.Atwal JK, Massie B, Miller FD, Kaplan DR. 2000. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron 27, 265–277. ( 10.1016/S0896-6273(00)00035-0) [DOI] [PubMed] [Google Scholar]
- 31.Jin K, Mao XO, Zhu Y, Greenberg DA. 2002. MEK and ERK protect hypoxic cortical neurons via phosphorylation of Bad. J. Neurochem. 80, 119–125. ( 10.1046/j.0022-3042.2001.00678.x) [DOI] [PubMed] [Google Scholar]
- 32.Barco A, Marie H. 2011. Genetic approaches to investigate the role of CREB in neuronal plasticity and memory. Mol. Neurobiol. 44, 330–349. ( 10.1007/s12035-011-8209-x) [DOI] [PubMed] [Google Scholar]
- 33.Sassone-Corsi P. 1998. Coupling gene expression to cAMP signalling: role of CREB and CREM. Int. J. Biochem. Cell Biol. 30, 27–38. ( 10.1016/S1357-2725(97)00093-9) [DOI] [PubMed] [Google Scholar]
- 34.Smyth GK. 2005. Limma: linear models for microarray data. In Bioinformatics and computational biology solutions using R and bioconductor (eds R Gentleman, VJ Carey, W Huber, RA Irizarry, S Dudoit), pp. 397–420. Berlin, Germany: Springer. [Google Scholar]
- 35.Lau P, Verrier JD, Nielsen JA, Johnson KR, Notterpek L, Hudson LD. 2008. Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J. Neurosci. 28, 11 720–11 730. ( 10.1523/JNEUROSCI.1932-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horesh D, Sapir T, Francis F, Grayer Wolf S, Caspi M, Elbaum M, Chelly J, Reiner O. 1999. Doublecortin, a stabilizer of microtubules. Hum. Mol. Genet. 8, 1599–1610. ( 10.1093/hmg/8.9.1599) [DOI] [PubMed] [Google Scholar]
- 37.Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M, Lötvall J, Tailleux L. 2010. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS ONE 5, e15353 ( 10.1371/journal.pone.0015353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE. 2007. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev. Neurobiol. 67, 1815–1829. ( 10.1002/dneu.20559) [DOI] [PubMed] [Google Scholar]
- 39.Schneider A, Simons M. 2013. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 352, 33–47. ( 10.1007/s00441-012-1428-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldberg MP, Choi DW. 1993. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci. 13, 3510–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Cristobal J, Cardenas A, Lizasoain I, Leza JC, Fernandez-Tome P, Lorenzo P, Moro MA. 2002. Inhibition of glutamate release via recovery of ATP levels accounts for a neuroprotective effect of aspirin in rat cortical neurons exposed to oxygen–glucose deprivation. Stroke 33, 261–267. ( 10.1161/hs0102.101299) [DOI] [PubMed] [Google Scholar]
- 42.Brown IR. 2007. Heat shock proteins and protection of the nervous system. Ann. N Y Acad. Sci. 1113, 147–158. ( 10.1196/annals.1391.032) [DOI] [PubMed] [Google Scholar]
- 43.Tytell M. 2005. Release of heat shock proteins (Hsps) and the effects of extracellular Hsps on neural cells and tissues. Int. J. Hyperthermia 21, 445–455. ( 10.1080/02656730500041921) [DOI] [PubMed] [Google Scholar]
- 44.Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. 2001. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 914, 66–73. ( 10.1016/S0006-8993(01)02774-3) [DOI] [PubMed] [Google Scholar]
- 45.Tytell M, Greenberg SG, Lasek RJ. 1986. Heat shock-like protein is transferred from glia to axon. Brain Res. 363, 161–164. ( 10.1016/0006-8993(86)90671-2) [DOI] [PubMed] [Google Scholar]
- 46.Kassmann CM, et al. 2007. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 39, 969–976. ( 10.1038/ng2070) [DOI] [PubMed] [Google Scholar]
- 47.Basso M, et al. 2013. Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J. Biol. Chem. 288, 15 699–15 711. ( 10.1074/jbc.M112.425066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomes C, Keller S, Altevogt P, Costa J. 2007. Evidence for secretion of Cu,Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci. Lett. 428, 43–46. ( 10.1016/j.neulet.2007.09.024) [DOI] [PubMed] [Google Scholar]
- 49.Kandel ER. 2012. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain 5, 14 ( 10.1186/1756-6606-5-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakamoto K, Karelina K, Obrietan K. 2011. CREB: a multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 116, 1–9. ( 10.1111/j.1471-4159.2010.07080.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walton MR, Dragunow I. 2000. Is CREB a key to neuronal survival? Trends Neurosci. 23, 48–53. ( 10.1016/S0166-2236(99)01500-3) [DOI] [PubMed] [Google Scholar]
- 52.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. 1998. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20, 709–726. ( 10.1016/S0896-6273(00)81010-7) [DOI] [PubMed] [Google Scholar]
- 53.Lonze BE, Ginty DD. 2002. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623. ( 10.1016/S0896-6273(02)00828-0) [DOI] [PubMed] [Google Scholar]
- 54.Riccio A, Alvania RS, Lonze BE, Ramanan N, Kim T, Huang Y, Dawson TM, Snyder SH, Ginty DD. 2006. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol. Cell 21, 283–294. ( 10.1016/j.molcel.2005.12.006) [DOI] [PubMed] [Google Scholar]
- 55.Du K, Montminy M. 1998. CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 273, 32 377–32 379. ( 10.1074/jbc.273.49.32377) [DOI] [PubMed] [Google Scholar]
- 56.Beitner-Johnson D, Rust RT, Hsieh TC, Millhorn DE. 2001. Hypoxia activates Akt and induces phosphorylation of GSK-3 in PC12 cells. Cell Signal. 13, 23–27. ( 10.1016/S0898-6568(00)00128-5) [DOI] [PubMed] [Google Scholar]
- 57.Doble BW, Woodgett JR. 2003. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186. ( 10.1242/jcs.00384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rockenstein E, Torrance M, Adame A, Mante M, Bar-on P, Rose JB, Crews L, Masliah E. 2007. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation. J. Neurosci. 27, 1981–1991. ( 10.1523/JNEUROSCI.4321-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chin PC, Majdzadeh N, D'Mello SR. 2005. Inhibition of GSK3beta is a common event in neuroprotection by different survival factors. Brain Res. Mol. Brain Res. 137, 193–201. ( 10.1016/j.molbrainres.2005.03.004) [DOI] [PubMed] [Google Scholar]
- 60.Dhanasekaran DN, Reddy EP. 2008. JNK signaling in apoptosis. Oncogene 27, 6245–6251. ( 10.1038/onc.2008.301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leppa S, Bohmann D. 1999. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene 18, 6158–6162. ( 10.1038/sj.onc.1203173) [DOI] [PubMed] [Google Scholar]
- 62.Oliva AA, Jr, Atkins CM, Copenagle L, Banker GA. 2006. Activated c-Jun N-terminal kinase is required for axon formation. J. Neurosci. 26, 9462–9470. ( 10.1523/JNEUROSCI.2625-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kreutz MR, Bien A, Vorwerk CK, Böckers TM, Seidenbecher CI, Tischmeyer W, Sabel BA. 1999. Co-expression of c-Jun and ATF-2 characterizes the surviving retinal ganglion cells which maintain axonal connections after partial optic nerve injury. Brain Res. Mol. Brain Res. 69, 232–241. ( 10.1016/S0169-328X(99)00113-8) [DOI] [PubMed] [Google Scholar]
- 64.Robinson GA. 1996. Changes in the expression of transcription factors ATF-2 and Fra-2 after axotomy and during regeneration in rat retinal ganglion cells. Brain Res. Mol. Brain Res. 41, 57–64. ( 10.1016/0169-328X(96)00070-8) [DOI] [PubMed] [Google Scholar]
- 65.Arlt A, Schafer H. 2011. Role of the immediate early response 3 (IER3) gene in cellular stress response, inflammation and tumorigenesis. Eur. J. Cell Biol. 90, 545–552. ( 10.1016/j.ejcb.2010.10.002) [DOI] [PubMed] [Google Scholar]
- 66.Gorski JA, Balogh SA, Wehner JM, Jones KR. 2003. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience 121, 341–354. ( 10.1016/S0306-4522(03)00426-3) [DOI] [PubMed] [Google Scholar]
- 67.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. 2005. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 192, 348–356. ( 10.1016/j.expneurol.2004.11.016) [DOI] [PubMed] [Google Scholar]
- 68.Shimizu E, et al. 2003. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry 54, 70–75. ( 10.1016/S0006-3223(03)00181-1) [DOI] [PubMed] [Google Scholar]
- 69.Bozdagi O, Rich E, Tronel S, Sadahiro M, Patterson K, Shapiro ML, Alberini CM, Huntley GW, Salton SRJ. 2008. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J. Neurosci. 28, 9857–9869. ( 10.1523/JNEUROSCI.3145-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alder J, et al. 2003. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J. Neurosci. 23, 10 800–10 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thakker-Varia S, Krol JJ, Nettleton J, Bilimoria PM, Bangasser DA, Shors TJ, Black IB, Alder J. 2007. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J. Neurosci. 27, 12 156–12 167. ( 10.1523/JNEUROSCI.1898-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hughes EG, Kang SH, Fukaya M, Bergles DE. 2013. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 16, 668–676. ( 10.1038/nn.3390) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.