Abstract
Homeostasis relies heavily on effective cell-to-cell communication. In the central nervous system (CNS), probably more so than in other organs, such communication is crucial to support and protect neurons especially during ageing, as well as to control inflammation, remove debris and infectious agents. Emerging evidence indicates that extracellular vesicles (EVs) including endosome-derived exosomes and fragments of the cellular plasma membrane play a key role in intercellular communication by transporting messenger RNA, microRNA (miRNA) and proteins. In neurodegenerative diseases, secreted vesicles not only remove misfolded proteins, but also transfer aggregated proteins and prions and are thus thought to perpetuate diseases by ‘infecting’ neighbouring cells with these pathogenic proteins. Conversely, in other CNS disorders signals from stressed cells may help control inflammation and inhibit degeneration. EVs may also reflect the status of the CNS and are present in the cerebrospinal fluid indicating that exosomes may act as biomarkers of disease. That extracellular RNA and in particular miRNA, can be transferred by EV also indicates that these vesicles could be used as carriers to specifically target the CNS to deliver immune modulatory drugs, neuroprotective agents and anti-cancer drugs. Here, we discuss the recent evidence indicating the potential role of exosomes in neurological disorders and how knowledge of their biology may enable a Trojan-horse approach to deliver drugs into the CNS and treat neurodegenerative and other disorders of the CNS.
Keywords: extracellular vesicles, exosomes, neurodegeneration, therapy, drug delivery
1. Introduction into extracellular vesicles
Extracellular vesicles (EVs) are a heterogeneous group of membrane vesicles of endosomal and plasma membrane that are classified based on biogenesis but also upon the cell type from which they originate (table 1).
Table 1.
Terminology of extracellular vesicles (EVs) and subclasses.
| name | size | description/formation | contents |
|---|---|---|---|
| EVs | 40 nm–1 µm | all vesicles shed by cells apart from oncosomes and apoptotic material | proteins, lipids, nucleic acids (mRNA, small RNA) [1] |
| exosomes | 40–100 nm | originate through inward budding from the limiting membrane of MVBs and are released upon fusion with the plasma membrane [2] | cholesterol, ceramide, tetraspanins, GPI proteins, small RNA [3] |
| ectosomes, microvesicles (MV), shed vesicles/particles | 100–200 nm | formed directly at the plasma membrane [4] | cholesterol, mRNA tetraspanins, GPI proteins [5] |
| oncosomes | 1–10 µm | tumour-derived microvesicles | reflect largely plasma membrane domains of cancer cells [6] |
Exosomes are perhaps the best-known extracellular membranous vesicles of endocytic origin and typically measure 40–100 nm in diameter as judged by electron microscopy (EM). Exosomes were first reported in 1983 by Johnstone and co-workers [2] while culturing reticulocytes. Reticulocytes are immature erythrocytes containing cytoplasmic ribosomes and some remnants of organelles, such as mitochondria, endoplasmic reticulum, Golgi apparatus and the endosomal system. During their differentiation into mature erythrocytes, these ribosomes and other organelles are lost and several plasma membrane proteins are cleared resulting in a decrease in size and loss of some specific plasma membrane activities [7]. Exosome secretion has been described to be the mechanism involved for shedding this ‘unwanted’ material [7,8]. Reticulocytes themselves appear to benefit from exosome secretion, but to date the role and the fate of exosomes after secretion in vivo remains unclear. Exosomes contain proteins, lipids and microRNAs (miRNAs) that can mediate various signalling functions [9]. Exosomes and other EVs have been found in cell-culture media and many bodily fluids suggesting that most, if not all, cell types can produce exosomes. Exosomes derive from intraluminal vesicles (ILVs) that are formed at the limiting membrane of multivesicular bodies (MVBs) through inward budding. Therefore, the luminal content of ILVs is presumably comparable to that of the cell's cytoplasm containing typical cytoplasmic biomolecules, including RNAs and protein [10]. While the true function of EVs and exosomes are still debated [11], it is clear that these vesicles can alter the physiology of the producing (autocrine) and recipient (paracrine) cells and have a major role in immune responses [12].
Exosomes have been shown to be released in vitro by a wide variety of cell types of haematological origin such as B lymphocytes, dendritic cells (DCs), mast cells, T lymphocytes and platelets, the latter being the precursors of the bulk of EVs found in human serum. Exosomes are also released from cells of non-haematological origin, including epithelial cells, tracheobronchial cells, hepatocytes and, the topic of this review, neuronal and glial cells [13–15]. Of direct relevance to central nervous system (CNS) disorders, tumour-derived cell-lines, including tumour cells derived from brain cells [16], produce large amounts of exosomes. In addition to neurons and glia, production of EVs from endothelial cells in the brain could potentially be a route for ‘externalizing’ brain-specific markers into the blood [17]. The notion that CNS-derived exosomes are released into physiological biofluids such as the cerebrospinal fluid (CSF) [18] and blood suggests that exosomes can be used as diagnostic tools due to their disease-specific content. Despite recent advances, neuronal cells seem to produce a rather limited amount of EVs compared with other cell types [19] and their composition and function are only recently becoming better understood [20].
2. Detection methods
Many optical and non-optical methods are applied for the detection and characterization of exosomes [21]. However, since the discovery of exosomes [2], the detection of EVs and exosomes has been greatly hampered due to a multitude of reasons some of which have led to misconceptions about their composition and function. Only slowly these problems are being recognized in the EV community. Owing to the biological complexity of body fluids, isolation of circulating EVs and exosomes has proved difficult. The first complication is size; exosomes are typically 100 nm and the use of standard differential ultracentrifugation techniques to isolate these EVs risks the possible co-isolation of many other subcellular particles of similar size, including lipoprotein particles, small platelets, cellular debris, protein complexes, fibrinogen and albumin. As a consequence, recovery of exosomes is hard to quantify and current isolation protocols have not been standardized [21]. A major task is to establish robust methods that discriminate between exosomes, microparticles and other types of EVs in biofluids such as urine, blood and CSF that are frequently used for biomarker studies. Differences in properties such as size, morphology, buoyant density and protein composition seem insufficient for a clear distinction [12]. Further characterization of isolated EVs by density requires supplementary techniques such as biochemical approaches, notably Western blotting. Purified exosomes float in sucrose gradients at 1.13–1.19 g ml−1 and are most often analysed by EM without sectioning on EM grids. Exosomes collapse during sample dehydration, resulting in a cup-shaped morphology, which is often erroneously used as a typical feature of exosomes [22]. Quickly frozen, vitrified vesicles analysed by EM show that exosomes are naturally rounded in shape [23]; nanoparticle tracking analysis allows determination of the size distribution of exosomes based on the Brownian motion of vesicles in suspension [24]. Finally, flow cytometers can be used but special high-resolution flow cytometry-based methods have only recently been developed for quantitative analysis of individual (immunolabelled)-nanosized vesicles [25].
3. Extracellular vesicles in the healthy central nervous system
While many studies refer to microparticles, exosomes or microvesicles, for the purpose of this review we will generally use the term EVs as defined by the International Society of Extracellular Vesicles [1].
(a). Oligodendrocytes and myelin
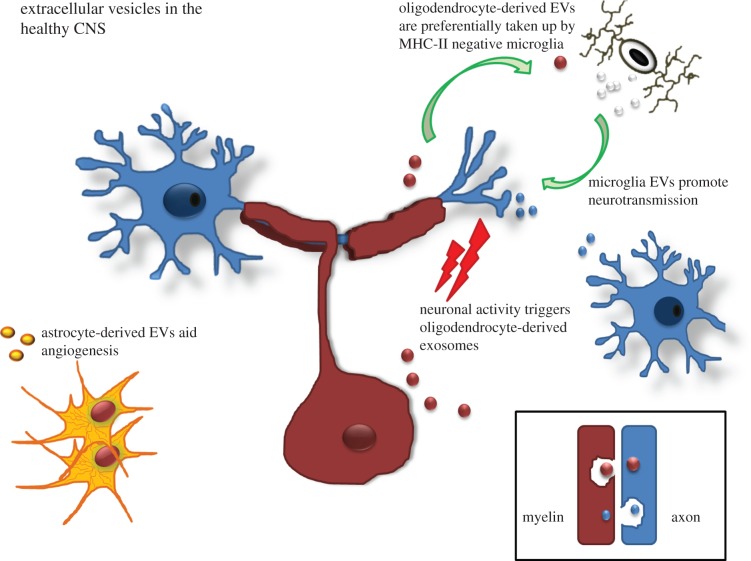
During development, oligodendrocyte progenitor cells differentiate into mature oligodendrocytes that enwrap axons forming myelin sheaths necessary for salutatory conduction of impulses. The process of myelination, which relies on two-way signalling from the unmyelinated axons and oligodendrocytes, is critical for maintaining axon integrity and axonal survival. This is clearly exemplified in demyelinating diseases where myelin damage is strongly associated with neuronal and axonal degeneration. That EVs play a role in maintaining axonal integrity is indicated by circumstantial evidence, such as the presence of MVBs containing proteolipid protein (PLP) in the innermost myelin layers in close contact with axons during myelination [26]. Intriguingly, Rab35 was identified as one of the most prevalent Rab proteins in exosomes secreted by oligodendroglia cells [27]. Further studies by these authors revealed that the Rab GTPase-activating proteins TBC1D10B, RN-tre, TBC1D10A, TBC1D10C and TBC1D15 induced a lower release of PLP via EV. However, these regulators are not universally applicable to cells of different origin. In vitro application of oligodendroglial EVs improves neuronal viability while inhibiting differentiation of oligodendrocytes and myelin formation [26,28]. Also, production of oligodendrocyte-derived EVs is dramatically reduced by conditioned neuronal medium. This suggests a role for EVs in the bidirectional communication between neurons and oligodendrocytes. Detailed studies of oligodendrocyte-derived EVs reveal the presence of enzymes, chaperones and signalling molecules in addition to myelin components and exosomes markers [15,29] (table 2). While many factors may trigger EV release from oligodendrocytes, one is the neuronal activity-dependent release of the neurotransmitter glutamate that triggers oligodendroglial EV secretion through NMDA and AMPA receptors [26]. That oligodendroglia EVs are preferentially taken up by microglia that do not have antigen-presenting capacity, indicates their role in removing debris in a silent manner avoiding induction of the inflammatory response [30]. As well as secreting EVs, oligodendrocytes take up EVs released by neighbouring glia and endothelial cells [31]. These findings indicate that oligodendroglial EVs participate in a novel mode of bidirectional neuron–glia communication contributing to neuronal integrity, at least in an experimental setting. The exact impact of ‘pathogenic’ EV release from oligodendrocytes during disease in vivo requires more detailed studies.
Table 2.
EV composition in the CNS in health and disease.
| cell type | health | disease |
|---|---|---|
| neurons | miRNA 124a, adhesion protein L1 Tsg101, Alix, GADPH, ubiquitin, HSP70, AMPA receptors, adapter protein Ndfip1 | APP, APP metabolites and cleaving enzymes, prion proteins, aggregated proteins, including Aβ, α-synuclein, phosphorylated tau |
| oligodendrocytes | myelin proteins, HSP70, HSP90 and HSP71, metabolites, glycolytic enzymes, mRNA and miRNA | cholesterol |
| astrocytes | HSP70, EAAT, mitochondria, growth factors, angiogenic factors | HSP70, mutant SOD1, PARA4, ceramide, miRNA |
| microglia | late endosomal markers, MHC-class II, CD13 | interleukins, co-stimulatory molecules IL-6, iNOS, COX-2 |
(b). Neurons and axons
In healthy neurons, EVs may act as a well-controlled mechanism for local and possibly systemic inter-neuronal transfer of information within functional brain networks. In the CNS, movements of MVBs to synapses are tightly linked to synaptic plasticity [32] and exosomes deriving from these compartments may exert alternating functions. Owing to their molecular make-up, EVs are believed to relay complex messages, superior to those of direct cell-to-cell contacts or secreted soluble factors [20].
Several lines of evidence reveal that neurons secrete EVs and that depolarization of neurons in vitro dramatically increases this release. Such neuronal-derived exosomes, which contain specific neuronal markers (table 2), are detectable in the CSF [33] indicating neuronal release of small vesicles into the extracellular space. Whether such EVs reach the bloodstream is still unclear, a finding that will be crucial if EVs can act as biomarkers of CNS disorders as maybe inferred from prion-infected cells [34]. In the CNS, neuronal EVs shed at the synapses are internalized by neighbouring cells by endocytosis [10]. Support for uptake of EVs was demonstrated upon injection of oligodendroglia-derived exosomes into the mouse brain, resulting in the functional retrieval of the EV cargo in neurons. As mentioned above, supply of cultured neurons with oligodendroglial EVs improves neuronal viability under conditions of cell stress supporting the contention that EVs may play a key role in neuronal glia interactions during disease.
Moreover, secreted Wnt seems to be transported across synapses by Evi-containing EVs with characteristics of exosomes. The exosome-like vesicles contain the Wnt-binding protein Evenness Interrupted/Wntless/Sprinter (Evi/Wls/Srt) and in the Drosophila larval neuromuscular junctions, presynaptic vesicular release of Evi is required for the secretion of the Wnt, Wingless (Wg). Secreted Evi may also act cell-autonomously in the postsynaptic Wnt-receiving cell to target dGRIP, a Wg-receptor-interacting protein, to postsynaptic sites [35]. Although MVBs are suggested to move into the presynaptic structure [36], little is known of how MVBs fuse with the plasma membrane to transmit Wnt signals; possibly RAB11 and syntaxin 1A may have a role [37].
(c). Astrocytes
In the CNS, astrocytes form the blood-brain barrier (BBB), regulate synaptic transmission, as well as being intricately involved in neuronal growth and survival by producing neural growth factors. An important role is in scavenging extracellular glutamate through membrane excitatory amino acid transporters (EAAT). While the functions make use of cell–cell contact and production of, e.g. growth factors, emerging evidence indicates that these functions may also take place via release of EVs. As with other cell types contents of astrocyte-derived EVs vary depending on the environment (table 2) but are known to include factors involved in angiogenesis, MMPs, mitochondria, lipids [38] as well as EAAT. Under stress conditions, astrocytes also release heat shock proteins and synapsin I to maintain homeostasis [39].
(d). Microglia
Early studies demonstrated that the protein content of microglia-derived EVs includes enzymes, chaperones, tetraspanins and membrane receptors previously reported in B cells and DC-derived exosomes. In addition, microglia-derived MV expressed CD13 and MCT-1 [40]. Intriguingly, microglia-derived EVs stimulate excitatory transmission of neurons in vitro and in vivo [41]. As discussed above, internalization of EVs by microglia occurs by a macropinocytotic mechanism without inducing a concomitant inflammatory response [30]. Microglia-generated EVs can contain MHC-class II antigens following interferon-γ treatment [40], while DC-derived EVs that contain the invariant CD74 chain may activate NF-κB signalling in microglia [42]. These studies combined clearly show that under certain conditions such EVs could augment inflammatory responses in the CNS. Upon ATP stimulation, microglia release EVs carrying IL-1β and the IL-1β-processing enzyme caspase-1 [43].
4. Extracellular vesicles in central nervous system diseases
(a). Infections
Viral infections alter the host cell composition and such changes are reflected in the composition of EV release from infected cells [44]. Several studies on viruses known to infect the CNS, including HIV-1, HTLV-1 and Epstein-Barr virus, reveal that EVs from infected cells contain viral miRNAs, viral transactivators and cytokines that can control the course of infection. That such EVs are pathogenic has been demonstrated in vitro, where treatment of cultured astrocytes with pathogenic HIV Tat protein decreases neuronal viability.
(b). Prion disorders
During prion disorders, the EVs released have a distinct signature containing cellular prion protein, PrPC, and the abnormal infectious form, as well as specific miRNA that in some cases have specific ultrastructural features [45]. These EVs that carry proteinase-K resistant PrPsc are infectious, indicating that EVs may well play a major role in the spread of prion proteins throughout the host [34,46,47].
(c). Neurodegenerative diseases
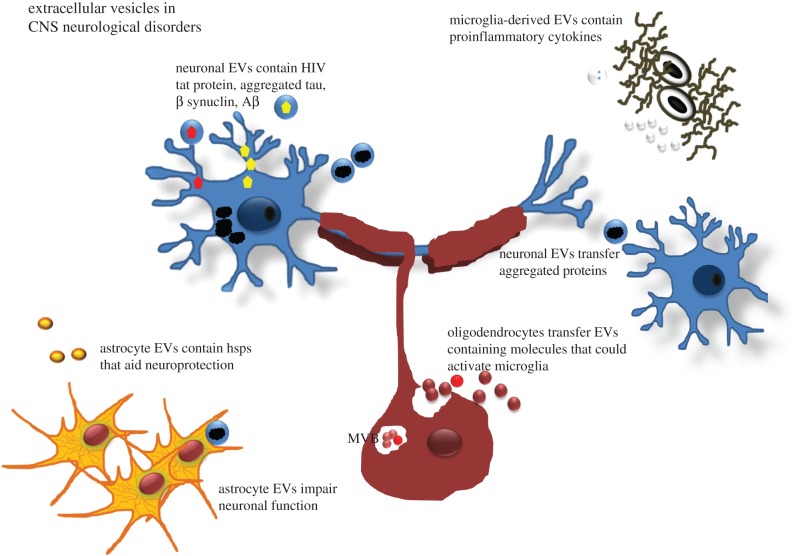
Apart from mediating normal brain function (for which there is still little evidence), it is clear that EVs have the ability to ‘spread’ pathology in the brain. This is particularly relevant to prion disorders (see above) and neurodegenerative disorders, where EVs have been reported to sequester and spread pathogenic proteins such as α-synuclein, amyloid precursor protein (APP) and phosphorylated Tau, which are involved in Parkinson's and Alzheimer's diseases, respectively [48,49]. In support of this, a recent study showed that myeloid-derived MVs from Alzheimer's patients are neurotoxic in vitro. Such MVs promote the formation of soluble amyloid-β (Aβ) species as well as the uptake of neurotoxic Aβ into MVs [50]. In contrast to being pathogenic, there is also evidence that sphingolipid-modulated EV secretion promotes clearance of Aβ by microglia, an event relevant for Alzheimer's disease. In this case, neuron-derived EVs drive conformational changes in Aβ to form non-toxic amyloid fibrils and promote uptake of Aβ by microglia [51]. These authors also showed that EV secretion was increased following treatment with SMS2 siRNA and enhanced Aβ uptake into microglial cells thereby significantly decreasing extracellular levels of Aβ. Amyloid protein is well known to induce neuronal cell death, and amyloid peptides are known to activate caspase 3 and induced apoptosis in primary cultured astrocytes. That such apoptosis could also be mediated by EVs including exosomes indicates that they might well be crucial in the development of Alzheimer's disease (for an overview, see figures 1 and 2).
Figure 1.
The role of extracellular vesicles in the healthy CNS. EVs carry signatures of the cell in question as well as specific EV-related factors. The impact of such release depends upon the cell type releasing the EVs and the cell type taking up the particles (see text for more details). (Online version in colour.)
Figure 2.
Extracellular vesicles in neurological disorders: proposed actions. In neurodegenerative disorders, neurons, and in some cases astrocytes, produce and release aggregated proteins such as α-synuclein, APP and phosphorylated tau and, in the case of prion disorders, pathogenic PrPSc protein. The EVs released may act as ‘seeds’ that spread the damage throughout the brain. In demyelinating disease, myelin-stressed oligodendrocytes produce altered myelin proteins and heat shock proteins (hsps) that may (hypothetically) be released in EVs. The ‘disease-associated’ proteins activate microglia that may augment disease or alternatively affect neurons and axons leading to dysfunction. (Online version in colour.)
Activation of innate immune receptors by host-derived factors exacerbates CNS damage, but the identity of these factors remains elusive. Apart from secretion of misfolded protein aggregates, unconventional release of pathogenic miRNAs, in particular miRNA let-7, may also have a role. miR-let7 is a highly abundant regulator of gene expression in the CNS, which appears to activate Toll-like receptor 7 (TLR7), inducing neurodegeneration. Notably, CSF from individuals with Alzheimer's disease contain increased amounts of miR-let7b, and extracellular introduction of let7b into the CSF of wild-type mice by intrathecal injection causes neurodegeneration while this is not observed in mice lacking TLR7. These studies suggest that miRNAs can function as signalling molecules contributing to the spread of CNS damage, a process possibly mediated by RNA protected in and transmitted by EVs such as exosomes [52,53].
(d). Multiple sclerosis
Recent evidence indicates perturbed interactions between axons and myelin/oligodendrocytes in the early stages of MS [54]. In an intriguing manner, this links with the known emergence of oligodendrocyte stress at the earliest stages of lesion development. Altered communication between axons and myelin/oligodendrocytes, placing the latter under stress, may well therefore be a key part of the early changes in MS [55,56]. It is known that stressed oligodendrocytes communicate in part by secretion of EVs including exosomes containing proteins, lipids and regulatory RNAs. That oligodendrocytes in MS express HSPB5, that such expression is related to activated microglia, and that HSPB5 activates microglia in vitro indicates that, hypothetically, such communication between oligodendrocytes and microglia may well occur via EV transfer [57] (figure 2), although this remains to be determined. In human MS and experimental autoimmune encephalomyelitis (EAE), an animal model of MS, the numbers of EVs increase relative to microglia activation. That some of these EVs released are beneficial has been shown in EAE, where in this case exosomes in the serum of young mice have been shown to protect against disease indicating a rejuvenating role [58]. Such a protective role has also been observed in serum from pregnant animals that may explain the temporary resolution of MS during pregnancy [59]. In part, this ability of exosomes to aid oligodendrocyte differentiation is dependent on exosomes delivery of miR-219 that suppresses factors known to inhibit oligodendrocyte progenitors [58]. In addition, EVs released by DC contain miRNA species that reduce oxidative stress and improve remyelination following acute lysolecithin-induced demyelination. Such EVs are preferentially taken up by oligodendrocytes and promote remyelination, although it is unclear whether microglia can also function in this way.
(e). Brain tumours
Small vesicles emanating from cancer cells, either arising as primary brain tumours or as metastases, contain proteins associated with malignancy. For this reason, these exosomes are often referred to as oncosomes and act as biomarkers of the disease. For example, oncosomes may be comprised Myc, Ras, HER2 and other cancer-related molecules, including nucleic acids and proteins. Proteomic studies have been performed on metastatic and primary brain tumours and represent an attractive platform to detect and monitor brain tumours as well as study oncogenic pathways and responses to therapies. (For excellent reviews, refer to [60–62].)
(f). Lipid storage diseases
To date, little is known about the role of EVs in lipid storage diseases. In one disorder, namely Niemann-Pick type C1 disease, it has been suggested that EV release of cholesterol may be beneficial [63]. In vitro the authors showed that such release may serve as a cellular mechanism to partially bypass the traffic block due to toxic lysosomal cholesterol accumulation. Furthermore, it was also suggested that secretion of cholesterol by EVs helps to maintain cellular cholesterol homeostasis.
5. Exosomes as biomarkers of neurodegenerative diseases
Clinical symptoms present rather late in the pathogenesis of neurological disorders. Although underlying molecular responses are present years before the clinical onset [64], current diagnostic tools are not sensitive enough to detect these early changes.
As discussed above, EVs reflect the state of cells in the brain, both under healthy and damaged conditions, and are present in the brain's extracellular fluid. Therefore, EVs might contain crucial information about these early biochemical changes, and thus represent a new reservoir for biomarker discovery. The CSF has been shown to be a rich source of biomarkers in chronic neurological disorders, since the CSF compartment is in close anatomical contact with the brain interstitial fluid. A brief study using five patients undergoing thoraco-abdominal aortic aneurysm repair, identified exosomes in the CSF using specific antibodies in Western blotting and immune-EM. The latter technique is necessary to examine their structure (cup-shaped) as well as expression of markers [65]. In AD patients, exosomes in the CSF reveal disease-associated proteins. The pathogenesis of AD is related to the hyperphosphorylation of the protein tau. In mild AD, the proportion of phosphotau in the exosomal fraction of the CSF was significantly higher compared with non-AD controls. A preliminary analysis of proteins co-purified with tau in secreted exosomes identified several known to be involved in tau misprocessing [48]. Likewise, in MS patients, higher levels of microglia-derived exosomes can be detected compared to age-matched controls. MS can be characterized by its fulminant activation of microglia resulting in myelin damage. Interestingly, the concentration of these microglia-derived exosomes increased upon brain inflammation and was closely related with disease course, pointing to a clinical value to record disease activity [66]. In brain tumours, CSF-derived exosomes are postulated to give insights into the origin of the malignancy, possible mutations and the response to therapy [67,68]. Examples of molecules found tumour-derived exosomes are oncogenetic growth factors, suppressor genes and miRNAs [68]. Changes in the regulation of specific miRNAs has been described in brain tumours, but also in neurodegenerative diseases and neuropsychiatric disorders, including schizophrenia [64,67]. Interestingly, miRNAs are not only detectable in the CSF, but are also present in serum, plasma and urine. Exosomes are able to transport these brain-specific miRNAs across the endothelial cellular layer of the BBB into the circulating blood [17]. Recently, a comprehensive inventory of the EV proteome in human CSF was compiled, revealing enrichment of exosome markers, heat shock proteins, as well as brain-derived proteins, underscoring the biomarker potential of EVs [33]. In this way, brain-specific information can be obtained in a non-invasive manner, underlining exosome-profiling as a perfect candidate for diagnostic tool in the early onset of neurological diseases.
6. Therapies
Since it is likely that EVs contribute to the local propagation of neurodegenerative diseases, targeting EVs or exploiting the nature of EVs as natural carriers of miRNAs and drug delivery devices has been investigated. Neurons communicate through the secretion of EVs contributing to local synaptic plasticity, but EVs may also allow longer range communication within the CNS and influence neuronal networks located at a distance [20]. Targeting EVs directly to sites for inhibiting deleterious effects seems an attractive approach. Adeno-associated viruses (AAVs) encapsulated in EVs that harbour viral capsid proteins can deliver genetic cargo into recipient cells. Gene delivery is dependent on the AAV transgene and not EV-bound mRNA or protein transfer [69]. Indeed, viruses have frequently been used in modified form for drug delivery purposes, however, we suggested previously that naturally produced exosomes equipped with specific viral proteins may serve as optimal delivery devices for functional RNA [70,71]. In an experimental model of stroke in rats, intravenous administration of cell-free MSC-generated exosomes has been shown to greatly improve functional recovery as well as enhancing neurite remodelling, neurogenesis and angiogenesis [72]. EV-mediated delivery offers multiple advantages as these vesicles are biocompatible, can be autologous (i.e. patient-derived) and appear to have the unique ability to cross biological barriers, notably the BBB. In one breakthrough study, exosomes were harnessed with exogenous siRNA by electroporation and engineered to expose a brain-specific peptide (rabies virus glycoprotein-derived peptide). Specific mRNA knockdown was observed throughout the brain but was negligible in the liver and spleen. The therapeutic potential of EV-mediated siRNA delivery was demonstrated by protein (62%) knockdown of BACE1, a therapeutic target in Alzheimer's disease. These findings represented a first in vivo example of how to exploit exosome physiology in a therapeutic setting [73]. Despite the current realization that siRNA loading into exosomes is at best very inefficient [74] and although many technical hurdles need to be overcome, targeting of exosomes to the brain, a major previous biological barrier, seems at least possible.
In another recent study, exosome-mediated transfer of miRNA (in particular, miR-124a) was shown to have a role in neuron to astrocyte signalling. Exosomes isolated from neuron conditioned medium contained small RNAs and were internalized into astrocytes, increasing astrocyte miR-124a and GLT1 protein levels. GLT1 in humans is an important glutamate transporter and its expression is selectively lost in amyotrophic lateral sclerosis (ALS) also known as Lou Gehrig's disease [75]. Intrastriatal injection of specific antisense against miR-124a into adult mice lead to a reduction in GLT1 protein expression and glutamate uptake levels in striatum without reducing GLT1 mRNA levels. miR-124a is selectively reduced in the spinal cord tissue of end-stage SOD1 G93A mice, a mouse model of ALS. Strikingly, exogenous delivery of miR-124a in vivo through stereotaxic injection seemed to prevent further pathological loss of GLT1 proteins in SOD1 G93A mice [76].
In conclusion, there is strong evidence that EVs, and in particular endosome-derived exosomes, contribute to homeostasis in the CNS. In CNS-associated diseases EV biogenesis, transfer or composition can alter, causing pathology. A deeper understanding of EV-mediated cell–cell communication and details on their biogenesis and release may lead to improved diagnosis and novel therapeutic options in CNS diseases.
Acknowledgements
The authors acknowledge the support of the UK MS Society and the Dutch MS society (Stichting MS research for L.P.) and the NWO for a Veni-scholarship awarded to D.M.P.
References
- 1.Witwer KW, et al. 2013. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2, 1–25. ( 10.3402/jev.v2i0.20360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan BT, Johnstone RM. 1983. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33, 967–978. ( 10.1016/0092-8674(83)90040-5) [DOI] [PubMed] [Google Scholar]
- 3.Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldrop JM, Pegtel DM. 2010. Exosomes: fit to deliver small RNA. Commun. Integr. Biol. 3, 447–450. ( 10.4161/cib.3.5.12339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D'Souza-Schorey C. 2009. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 19, 1875–1885. ( 10.1016/j.cub.2009.09.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocucci E, Racchetti G, Meldolesi J. 2009. Shedding microvesicles: artefacts no more. Trends Cell Biol. 19, 43–51. ( 10.1016/j.tcb.2008.11.003) [DOI] [PubMed] [Google Scholar]
- 6.Di Vizio D, et al. 2009. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 69, 5601–5609. ( 10.1158/0008-5472.CAN-08-3860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. 1987. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262, 9412–9420. [PubMed] [Google Scholar]
- 8.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. 2000. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 113, 3365–3374. [DOI] [PubMed] [Google Scholar]
- 9.Mathivanan S, Ji H, Simpson RJ. 2010. Exosomes: extracellular organelles important in intercellular communication. J. Proteomics 73, 1907–1920. ( 10.1016/j.jprot.2010.06.006) [DOI] [PubMed] [Google Scholar]
- 10.Raposo G, Stoorvogel W. 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383. ( 10.1083/jcb.201211138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieuwland R, Sturk A. 2010. Why do cells release vesicles? Thromb. Res. 125, S49–S51. ( 10.1016/j.thromres.2010.01.037) [DOI] [PubMed] [Google Scholar]
- 12.Bobrie A, Colombo M, Raposo G, Thery C. 2011. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 12, 1659–1668. ( 10.1111/j.1600-0854.2011.01225.x) [DOI] [PubMed] [Google Scholar]
- 13.Faure J, et al. 2006. Exosomes are released by cultured cortical neurones. Mol. Cell Neurosci. 31, 642–648. ( 10.1016/j.mcn.2005.12.003) [DOI] [PubMed] [Google Scholar]
- 14.Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G. 2004. Cells release prions in association with exosomes. Proc. Natl Acad. Sci. USA 101, 9683–9688. ( 10.1073/pnas.0308413101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C, Möbius W, Berger H, Nave K-A, Schild H, Trotter J. 2007. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteom. Clin. Appl. 1, 1446–1461. ( 10.1002/prca.200700522) [DOI] [PubMed] [Google Scholar]
- 16.Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD. 2009. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 23, 1541–1557. ( 10.1096/fj.08-122184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haqqani AS, Delaney CE, Tremblay T-L, Sodja C, Sandhu JK, Stanimirovic DB. 2013. Method for isolation and molecular characterization of extracellular microvesicles released from brain endothelial cells. Fluids Barriers CNS 10, 4 ( 10.1186/2045-8118-10-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng L, Quek CYJ, Sun X, Bellingham SA, Hill AF. 2013. The detection of microRNA associated with Alzheimer's disease in biological fluids using next-generation sequencing technologies. Front. Genet. 4, 150 ( 10.3389/fgene.2013.00150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachenal G, et al. 2011. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell Neurosci. 46, 409–418. ( 10.1016/j.mcn.2010.11.004) [DOI] [PubMed] [Google Scholar]
- 20.Chivet M, Javalet C, Hemming F, Pernet-Gallay K, Laulagnier K, Fraboulet S, Sadoul R. 2013. Exosomes as a novel way of interneuronal communication. Biochem. Soc. Trans. 41, 241–244. ( 10.1042/BST20120266) [DOI] [PubMed] [Google Scholar]
- 21.Van der Pol E, Hoekstra AG, Sturk A, Otto C, van Leeuwen TG, Nieuwland R. 2010. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J. Thromb. Haemost. 8, 2596–2607. ( 10.1111/j.1538-7836.2010.04074.x) [DOI] [PubMed] [Google Scholar]
- 22.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. 1996. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172. ( 10.1084/jem.183.3.1161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conde-Vancells J, et al. 2008. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J. Proteome Res. 7, 5157–5166. ( 10.1021/pr8004887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balaj L, Lessard R, Dai L, Cho Y-J, Pomeroy SL, Breakfieeld XO, Skog J. 2011. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2, 180 ( 10.1038/ncomms1180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolte-'t Hoen EN, van der Vlist EJ, de Boer-Brouwer M, Arkesteijn GJ, Stoorvogel W, Wauben MH. 2013. Dynamics of dendritic cell-derived vesicles: high-resolution flow cytometric analysis of extracellular vesicle quantity and quality. J. Leukoc. Biol. 93, 395–402. ( 10.1189/jlb.0911480) [DOI] [PubMed] [Google Scholar]
- 26.Frühbeis C, et al. 2013. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 11, e1001604 ( 10.1371/journal.pbio.1001604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu C, et al. 2010. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 189, 223–232. ( 10.1083/jcb.200911018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakhti M, Winter C, Simons M. 2011. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 286, 787–796. ( 10.1074/jbc.M110.190009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frühbeis C, Fröhlich D, Krämer-Albers E-M. 2012. Emerging roles of exosomes in neuron–glia communication. Front Physiol. 3, 119 ( 10.3389/fphys.2012.00119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch U-K, Simons M. 2011. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 124, 447–458. ( 10.1242/jcs.074088) [DOI] [PubMed] [Google Scholar]
- 31.Pusic AD, Pusic KM, Clayton BLL, Kraig RP. 2013. IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J. Neuroimmunol. 266, 12–23. ( 10.1016/j.jneuroim.2013.10.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Von Bartheld CS, Altick AL. 2011. Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Prog. Neurobiol. 93, 313–340. ( 10.1016/j.pneurobio.2011.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiasserini D, van Weering JRT, Piersma SR, Pham TV, Malekzadeh A, Teunissen CE, de Wit H, Jiménez CR. 2014. Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J. Proteomics 106C, 191–204. ( 10.1016/j.jprot.2014.04.028) [DOI] [PubMed] [Google Scholar]
- 34.Bellingham SA, Coleman BM, Hill AF. 2012. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 40, 10 937–10 949. ( 10.1093/nar/gks832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. 2009. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139, 393–404. ( 10.1016/j.cell.2009.07.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chia PH, Li P, Shen K. 2013. Cell biology in neuroscience: cellular and molecular mechanisms underlying presynapse formation. J. Cell Biol. 203, 11–22. ( 10.1083/jcb.201307020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, Budnik V. 2012. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J. Biol. Chem. 287, 16 820–16 834. ( 10.1074/jbc.M112.342667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guescini M, Genedani S, Stocchi V, Agnati LF. 2010. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 117, 1–4. ( 10.1007/s00702-009-0288-8) [DOI] [PubMed] [Google Scholar]
- 39.Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE. 2007. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev. Neurobiol. 67, 1815–1829. ( 10.1002/dneu.20559) [DOI] [PubMed] [Google Scholar]
- 40.Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, Santambrogio L. 2005. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J. Immunol. 175, 2237–2243. ( 10.4049/jimmunol.175.4.2237) [DOI] [PubMed] [Google Scholar]
- 41.Antonucci F, et al. 2012. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 31, 1231–1240. ( 10.1038/emboj.2011.489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teo BHD, Wong SH. 2010. MHC class II-associated invariant chain (Ii) modulates dendritic cells-derived microvesicles (DCMV)-mediated activation of microglia. Biochem. Biophys. Res. Commun. 400, 673–678. ( 10.1016/j.bbrc.2010.08.126) [DOI] [PubMed] [Google Scholar]
- 43.Turola E. 2012. Microglial microvesicle secretion and intercellular signaling. Front. Physiol. 3, 149 ( 10.3389/fphys.2012.00149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sampey GC, Meyering SS, Asad Zadeh M, Saifuddin M, Hakami RM, Kashanchi F. 2014. Exosomes and their role in CNS viral infections. J. Neurovirol. 20, 199–208. ( 10.1007/s13365-014-0238-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coleman BM, Hanssen E, Lawson VA, Hill AF. 2012. Prion-infected cells regulate the release of exosomes with distinct ultrastructural features. FASEB J. 26, 4160–4173. ( 10.1096/fj.11-202077) [DOI] [PubMed] [Google Scholar]
- 46.Porto-Carreiro I, Février B, Paquet S, Vilette D, Raposo G, Fevrier B. 2005. Prions and exosomes: from PrPc trafficking to PrPsc propagation. Blood Cells Mol. Dis. 35, 143–148. ( 10.1016/j.bcmd.2005.06.013) [DOI] [PubMed] [Google Scholar]
- 47.Aguzzi A, Rajendran L. 2009. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron 64, 783–790. ( 10.1016/j.neuron.2009.12.016) [DOI] [PubMed] [Google Scholar]
- 48.Saman S, et al. 2012. Exosome-associated Tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 287, 3842–3849. ( 10.1074/jbc.M111.277061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. 2006. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc. Natl Acad. Sci. USA 103, 11 172–11 177. ( 10.1073/pnas.0603838103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joshi P, et al. 2014. Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ. 21, 582–593. ( 10.1038/cdd.2013.180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuyama K, Sun H, Mitsutake S, Igarashi Y. 2012. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 287, 10 977–10 989. ( 10.1074/jbc.M111.324616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehmann SM, et al. 2012. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 15, 827–835. ( 10.1038/nn.3113) [DOI] [PubMed] [Google Scholar]
- 53.Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu X-J, Ji R-R. 2014. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 82, 47–54. ( 10.1016/j.neuron.2014.02.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dhaunchak AS, Becker C, Schulman H, De Faria O, Rajasekharan S, Banwell B, Colman DR, Bar-Or A. 2012. Implication of perturbed axoglial apparatus in early pediatric multiple sclerosis. Ann. Neurol. 71, 601–13. ( 10.1002/ana.22693) [DOI] [PubMed] [Google Scholar]
- 55.Van Noort JM, van den Elsen PJ, van Horssen J, Geurts JJG, van der Valk P, Amor S. 2011. Preactive multiple sclerosis lesions offer novel clues for neuroprotective therapeutic strategies. CNS Neurol. Disord. Drug Targets 10, 68–81. ( 10.2174/187152711794488566) [DOI] [PubMed] [Google Scholar]
- 56.Van Noort JM, Bsibsi M, Gerritsen WH, van der Valk P, Bajramovic JJ, Steinman L, Amor S. 2010. AlphaB-crystallin is a target for adaptive immune responses and a trigger of innate responses in preactive multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 69, 694–703. ( 10.1097/NEN.0b013e3181e4939c) [DOI] [PubMed] [Google Scholar]
- 57.Peferoen L, Kipp M, van der Valk P, van Noort JM, Amor S. 2014. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 141, 302–313. ( 10.1111/imm.12163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pusic AD, Kraig RP. 2014. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 62, 284–299. ( 10.1002/glia.22606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams Z, Ben-Dov IZ, Elias R, Mihailovic A, Brown M, Rosenwaks Z, Tuschl T. 2013. Comprehensive profiling of circulating microRNA via small RNA sequencing of cDNA libraries reveals biomarker potential and limitations. Proc. Natl Acad. Sci. USA 110, 4255–4260. ( 10.1073/pnas.1214046110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noerholm M, Balaj L, Limperg T, Salehi A, Zhu LD, Hochberg FH, Breakfield XO, Carter BS, Skog J. 2012. RNA expression patterns in serum microvesicles from patients with glioblastoma multiforme and controls. BMC Cancer 12, 22 ( 10.1186/1471-2407-12-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al-Nedawi K, Meehan B, Rak J. 2009. Microvesicles: messengers and mediators of tumor progression. Cell Cycle 8, 2014–2018. ( 10.4161/cc.8.13.8988) [DOI] [PubMed] [Google Scholar]
- 62.Gonda DD, Akers JC, Kim R, Kalkanis SN, Hochberg FH, Chen CC, Carter BS. 2013. Neuro-oncologic applications of exosomes, microvesicles, and other nano-sized extracellular particles. Neurosurgery 72, 501–510. ( 10.1227/NEU.0b013e3182846e63) [DOI] [PubMed] [Google Scholar]
- 63.Strauss K, Goebel C, Runz H, Mobius W, Weiss S, Feussner I, Simons M, Schneider A. 2010. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J. Biol. Chem. 285, 26 279–26 288. ( 10.1074/jbc.M110.134775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rao P, Benito E, Fischer A. 2013. MicroRNAs as biomarkers for CNS disease. Front. Mol. Neurosci. 6, 39 ( 10.3389/fnmol.2013.00039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, Chalmers RTA, Webb DJ, Dear JW. 2012. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J. Transl. Med. 10, 5 ( 10.1186/1479-5876-10-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verderio C, et al. 2012. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 72, 610–624. ( 10.1002/ana.23627) [DOI] [PubMed] [Google Scholar]
- 67.De Smaele E, Ferretti E, Gulino A. 2010. MicroRNAs as biomarkers for CNS cancer and other disorders. Brain Res. 1338, 100–111. ( 10.1016/j.brainres.2010.03.103) [DOI] [PubMed] [Google Scholar]
- 68.D'Asti E, Garnier D, Lee TH, Montermini L, Meehan B, Rak J. 2012. Oncogenic extracellular vesicles in brain tumor progression. Front. Physiol. 3, 294 ( 10.3389/fphys.2012.00294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maguire CA, et al. 2012. Microvesicle-associated AAV vector as a novel gene delivery system. Mol. Ther. 20, 960–971. ( 10.1038/mt.2011.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MAJ, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. 2010. Functional delivery of viral miRNAs via exosomes. Proc. Natl Acad. Sci. USA 107, 6328–6333. ( 10.1073/pnas.0914843107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koppers-Lalic D, Hogenboom MM, Middeldorp JM, Pegtel DM. 2013. Virus-modified exosomes for targeted RNA delivery; a new approach in nanomedicine. Adv. Drug Deliv. Rev. 65, 348–356. ( 10.1016/j.addr.2012.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. 2013. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 33, 1711–1715. ( 10.1038/jcbfm.2013.152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345. ( 10.1038/nbt.1807) [DOI] [PubMed] [Google Scholar]
- 74.Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, Schiffelers RM, Raemdonck K, Vader P. 2013. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 172, 229–238. ( 10.1016/j.jconrel.2013.08.014) [DOI] [PubMed] [Google Scholar]
- 75.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. 1995. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol. 38, 73–84. ( 10.1002/ana.410380114) [DOI] [PubMed] [Google Scholar]
- 76.Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, Rothstein J, Yang Y. 2013. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J. Biol. Chem. 288, 7105–7116. ( 10.1074/jbc.M112.410944) [DOI] [PMC free article] [PubMed] [Google Scholar]