Overview
While the transition to molecularly defined patient subgroups in advanced non-small cell lung cancer (NSCLC) often leads to dramatic and prolonged responses to an inhibitor of an identified oncogenic mutation, acquired resistance eventually ensues. The optimal approach to management in that setting remains the subject of ongoing research, though it is possible to identify several points that distinguish it from traditional tenets based on conventional chemotherapy. Such patients are not equivalent to those who have progressed on first line chemotherapy, and consideration of initiation of chemotherapy-based regimens as if the patient were being treated first line in the absence of a oncogenic mutation is a reasonable consideration. Acquired resistance is often partial, so that continued treatment with the same targeted therapy or another against the same target is a strategy favored by many experts, in part to minimize the risk of “rebound progression” that may occur when the targeted therapy is withdrawn. Progression within the central nervous system (CNS) may occur because of poor penetration of the systemic targeted therapy into the CNS, rather than true cellular resistance to the therapy itself; accordingly, local therapy for “brain only” progression with sustained targeted therapy for extracranial disease can be associated with prolonged disease control. Finally, patients with acquired resistance to a targeted therapy are ideal candidates for clinical trials when available, particularly when repeat biopsies of progressing lesions can help elucidate mechanisms of resistance and thereby lead to histologically and molecularly informed treatment decisions.
Introduction
The management of advanced non-small cell lung cancer (NSCLC) has transitioned into an algorithm directed significantly by the presence or absence of an oncogenic mutation (where mutation refers to nucleotide substitutions, insertions, deletions, or chromosomal rearrangements or duplications) that can be effectively inhibited with a specific molecularly targeted agent. We have achieved a clear consensus that the most effective initial intervention for patients with an activating epidermal growth factor receptor (EGFR) gene mutation or anaplastic lymphoma kinase (ALK) gene rearrangement should be an EGFR tyrosine kinase inhibitor (TKI) or the ALK inhibitor crizotinib, respectively. These therapies, generally administered at the earliest opportunity as the next line of therapy after the oncogenic mutation has been identified, offer the greatest probability of a dramatic and prolonged response as compared to conventional chemotherapy. Responses to these generally well-tolerated therapies often last for a year and sometimes much longer. However, patients with these oncogenic mutations eventually demonstrate progression of their disease, a clinical setting described as acquired resistance to the previously highly effective targeted therapy.
The optimal approach to management of such patients remains undefined. While clinical practice varies in how best to treat patients who experience acquired resistance, the only consensus among leading researchers is that this is not a setting analogous to second line management of advanced NSCLC in patients with a cancer that does not have an “oncogene addiction” to a molecular signaling cascade driven by a specific oncogene. There is good reason to believe that the resistance observed in such patients is only partial, so that ongoing inhibition may prove to be beneficial. Though the practice of continuing a therapy on which a patient has demonstrated prior progression runs counter to the basic tenets of oncology, we now recognize many settings in which ongoing therapy despite prior progression with that class of therapy may be beneficial. This is presumably because a subset of cancer cells remains effectively inhibited by the targeted therapy, and/or the suppression is at least somewhat effective in a broader population of cancer cells. For example, objective clinical benefit has been established for continuing trastuzumab in patients with HER2-amplified breast cancer who have progressed on this agent (1) or pursuing additional inhibitors of BCR-ABL in patients with chronic myelogenous leukemia who have demonstrated progression on imatinib (2, 3), providing a helpful heuristic for guiding practice in management of acquired resistance in advanced NSCLC that has an established oncogene addiction to a specific oncogenic mutation.
Heterogeneiety of Presentations with Acquired Resistance to Targeted Therapy in Advanced NSCLC
Compounding the challenge of defining an optimal strategy for progression in patients with advanced NSCLC and an identified oncogenic mutation is the heterogeneity in the clinical picture for patients considered as demonstrating “acquired resistance”. Though the initial criteria offered by Jackman and colleagues (4)(Table 1) provide a basic definition for acquired resistance to an EGFR TKI, others use less formal definitions or a more strict approach that may require non-progression over 6 months or longer. In addition, patients may demonstrate a pattern of progression that is a single focus or few foci of progression against a background of still very well suppressed disease, more diffuse but still relatively indolent progression that may not be clinically significant, or a diffuse and rapidly progressing process. Progression may potentially be predicated on development of a new mutation, loss of the prior oncogenic mutation, or other changes that still remain to be defined. Though they show the promise of augmenting our understanding of this process quite significantly, repeat biopsies to clarify the underlying molecular mechanism(s) are done only infrequently and have demonstrated a wide array of mechanisms that are discussed further below.
Table 1. Defining Acquired Resistance (specifically to EGFR TKI) (4).
|
Though the biology of EGFR mutations and ALK rearrangements are distinct, the patterns of progression seen in the setting of acquired resistance have been similar and allow us to create some shared principles to guide the emerging principles of clinical management to follow.
Acquired Resistance to EGFR TKIs
Though much has been learned over past years about first-line therapy with EGFR TKI for EGFR-mutant lung cancer, much remains to be learned about the optimal management of patients after resistance develops. Most agree that chemotherapy is the current standard of care for these patients, particularly with data suggesting an increased sensitivity to chemotherapy in the first-line setting (5). However, little prospective evidence exists describing the effectiveness of chemotherapy in EGFR-mutant lung cancer patients following TKI failure. One arm of the TORCH trial prospectively delivered cisplatin/gemcitabine following first-line erlotinib (6) – in 13 patients with EGFR mutations receiving chemotherapy after erlotinib, 2 objective responses were seen (15%) with a median PFS of 4 months (7). Retrospective series have similarly described response rates of only 15-18% to chemotherapy alone after TKI resistance (8, 9). Given that many patients develop slow or asymptomatic progression on EGFR TKI (10). oncologists are increasingly electing to continue single-agent TKI past progression in order to delay transition to chemotherapy (11). The effectiveness of this approach for prolonging disease control is being prospectively studied in the ongoing ASPIRATION study (12).
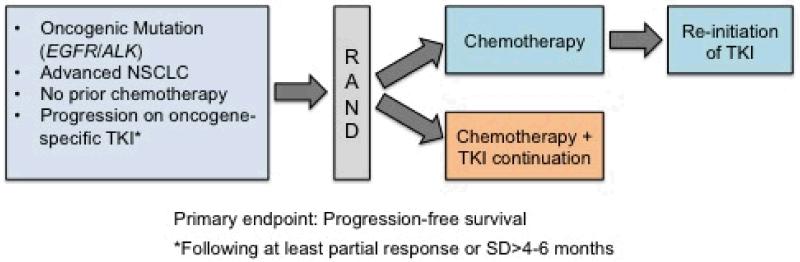
One reason oncologists avoid stopping EGFR TKI after resistance develops is concern over a “flare” of cancer growth when TKI is withdrawn, felt to be due to regrowth of faster-growing TKI-sensitive cells (13). In one study of patients stopping EGFR TKI for clinical trial accrual, 23% of patients developed severe flare requiring hospitalization after a median of 8 days off TKI (14). This has led many to advocate continuing the initial targeted therapy up to the time that a new treatment is initiated, rather than favoring a washout period of several weeks, or to continue the TKI in addition to chemotherapy, an approach that augmented the effectiveness of chemotherapy in cell line models (13), The first prospective study of EGFR TKI plus chemotherapy after TKI resistance used single-agent pemetrexed plus erlotinib or gefitinib (15) and described a response rate of 26% and a 7 month median PFS. A retrospective study has also shown higher response rates in this setting when chemotherapy is given with TKI (9). In a randomized study conducted in the first-line setting, erlotinib plus chemotherapy was found to be somewhat more toxic than chemotherapy, but no antagonism was seen; efficacy was equal in patients with untreated EGFR-mutant lung cancer (16). To test whether EGFR TKI increases the activity of chemotherapy in the acquired resistant seeing, the IMPRESS study is randomizing patients to chemotherapy with or without gefitinib after acquired resistance, and a study using erlotinib is planned. The general schema for these trials and a similar one in development with ALK-positive advanced NSCLC and acquired resistance to crizotinib is illustrated in Figure 1.
Figure 1.
Several clinical trials exploring the benefit of tyrosine kinase inhibitor (TKI) continuation with the addition of chemotherapy following progression on TKI alone are underway or proposed. The IMPRESS trial (NCT01544179; PIs, T. Mok and J.C. Soria) is currently accruing advanced NSCLC patients with EGFR activating mutations that have experienced disease progression on gefitinib and will randomize to cisplatin/pemetrexed alone or cisplatin/pemetrexed with continuation of gefitinib. A similar trial in EGFR mutation positive patients who have experienced disease progression on erlotinib will randomize patients to cisplatin or carboplatin plus pemetrexed alone or in combination with continuation of erlotinib (PI, L. Horn). This trial will allow reintroduction erlotinib in the chemotherapy arm following disease progression. The proposed SWOG 1300 (PI, D.R. Camidge) will randomize ALK positive patients with disease progression on crizotinib to either pemetrexed alone or in combination with crizotinib. Reintroduction of crizotinib following disease progression on pemetrexed will be allowed within the trial.
Targeted therapies studied after TKI resistance have often focused on the T790M mutation, a gatekeeper mutation acquired in 49-68% of cancers after TKI resistance (17, 18). Second-generation irreversible EGFR inhibitors such as afatinib and dacomitinib appeared to inhibit T790M in preclinical models, but have generated response rates of less than 10% in prospective trials in erlotinib or gefitinib resistant patients (19, 20). A more impressive response rate of 36% was seen when afatinib was combined with cetuximab, a monoclonal antibody against EGFR, in EGFR-mutant, EGFR TKI resistant patients and interestingly was not dependent on the presence of T790M (21); yet development of this combination has been slowed in part by toxicity. A third generation of EGFR TKI has been identified with selective activity against T790M and minimal inhibition of wild-type EGFR (22), but these agents are very early in their clinical development.
Other trials for acquired EGFR resistance have focused on less common resistance mechanisms. Several trials are studying the combination of EGFR TKIs with MET kinase inhibitors (23), though the incidence of MET amplification in clinical specimens appears to be less than 10% (17, 18). One series found PIK3CA mutations in 5% of cases of acquired resistance (18), and several trials combining EGFR-TKI with PI3K inhibitors are underway (24). The HSP90 inhibitor AUY922 has shown signs of activity in some patients with acquired resistance (25), and is also being studied in combination with erlotinib (26). The most surprising finding may be histologic transformation to small cell lung cancer, seen in 3-14% of cases of acquired resistance (17, 18), supporting a clinical role for repeat biopsies for these patients to determine the potential utility of a chemotherapy approach for that histology.
Acquired Resistance to Crizotinib in ALK-Positive NSCLC
The identification of ALK gene rearrangements in patients with non-small cell lung cancer has led to significant improvements in clinical outcomes for this subset of lung cancer patients. Treatment with crizotinib leads to objective response rates (ORR) of approximately 50-60%, progression free survival of 7-10 months and evidence of prolonged survival (27, 28). Recent results also demonstrate that that crizotinib is superior to treatment with single agent chemotherapy (29). Despite the clear benefits of crizotinib in ALK+ lung cancer, patients treated with crizotinib ultimately experience disease progression related to poor central nervous system penetration or because of cellular resistance.
Both in vitro and patient-based studies have yielded insights into mechanisms of crizotinib resistance in ALK+ lung cancer. One of the earliest identified mechanisms of resistance were ALK kinase domain mutations. In contrast to EGFR mutation positive lung cancer, where the vast majority of resistance mutations are T790M, numerous ALK kinase domain mutations have been identified in patients with ALK gene rearrangements with only a slight preponderance of the gatekeeper mutation, L1196M, which is analogous to T790M in EGFR (30). Indeed, mutations have been identified in clinical tumor samples in 9 different amino acid positions in exons 22, 23, and 25 of the ALK kinase domain (31). Additional mutations have been identified using in vitro studies in exons 21-25 of ALK corresponding to the kinase domain.
The increased complexity of resistance mutations has several implications for patients and physicians. First, it is more difficult to develop robust assays that can encompass and detect all known resistance mutations. Second, it is likely that tumors may harbor more than one mutation at resistance. The first published case of crizotinib-resistance demonstrated two different mutations (C1156Y and L1196M) in the same tumor sample (32). In CML, patient samples derived at the time of dasatinib or nilotinib resistance demonstrated up to 10 different resistance mutations by mass-spectrometry, many of which were missed by direct sequencing techniques (33). Thus, samples that harbor multiple mutations may register as false negatives by direct sequencing due to allelic dilution if there is not a dominant mutation in a large percentage of the tumor cells. Finally, many of the resistance mutations identified thus far in ALK do not seem to confer a fitness disadvantage in the absence of an ALK inhibitor as the EGFR resistance mutation, T790M, does (34-36). Thus the re-response observed in some EGFR mutation positive patients may not be as common in ALK+ patients re-challenged with crizotinib. A recent case report of an ALK+ patient with a response at re-challenge demonstrates that this can also occur ALK+ lung cancer, although the mechanism of crizotinib for this patient was not known (37).
Increase in copy number of the ALK gene fusion was initially identified in ALK+ cell lines made resistant to crizotinib.(38) Evidence of copy number gain has also identified in patient samples from crizotinib resistant patient samples suggesting that this may play a role in cellular resistance (34, 39). In the case of a kinase domain mutation or copy number gain of the ALK gene fusion, ALK signaling would be retained and is expected that tumor cells might still harbor oncogene addiction to the ALK gene fusion. Thus, more potent second-generation ALK inhibitors might overcome these cellular resistance mechanisms. This type of resistance has been termed ALK-dominant resistance (40).
The final class of resistance can be classified as ALK non-dominant resistance defined by emergence of other signaling pathways to ALK signaling dependence, rendering the inhibition of ALK insufficient to inhibit cancer cell growth. Multiple alternate signaling pathways have been identified. The presence of activating mutations in EGFR or KRAS in both crizotinib-naïve and crizotinib treated patients. In vitro studies demonstrate that EGFR and other HER family receptor tyrosine kinases can also mediate resistance through ligand-mediated activation of these receptors. Addition of an EGFR TKI was able to resensitize cancer cells to crizotinib.(39, 41-43) Additional support for this mechanism of resistance has been demonstrated in crizotinib-resistant tumor samples showing increased phosphorylated EGFR compared to pre-crizotinib tumor samples (39). Activation of the KIT receptor tyrosine kinase by the stem cell factor (SCF) has been shown to mediate crizotinib resistance in vitro and evidence of KIT gene amplification by FISH as well as increased SCF staining by immunohistochemistry (39).
Tumor heterogeneity may lead to further complexity when trying to overcome crizotinib resistance in ALK+ lung cancer. Indeed, tumor heterogeneity with respect to cellular resistance has already been observed. Two different kinase domain mutations were identified in one patient sample with some of the tumor cells showing no evidence of mutation (32). Copy number gain and mutation have been found in the same sample, although it is unclear whether both aberrations were present in the same cell (34). Finally one patient who underwent two biopsies of separate lesions showed different molecular results at each site of biopsy (34). This leads to the inevitable question of whether the molecular results on a given biopsy are representative for the entirety of the disease burden, and whether current limited molecular testing is revealing all sources of cellular resistance.
Next generation ALK inhibitors such as CH5424802 demonstrate preclinical activity against cancer cells harboring EML4-ALK gene fusions and have activity against many of the resistance mutations identified in the ALK kinase domain (39). Early preclinical data with LDK378, AP26113, and CH5424802 suggest that these drugs have activity in both crizotinib naïve and crizotinib-resistant patients and each drug has anecdotal data for response of brain metastases (44-46). Next generation ALK inhibitors might be the optimal choice for ALK-dominant resistance where tumors still rely predominantly on ALK signaling as the oncogenic mutation.
EML4-ALK is a client of HSP90 and several drugs in this class have shown clinical activity (47, 48). Notably, AUY-922 has also shown clinical activity against EGFR mutation positive lung cancer or tumors that are wild-type for EGFR, KRAS, and ALK making this a potentially attractive agent to study in ALK-non-dominant resistance (49). Pemetrexed-containing regimens appears to show notable activity in ALK+ lung cancer and thus represent reasonable standard of care options for patients where a clinical trial is not feasible or available (29, 50). Whether to continue crizotinib in the presence of chemotherapy remains an unanswered question, but a clinical trial has been proposed to help answer this question (Figure 1). Use of local ablative therapy also seems an attractive option to extend the clinical benefit of crizotinib in cases where disease progression is limited to one or a few lesions (oligoprogressive disease) (51).
Translating Early Research into Practical Management
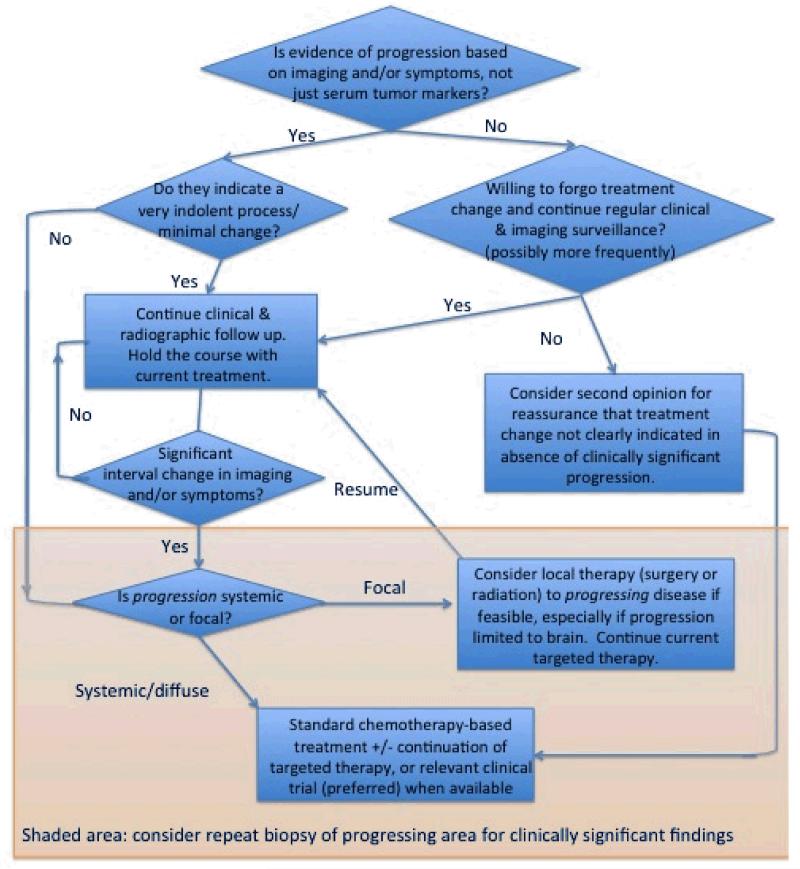
While there are not randomized trial data to develop a clear evidence-based recommendation for clinical management of acquired resistance, there is converging evidence that supports an individualized approach based on the pattern of progression in terms of both pace and extent of progressing disease, as illustrated in Figure 2.
Figure 2.
Proposed algorithm for management of acquired resistance to a targeted therapy in advanced NSCLC.
A key insight into the management of acquired resistance, whether to an EGFR inhibitor, ALK inhibitor, or other oncogenic mutations that may emerge in the future, is that this resistance is often incomplete, so that a subset of the existing cancer cells remain suppressed by the targeted therapy on which progression has been demonstrated. Prior to any therapeutic changes being made, it is important to distinguish between detectable and clinically significant progression, since many patients with acquired resistance may demonstrate minimal, asymptomatic progression that still represents a significant net decrease in tumor burden compared to the status of the patient prior to initiation of the targeted therapy in question. Findings such as the rebound progression that are sometimes seen with discontinuation of a targeted therapy on which a patient has demonstrated recent progression, followed by improved disease control again with reintroduction of the same agent or another in the same class, highlight that continuation of the targeted therapy is often still effectively suppressing at least a subset of the disease that remains responsive to it, even as progression demonstrates that a subset of the cancer cell population has developed resistance. Moreover, this resistance may not only be partial in terms of appearing in a subset of cancer cells within a patient’s overall disease burden, but also may be partial within these cells, so that these cells may still be relatively inhibited by ongoing targeted therapy, compared with withdrawal of the targeted therapy entirely.
The common finding of only one or a few areas of progression against a background of ongoing disease control elsewhere suggests the potential value of local therapy, (e.g. radiation, surgery, or radiofrequency ablation), to the very limited extent of progressive disease, while continuing the targeted therapy that is effectively controlling disease elsewhere. This practice is best established in the management of brain metastases, where local failure can be in part due to the limited penetration of systemic agents through the blood brain barrier. Several groups have described success with continued targeted therapy after delivery of radiation to the brain. There is optimism that this approach can be generalized to instance of focal progression outside of the CNS, though experience to date is limited.
For those patients with systemic progression, the question of whether to discontinue the targeted therapy or continue it in combination with alternative systemic therapy, commonly chemotherapy-based, remains a matter of clinical judgment. In the absence of meaningful data to address this question, the authors differ in their own favored approaches and feel that this is a question that is left to the judgment of the treating oncologist. The clearest consensus is that clinical trials to address such questions are especially welcome.
There are no data to support a specific systemic therapy to initiate, though the authors favor an approach essentially identical to the decision-making strategy that guides the recommendation for first line treatment in a patient without an identified mutation, so that a platinum doublet-based chemotherapy combination is most often favored, modified as needed by the comorbidities, performance status, and treatment preferences of the patient.
Discontinuation of the targeted therapy to which acquired resistance has developed is a particularly reasonable consideration in the subgroup of patients who demonstrate rapid and diffuse progression, suggestive that the targeted therapy is providing little or no inhibitory effect. In such patients, a repeat biopsy may reveal clinically relevant findings of changes in histology or new mutations that may potentially be treated effectively with commercially available or investigational agents.
The introduction of targeted therapies for defined populations with the relevant molecular target have transformed our expectations about what is possible in treating advanced NSCLC, but this remains limited by the essentially invariable development of acquired resistance. While the role of a repeat biopsy at the present time may or may not yield an “actionable” result, our understanding of the mechanisms underpinning acquired resistance have been facilitated greatly by these limited efforts in recent years. By concentrating efforts on repeat biopsies and the molecular evolution of progressing lesions in patients with an identified oncogenic mutation who develop acquired resistance, we can realistically expect to confer additional significant clinical benefits to these patients as we gain a remarkably richer understanding of the complex biology of the molecular evolution of lung cancer that can translate to broader patient populations as well.
Key Points.
Acquired resistance to a specific inhibitor of an identified oncogenic mutation in advanced NSCLC is a clinical entity that is distinct from conventional management of progression after first line traditional combination chemotherapy.
Because acquired resistance is often partial, with discontinuation potentially leading to accelerated progression, it may therefore be advisable to continue the targeted therapy beyond progression, up to the time of initiation of subsequent treatment or continued concurrent with it.
For progression in an isolated area, particularly if limited to the CNS, local therapy to the area of progression with continued targeted therapy may lead to prolonged disease control.
Patients with acquired resistance are ideal candidates for clinical trials when available.
Though not yet considered standard of care, repeat biopsies of areas of progression may potentially help inform recommendations for subsequent therapy and lead to a much greater understanding of the mechanisms of resistance and future directions for optimized treatment in this setting.
Contributor Information
Howard West, Swedish Cancer Institute, 1221 Madison St., Suite 200, Seattle, WA 98104.
Geoffrey R. Oxnard, Dana Farber Cancer Institute, Boston, MA, Geoffrey_Oxnard@DFCI.HARVARD.EDU, Tel: 617-632-6049.
Robert C. Doebele, University of Colorado Cancer Center, robert.doebele@ucdenver.edu, Tel: 303.724.2980.
References
- 1.von Minckwitz G dBA, Schmidt M, Maass N, Cufer T, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German Breast Group 26/Breast International Group 03-05 study. J Clin Oncol. 2009;27(12):1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HGF, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;353:2542–51. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian HM, Talpaz M, O’Brien S, Giles F, Garcia-Manero G, Faderl S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003 Jan 15;101(2):473–5. doi: 10.1182/blood-2002-05-1451. 2003. [DOI] [PubMed] [Google Scholar]
- 4.Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Jänne PA, et al. Clinical Definition of Acquired Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-Small-Cell Lung Cancer. Journal of Clinical Oncology. 2010 Jan 10;28(2):357–60. doi: 10.1200/JCO.2009.24.7049. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009 Aug 19;361(10):947–57. doi: 10.1056/NEJMoa0810699. 2009. [DOI] [PubMed] [Google Scholar]
- 6.Gridelli C, Ciardiello F, Gallo C, Feld R, Butts C, Gebbia V, et al. First-Line Erlotinib Followed by Second-Line Cisplatin-Gemcitabine Chemotherapy in Advanced Non-Small-Cell Lung Cancer: The TORCH Randomized Trial. Journal of Clinical Oncology. 2012 Aug 20;30(24):3002–11. doi: 10.1200/JCO.2011.41.2056. 2012. [DOI] [PubMed] [Google Scholar]
- 7.GR O, editor. C. G. Response rate 15% and median progression-free survival of 4 months with chemotherapy following erlotinib in EGFR mutation-positive patients on TORCH trial. 2012. [Google Scholar]
- 8.Wu J-Y, Shih J-Y, Yang C-H, Chen K-Y, Ho C-C, Yu C-J, et al. Second-line treatments after first-line gefitinib therapy in advanced nonsmall cell lung cancer. International Journal of Cancer. 2010;126(1):247–55. doi: 10.1002/ijc.24657. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg SB, Oxnard GR, Digumarthy S, Muzikansky A, Jackman DM, Lennes IT, et al. Chemotherapy with erlotinib or chemotherapy alone in advanced NSCLC with acquired resistance to EGFR tyrosine kinase inhibitors (TKI) ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):7524. doi: 10.1634/theoncologist.2013-0168. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17(6):1616–22. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxnard GR, Lo P, Jackman DM, Butaney M, Heon S, Johnson BE, et al. Delay of chemotherapy through use of post-progression erlotinib in patients with EGFR-mutant lung cancer. ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):7547. 2012. [Google Scholar]
- 12.Park K, Tsai C-M, Ahn M-j, Yu C-J, Kim S-W, Sriuranpong V, et al. ASPIRATION: Phase II study of continued erlotinib beyond RECIST progression in Asian patients (pts) with epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):TPS7614. 2012. [Google Scholar]
- 13.Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Somwar R, Wang L, et al. Optimization of Dosing for EGFR-Mutant Non-Small Cell Lung Cancer with Evolutionary Cancer Modeling. Sci Transl Med. 2011;3(90):90ra59. doi: 10.1126/scitranslmed.3002356. Pubmed Central PMCID: 21734175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaft JE, Oxnard GR, Miller VA, Kris MG, Sima CS, Riely GJ. Disease flare after tyrosine kinase inhibitor (TKI) discontinuation in patients with EGFR mutant lung cancer and acquired resistance. ASCO Meeting Abstracts. 2011;29(15_suppl):e18001. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshimura N, Okishio K, Mitsuoka S, Kimura T, Kawaguchi T, Kobayashi M, et al. Prospective Assessment of Continuation of Erlotinib or Gefitinib in Patients with Acquired Resistance to Erlotinib or Gefitinib Followed by the Addition of Pemetrexed. Journal of Thoracic Oncology. 2012 doi: 10.1097/JTO.0b013e3182762bfb. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Jänne PA, Wang X, Socinski MA, Crawford J, Stinchcombe TE, Gu L, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 Trial. Journal of Clinical Oncology. 2012 Apr 30; doi: 10.1200/JCO.2011.40.1315. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski M, et al. Rebiopsy of Lung Cancer Patients with Acquired Resistance to EGFR Inhibitors and Enhanced Detection of the T790M Mutation Using a Locked Nucleic Acid-Based Assay. Clin Cancer Res. 2011;17(5):1169–80. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. PubMed PMID: 21430269. Epub 2011/03/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell A, Reckamp KL, Camidge DR, Giaccone G, Gadgeel SM, Khuri FR, et al. PF-00299804 (PF299) patient (pt)-reported outcomes (PROs) and efficacy in adenocarcinoma (adeno) and nonadeno non-small cell lung cancer (NSCLC): A phase (P) II trial in advanced NSCLC after failure of chemotherapy (CT) and erlotinib (E) ASCO Meeting Abstracts. 2010 May 20;28(15_suppl):7596. 2010. [Google Scholar]
- 20.Miller VA, Hirsh V, Cadranel J, Chen Y-M, Park K, Kim S-W, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. The Lancet Oncology. 2012;(0) doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 21.Janjigian YY, Groen HJM, Horn L, Smit EF, Yali Fu FW, Shahidi M, et al. Activity and tolerability of afatinib (BIBW 2992) and cetuximab in NSCLC patients with acquired resistance to erlotinib or gefitinib. ASCO Meeting Abstracts. 2011;29(15_suppl):7525. [Google Scholar]
- 22.Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009 Dec 24;462(7276):1070–4. doi: 10.1038/nature08622. PubMed PMID: 20033049. Pubmed Central PMCID: 2879581. Epub 2009/12/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakelee HA, Gettinger SN, Engelman JA, Janne PA, West HJ, Subramaniam DS, et al. A phase Ib/II study of XL184 (BMS 907351) with and without erlotinib (E) in patients (pts) with non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2010 Jun 14;28(15S):3017. 2010. [Google Scholar]
- 24.Cohen RB, Janne PA, Engelman JA, Martínez P, Nishida Y, Gendreau S, et al. A phase I safety and pharmacokinetic (PK) study of PI3K/TORC1/TORC2 inhibitor XL765 (SAR245409) in combination with erlotinib (E) in patients (pts) with advanced solid tumors. ASCO Meeting Abstracts. 2010;28(15s):3015. [Google Scholar]
- 25.Garon EB, Moran T, Barlesi F, Gandhi L, Sequist LV, Kim S-W, et al. Phase II study of the HSP90 inhibitor AUY922 in patients with previously treated, advanced non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):7543. 2012. [Google Scholar]
- 26.Johnson ML, Yu HA, Hart EM, Worden R, Rademaker A, Gupta R, et al. A phase I dose-escalation study of the HSP90 inhibitor AUY922 and erlotinib for patients with EGFR-mutant lung cancer with acquired resistance (AR) to EGFR tyrosine kinase inhibitors (EGFR TKIs) ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):3083. 2012. [Google Scholar]
- 27.Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. The lancet oncology. 2012 Oct;13(10):1011–9. doi: 10.1016/S1470-2045(12)70344-3. PubMed PMID: 22954507. Epub 2012/09/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. The lancet oncology. 2011 Oct;12(11):1004–12. doi: 10.1016/S1470-2045(11)70232-7. PubMed PMID: 21933749. Pubmed Central PMCID: 3328296. Epub 2011/09/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Phase III study of crizotinib versus pemetrexed or docetaxel chemotherapy in patients with advanced ALK-positive non-small cell lung cancer (NSCLC) (PROFILE 1007) ESMO Congress. 2012 LBA1_PR. 2012. [Google Scholar]
- 30.Lovly CM, Pao W, Escaping ALK. Inhibition: Mechanisms of and Strategies to Overcome Resistance. Science translational medicine. 2012 Feb 8;4(120):120ps2. doi: 10.1126/scitranslmed.3003728. PubMed PMID: 22323827. Epub 2012/02/11. eng. [DOI] [PubMed] [Google Scholar]
- 31.Camidge DR, Doebele RC. Treating ALK-positive lung cancer-early successes and future challenges. Nature reviews Clinical oncology. 2012 Apr 3; doi: 10.1038/nrclinonc.2012.43. PubMed PMID: 22473102. Epub 2012/04/05. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. The New England journal of medicine. 2010 Oct 28;363(18):1734–9. doi: 10.1056/NEJMoa1007478. PubMed PMID: 20979473. Epub 2010/10/29. eng. [DOI] [PubMed] [Google Scholar]
- 33.Parker WT, Lawrence RM, Ho M, Irwin DL, Scott HS, Hughes TP, et al. Sensitive detection of BCR-ABL1 mutations in patients with chronic myeloid leukemia after imatinib resistance is predictive of outcome during subsequent therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011 Nov 10;29(32):4250–9. doi: 10.1200/JCO.2011.35.0934. PubMed PMID: 21990409. Epub 2011/10/13. eng. [DOI] [PubMed] [Google Scholar]
- 34.Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012 Mar 1;18(5):1472–82. doi: 10.1158/1078-0432.CCR-11-2906. PubMed PMID: 22235099. Pubmed Central PMCID: 3311875. Epub 2012/01/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer research. 2011 Jul 26; doi: 10.1158/0008-5472.CAN-11-1340. PubMed PMID: 21791641. Epub 2011/07/28. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Science translational medicine. 2011 Jul 6;3(90):90ra59. doi: 10.1126/scitranslmed.3002356. PubMed PMID: 21734175. Epub 2011/07/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Browning ET, Weickhardt AJ, Camidge DR. Response to crizotinib rechallenge after initial progression and intervening chemotherapy in ALK+ lung cancer. Journal of Thoracic Oncology. doi: 10.1097/JTO.0b013e31827a892c. in press. [DOI] [PubMed] [Google Scholar]
- 38.Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proceedings of the National Academy of Sciences of the United States of America. 2011 May 3;108(18):7535–40. doi: 10.1073/pnas.1019559108. PubMed PMID: 21502504. Pubmed Central PMCID: 3088626. Epub 2011/04/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Science translational medicine. 2012 Feb 8;4(120):120ra17. doi: 10.1126/scitranslmed.3003316. PubMed PMID: 22277784. Pubmed Central PMCID: 3385512. Epub 2012/01/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camidge DR, Doebele RC. Treating ALK-positive lung cancer--early successes and future challenges. Nature reviews Clinical oncology. 2012 May;9(5):268–77. doi: 10.1038/nrclinonc.2012.43. PubMed PMID: 22473102. Epub 2012/04/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008 Jul 1;14(13):4275–83. doi: 10.1158/1078-0432.CCR-08-0168. PubMed PMID: 18594010. Pubmed Central PMCID: 3025451. Epub 2008/07/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011 Sep 15;71(18):6051–60. doi: 10.1158/0008-5472.CAN-11-1340. PubMed PMID: 21791641. Pubmed Central PMCID: 3278914. Epub 2011/07/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanizaki J, Okamoto I, Okabe T, Sakai K, Tanaka K, Hayashi H, et al. Activation of HER family signaling as a mechanism of acquired resistance to ALK inhibitors in EML4-ALK-positive non-small cell lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012 Nov 15;18(22):6219–26. doi: 10.1158/1078-0432.CCR-12-0392. PubMed PMID: 22843788. Epub 2012/07/31. eng. [DOI] [PubMed] [Google Scholar]
- 44.Shaw AT, Camidge DR, Felip E, Sharma S, Tan DSW, Kim D, et al. RESULTS OF A FIRST-IN-HUMAN PHASE I STUDY OF THE ALK INHIBITOR LDK378 IN ADVANCED SOLID TUMORS. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2012;23(supplement 9):ix153. [Google Scholar]
- 45.Nishio M, Kiura K, Nakagawa K, Seto T, Inoue A, Maemondo M, et al. A PHASE I/II STUDY OF ALK INHIBITOR CH5424802 IN PATIENTS WITH ALK-POSITIVE NSCLC; SAFETY AND EFFICACY INTERIM RESULTS OF THE PHASE II PORTION. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2012;23(Supplement 9):ix153. [Google Scholar]
- 46.Gettinger S, Weiss GJ, Salgia R, Bazhenova L, Narasimhan NI, Dorer J, et al. A FIRST-IN-HUMAN DOSE-FINDING STUDY OF THE ALK/EGFR INHIBITOR AP26113 IN PATIENTS WITH ADVANCED MALIGNANCIES. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2012;23(supplement 9):ix152. [Google Scholar]
- 47.Normant E, Paez G, West KA, Lim AR, Slocum KL, Tunkey C, et al. The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and induces tumor regression in ALK-driven NSCLC models. Oncogene. 2011 Jun 2;30(22):2581–6. doi: 10.1038/onc.2010.625. PubMed PMID: 21258415. Epub 2011/01/25. eng. [DOI] [PubMed] [Google Scholar]
- 48.Sequist LV, Gettinger S, Senzer NN, Martins RG, Janne PA, Lilenbaum R, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010 Nov 20;28(33):4953–60. doi: 10.1200/JCO.2010.30.8338. PubMed PMID: 20940188. Epub 2010/10/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garon EB, Moran T, Barlesi F, Gandhi L, Sequist L, Kim SW, et al. Phase II study of the HSP90 inhibitor AUY922 in patients with previously treated, advanced non-small cell lung cancer (NSCLC) Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(suppl) abst 7543. [Google Scholar]
- 50.Camidge DR, Kono SA, Lu X, Okuyama S, Baron AE, Oton AB, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2011 Apr;6(4):774–80. doi: 10.1097/JTO.0b013e31820cf053. PubMed PMID: 21336183. Epub 2011/02/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA, Jr., et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2012 Dec;7(12):1807–14. doi: 10.1097/JTO.0b013e3182745948. PubMed PMID: 23154552. Pubmed Central PMCID: 3506112. Epub 2012/11/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]