Abstract
Rearrangements of the anaplastic lymphoma kinase (ALK) gene occur infrequently in non-small cell lung cancer (NSCLC), but provide an important paradigm for oncogene-directed therapy in this disease. Crizotinib, an orally bioavailable inhibitor of ALK, provides significant benefit for patients with ALK positive (ALK+) NSCLC in association with characteristic, mostly mild, toxicities and is now FDA approved in this molecularly defined subgroup of lung cancer. Many new ALK inhibitors are being developed and understanding the challenges of determining and addressing the side-effects that are likely to be ALK specific, maximizing the time of benefit on targeted agents, and understanding the mechanisms that underlie drug resistance will be critical in informing the optimal therapy of ALK+NSCLC in the future.
Keywords: ALK, Lung cancer, crizotinib
Introduction
The Anaplastic Lymphoma Kinase (ALK) gene was first identified in its aberrant, disease-causing form. A reciprocal translocation between chromosomes 2 and 5 (t(2;5) (p23;q35)) had been noted in a subset of anaplastic large cell lymphomas (ALCL). 1 Later this was proven to generate a fusion gene combining the 5′ end of nucleophosmin (NPM) with the 3′ kinase encoding region of a novel gene, subsequently named ALK. 2 However, it is the potential of aberrant ALK to act as an oncogene in many different cancers, especially non-small cell lung cancer (NSCLC) that has heightened interest in ALK as a therapeutic target (Box 1). 3 In 2011, on the basis of dramatic and prolonged objective responses, the FDA approved crizotinib as the first licensed ALK inhibitor for ALK positive (ALK+) NSCLC, effectively defining the relevance of a disease subtype in lung cancer by its response to a targeted therapy. 4,5 Despite the early successes of crizotinib, it is clear that many challenges still lie ahead for those who treat, or are affected by, ALK+ NSCLC. This review will examine both what we already know and the major emerging questions associated with optimal management of this disease.
Box 1: Activation of ALK as an oncogene in NSCLC and other cancers.
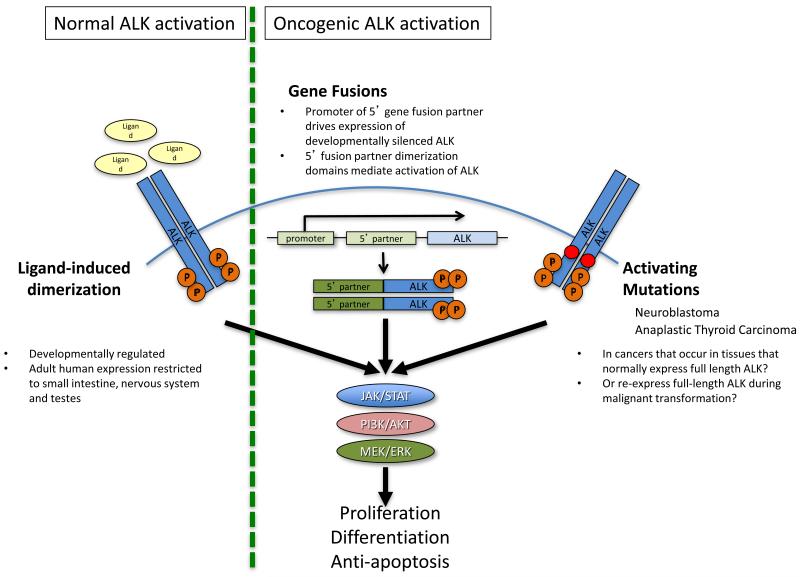
Native ALK is a tyrosine kinase primarily involved in developmental processes. It is only expressed at low levels in adults, in the gut, testis and nervous system, where it’s normal function remains under investigation. 20 ALK activation has been described in a number of different cancers including subsets of non-Hodgkins lymphoma, inflammatory myofibroblastic tumors, rhabdomyosarcomas, NSCLC, renal cancer, anaplastic thyroid cancers and neuroblastomas. 15-20,95,96 In cancers arising from tissues in in which full-length ALK is normally expressed, or, conceivably, where it becomes re-expressed during malignant dedifferentiation oncogenic activation may involve point mutations in the ALK kinase domain (e.g. neuroblastoma and anaplastic thyroid cancer). 15-19 In most tumors, however, including NSCLC, in order for ALK to function as an oncogenic driver, gene fusions that can induce both de novo ALK expression and activation are required. Multiple different 5′ fusion partners for oncogenic ALK exist in different cancers that all share the following characteristics: a) a promoter region that is functional in the tissue of interest and b) an encoded region capable of mediating oligomerization of the fusion protein, activating the ALK kinase domain in a manner comparable to the ligand-mediated oligomerization of native ALK. 20 In addition, there are presumably key structural constraints that must be met in both the 5′ partner and the specific breakpoint selected in the gene for the resulting fusion protein to be stable. While tissue specific expression of the 5′ partner could influence the ‘preferred fusion partnerings’ seen in some ALK+ malignancies, in many cases the 5′ partners represent very widely expressed proteins. Therefore, why EML4 is the dominant fusion partner in NSCLC, and not, for example, clathrin heavy chain (CLTC, a ubiquitously expressed endocytic scaffold protein) or any of several other very commonly expressed genes that have been reported as 5′ partners in other ALK+ malignancies, remains unknown. 78,79
The biology of native ALK
ALK is located on chromosome 2 and encodes a transmembrane receptor tyrosine kinase in the insulin receptor superfamily, with homology to the leukocyte tyrosine kinase. 6,7 Expression of native ALK in adult human tissues appears restricted to the small intestine, testis and nervous system. 2 In mice, expression in aspects of the embryonic and neonatal central and peripheral nervous systems suggests a role in neurological development. 6-8 In Drosophila, ALK has a proven role in the development of both the visual system and in visceral muscle patterning. 9-13 As with many other transmembrane tyrosine kinases, dimerization is thought to mediate the normal activation of the receptor. 14
Activating ALK in NSCLC
Although primary activating mutations in ALK do occur, in most ALK+ cancers including NSCLC activation of ALK is through the formation of fusion genes (Box 1, Figure 1). 15-20 Multiple 5′ fusion partners for ALK occur in different ALK+ cancers. 20 In NSCLC, the dominant 5′ fusion partner is EML4, but other rarer partners, notably KIF5B and TFG have been described. 3,21,22 All 5′ fusion partners in oncogenic ALK rearrangements share certain key characteristics (Box 1). The 5′ partner and its associated promoter are known to influence both the expression levels and sometimes the sub-cellular location of the fusion protein. 23,24 Whether such variability introduces significant biological differences in terms of the downstream consequences of ALK activation is currently unclear.
Figure 1. ALK activation mechanisms.
Activation of ALK signaling is rare in most adult tissues but is active in the development of the gut and nervous system. Activation of native ALK is via ligand-induced dimerization and resultant autophosphorylation. In drosophila the ligand for ALK is Jelly Belly, whereas in mammals pleiotrophin and midkine have been reported as ligands for ALK. 11,12,86,87 In most ALK+ cancers, ALK expression is re-instituted through the active promoter of a 5′ partner that fuses with the kinase-encoding region of ALK. The resulting fusion gene then generates a fusion protein that can dimerize via domains in the 5′ partner mimicking ligand induced activation. 14 Rarely, mutations in the kinase domain of full length ALK can also promote primary oncogenic activation of ALK (Box 1). ALK phosphorylation results in activation of downstream signaling pathways including JAK/STAT, PI3K/AKT, and MEK/ERK, which can promote cell proliferation, differentiation, and provide anti-apoptotic signals. 88
The natural history of ALK positive NSCLC
ALK rearrangements in NSCLC are more common among those with adenocarcinoma histology, in never smokers and in those who are known to be wildtype for EGFR and KRAS (Box 2). 25 However, multiple ALK+ NSCLC cases that do not fit this clinical stereotype also exist. 26,27 Several different histological patterns such as signet-ring histology have been reported in association with ALK positivity but these are not specific. 28-30
Box 2: Features associated with ALK+ NSCLC*.
Median age of onset in 5th or 6th decade, but with a broad age range extending from 2nd to 8th decade in adults 26
Adenocarcinoma histology (especially with signet ring histology) 26
Never/light smoking status 26
Excess of hepatic metastases, pleural and pericardial effusions at presentation with advanced disease 41
Prolonged progression free survival and high response rate to pemetrexed 39,40
ALK rearrangements may originate in a ‘near-diploid’ state, before significant chromosomal aneusomy occurs 84
*Exceptions to all of the listed clinical features occur
Given the lack of association with smoking, tobacco-related carcinogens are unlikely to underlie the etiology of most ALK+ lung cancers. While a number of general risk factors for non-smoking related lung cancer have previously been identified, including radon gas, no molecularly categorized epidemiological studies have been undertaken to explore the role of such factors specifically in ALK+ disease. 31
The impact of ALK positivity on prognosis has been explored in resection series, where both better and worse survival outcomes compared to ALK negative groups have been reported. 32-35 In the advanced disease setting, if anything ALK positivity appears to be associated with a neutral or slightly better prognosis than EGFR/ALK wildtype control groups. 36-38 However, although crizotinib naïve, high proportions of patients received pemetrexed within these studies (52-67%), a cytotoxic that has since been recognized to be particularly active in ALK+ NSCLC (see below). Consequently, we may never know the true prognostic value of ALK positivity in the absence of any key treatment in this setting.
The time to progression of advanced ALK+ NSCLC with most platinum-based first-line combination therapy appears similar to both EGFR mutant and EGFR/ALK wildtype patients. 29 Time to progression and response to erlotinib are comparable to EGFR/ALK wildtype patients but significantly lower than in EGFR mutant patients. 29 In contrast, pemetrexed, a multi-targeted anti-folate chemotherapy, appears to show exaggerated activity in ALK+ NSCLC. Among NSCLC patients treated with pemetrexed in different lines of therapy and in both mono- and combination therapy regimens, the median progression free survival (PFS) in ALK+ patients was 9 months (range: 1.5-21 months), compared to 4 months (range: 1-12.5 months) in an EGFR/ALK/KRAS wildtype control group. 39 Within a multivariate analysis adjusting for line of therapy, mono- vs. platinum and non-platinum combination therapy, age, sex, histology and smoking status, only ALK positivity was associated with a lower risk of progression on pemetrexed (HR = 0.36 (95% CI: 0.17-0.73, p=0.0051). 39 In addition, ALK positivity appears to be associated with a significantly higher response rate to pemetrexed (46.7%) than that seen in EGFR mutant or a double negative control group (4.7 and 16.7%, respectively; p=0.001). 40
Two initial hypotheses have been raised to explain ALK+ NSCLC’s apparent ‘super-sensitivity’ to pemetrexed. Firstly, that ALK positivity is associated with lower levels of thymidylate synthase, one of the targets of pemetrexed. 40 Secondly, that ATIC (5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/inosine monophosphate cyclohydrolase), a bifunctional enzyme that catalyzes the last two steps of purine biosynthesis may be both a substrate for ALK-mediated phosphorylation, increasing the cells dependence upon this pathway, and a direct target for pemetrexed. 39 Whether this apparent activity will affect the outcomes of the ongoing confirmatory randomized studies in advanced ALK+ NSCLC comparing crizotinib to either pemetrexed or docetaxel in the second line setting (PROFILE 1007; www.clinicaltrials.gov identifier: NCT00932893), or to a platinum-pemetrexed doublet in the first line setting (PROFILE 1014; www.clinicaltrials.gov identifier: NCT01154140) remains to be seen. Additional studies will also be required to determine if pemetrexed should now be prioritized as a cytotoxic in metastatic ALK+ NSCLC in the absence of access to crizotinib, after failure of crizotinib, and/or in the locally advanced, adjuvant or neoadjuvant setting, either with or without concurrent radiation therapy.
ALK positivity seems to be associated with a particular pattern of metastatic spread. At the time of diagnosis of metastatic disease, ALK+ patients, but not those with EGFR or KRAS mutations were associated with a higher incidence of pericardial (Odds ratio 4.61, p=0.02) and pleural spread (Odds ratio 4.8, p<0.001) than a triple negative control group. 41 The incidence of hepatic disease in ALK+ and EGFR mutant patients was also significantly higher than in the control group (Odds ratio 5.5, p=0.003). 41
Early successes: Crizotinib’s activity in single arm studies
Crizotinib (PF-02341066, Xalkori®) is a small molecule, orally bioavailable tyrosine kinase inhibitor (TKI) of both ALK and MET. 42 Within the first-in-man study of crizotinib, a series of molecularly defined expanded cohorts were recruited at the recommended phase II dose of 250mg BID in patients whose tumors were prescreened for evidence of either ALK or MET activation. 4 In addition to its activity in ALK positive NSCLC, clinical benefit of crizotinib has also been documented in both ALK positive lymphomas and sarcomas and in MET positive NSCLC and esophageal carcinomas. 90-93 ALK positivity within all of the initial studies was determined using break-apart FISH technology (Box 2). In the most recent NSCLC dataset from the phase I study, covering 119 patients of whom 116 were assessable for response, the objective response rate was 61%. 43 The high response rate appeared largely independent of age, sex, performance status or line of therapy. Median PFS was 10 months and although median overall survival data are not yet mature, the estimated overall survival rates at 6 and 12 months were 90% and 81%, respectively. 43
Very similar results were obtained from the 136 ALK positive patients treated within the subsequent PROFILE 1005 single arm phase II study. 5 Within this study there was also evidence from patient reported outcomes of significant and sustained symptomatic benefit in pain, dyspnea, cough and fatigue after 6 weeks of therapy. 5 Although crizotinib received accelerated approval in the US in August 2011, this approval is conditional upon the results of ongoing randomized studies comparing crizotinib to standard chemotherapies (PROFILE 1014 and 1007). In both of these studies, patients randomized to the chemotherapy arm will have the option of crossing over to crizotinib upon progression. Consequently, the primary endpoint for both trials is PFS and not overall survival (OS). A prospective study that could formally address the effect of crizotinib on OS in advanced ALK+ NSCLC is unlikely to occur. However, when crizotinib treated patients were retrospectively compared with matched crizotinib naïve controls, the one and two-year survival rates significantly favored the crizotinib treated group (70% vs. 44% and 55% vs. 12%, respectively, p=0.004). 36
Early successes: Crizotinib’s tolerability in single arm studies
Although up to 96% of ALK positive NSCLC patients in the early studies of crizotinib reported treatment-related adverse events, the majority of these were only grade 1 or 2 in severity (Table 1). 4,5,43 Given the role of ALK in the development of the visual system and gut, it is tempting to speculate that several of the common side effects reflect direct anti-ALK effects on the native protein. Peripheral edema may be a notable exception, having also been described in association with MET inhibition. 44 The visual disturbances associated with crizotinib usually commence within days of starting the drug and involve brief light trails, flashes or image persistence occurring at the edges of the visual field. Most commonly, these occur in association with changes in lighting. Studies in rats have demonstrated that crizotinib causes significant reductions in the rate of retinal dark adaptation, but not the ability to achieve full dark adaptation, offering a partial explanation for these clinical findings. 45 Severe side effects are rare (Table 1). 4,5,43 Drug holidays, followed by rechallenge at a lower dose have been reported to allow ongoing treatment in some cases of severe neutropenia or transaminitis, but permanent drug discontinuation is occasionally required. 4,43
Table 1. Common, severe and/or characteristic side-effects attributed to crizotinib 4,5,43,46,47,85.
| Common treatment related adverse events (all grades) occurring in ≥10% of patients | All Grades |
|---|---|
|
| |
| Vision Disorder (usually involving brief light trails, flashes or image persistence occurring at the edges of the visual field. Most commonly, these occur in association with light adaptation) | 62% |
| Gastrointestinal Disorders | |
| Nausea | 53% |
| Diarrhea | 43% |
| Vomiting | 40% |
| Constipation | 27% |
| Decreased Appetite | 19% |
| Esophageal Disorders | 11% |
| General Disorders | |
| Edema | 28% |
| Fatigue | 20% |
| Other | |
| Dizziness | 16% |
| Neuropathy | 13% |
| Dysgeusia | 12% |
| Rash | 10% |
| Investigations | |
| Alanine Aminotransferase Increased | 13% |
|
| |
| Severe (grade 3 or 4) treatment related adverse events (all <1%-5% of cases) | |
|
| |
| Stomatitis | |
| Constipation | |
| Fatigue | |
| Dyspnea | |
| Neuropathy | |
| Pneumonitis | |
| Aspartate Aminotransferase Increased | |
| Alanine Aminotransferase Increased | |
| Neutropenia | |
| Lymphopenia | |
| Hypophosphatemia | |
|
| |
| Additional recently described common and/or characteristic side effects | |
|
| |
| Renal cysts (rare) | |
| Asymptomatic bradycardia (frequency unknown) | |
| Rapid onset low testosterone in men (common) | |
Rapid onset hypogonadism in the majority of male patients taking crizotinib has now been reported, suggesting that serum testosterone levels should be routinely checked and replaced as appropriate on therapy. 46 Cases of crizotinib-induced asymptomatic profound bradycardia have also recently been described, the clinical significance of which remains uncertain. 47
Coming challenges: The need for newer ALK directed therapies - pharmacology, tolerability and resistance
Given the early successes, in terms of both activity and tolerability, of crizotinib in ALK+ NSCLC, it may seem strange to even consider the need for newer ALK directed therapies in this disease. However, several opportunities present themselves as suitable for improvement. For example, despite crizotinib having a half-life of over 50 hours, it is dosed twice a day primarily to abrogate Cmax related side-effects within the phase I study. 4, 83 Consequently, re-exploring crizotinib or exploring another ALK inhibitor as a once a day regimen may be attractive to many patients. Although crizotinib appears to be relatively well tolerated, it is not yet clear which of crizotinib’s side-effects reflect activity against native ALK as opposed to other molecular targets (Table 1). Therefore newer ALK inhibitors with narrower, or at least different, spectra of activity, may offer different and in some cases more desirable side-effect profiles. However, the dominant reason to consider newer ALK-directed therapies is to address the challenge of intrinsic and acquired resistance to crizotinib in ALK+ NSCLC.
Coming challenges: Resistance to crizotinib in the central nervous system (CNS)
Despite the dramatic activity of crizotinib in ALK+ NSCLC, the disease will eventually progress in all cases. While on many occasions resistance to crizotinib will be manifested as systemic progression, in some situations CNS only progression occurs. 43 Accurate details on the exact proportion of times this occurs are lacking as standardized baseline and surveillance scans of the CNS were not part of any of the crizotinib trials conducted to date. Whether CNS progression represents the chance location of a change in the dominant biology of ALK+ NSCLC and/or unaffected growth secondary to relative under-exposure to the drug in the CNS is uncertain. Data do exist to support a pharmacokinetic explanation in some cases. In one patient with CNS progression, CSF drug levels were noted to be <0.3% of those seen in the blood, levels predicted to be too low to be effective against any ALK fusion proteins present. 48
Consequently, further exploration of the potential for CNS progression to reflect a condition when local CNS treatment, e.g. radiation therapy, should be combined with ongoing use of the crizotinib to continue to suppress systemic disease is warranted. In addition, the potential for intrathecal delivery of crizotinib and formal assessment of meaningful CNS exposure with newer ALK-directed therapies should be considered as a means of assessing this potentially distinct mechanism of ‘acquired’ resistance to crizotinib.
Coming challenges: Resistance to crizotinib systemically
Biological drug resistance, whether acquired or intrinsic, poses a significant challenge to oncogene-targeted therapy. Based on the previous paradigm of EGFR mutant NSCLC, kinase domain mutations were expected to provide a mechanism of resistance to crizotinib in ALK+ NSCLC. In fact, the first published report of crizotinib’s clinical activity was accompanied by a case report of acquired resistance secondary to two new kinase domain mutations, L1196M and C1156Y, each occurring in different clones from the same patient. 4,49 The L1196M substitution occurs at the gatekeeper position, homologous to the T790M in EGFR or the T315I substitutions in BCR-ABL. 50,51 Five additional ALK kinase domain mutations (L1152R, G1269A, S1206Y, G1202R and 1151Tins) have since been reported from NSCLC patients with crizotinib resistance, with several others, including D1203N and F1174C, already identified (Doebele, unpublished data). 52,53,94 Multiple other mutations that confer crizotinib resistance can be generated through in vitro mutagenesis screens on EML4-ALK positive cell lines. 54,55 Of note, one of these, F1174L, has now been identified clinically in a patient with ALK+ inflammatory myofibroblastic tumor (IMT), harboring a RANBP2-ALK translocation who developed crizotinib resistance. 56 F1174L is also a known activating mutation of full length ALK in neuroblastoma. 17,18 Increases in our understanding of crizotinib resistance mechanisms in ALK+ disease are therefore likely to be important across histologically diverse tumors.
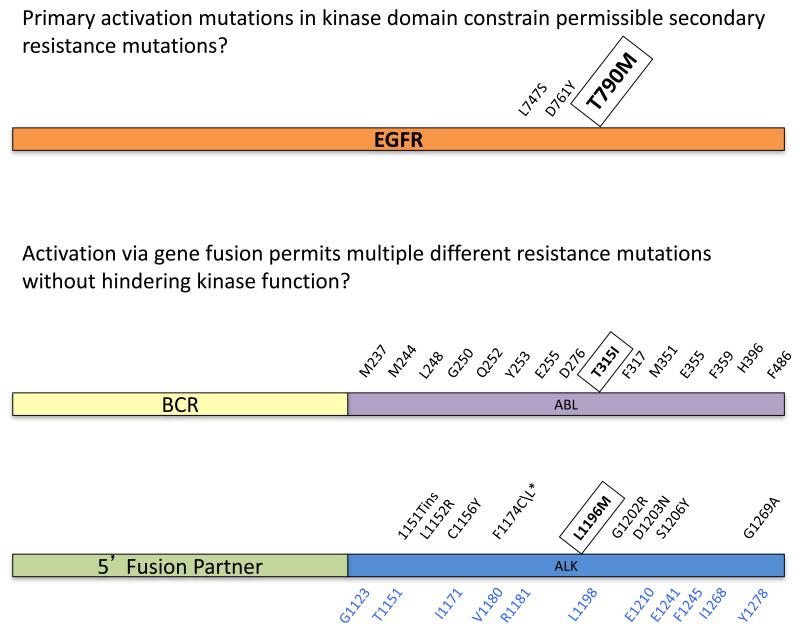
Collectively, the available clinical and preclinical data suggest multiple different kinase mutations occurring at comparable frequencies may generate crizotinib resistance. This stands in contrast to the EGFR paradigm where T790M predominates and other EGFR TKI resistance mutations occur only rarely. 57,58,59 Crizotinib resistance mutations appear more analogous to the broad spectrum of mutations observed in BCR-ABL following treatment with imatinib. 60 Potentially, the ability of the EGFR to tolerate additional mutations in its kinase domain may be constrained by the presence of the common activating mutations. 61 As EML4-ALK, like BCR-ABL, is not activated via mutations, the kinase domain may be less constrained and able to tolerate multiple different new mutations that impair crizotinib binding without significantly impacting the kinase’s underlying activity (Figure 2).
Figure 2. Resistance mutation spectra in EGFR mutant, ALK+ and BCR-ABL+ cancers following treatment with relevant kinase inhibitors.
The spectra of mutations found in EGFR mutation positive NSCLC, ALK+ cancers, and BCR-ABL+ chronic myeloid leukemia are shown. We hypothesize that activating mutations such as those found in EGFR constrain the resistance mutations to those that allow constitutive activation. In situations where activation of the kinase is driven by dimerization via a gene fusion partner (e.g., BCR-ABL and EML4-ALK), the kinase domain is less constrained and can accommodate a wider array of mutations. The gatekeeper mutation, which sits at a critical position in the ATP/kinase inhibitor pocket, in each oncogene is enclosed with a box. The predominant resistance mutation in EGFR following therapy with erlotinib or gefitinib is T790M (shown in larger font) with only rare reports of other resistance mutations occurring. 57-59 The most common resistance mutations in BCR-ABL are shown. 89 T315I, the most frequent of these, only occurs <15% of the time. The true prevalence of the different ALK mutations that produce resistance to crizotinib in patients is still unknown but a single dominant form comparable to the status of T790M does not seem apparent among the patients studied to date. 49,53,56,94 Almost all of the resistance mutations found in patients have also been identified using in vitro screens. 54,55 Additional resistant mutations identified through these screens but not yet identified in patients are shown in blue. The asterisk denotes a mutation, F1174L, that was found in an IMT tumor with the RANB2-ALK gene fusion treated with crizotinib; all other mutations were found in ALK+ NSCLC patients. 56
In support of this, several groups have demonstrated that ALK gene rearrangements with resistance mutations enhance the growth of cells compared to unmutated ALK rearrangements. 53,56 In contrast, a secondary T790M mutation reduces the growth of EGFR mutant cells. 62 This could have important clinical implications for ALK+ patients. Whereas the less ‘fit’ T790M clones may be competed out by re-emerging sensitive subclones in the absence of an EGFR TKI, crizotinib resistant ALK mutated clones may not regress so easily in the absence of an ALK TKI. 63
The different ALK mutations identified thus far show variability in their sensitivity to crizotinib and to other ALK inhibitors suggesting that a single next generation ALK inhibitor may not be able to effectively inhibit the entire spectrum of resistance mutations. 54,55,94 Complicating this strategy further is the possibility of intrapatient heterogeneity in terms of different resistance mutations. 49 With BCR-ABL up to a ten different resistance mutations have been detected in a single patient using mass spectrometry following progression on imatinib and with comparable analyses similar complexity may emerge in ALK+ disease after crizotinib therapy (Figure 2). 64 With differences in CNS penetration of some newer ALK-directed drugs we may even find that achievable exposures will suppress some mutations, but allow others to grow in different parts of the body with the exact pattern varying depending on the drug involved. Copy number gain (CNG) of the ALK gene fusion, either alone or in combination with a kinase domain mutation, has also been observed both in vitro and in patients as an additional mechanism of crizotinib resistance. 53,65,94
Approximately 13% of patients with an ALK gene fusion demonstrate the presence of an additional mutated oncogene, specifically EGFR, KRAS, BRAF or MET, in their pre-crizotinib sample. 66 Potentially, selection of these or other alternate means of oncogene activation could provide a mechanism of resistance to ALK inhibitors distinct from ALK kinase domain mutations and ALK CNG. Of note, MET is unlikely to function as a driver of resistance to crizotinib as it is a target of the same drug, however it could act as a driver of resistance for other non-MET directed ALK inhibitors. In DFC1032, a cell line derived from an ALK+, treatment naïve patient, both an ALK gene rearrangement and activation of EGFR and HER2 were noted. 67 In another cell line, derived from a patient with acquired resistance to crizotinib (DFC1076) both an L1152R resistance mutation in ALK and enhanced baseline EGFR and MET phosphorylation were noted. 52 No HER family CNG or mutations were detected and ligand-mediated activation of a second driver pathway was therefore proposed as contributing to crizotinib resistance in both cases. In both cell lines growth inhibition by the combination of crizotinib and an irreversible inhibitor of EGFR and HER2 was more effective than either treatment alone. More recently, 3 of 11 ALK+ patients with resistance to crizotinib were shown to have a detectable EGFR mutation or a KRAS mutation in their post-crizotinib specimen. 53 Unlike with cell line data, the presence of different oncogenes in the same/subsequent biopsies could represent either direct mechanisms of resistance occurring within the same cells (second drivers), or the emergence of independent/divergent clones with separate drivers (Figure 3). Certainly, introduction of a mutated EGFR cDNA can induce crizotinib resistance in an ALK+ cell line, consistent with the possibility of an EGFR mutation acting as a second driver. 52 In contrast, expression of a mutant KRAS cDNA into an ALK+ cell line does not. 53 An explanation for this finding may lie in previous work demonstrating that activated KRAS does not always behave as a classical driver oncogene and its role in oncogenesis may be highly contextual. 97 In addition, in a cell line derived from an ALK+ patient at the time of progression on crizotinib only a KRAS mutation was detectable without evidence of a persisting ALK gene rearrangement, suggesting that two separate clonal populations each with a separate driver co-existed in the same patient. Case reports of patients demonstrating both ALK positivity and an EGFR mutation who have responded to an EGFR TKI alone, rather than requiring a combination of drugs, also suggest separate drug sensitive clonal populations can co-exist in ALK+ patients. 68 Recently, amplification of the KIT gene has been reported as an additional alternate oncogene that may drive resistance to crizotinib in selected cases. 94 KIT amplification (defined as a ratio of the KIT gene to centromere 4 of >5) was noted in 2 of 18 cases of acquired resistance to crizotinib (1 focally and 1 diffusely within the tumor) with accompanying preclinical evidence of the potential for KIT CNG to drive crizotinib resistance as a second driver in the presence of its cognate ligand stem cell factor (SCF). 94
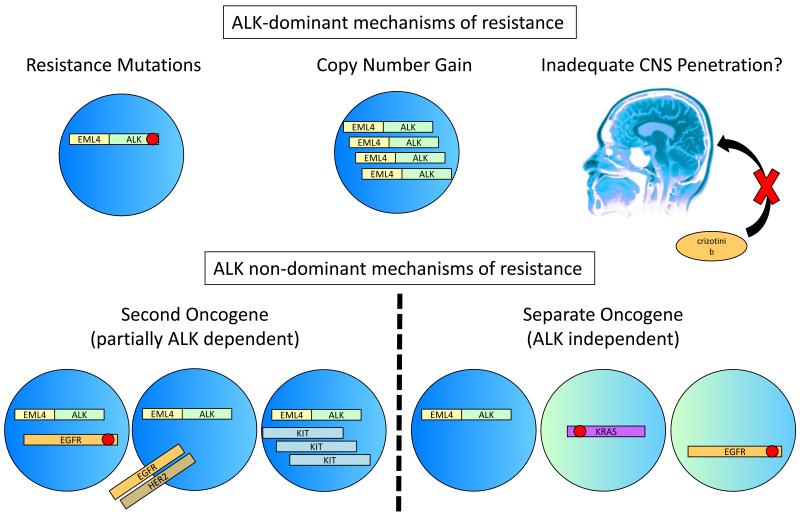
Figure 3. Mechanisms of resistance to crizotinib in ALK+ NSCLC.
Crizotinib resistance can be classified into two broad categories, those mechanisms that retain the dominance of ALK signaling and those that lose the dominance of ALK signaling either partially or completely. ALK-dominant resistance can occur through kinase domain mutations that inhibit crizotinib binding but permit ongoing constitutive activation of ALK. 49,53,56 ALK dominant resistance also occurs through copy number gain of the ALK gene fusion, which may co-exist with resistance mutations. 53, 65 Finally, poor penetration of crizotinib into the CNS may simply allow unaltered ALK+ cancer cells to grow because of inadequate local drug exposures. 48 For resistance that degrades the dominance of ALK signaling, a second oncogene may become active via mutation or another mechanism co-existing with oncogenic ALK in the same cells. 52,53,65,94 Alternatively, true ALK independent resistance may arise through the outgrowth of clones that do not harbor an ALK gene fusion and contain a different activated oncogene (separate oncogene). 53
From the resistance data collected this far it appears that systemic resistance to crizotinib in ALK+ NSCLC can be parsed into two groups, those that are still dominated by ALK signaling (kinase mutation and CNG) and those that are only partially dependent on, or independent of, ALK signaling (presence of a second or separate oncogene driver) (Figure 3). Based on one of the largest series to date, the sizes of these two groups may be approximately equal. 53 However, given that clinical and preclinical examples positive for both kinase domain mutations and evidence of second or separate drivers have now been described, it is important to recognize that multiple different resistance mechanisms may be generated and co-exist at different levels in the same crizotinib-resistant patient. 52,94 Next generation ALK inhibitors alone, unless they also target other relevant kinases, may overcome some resistance mutations or CNG through higher exposures and/or higher potency, but are unlikely to overcome resistance acquired through second or separate drivers. 53,54 In such cases, combination therapy may be required. 52,67 However, while the technology for detecting mutations or CNG is well established, identifying those who require specific combinations based on increased signaling of an alternate oncogene alone, as in the DFC1032/1076 cell line data, is not yet feasible for routine clinical samples. For example, in a recent study looking at phospho-EGFR levels by IHC in paired pre- and post-acquired resistance to crizotinib samples, although the study reported 4 of 9 cases with subjectively increased staining as evidence of EGFR pathway activation as a potential mechanism of resistance, levels remained the same in 3 cases and went down in two cases. 94 Although intriguing and consistent with preclinical evidence, several factors including standardization of sample fixation, preparation and IHC quantitation might influence changes in phoshpo-EGFR levels given the well-known lability of phospho-epitopes. 94 Two other pharmacological classes of agent are being actively explored for their activity in crizotinib-resistant ALK+ NSCLC: pemetrexed and HSP90 inhibitors. However, just as with the new ALK inhibitors, recognizing that different biological mechanisms of crizotinib resistance exist is likely to have major impact on the development of these agents in this setting.
Currently, the activity of pemetrexed has been demonstrated in ALK+ crizotinib naïve patients, but not in crizotinib resistant patients. 39,40 It is also not yet clear whether ALK positivity is simply associated with pemetrexed sensitivity, or whether ALK signaling per se drives that sensitivity. If it is the latter, then mechanisms of resistance that preserve aspects of ALK signaling might retain marked sensitivity to pemetrexed, while if resistance is through the emergence of ALK negative clones pemetrexed may be less effective.
An alternate strategy for overcoming ALK resistance is to target the chaperone pathway. HSP90 is a chaperonin that stabilizes proteins during their maturation within the cell. 69 Multiple different oncogenic proteins have been described as potential clients for HSP90, however not all of them are equally HSP90 dependent. ALK fusion proteins were first described as highly sensitive to HSP90 inhibition in preclinical models of NPM-ALK. 70 Subsequently, EML4-ALK was also shown to be highly sensitive to HSP90 inhibition both in vitro and in vivo in crizotinib naïve patients. 71-73 Unfortunately, with regard to their activity in crizotinib resistant disease, two such patients have already been noted to progress through HSP90 inhibitor therapy without evidence of benefit. 73 While HSP90 inhibitors have been shown to have activity across EML4-ALK+ cell lines harboring a range of different crizotinib resistance mutations, suggesting their clinical spectrum of activity may be broader than some specific ALK inhibitors in this context, notably the mechanism of crizotinib resistance had not been explored in either of the two patients described. 65,94 Similar to pemetrexed, it is likely that different mechanisms of resistance to crizotinib will result in retained or reduced sensitivity to HSP90 inhibitors compared to a crizotinib-naïve ALK+ population.
Whether effective levels of pemetrexed or HSP90 inhibitors will penetrate the CNS with regard to ALK+ brain metastases is unknown. Similarly, what biological mechanisms and how easily they will occur as a means of generating acquired resistance to these different classes of drugs in the post-crizotinib setting is also currently unknown. 74
Coming challenges: Ascribing value to local therapy for ‘oligo-progressive’ disease plus treatment with crizotinib beyond progression
Even in situations where the mechanism of resistance is unknown or without an obvious drug-based therapy to direct towards it, resistant disease may sometimes be amenable to local therapy, such as stereotactic body radiation therapy. The logic behind this approach is that if resistance arises clonally, in conjunction with close CT or PET/CT observation, isolated areas of growth may be identified and deleted prior to more widespread dissemination of the resistant disease. 75-77 Within the Phase I study of crizotinib, thirty-seven patients continued to receive crizotinib for >2 weeks post-progression at the investigator’s discretion, 11 for more than 6 months from the time of their initial protocol-defined progression. 43 Although no formal details are available, in many cases this continuation likely occurred after areas of localized progression in both the body and/or CNS were treated with radiotherapy. How we formalize the definition of ‘ongoing clinical benefit’ to determine the true utility of attempted clonal deletion and continuing crizotinib in this setting will be yet another challenge in the future for determining the optimal management of ALK+ disease.
Conclusions
ALK+ disease has emerged as a relevant clinical subtype of NSCLC based on its dramatic and prolonged benefit from crizotinib. With increased experience, key features associated with the disease are now being revealed (Box 2). The greatest challenges associated with optimally treating this disease in the future will include identifying and managing the side effects associated with therapy (Table 1), determining which of these side effects are likely to be class specific in the face of new ALK directed treatments being developed and addressing the multiple different ways in which ALK+ disease may become resistance to crizotinib (Figure 3).
Box 3: Finding ALK positive patients.
ALK rearrangements are found in approximately 3-7% of NSCLC, in series dominated by adenocarcinoma histology. 26 Within the initial crizotinib studies, all patients were proven to be ALK+ using the Vysis break apart FISH probe set and it was this technology that was filed as a companion diagnostic with the FDA. 4,5 In break-apart testing, the FISH probes flank the common breakpoint in ALK and separate when a rearrangement occurs. 25 However, several other techniques, notably immunohistochemistry (IHC) and reverse transcriptase polymerase chain reaction (RT-PCR), are being developed. Each has both pros and cons. 80,81 Whether physicians or payers in the USA and in other countries will restrict prescribing only to those proven to be ALK+ using a specific methodology, or whether prescribing in cases proven to be ALK+ by any technique will occur remains to be seen. In addition, as ALK positivity only occurs in a small proportion of NSCLC and testing resources may be limited, various screening strategies to determine who to test have also been proposed. 26 These range from clinical enrichment using the factors associated with ALK positivity listed in Box 2, to tiered molecular testing. 25, 82 In tiered testing, the commonest molecular abnormality in NSCLC is assessed first and then, if negative, the next most common marker is assessed, etc until a positive result is achieved. Much of the basis for adopting or not adopting any given technique will be its sensitivity and specificity, its reproducibility over time and between centers and its cost when optimized for performance and reproducibility. Much of the basis for adopting any given screening strategy will be in balancing a) the cost savings in terms of reducing the absolute numbers of patients screened and the cost per positive within an enriched population, and b) the number of true positives missed by any pre-selection approach. 27
Keypoints.
Crizotinib shows significant benefit in terms of both radiographic response and PFS in ALK+ NSCLC.
Crizotinib is generally well tolerated, but is associated with characteristic common mild (e.g. gastrointestinal disturbance, visual changes and low testosterone) and rare serious side effects (e.g. transaminitis).
Crizotinib-resistance in ALK+ NSCLC occurs through multiple different biological mechanisms including kinase domain mutations and copy number gain in the rearranged gene that preserve ALK dominance, and the emergence of ‘second’ or ‘separate’ oncogenic drivers which may weaken or negate ALK dominance, respectively.
In addition to a change in the biology of the cancer, cases of isolated CNS progression on crizotinib could reflect inadequate CNS penetration of the drug.
Newer ALK inhibitors, HSP90 inhibitors and pemetrexed have all shown preliminary clinical or preclinical activity in ALK+ NSCLC and are being investigated further in crizotinib resistant and crizotinib naive ALK+ disease.
The potential of these new agents to have activity on CNS disease and on crizotinib resistant cases manifested through different biological mechanisms will be critical in determining their future roles in the management of ALK+ NSCLC.
Acknowledgements
None
Funding: None
Biographies
Author biography
D. Ross Camidge, MD PhD:
Dr Ross Camidge gained his PhD in molecular biology in 1992 from Cambridge University and his MD in 1995 from Oxford University. Dr Camidge then dual trained in both medical oncology and clinical pharmacology at Edinburgh University in the UK before being recruited to the University of Colorado in 2005. Currently, Dr Camidge is the Director of the Thoracic Oncology Clinical Program at the University of Colorado.
Robert C. Doebele, MD PhD:
Dr. Robert C. Doebele obtained his PhD in Immunology and MD from the University of Pennsylvania School of Medicine. He completed his Internal Medicine and Oncology training at the University of Chicago. Dr. Doebele, a Boettcher Investigator, leads a research laboratory studying lung cancer biology and mechanisms of sensitivity and resistance to oncogene-targeted therapy in lung cancer and is a medical oncologist specializing in thoracic malignancies.
Footnotes
Review criteria
The relevant articles were identified by the authors using PubMed (Searchterms: Anaplastic lymphoma kinase, ALK and lung) and on the basis of their knowledge of the biology of ALK+ disease and the clinical development of ALK inhibitors. The published data were inclusive of articles published from January 1994 through to January 2012, and abstracts from major conferences (including the Annual Meeting of ASCO and European Society of Medical Oncology from 2009 to 2011).
Disclosures:
DRC has participated in ad hoc advisory boards and received honoraria from Ariad, Chugai, Eli Lilly, Novartis and Pfizer.
RCD has received research funding from Eli Lilly and Pfizer and honoraria from Pfizer and Abbott Laboratories.
References
- 1.Le Beau MM, Bitter MA, Larson RA, Doane LA, Ellis ED, Franklin WA, Rubin CM, Kadin ME, Vardiman JW. The t(2;5)(p23;q35): a recurring chromosomal abnormality in Ki-1-positive anaplastic large cell lymphoma. Leukemia. 1989;3:866–70. [PubMed] [Google Scholar]
- 2.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–4. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 3.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 4.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. New England Journal of Medicine. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crinò L, Kim D, Riely GJ, Janne PA, Blackhall FH, Camidge DR, Hirsh V, Mok T, Solomon BJ, Park K, Gadgeel SM, Martins R, Han J, De Pas TM, Bottomley A, Polli A, Petersen J, Tassell VR, Shaw AT. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. 2011;29(suppl) 2011 ASCO Annual Meeting. Abstract 7514. abstr 7514. [Google Scholar]
- 6.Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997 Jan 30;14(4):439–49. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 7.Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, Witte DP. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK) Oncogene. 1997 May 8;14(18):2175–88. doi: 10.1038/sj.onc.1201062. Erratum in: Oncogene 1997 Dec 4;15(23):2883. [DOI] [PubMed] [Google Scholar]
- 8.Vernersson E, Khoo NK, Henriksson ML, Roos G, Palmer RH, Hallberg B. Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene Expr Patterns. 2006 Jun;6(5):448–61. doi: 10.1016/j.modgep.2005.11.006. Epub 2006 Feb 2. [DOI] [PubMed] [Google Scholar]
- 9.Lorén CE, Scully A, Grabbe C, Edeen PT, Thomas J, McKeown M, Hunter T, Palmer RH. Identification and characterization of DAlk: a novel Drosophila melanogaster RTK which drives ERK activation in vivo. Genes Cells. 2001 Jun;6(6):531–44. doi: 10.1046/j.1365-2443.2001.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorén CE, Englund C, Grabbe C, Hallberg B, Hunter T, Palmer RH. A crucial role for the Anaplastic lymphoma kinase receptor tyrosine kinase in gut development in Drosophila melanogaster. EMBO Rep. 2003 Aug;4(8):781–6. doi: 10.1038/sj.embor.embor897. Epub 2003 Jul 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HH, Norris A, Weiss JB, Frasch M. Jelly belly protein activates the receptor tyrosine kinase Alk to specify visceral muscle pioneers. Nature. 2003 Oct 2;425(6957):507–12. doi: 10.1038/nature01916. [DOI] [PubMed] [Google Scholar]
- 12.Englund C, Lorén CE, Grabbe C, Varshney GK, Deleuil F, Hallberg B, Palmer RH. Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature. 2003 Oct 2;425(6957):512–6. doi: 10.1038/nature01950. [DOI] [PubMed] [Google Scholar]
- 13.Bazigou E, Apitz H, Johansson J, Lorén CE, Hirst EM, Chen PL, Palmer RH, Salecker I. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. 2007 Mar 9;128(5):961–75. doi: 10.1016/j.cell.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Souttou B, Carvalho NB, Raulais D, Vigny M. Activation of anaplastic lymphoma kinase receptor tyrosine kinase induces neuronal differentiation through the mitogen-activated protein kinase pathway. J Biol Chem. 2001 Mar 23;276(12):9526–31. doi: 10.1074/jbc.M007333200. Epub 2000 Dec 19. [DOI] [PubMed] [Google Scholar]
- 15.Mossé YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P, Laureys G, Speleman F, Kim C, Hou C, Hakonarson H, Torkamani A, Schork NJ, Brodeur GM, Tonini GP, Rappaport E, Devoto M, Maris JM. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008 Oct 16;455(7215):930–5. doi: 10.1038/nature07261. Epub 2008 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janoueix-Lerosey I, Lequin D, Brugières L, Ribeiro A, de Pontual L, Combaret V, Raynal V, Puisieux A, Schleiermacher G, Pierron G, Valteau-Couanet D, Frebourg T, Michon J, Lyonnet S, Amiel J, Delattre O. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008 Oct 16;455(7215):967–70. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, Wang L, Soda M, Kikuchi A, Igarashi T, Nakagawara A, Hayashi Y, Mano H, Ogawa S. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008 Oct 16;455(7215):971–4. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 18.George RE, Sanda T, Hanna M, Fröhling S, Luther W, 2nd, Zhang J, Ahn Y, Zhou W, London WB, McGrady P, Xue L, Zozulya S, Gregor VE, Webb TR, Gray NS, Gilliland DG, Diller L, Greulich H, Morris SW, Meyerson M, Look AT. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008 Oct 16;455(7215):975–8. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murugan AK, Xing M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res. 2011 Jul 1;71(13):4403–11. doi: 10.1158/0008-5472.CAN-10-4041. Epub 2011 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb TR, Slavish J, George RE, Look AT, Xue L, Jiang Q, Cui X, Rentrop WB, Morris SW. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther. 2009 Mar;9(3):331–56. doi: 10.1586/14737140.9.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi K, Choi YL, Togashi Y, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15(9):3143–9. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 23.Gascoyne RD, Lamant L, Martin-Subero JI, Lestou VS, Harris NL, Müller-Hermelink HK, Seymour JF, Campbell LJ, Horsman DE, Auvigne I, Espinos E, Siebert R, Delsol G. ALK-positive diffuse large B-cell lymphoma is associated with Clathrin-ALK rearrangements: report of 6 cases. Blood. 2003 Oct 1;102(7):2568–73. doi: 10.1182/blood-2003-03-0786. Epub 2003 May 22. [DOI] [PubMed] [Google Scholar]
- 24.Ma Z, Hill DA, Collins MH, Morris SW, Sumegi J, Zhou M, Zuppan C, Bridge JA. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003 May;37(1):98–105. doi: 10.1002/gcc.10177. [DOI] [PubMed] [Google Scholar]
- 25.Camidge DR, Kono SA, Flacco A, et al. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res. 2010 Nov 15;16(22):5581–90. doi: 10.1158/1078-0432.CCR-10-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weickhardt AJ, Camidge DR. The therapeutic potential of anaplastic lymphoma kinase inhibitors in lung cancer: rationale and clinical evidence. Clin. Invest. 2011;1(8):1119–1126. [Google Scholar]
- 27.Atherly AJ, Camidge DR. The cost effectiveness of screening lung cancer patients for targeted drug sensitivity markers. British Journal of Cancer. doi: 10.1038/bjc.2012.60. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3(1):13–7. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 29.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodig SJ, Mino-Kenudson M, Dacic S, Yeap BY, Shaw A, Barletta JA, Stubbs H, Law K, Lindeman N, Mark E, Janne PA, Lynch T, Johnson BE, Iafrate AJ, Chirieac LR. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009 Aug 15;15(16):5216–23. doi: 10.1158/1078-0432.CCR-09-0802. Epub 2009 Aug 11. Erratum in: Clin Cancer Res. 2009 Nov 15;15(22):7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian J, Govindan R. Lung cancer in ‘Never-smokers’: a unique entity. Oncology (Williston Park) 2010 Jan;24(1):29–35. [PubMed] [Google Scholar]
- 32.Zhang X, Zhang S, Yang X, Yang J, Zhou Q, Yin L, An S, Lin J, Chen S, Xie Z, Zhu M, Zhang X, Wu YL. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer. 2010 Jul 13;9:188. doi: 10.1186/1476-4598-9-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varella-Garcia M, Cho Y, Lu X, Barón AE, Terracciano L, Camidge DR, Bunn PA, Franklin WA, Cappuzzo F, Doebele RC. ALK gene rearrangements in unselected Caucasians with non-small cell lung carcinoma (NSCLC); ASCO Proceedings; 2010; Abstract 10533. [Google Scholar]
- 34.Yang P, Kulig K, Boland JM, Erickson-Johnson MR, Oliveira AM, Wampfler J, Jatoi A, Deschamps C, Marks R, Fortner C, Stoddard S, Nichols F, Molina J, Aubry MC, Tang H, Yi ES. Worse Disease-Free Survival in Never-Smokers with ALK+ Lung Adenocarcinoma. J Thorac Oncol. 2012 Jan;7(1):90–97. doi: 10.1097/JTO.0b013e31823c5c32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon B, Shaw AT. Are Anaplastic Lymphoma Kinase Gene Rearrangements in Non-small Cell Lung Cancer Prognostic, Predictive, or Both? J Thorac Oncol. 2012 Jan;7(1):5–7. doi: 10.1097/JTO.0b013e31823f1289. [DOI] [PubMed] [Google Scholar]
- 36.Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, Shapiro GI, Costa DB, Ou SH, Butaney M, Salgia R, Maki RG, Varella-Garcia M, Doebele RC, Bang YJ, Kulig K, Selaru P, Tang Y, Wilner KD, Kwak EL, Clark JW, Iafrate AJ, Camidge DR. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncology. 2011 Sep 16; doi: 10.1016/S1470-2045(11)70232-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JK, Park HS, Kim DW, Kulig K, Kim TM, Lee SH, Jeon YK, Chung DH, Heo DS, Kim WH, Bang YJ. Comparative analyses of overall survival in patients with anaplastic lymphoma kinase-positive and matched wild-type advanced nonsmall cell lung cancer. Cancer. 2011 Nov 15; doi: 10.1002/cncr.26668. doi: 10.1002/cncr.26668. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Wu SG, Kuo YW, Chang YL, Shih JY, Chen YH, Tsai MF, Yu CJ, Yang CH, Yang PC. EML4-ALK Translocation Predicts Better Outcome in Lung Adenocarcinoma Patients with Wild-Type EGFR. J Thorac Oncol. 2012 Jan;7(1):98–104. doi: 10.1097/JTO.0b013e3182370e30. [DOI] [PubMed] [Google Scholar]
- 39.Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011;6(4):774–80. doi: 10.1097/JTO.0b013e31820cf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JO, Kim TM, Lee SH, et al. Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J Thorac Oncol. 2011;6(9):1474–80. doi: 10.1097/JTO.0b013e3182208fc2. [DOI] [PubMed] [Google Scholar]
- 41.Doebele RC, Lu X, Sumey C, Maxson DA, Weickhardt AJ, Oton AB, Bunn PA, Barón AE, Franklin WA, Aisner DL, Varella-Garcia M, Camidge DR. Oncogene Status Predicts Patterns of Metastatic Spread in Treatment-Naïve Non-Small Cell Lung Cancer. Cancer. 2012 Jan 26; doi: 10.1002/cncr.27409. doi: 10.1002/cncr.27409. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui JJ, Tran-Dubé M, Shen H, Nambu M, Kung PP, Pairish M, Jia L, Meng J, Funk L, Botrous I, McTigue M, Grodsky N, Ryan K, Padrique E, Alton G, Timofeevski S, Yamazaki S, Li Q, Zou H, Christensen J, Mroczkowski B, Bender S, Kania RS, Edwards MP. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK) J Med Chem. 2011 Sep 22;54(18):6342–63. doi: 10.1021/jm2007613. Epub 2011 Aug 18. [DOI] [PubMed] [Google Scholar]
- 43.Camidge DR, Bang Y, Kwak EL, et al. Progression-free survival from a phase I study of crizotinib (PF-02341066) in patients with ALK-positive non-small cell lung cancer; 2011 ASCO Annual Meeting; Abstract 2501. [Google Scholar]
- 44.Spigel DR, Ervin TJ, Ramlau R, et al. Final efficacy results from OAM4558g, a randomized phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC. J Clin Oncol. 2011;29(suppl) abstract 7505. [Google Scholar]
- 45.Matsumoto DC, Liu C-N, Somps C, Wilner K, Skillings J, Burns-Naas LA. Effect on retinal function as a mechanism for vision disorders with crizotinib (PF-02341066); Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; Orlando, Florida. 2011 Apr 2-6; Philadelphia (PA): AACR; 2011. Abstract 4385. [Google Scholar]
- 46.Weickhardt AJ, Rothman MS, Salian-Mehta S, Kiseljak-Vassiliades K, Oton AB, Doebele RC, Wierman ME, Camidge DR. Rapid Onset Hypogonadism Secondary To Crizotinib Use In Men With Metastatic Non-Small Cell Lung Cancer. Cancer. 2012 doi: 10.1002/cncr.27450. In press. [DOI] [PubMed] [Google Scholar]
- 47.Ou SH, Azada M, Dy J, Stiber JA. Asymptomatic profound sinus bradycardia (heart rate ≤45) in non-small cell lung cancer patients treated with crizotinib. J Thorac Oncol. 2011 Dec;6(12):2135–7. doi: 10.1097/JTO.0b013e3182307e06. [DOI] [PubMed] [Google Scholar]
- 48.Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, Wilner KD. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–5. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 49.Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–9. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 50.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A Novel ALK Secondary Mutation and EGFR Signaling Cause Resistance to ALK Kinase Inhibitors. Cancer Res. 2011;71:6051–60. doi: 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, Kondo KL, Linderman D, Heasley LE, Franklin WA, Varella-Garcia M, Camidge DR. Mechanisms of Resistance to Crizotinib in Patients with ALK Gene Rearranged Non-Small Cell Lung Cancer. Clinical Cancer Research. 2012 Jan 10; doi: 10.1158/1078-0432.CCR-11-2906. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S, Wang F, Keats J, Zhu X, Ning Y, Wardwell SD, et al. Crizotinib-Resistant Mutants of EML4-ALK Identified Through an Accelerated Mutagenesis Screen. Chem Biol Drug Des. 2011;78:999–1005. doi: 10.1111/j.1747-0285.2011.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heuckmann JM, Holzel M, Sos ML, Heynck S, Balke-Want H, Koker M, et al. ALK Mutations Conferring Differential Resistance to Structurally Diverse ALK Inhibitors. Clin Cancer Res. 2011;17:7394–401. doi: 10.1158/1078-0432.CCR-11-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasaki T, Okuda K, Zheng W, Butrynski J, Capelletti M, Wang L, et al. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res. 2010;70:10038–43. doi: 10.1158/0008-5472.CAN-10-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, Chiang A, Yang G, Ouerfelli O, Kris MG, Ladanyi M, Miller VA, Pao W. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006 Nov 1;12(21):6494–501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 59.Costa DB, Nguyen KS, Cho BC, Sequist LV, Jackman DM, Riely GJ, Yeap BY, Halmos B, Kim JH, Jänne PA, Huberman MS, Pao W, Tenen DG, Kobayashi S. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clin Cancer Res. 2008 Nov 1;14(21):7060–7. doi: 10.1158/1078-0432.CCR-08-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Bubnoff N, Schneller F, Peschel C, Duyster J. BCR-ABL gene mutations in relation to clinical resistance of Philadelphia-chromosome-positive leukaemia to STI571: a prospective study. Lancet. 2002 Feb 9;359(9305):487–91. doi: 10.1016/S0140-6736(02)07679-1. [DOI] [PubMed] [Google Scholar]
- 61.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, Meyerson M, Eck MJ. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008 Feb 12;105(6):2070–5. doi: 10.1073/pnas.0709662105. Epub 2008 Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, Wang L, Amato KR, Arcila M, Sos ML, Socci ND, Viale A, de Stanchina E, Ginsberg MS, Thomas RK, Kris MG, Inoue A, Ladanyi M, Miller VA, Michor F, Pao W. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011 Jul 6;3(90):90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Becker A, Crombag L, Heideman DA, Thunnissen FB, van Wijk AW, Postmus PE, Smit EF. Retreatment with erlotinib: Regain of TKI sensitivity following a drug holiday for patients with NSCLC who initially responded to EGFR-TKI treatment. Eur J Cancer. 2011 Nov;47(17):2603–6. doi: 10.1016/j.ejca.2011.06.046. Epub 2011 Jul 23. [DOI] [PubMed] [Google Scholar]
- 64.Parker WT, Lawrence RM, Ho M, Irwin DL, Scott HS, Hughes TP, Branford S. Sensitive detection of BCR-ABL1 mutations in patients with chronic myeloid leukemia after imatinib resistance is predictive of outcome during subsequent therapy. J Clin Oncol. 2011 Nov 10;29(32):4250–9. doi: 10.1200/JCO.2011.35.0934. Epub 2011 Oct 11. [DOI] [PubMed] [Google Scholar]
- 65.Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A. 2011;108:7535–40. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kris MG, Johnson BE, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Aronson SL, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI’s Lung Cancer Mutation Consortium (LCMC) J Clin Oncol. 2011;29 abstr CRA7506. [Google Scholar]
- 67.Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, Choi HG, Kim J, Chiang D, Thomas R, Lee J, Richards WG, Sugarbaker DJ, Ducko C, Lindeman N, Marcoux JP, Engelman JA, Gray NS, Lee C, Meyerson M, Jänne PA. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008 Jul 1;14(13):4275–83. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Popat S, Vieira de Araújo A, Min T, Swansbury J, Dainton M, Wotherspoon A, Lim E, Nicholson AG, O’Brien ME. Lung adenocarcinoma with concurrent exon 19 EGFR mutation and ALK rearrangement responding to erlotinib. J Thorac Oncol. 2011 Nov;6(11):1962–3. doi: 10.1097/JTO.0b013e31822eec5e. [DOI] [PubMed] [Google Scholar]
- 69.Makhnevych T, Houry WA. The role of Hsp90 in protein complex assembly. Biochim Biophys Acta. 2011 Sep 16; doi: 10.1016/j.bbamcr.2011.09.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 70.Bonvini P, Gastaldi T, Falini B, Rosolen A. Nucleophosmin-anaplastic lymphoma kinase (NPM-ALK), a novel Hsp90-client tyrosine kinase: down-regulation of NPM-ALK expression and tyrosine phosphorylation in ALK(+) CD30(+) lymphoma cells by the Hsp90 antagonist 17-allylamino,17-demethoxygeldanamycin. Cancer Res. 2002 Mar 1;62(5):1559–66. [PubMed] [Google Scholar]
- 71.Normant E, Paez G, West KA, Lim AR, Slocum KL, Tunkey C, et al. The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and induces tumor regression in ALK-driven NSCLC models. Oncogene. 2011;30:2581–6. doi: 10.1038/onc.2010.625. [DOI] [PubMed] [Google Scholar]
- 72.Sequist LV, Gettinger S, Senzer NN, Martins RG, Jänne PA, Lilenbaum R, Gray JE, Iafrate AJ, Katayama R, Hafeez N, Sweeney J, Walker JR, Fritz C, Ross RW, Grayzel D, Engelman JA, Borger DR, Paez G, Natale R. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010 Nov 20;28(33):4953–60. doi: 10.1200/JCO.2010.30.8338. Epub 2010 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong K, Koczywas M, Goldman JW, Paschold EH, Horn L, Lufkin JM, Blackman RK, Teofilovici F, Shapiro G, Socinski MA. An open-label phase II study of the Hsp90 inhibitor ganetespib (STA-9090) as monotherapy in patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2011;29(suppl) abstr 7500. [Google Scholar]
- 74.Chen Z, Sasaki T, Tan X, Carretero J, Shimamura T, Li D, Xu C, Wang Y, Adelmant GO, Capelletti M, Lee HJ, Rodig SJ, Borgman C, Park SI, Kim HR, Padera R, Marto JA, Gray NS, Kung AL, Shapiro GI, Jänne PA, Wong KK. Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4-ALK fusion oncogene. Cancer Res. 2010 Dec 1;70(23):9827–36. doi: 10.1158/0008-5472.CAN-10-1671. Epub 2010 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knowlton CA, Brady LW, Heintzelman RC. Radiotherapy in the treatment of gastrointestinal stromal tumors. Rare Tumors. 2011;3:e35, p111–113. doi: 10.4081/rt.2011.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raut CP, Posner M, Desai J, Morgan JA, George S, Zahrieh D, Fletcher CD, Demetri GD, Bertagnolli MM. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006 May 20;24(15):2325–31. doi: 10.1200/JCO.2005.05.3439. [DOI] [PubMed] [Google Scholar]
- 77.Al-Batran SE, Hartmann JT, Heidel F, Stoehlmacher J, Wardelmann E, Dechow C, Düx M, Izbicki JR, Kraus T, Fischer T, Jäger E. Focal progression in patients with gastrointestinal stromal tumors after initial response to imatinib mesylate: a three-center-based study of 38 patients. Gastric Cancer. 2007;10(3):145–52. doi: 10.1007/s10120-007-0425-8. Epub 2007 Sep 26. [DOI] [PubMed] [Google Scholar]
- 78.Bridge JA, Kanamori M, Ma Z, Pickering D, Hill DA, Lydiatt W, Lui MY, Colleoni GW, Antonescu CR, Ladanyi M, Morris SW. Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol. 2001 Aug;159(2):411–5. doi: 10.1016/S0002-9440(10)61711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colleoni GW, Bridge JA, Garicochea B, Liu J, Filippa DA, Ladanyi M. ATIC-ALK: A novel variant ALK gene fusion in anaplastic large cell lymphoma resulting from the recurrent cryptic chromosomal inversion, inv(2)(p23q35) Am J Pathol. 2000 Mar;156(3):781–9. doi: 10.1016/S0002-9440(10)64945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Camidge DR, Hirsch FR, Varella-Garcia M, Franklin WA. Finding ALK-positive lung cancer: what are we really looking for? J Thorac Oncol. 2011c Mar;6(3):411–3. doi: 10.1097/JTO.0b013e31820cf068. [DOI] [PubMed] [Google Scholar]
- 81.Shaw AT, Solomon B, Kenudson MM. Crizotinib and Testing for ALK. J Natl Compr Canc Netw. 2011b Dec 1;9(12):1335–41. doi: 10.6004/jnccn.2011.0115. [DOI] [PubMed] [Google Scholar]
- 82.Horn L, Pao W. EML4-ALK: honing in on a new target in non-small-cell lung cancer. J Clin Oncol. 2009 Sep 10;27(26):4232–5. doi: 10.1200/JCO.2009.23.6661. Epub 2009 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tan W, Wilner KD, Bang Y, et al. Pharmacokinetics (PK) of PF-02341066, a dual ALK/MET inhibitor after multiple oral doses to advanced cancer patients. J Clin Oncol. 2010;28(suppl) abstract 2596. [Google Scholar]
- 84.Camidge DR, Theodoro M, Maxson DA, Skokan M, O’Brien T, Lu X, Doebele RC, Barón AE, Varella-Garcia M. Correlations between the percentage of tumor cells showing an ALK gene rearrangement, ALK signal copy number and response to crizotinib therapy in ALK FISH positive non-small cell lung cancer. Cancer. 2012 Jan 26; doi: 10.1002/cncr.27411. doi: 10.1002/cncr.27411. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pfizer Crizotinib US Package Insert. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202570s000lbl.pdf.
- 86.Stoica GE, Kuo A, Aigner A, Sunitha I, Souttou B, Malerczyk C, Caughey DJ, Wen D, Karavanov A, Riegel AT, Wellstein A. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001 May 18;276(20):16772–9. doi: 10.1074/jbc.M010660200. Epub 2001 Feb 8. [DOI] [PubMed] [Google Scholar]
- 87.Stoica GE, Kuo A, Powers C, Bowden ET, Sale EB, Riegel AT, Wellstein A. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002 Sep 27;277(39):35990–8. doi: 10.1074/jbc.M205749200. Epub 2002 Jul 16. [DOI] [PubMed] [Google Scholar]
- 88.McDermott U, Iafrate AJ, Gray NS, Shioda T, Classon M, Maheswaran S, Zhou W, Choi HG, Smith SL, Dowell L, Ulkus LE, Kuhlmann G, Greninger P, Christensen JG, Haber DA, Settleman J. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008 May 1;68(9):3389–95. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 89.Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007 Nov;8(11):1018–29. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- 90.Gambacorti-Passerini C, Messa C, Pogliani EM. Crizotinib in anaplastic large-cell lymphoma. N Engl J Med. 2011 Feb 24;364(8):775–6. doi: 10.1056/NEJMc1013224. [DOI] [PubMed] [Google Scholar]
- 91.Butrynski JE, D’Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ, Ramaiya N, Kwak EL, Clark JW, Wilner KD, Christensen JG, Jänne PA, Maki RG, Demetri GD, Shapiro GI. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010 Oct 28;363(18):1727–33. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ou SH, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, Camidge DR, Solomon BJ, Maki RG, Bang YJ, Kim DW, Christensen J, Tan W, Wilner KD, Salgia R, Iafrate AJ. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011 May;6(5):942–6. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- 93.Lennerz JK, Kwak EL, Ackerman A, Michael M, Fox SB, Bergethon K, Lauwers GY, Christensen JG, Wilner KD, Haber DA, Salgia R, Bang YJ, Clark JW, Solomon BJ, Iafrate AJ. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011 Dec 20;29(36):4803–10. doi: 10.1200/JCO.2011.35.4928. Epub 2011 Oct 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, Jessop NA, Wain JC, Yeo AT, Benes C, Drew L, Saeh JC, Crosby K, Sequist LV, Iafrate AJ, Engelman JA. Mechanisms of Acquired Crizotinib Resistance in ALK-Rearranged Lung Cancers. Sci Transl Med. 2012 Feb 8;4(120):120ra17. doi: 10.1126/scitranslmed.3003316. Epub 2012 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sugawara E, Togashi Y, Kuroda N, Sakata S, Hatano S, Asaka R, Yuasa T, Yonese J, Kitagawa M, Mano H, Ishikawa Y, Takeuchi K. Identification of anaplastic lymphoma kinase fusions in renal cancer: Large-scale immunohistochemical screening by the intercalated antibody-enhanced polymer method. Cancer. 2012 Jan 17; doi: 10.1002/cncr.27391. doi: 10.1002/cncr.27391. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 96.van Gaal JC, Flucke UE, Roeffen MH, de Bont ES, Sleijfer S, Mavinkurve-Groothuis AM, Suurmeijer AJ, van der Graaf WT, Versleijen-Jonkers YM. Anaplastic lymphoma kinase aberrations in rhabdomyosarcoma: clinical and prognostic implications. J Clin Oncol. 2012 Jan 20;30(3):308–15. doi: 10.1200/JCO.2011.37.8588. Epub 2011 Dec 19. [DOI] [PubMed] [Google Scholar]
- 97.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009 Jun 2;15(6):489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]