Abstract
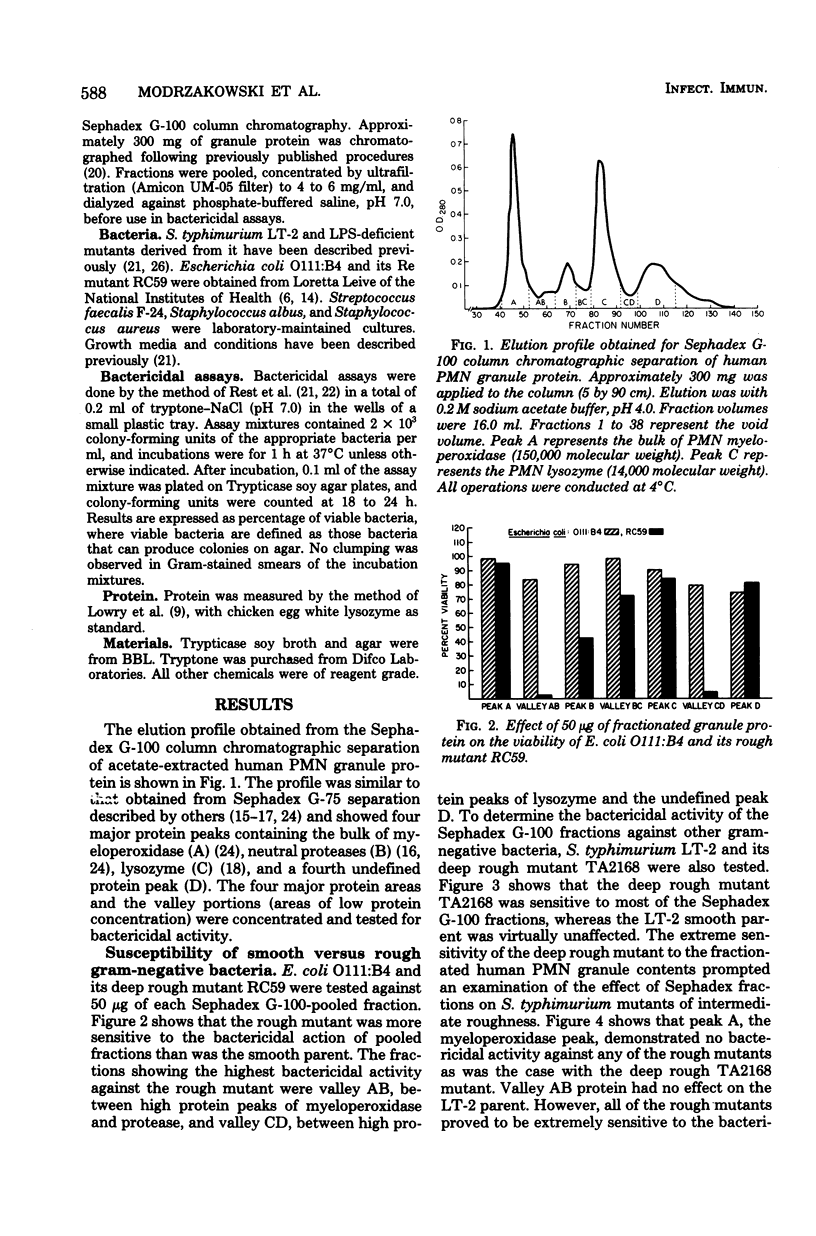
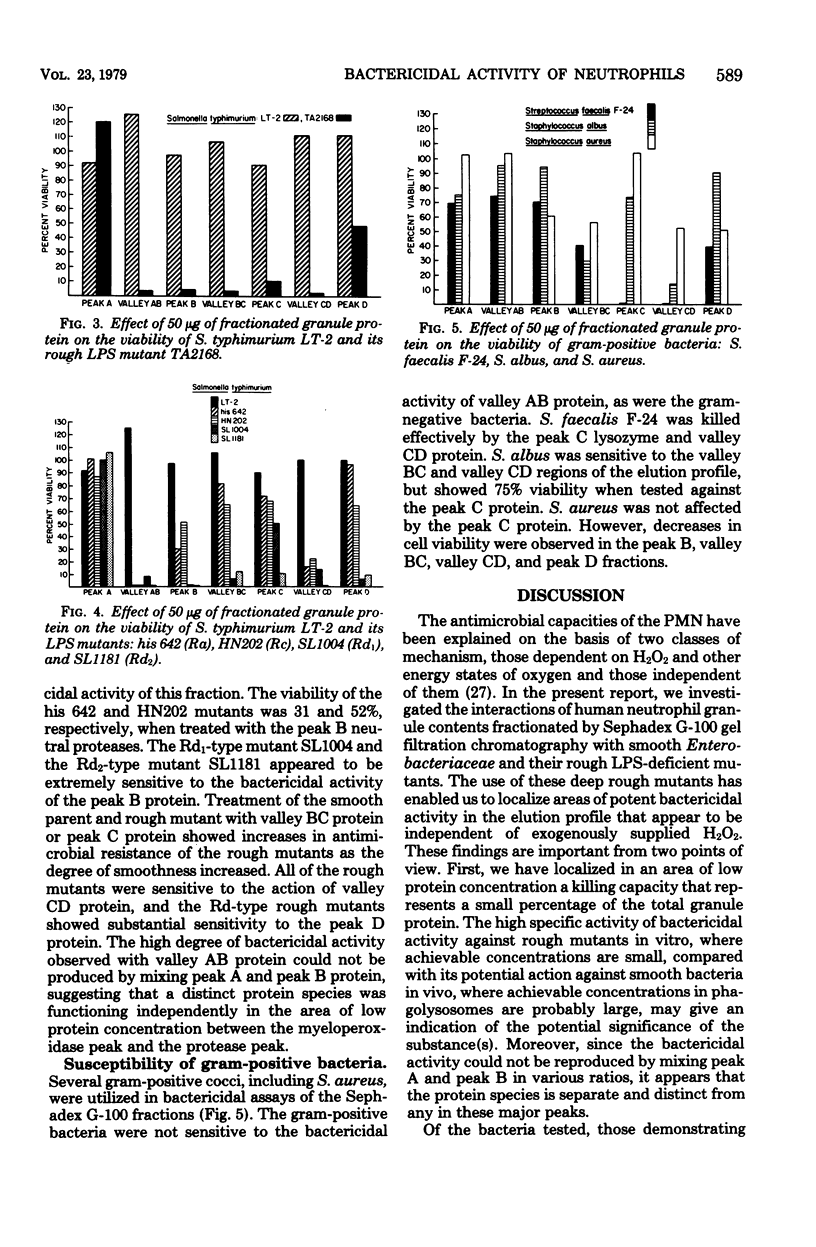
Proteins from human polymorphonuclear leukocyte granules were extracted with 0.2 M acetate, pH 4.0, and fractionated by Sephadex G-100 column chromatography. The fractions demonstrated selective bactericidal action against a deep rough cell wall mutant of Escherichia coli O111:B4 with rough lipopolysacharide and cell wall mutants of Salmonella typhimurium LT-2 with lipoplysacharide of Ra, Rc, Rd1, Rd2, and Re types. Smooth parent strains were most resistant to the bactericidal action. Fractions with greatest activity for the mutants were from valley regions (regions of low protein concentration) between three high protein peaks comprising myeloperoxidase, protease, and lysozyme, respectively. Susceptibility of the mutants to bactericidal action increased as sugar residues decreased in lipopolysaccharide. Gram-positive bacteria were susceptible to different fractions than were the gram-negative bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Friedberg D., Friedberg I., Shilo M. Interaction of Gram-Negative Bacteria with the Lysosomal Fraction of Polymorphonuclear Leukocytes II. Changes in the Cell Envelope of Escherichia coli. Infect Immun. 1970 Mar;1(3):311–318. doi: 10.1128/iai.1.3.311-318.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg D., Shilo M. Interaction of Gram-Negative Bacteria with the Lysosomal Fraction of Polymorphonuclear Leukocytes I. Role of Cell Wall Composition of Salmonella typhimurium. Infect Immun. 1970 Mar;1(3):305–310. doi: 10.1128/iai.1.3.305-310.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Blondin J. The effect of human granulocyte elastase on bacterial suspensions. Lab Invest. 1973 Oct;29(4):454–457. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lahav M., Ne'eman N., Adler E., Ginsburg I. Effect of leukocyte hydrolases on bacteria. I. Degradation of 14C-labeled Streptococcus and Staphylococcus by leukocyte lysates in vitro. J Infect Dis. 1974 May;129(5):528–537. doi: 10.1093/infdis/129.5.528. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. Intraphagosomal pH of human polymorphonuclear neutrophils. Proc Soc Exp Biol Med. 1970 Jun;134(2):447–449. doi: 10.3181/00379727-134-34810. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Olsson I., Venge P. Cationic proteins of human granulocytes. I. Isolation of the cationic proteins from the granules of leukaemic myeloid cells. Scand J Haematol. 1972;9(3):204–214. doi: 10.1111/j.1600-0609.1972.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Olsson I., Venge P. Cationic proteins of human granulocytes. II. Separation of the cationic proteins of the granules of leukemic myeloid cells. Blood. 1974 Aug;44(2):235–246. [PubMed] [Google Scholar]
- Oram J. D., Reiter B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim Biophys Acta. 1968 Dec 23;170(2):351–365. doi: 10.1016/0304-4165(68)90015-9. [DOI] [PubMed] [Google Scholar]
- Pryzwansky K. B., Martin L. E., Spitznagel J. K. Immunocytochemical localization of myeloperoxidase, lactoferrin, lysozyme and neutral proteases in human monocytes and neutrophilic granulocytes. J Reticuloendothel Soc. 1978 Sep;24(3):295–310. [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Bactericidal activity of specific and azurophil granules from human neutrophils: studies with outer-membrane mutants of Salmonella typhimurium LT-2. Infect Immun. 1978 Jan;19(1):131–137. doi: 10.1128/iai.19.1.131-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Spitznagel J. K. Subcellular distribution of superoxide dismutases in human neutrophils. Influence of myeloperoxidase on the measurement of superoxide dismutase activity. Biochem J. 1977 Aug 15;166(2):145–153. doi: 10.1042/bj1660145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler-Ludwig R., Schmalzl F., Braunsteiner H. Esterases in human neutrophil granulocytes: evidence for their protease nature. Br J Haematol. 1974 May;27(1):57–64. doi: 10.1111/j.1365-2141.1974.tb06774.x. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., MacAlister T., Costerton J. W., Cheng K. J. Permeability of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium to antibiotics, lysozyme, and other agents. Can J Microbiol. 1974 Aug;20(8):1135–1145. doi: 10.1139/m74-176. [DOI] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., Barrett A. J. Lysis and killing of bacteria by lysosomal proteinases. Infect Immun. 1976 Aug;14(2):555–563. doi: 10.1128/iai.14.2.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



