Abstract
Despite remarkable achievements in the treatment of breast cancer, some obstacles still remain. Gold nanoparticles may prove valuable in addressing these problems owing to their unique characteristics, including their enhanced permeability and retention in tumor tissue, their light absorbance and surface plasmon resonance in near-infrared light, their interaction with radiation to generate secondary electrons, and their ability to be conjugated with drugs or other agents. Herein, we discuss some basic concepts of gold nanoparticles, and early results from studies regarding their use in breast cancer, including toxicity and side effects. We also discuss these particles’ potential clinical applications.
Keywords: Gold nanoparticle, Breast neoplasms, Theranostics, Nanotechnology
1. Introduction
Intensive screening and advanced treatment modalities have reduced the incidence of breast cancer and the rate of breast cancer–related mortality [1,2]. A relatively new concept of breast cancer as a “chronic disease” reflects not only increased survival rates but also the importance of patients’ quality of life. Courtesy of gene expression profiling having prognostic or predictive significance personalized therapy enables tailored treatment avoiding chemotherapy in subgroups unlikely to have much benefit. In addition, minimally invasive approaches to treating early-stage breast cancer now consider the patient’s cosmetic appearance and minimize the lifelong sequelae of lymphedema. However, many challenges in treating breast cancer patients remain, including reducing treatment-related adverse events, managing triple-negative breast cancer despite poor outcomes and the lack of a therapeutic target, and balancing treatment toxicity with quality of life in patients with metastatic cancer who have already received extensive therapy.
To overcome these obstacles, researchers have introduced the use of nanotechnology in breast cancer diagnosis and treatment [3]. In this context, nanotechnology involves the use of nanoparticles that are small bits of matter of about 1–100 nm in two or three dimensions, according to the American Society for Testing and Materials. Matter on the nanoscale often offers unique properties not seen in bulk matter or in solutions, and their interaction with body tissues differs from that of drugs or transplants. Several technological advances now make it possible to design and characterize nanoparticles by using a large variety of organic and inorganic materials to obtain the desired properties. The research literature on cancer nanotechnology has exploded over the last decade. Our focus here is on one of the more promising variants for the treatment of breast cancer—gold nanoparticles. In this review, we summarize the characteristics of gold nanoparticles, their contributions to tumor destruction, their toxicity, and their potential in breast cancer treatment.
2. Characteristics of gold nanoparticles
2.1. Physical attributes
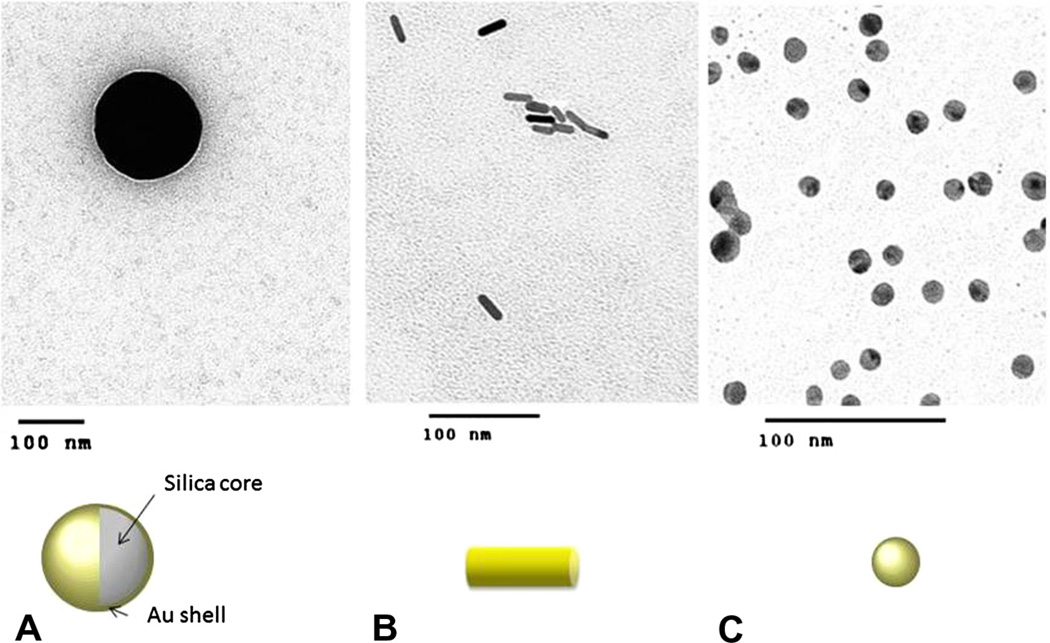
Gold nanoparticles can vary in size, shape, and structure (Fig. 1), and researchers have developed diverse formulations of gold nanoparticles for different treatment purposes. Gold nanospheres (AuNPs), which are produced by the reduction of chloroauric acid, are solid balls of gold that range in diameter from only a few to more than 100 nm and are useful for imaging and radiation dose enhancement. Gold nanoshells (AuNSs) are spherical structures comprising a silica core and thin layer of gold, 50–150 nm in size. Their optical properties can be tuned by modifying the core diameter and shell thickness. Gold nanorods (AuNRs) are synthesized from chloroauric acid with a gold seed and a stabilizing agent, usually cetyltrimethylammonium bromide (CTAB) [4]. The absorption wavelength of AuNRs has two peaks depending on the orientation of the particle to an incident beam of light. Size of AuNRs is typically 25–45 nm in longest dimension, and manipulating these plasmonic particles’ length-to-diameter ratio (i.e., aspect ratio) changes their peak absorbance wavelength. Depending on their surface charge and functional groups, AuNRs can have higher cellular uptake [5,6]. Nanocages and hollow gold nanospheres are other forms of gold nanoparticles that have excellent plasmonic photothermal activities. Depending on the nature of preclinical or eventual clinical application, these differences in size, shape and surface properties of gold nanoparticles are exploited by researchers for specific cancer therapy scenarios. In addition to presence of an inorganic metallic substrate, this tunability to create spheres, shells, rods, and cages of varying sizes and shapes distinguishes gold nanoparticles from other commonly used non-metallic nanoparticles such as liposomes and polymers.
Fig. 1.
Some types of gold nanoparticles of interest for breast cancer research. (A) Gold nanoshells usually have a silica core coated with a thin layer of gold. Hollow gold nanoshells can also be made. (B) Gold nanorods are cylindrical, solid gold nanoparticles. (C) Solid gold nanoparticles are spherical nanoparticles comprising gold alone.
2.2. Passive and targeted accumulation in tumors
Gold nanoparticles can easily permeate tumor vasculature and remain in tumors owing to the enhanced permeability and retention (EPR) effect, as gaps in tumor vasculature, whose sizes range from 100 nm to 2 µm, are larger than the gaps in the endothelial lining of normal capillaries. Gold nanoparticles easily pass through these gaps and, because tumors lack lymphatic clearance and have a disordered extracellular matrix, are able to remain in the tumor tissue. However, the tumor uptake of gold nanoparticles in vivo is significantly lessened by the opsonization of the nanoparticles with plasma proteins and their subsequent phagocytosis by reticuloendothelial system (RES) components such as monocytes and macrophages. Thus, most injected gold nanoparticles are eventually sequestered in the liver and spleen. Coating the nanoparticles with the polymer polyethylene glycol (PEG) [7] acts like a “stealth” cloak, preventing the uptake of nanoparticles by the RES, thus prolonging their circulation time and increasing their concentration in tumor tissue.
Gold nanoparticles can accumulate in tumor across the blood– brain barrier in brain tumor models, as contrasted with normal brain tissue [8]. Furthermore, conjugated gold nanoparticles can be delivered into brain parenchyma using a carrier-mediated influx of endothelial cells [9]. Such mechanisms can be used to carry drugs to specific targets within the central nervous system. In larger tumors, hypovascular cores confine the neovasculature’s leakage of gold nanoparticles to the periphery of the tumor; gold nanoparticle-loaded macrophages and T cells can be used to overcome the difficulties of treating the hypovascular area.
2.3. Hyperthermic effect
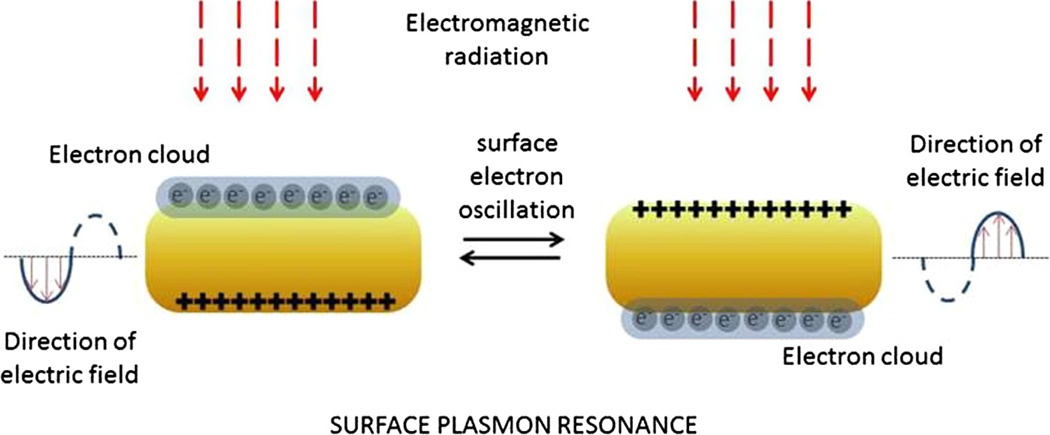
Gold nanoparticles such as AuNRs or AuNSs have optical properties of light absorbance and scattering in near-infrared (NIR) wavelengths (650–900 nm) [10]. With exposure to electromagnetic radiation, especially an NIR laser, gold nanoparticles can generate heat via the surface plasmon resonance effect (Fig. 2). Because its peak absorbance wavelength is in the visible range (450–600 nm), NIR light is transmitted through normal tissue components with minimal absorption [11,12]. Therefore, gold nanoparticles stimulated with NIR laser illumination can induce hyperthermia [13] in tumor tissue with little damage to normal tissues. In a pivotal study of mice with subcutaneously implanted colon cancer cells, intravenous administration of AuNS-PEG conjugates resulted in the passive accumulation of the AuNSs within the tumors, and subsequent illumination of these tumors with an 808-nm NIR laser successfully ablated the tumors. Compared with mock treatment, this treatment extended the survival of mice. The NIR laser-induced skin reaction in the AuNS-treated mice was no different from that in mice undergoing mock treatment, except that the AuNS-treated mice had a greater skin reaction at the tumor site [14].
Fig. 2.
Interactions of light with gold nanorods. Although only oscillations of the electron cloud across the shorter diameter of the gold nanorods are shown, oscillations also occur across the longer diameter. Because the path lengths are different, the resonant wavelengths are also different for oscillations across the two axes.
2.4. Radiosensitizer properties
Owing to the high atomic number of gold, gold nanoparticles can also be used as imaging contrast agents and radiosensitizers [15,16]. Several studies have shown that nanoparticles can be used to enhance the effects of radiation [17,18]. This dose-enhancement effect results from several sources, including the nanoparticles’ increased absorption of ionizing radiation energy and their production of secondary electrons that trigger the creation of reactive oxygen species, which cause additional damage to the tumor cell DNA [19]. These effects may not be limited to the DNA but also to the cell membrane and mitochondria [20].
2.4.1. Cellular uptake, pharmacokinetics, clearance, and toxicity concerns
The cellular uptake and localization of gold nanoparticle in tumor cells varies according to particle type, size, and/or surface molecule. In one study, smaller (2- or 6-nm) AuNPs coated with tiopronin were found in both the cytoplasm and nucleus, whereas 15-nm particles were found only in the cytoplasm [21]. In another study employing sequential transmission electron microscopy (TEM) of MDA-MB-231 cells, AuNRs were taken up by receptor-mediated endocytosis and formed endocytotic vesicles that evolved into lysosomes and autophagosomes. Reuptake of the eliminated particles was observed after exocytosis [22].
Gold nanoparticles used for imaging and therapeutic research tend to have diameters of at least 5 nm; like bulk gold, these larger gold particles are generally assumed to be chemically inert. One study found that molecules 4 or 10 nm in diameter, which were more easily absorbed than molecules 28 or 58 nm in diameter, accumulated in the liver, kidney, spleen, and other organs, including the brain [23]. Various factors, such as surface charge and size, are associated with the clearance of these molecules from the body. A positive surface charge stimulates the molecules’ binding to plasma protein, resulting in RES sequestration or impeding renal excretion owing to the larger hydrodynamic diameter. Particles with hydrodynamic diameters of less than 5–6 mm show enhanced renal clearance. Coating the particles with materials smaller than PEG (e.g. cystein [24], dithiolated polyaminocarboxylate [25]) to neutralize the surface charge might be one solution to enhancing the renal clearance of nanoparticles. Although numerous studies have investigated the toxicity of gold nanoparticles in animals, the toxicity of gold nanoparticles in humans has not been thoroughly assessed. One phase I trial for dose escalation studied the use of one type of conjugated gold nanoparticle: CYT-6091, a pegylated 27-nm AuNP with recombinant human tumor necrosis factor alpha (rhTNF) [26]. Patients with solid tumors nonresponsive to conventional treatment were enrolled, and possible tissue samples were collected. Twenty-four hours after they were administered, the AuNPs were found in tumor tissue. AuNPs were not found in normal parenchyma in breast cancer patients but were found in normal liver tissue in patients with liver tumors.
Gold nanoparticles cannot be effectively absorbed by oral entry. In contrast, 90% of intravenously delivered nanoparticles remain in the circulation for at least 1 week, and more than 70% of the particles eventually accumulate in the liver. From a toxicity standpoint, these particles’ persistence in the body is particularly pertinent because their large surface area-to-mass ratio may render them more active biologically [27]. However, in a study of repeated AuNPs treatments [28], the proportions of the accumulated amounts decreased as the dose increased, suggesting the activation of a clearance mechanism.
The findings of previous studies suggest that gold nanoparticles have little toxicity. In one such study, AuNPs of various sizes (4 nm, 12 nm and 18 nm) coated with cystein, citrate, biotin, or CTAB were incubated with human leukemia cells. The nanoparticles did not influence cellular mortality, but the CTAB was found to be cytotoxic [29]. Another experiment revealed that incubating human blood cells with 30-nm AuNPs resulted in concentration-dependent hemolysis but no platelet aggregation or change in ROS generation [30]. In rabbits, AuNPs did not produce evidence of acute toxicity within 24 h, nor was any organ observed [31]. The findings of another in vivo study indicated that exposure to silver nanoparticles, but not gold nanoparticles, resulted in the malformation and death of zebrafish embryos [32]. In recent animal studies of the biodistribution and long-term toxicity of AuNSs, dogs injected with AuNSs had transient weight loss, which recovered within 37 days, and no significant abnormalities in blood chemistry and hematologic analysis. Pathologic evaluations performed 10 months later revealed black pigmentation (indicating gold accumulation) in the Kupffer cells in the liver, in the red pulp of the spleen, and in the lymph nodes. The intensity of the pigment accumulation was mild in the low-dose group and moderate in the high-dose group [33]. The toxicity of AuNRs may result from unbound CTAB. Potential substitutes for CTAB include transferrin, polyacrylic acid, polystyrene sulfonate, and PEG.
3. Gold nanoparticles in breast cancer research
3.1. Radiation dose enhancement
Hainfeld et al. were the first to use gold nanoparticles to enhance radiation dose. The researchers injected 1.9-nm gold nanoparticles (2.7 g Au/kg body weight) into mice bearing EMT-6 mammary carcinomas in the thighs and then irradiated the tumor 2 min later (30 Gy, 250 peak kilovoltage) [34]. Compared with the control mice, the mice injected with the gold nanoparticles had smaller tumors and a higher 1-year survival rate (86% versus 20%). Kilovoltage (kV) radiation has been shown to enhance DNA double-strand breakage in the presence of gold in multiple cell lines and a range of radiation voltages due to photoelectric effect. However, kV radiation is rarely used therapeutically. Externalbeam radiation therapy for cancer almost exclusively requires megavoltage (MV) radiation to penetrate deep into the body without depositing excessive dose within the skin and subcutaneous tissues. Recent findings showed dose enhancement in MDA-MB-231 breast cancer cell lines not only from kV radiation but also from MV X-rays [35]. Nanoscale dose effect of AuNPs from Auger electron and biological effect such as influence on cell cycle or reactive oxygen species production are believed to contribute to dose enhancement in MV radiation [36].
Enhancing the radiation dose to the tumor can be crucial in the treatment of patients with breast cancer and its metastases, particularly patients undergoing reirradiation for a local recurrence after breast-conserving surgery [37] and adjuvant therapy for stereotactic radiosurgery after prior whole-brain irradiation. Gold nanoparticles might offer great promise for situations such as these and should be more thoroughly investigated for possible translation to clinical settings.
3.2. Nanoparticle-induced hyperthermia: thermal ablation and radiosensitization
The potential value of thermal ablation of tumors in early breast cancer has been the subject of some debate because surgery is required for sentinel lymph node biopsy and because purely thermal ablation of a primary tumor does not allow accurate histopathological classification or evaluation of margin widths. However, its potential for complete ablation with good cosmetic results is considered promising. Although the use of hyperthermia with radiofrequency ablation for breast cancer has achieved complete ablation rates of 76–100% in some studies, reported side effects have included pain, bleeding, infection, and skin burns. Although use of NIR lasers requires an invasive catheter device for lesions located more than 1 cm below the skin surface, thermal ablation with gold nanoparticles and an NIR laser could reduce the risk of adverse effects on the surrounding normal tissue because of the gold being concentrated in the tumor. Use of injected gold nanoparticles can also result in more even heat distribution than external application of heat, in which heat loss from cooling by the blood flow of adjacent capillaries acts as a “heat sink” and causes lower perivascular temperatures. Conversely, because nanoparticles tend to aggregate in the perivascular spaces, they tend to get heated more, causing additional damage to the vasculature feeding the tumor.
Thermal therapy with NIR laser and gold nanoparticles has proven effective in breast cancer cell lines in vitro [38–42]. In one study, SK-BR-3 breast cancer cells incubated with PEG-conjugated AuNS were treated with an 820-nm NIR laser. Fewer AuNS-treated breast cancer cells survived after laser treatment than cells incubated without AuNSs [38]. AuNSs conjugated with a human epidermal growth factor receptor 2 (HER-2) antibody and PEG showed enhanced uptake in SK-BR-3 cells, increased contrast on microscopy, and greater cytotoxicity after NIR laser application [39–41]. In another study, 30- to 40-nm antibody-conjugated AuNPs aggregated on the membranes of MDA-MB-231 cells, where they formed 100- to 200-nm clusters of gold. The peak absorption wavelength of these gold clusters showed a red shift. Exciting these clusters with NIR lasers caused microbubble formation and was cytotoxic [42].
Apart from destruction of cancer cells with lethal levels of heat, apoptosis of cancer cells can be also be induced by mild hyperthermia. Mild hyperthermia, defined in cancer treatment as a rise in tissue temperature to 40–43 °C, can be applied adjuvantly to enhance the effects of radiotherapy or chemotherapy [43]. Mild hyperthermia can also inhibit the repair of sublethal radiation damage, which is characterized by the efficient repair of radiation-induced DNA strand breaks between consecutive fractions of radiation. In one randomized controlled study of patients who had superficial tumors and underwent radiotherapy with or without hyperthermia, hyperthermia significantly improved local control, especially among patients who had previously undergone radiotherapy [44] (the percentages of patients with breast or chest wall tumors in the treatment and control groups were 63% and 66%, respectively). A review of several phase III randomized trials of radiotherapy plus hyperthermia for superficial recurrent cancer in the breast or chest wall revealed that the disease-free, but not overall, survival durations of patients who received hyperthermia were longer than those of patients who did not [45], suggesting that prospective studies of hyperthermia in combination with radiation would prove valuable.
AuNS-mediated hyperthermia preferentially sensitized stem cell–like mouse mammary tumor cells to radiotherapy in vivo. Interestingly, radiotherapy alone resulted in the reduction of tumor volume but a relative increase in the proportion of stem-like cells in the residual tumor, whereas the combination of hyperthermia and radiotherapy not only resulted in a larger reduction in tumor volume but also a reduction in the proportion of stem-like cells in the residual tumor. Furthermore, the residual tumors were less likely to regrow after transplantation into other mice, providing the impetus for researchers to design clinical trials of hyperthermia targeting traditionally radioresistant tumor subpopulations [46].
3.3. Tumor-targeting nanoparticles
Given their relatively large surface area and the EPR effect, decorating gold nanoparticles with a variety of therapeutic agents can enhance the delivery or controlled release of those agents to tumor. To date, gold nanoparticles have been coated with biological agents including tumor necrosis factor, antisense DNA, small interfering RNA(siRNA), paclitaxel, and docetaxel [47–51]. Once taken up by tumor cells and localized within endosomes, the lower pH within these endosomes enables release of the drug [52,53]. Or hyperthermia from NIR laser could also facilitate drug release at the targeted area [54]. For instance, You et al. found that, compared with free or liposomal doxurubicin, doxorubicin-conjugated hollow gold nanoshells (HAuNSs) stimulated with an NIR laser were more cytotoxic to MDA-MB-231 cells in vitro and further enhanced eradiation of tumors in vivo. Moreover, the stimulated gold conjugates were not only more effective at ablating tumors but also less cardiotoxic than liposomal doxorubicin, presumably because the conjugated form was associated with less free doxorubicin in the blood [55].
3.3.1. Trastuzumab conjugates
Theoretically, conjugating gold nanoparticles with trastuzumab has two potential advantages for treating breast cancer cells, one being to enhance the uptake of gold nanoparticles and the other to overcome trastuzumab resistance. Application of 300 kVp (peak kilovoltage) radiation to HER-2 overexpressing SK-BR-3 breast cancer cells led to 5.1 times more DNA double-strand breaks in trastuzumab-PEG-AuNPs than in PEG-AuNPs [56]. Trastuzumab-AuNR conjugates have also been shown to be taken up into tumor-cell endosomes and lysosomes rather than attaching to the cellular membrane [57]. Adding PEG to trastuzumab-AuNR conjugates can preserve their binding affinity during incubation in blood, and these conjugates were further shown to accumulate in BT474 xenograft tumor tissues in vivo [58]. Although trastuzumab has been shown to be effective against HER-2–overexpressing breast cancer, 15% of patients who receive trastuzumab as adjuvant therapy and 66–88% of patients who receive trastuzumab for metastatic disease eventually develop resistance to the agent. One proposed hypothesis to explain this resistance is that the overexpression of MUC4, which affects the binding capacity of the HER-2 receptor. However, MUC4 overexpression does not affect overall HER-2 expression levels [59]. In testing this hypothesis, one group treated JIMT-1 cells, a trastuzumab-resistant, MUC4-overexpressing breast cancer cell line, with trastuzumab-AuNS conjugates and found successful ablation after stimulation with an NIR laser. Higher uptake and enhanced laser ablation was also found in another HER-2-positive cell line resistant to both trastuzumab and lapatinib (a dual inhibitor of epidermal growth factor receptor and HER-2), BT474 AZ LR cells [60]. These promising results need to be confirmed in in vivo studies.
3.3.2. Targeting agents, gene therapy, and cytotoxic chemotherapeutic agents
The transferrin receptor participates in cellular proliferation, and overexpression of the transferrin receptor has been observed in breast tumors. Thus, the tumoral intake of AuNPs would be enhanced by conjugating them with transferrin [61]. Breast cancer cells also have a high expression level of receptors for luteinizing hormone-releasing hormone (LHRH). Jin et al. [62] conjugated gold-coated Fe3O4 nanoparticles with an LHRH analog and found that this conjugation did not affect the binding affinity or biological activity of the LHRH receptor in LβT2 mouse gonadotrope cells. Moreover, these conjugates had substantial dose-dependent effects on the survival and proliferation of MCF-7 and MDA-MB-231 breast cancer cells, both of which express the LHRH receptor. Gold nanoparticles have also been used to convey siRNA. In one such study, AuNRs conjugated with siRNA against protease-activated receptor-1 (PAR-1) inhibited the expression of PAR-1 on the surfaces of MDA-MB-231 cells, which the investigators suggested could be useful for decreasing metastatic activity [63].
Dumbbell-shaped Au-Fe3O4 nanoparticles conjugated with a trastuzumab-and-platinum complex showed target-specific delivery of platinum compounds to HER-2-positive SK-BR-3 cells. That cisplatin was released at lower pH levels led the investigators to suggest that the intracellular release of cisplatin (and hence its cytotoxicity) was stimulated by the lower pH in endosomes after uptake of the conjugates [52]. The lower pH levels in cellular endosomes could also facilitate the release of drugs from chloroquine-conjugated AuNPs, which also have antitumor activity in breast cancer, and lead to increased cytotoxicity in MCF-7 cells [53].
3.4. Gold nanoparticles as contrast agents: from microscopy to theranostics
Gold nanoparticles have been used to detect breast cancer in several diverse applications, ranging from the evaluation of pathology specimens to noninvasive in vivo imaging. Park et al. found that two-photon-induced photoluminescence (TPIP) could be used to visualize tumor cells embedded with gold in vivo [64]. With trastuzumab-PEG-AuNS treatment, HER-2 positive SK-BR-3 cell line was visualized using 10% of maximum laser power in TPIP imaging while normal breast cells were not visualized [65]. Moreover, in a study of the ex vivo assessment of the surgical resection margins of breast-conserving surgery, Bickford et al. found that reflectance confocal microscopy images of tissue treated with AuNS conjugated with PEG and an anti-HER-2 antibody produced results that corresponded to those of classical immunohistochemical and hematoxylin-and-eosin staining. It shortened the preparation time for imaging to 5 min, which enhances the potential for clinical translation wherein frozen section analysis can be undertaken in real-time during surgery [66].
3.4.1. In vivo imaging
Hainfeld et al. described gold nanoparticles as contrast agents for X-ray imaging in vivo. Intravenous injection of 1.9 nm AuNPs for the visualization of EMT-6 tumors in mice led to fair contrast enhancement for longer imaging durations than iodine, and the AuNPs allowed visualization of 100 µm vessels on X-ray images [67]. Researchers have also exploited the surface-enhanced Raman scattering of targeted AuNPs conjugates to detect tumors noninvasively in vivo [68]. Au3Cu1 nanoshells have also been found to be effective contrast agents for magnetic resonance imaging [69], as their porous interiors allows them to easily interact with water within the nanoshells. The use of nanoparticles is also being explored for photoacoustic or optoacoustic imaging [70], a novel non-ionizing imaging method that uses optical illumination and ultrasonic detection to produce deep tissue images based on their light absorption. Photoacoustic imaging with gold nanocages has been used successfully for sentinel lymph node mapping in a rat model [71].
3.4.2. Theranostics
The use of gold nanoparticles is also being explored in “theranostics,” applications in which diagnosis and treatment are combined. Multifunctional nanoparticles made of magnetic nanoparticles, AuNS, and upconversion nanoparticles could accumulate in tumors enabling detection by upconversion luminescence and MRI, and to increase survival from photothermal treatment combined with magnetic field [72]. In another study of gold nanostars tagged with a label for surface-enhanced Raman scattering (for visualization) and coated with a silica shell containing a methylene-blue photosensitizing drug for singlet-oxygen generation (for therapeutics), it demonstrated cytotoxicity upon stimulation with an infrared laser in BT549 breast tumor cells [73].
4. Future perspectives for breast cancer treatment
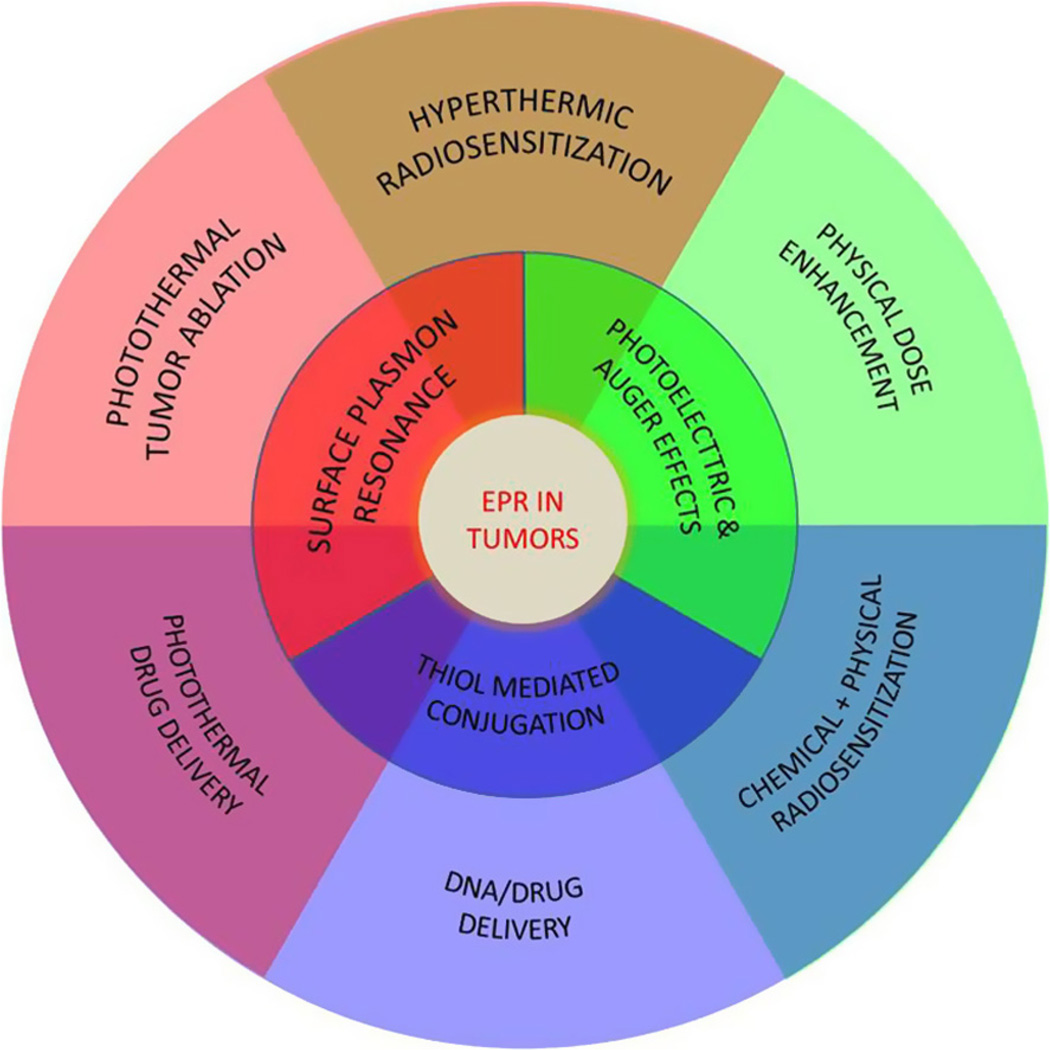
Gold nanoparticles show promise for both the imaging and treatment of breast cancer. (Fig. 3). From a logistical standpoint, most breast tumors occur relatively near the surface of the skin, where they can be easily accessed and treated despite the limited tissue penetration of an NIR laser. Intratumoral injection may also be advantageous for some superficially located tumors, as some studies have indicated that only 0.7–5% of an intravenously injected dose of gold nanoparticles accumulates in the tumor tissue [34,74,75]. Administration of nanoparticles may thus be beneficial not only for imaging or curative therapy of early-stage disease but also for palliative local control of more advanced breast tumors.
Fig. 3.
Properties of gold nanoparticles and their potential use in cancer treatment. The enhanced permeability and retention (EPR) effect traps the nanoparticles inside the tumor, where they can be activated using light (surface plasmon resonance), or used to increase the effect of radiation (secondary electrons) or deliver drugs/DNA. One or a combination of these strategies can be useful in cancer treatment.
From an imaging standpoint, contrast provided by gold (a higher atomic number element than calcium) would be even more pronounced on mammography. Additionally, intraoperative visualization of surgical resection margins based on tumor-specific nanoparticle uptake and non-contact large-field gold nanoparticle imaging techniques may aid surgeons in ensuring resection margin. The challenge is to find the ideal method of concentrating gold nanoparticles within tumor cells and not benign breast tissue.
From a therapeutic standpoint, there is always a need for minimally invasive techniques to treat breast cancers, particularly to maintain cosmesis within the treated breast and minimize the risk of lymphedema associated with axillary lymph node dissection. Although hyperthermic therapy is still considered experimental and requires considerably more research before it can be brought into widespread clinical use, hyperthermia using gold nanoparticles may well be useful in the future. Noninvasive imaging with gold nanoparticles may also help in deciding whether to forego axillary lymph node dissection for specific cases involving positive sentinel lymph nodes [76], which to date has not met with wide acceptance.
Gold nanoparticles may also be helpful in overcoming some of the challenges inherent to current breast cancer treatment modalities. The potential advantages include radiation dose enhancement for recurrent tumors in a previously radiated field or radiosensitization via hyperthermic treatment of similarly recurrent tumors. Combination of hyperthermia and photoelectric dose enhancement of radiation therapy could be effective for treatment of superficially positioned inflammatory breast cancers or recurrence in post-mastectomy chest wall. Sensitizing tumors to standard treatments via targeted delivery of chemotherapy, oligonucleotides, or biologic agents via EPR effect and tumor-specific targeting strategies might also overcome the challenge of treating triple-negative cancers through targeting of other cell-surface receptors that may not be drivers of proliferation and survival but yet overexpressed sufficiently to allow docking of conjugated nanoparticles.
5. Conclusions
Gold nanoparticles can accumulate in tumor tissue either passively via the EPR effect or actively via their conjugation with a targeted molecule. Through plasmon resonance, gold nanoparticles can induce hyperthermia with NIR laser. Gold’s high atomic number enables it to enhance the effect of radiotherapy, which can be amplified by mild, laser-induced hyperthermia. Conjugated with drugs, it can also be a drug carrier to enhance uptake of the drug to the tumor tissue; controlled release of the drug can be possible with magnetic nanoparticles or hyperthermic stimulation. Comprehensive studies of the use of gold nanoparticles in breast cancer have been conducted from cellular uptake and imaging in vitro to survival improvement in vivo. Although gold is less toxic than other metallic nanoparticles, problems stemming from its lack of clearance and more specific targeting of tumor cells must be resolved before gold nanoparticles can be introduced directly to the clinic. The results of early clinical trials of gold nanoparticles in lung cancer patients (NCT01679470) and head and neck cancer patients (NCT00848042) will provide new insights into these particles’ use and inform their application in breast cancer patients.
Supplementary Material
Acknowledgements
This work was supported by the Soon Chun Hyang University Research Fund and funded in part by grants from the National Institutes of Health (1R01CA132032, 1R01CA155446, and U01CA151886), Department of Defense (PC111832), and MD Anderson Institutional Research Grant to SK.
The authors also wish to thank Chris Wogan for her assistance with scientific writing, proofreading and finalizing this manuscript.
Abbreviations
- AuNPs
gold nanoparticles
- AuNSs
gold nanoshells
- AuNRs
gold nanorods
- CTAB
cetyltrimethylammonium bromide
- EPR
enhanced permeability and retention
- RES
reticuloendothelial system
- PEG
polyethylene glycol
- NIR
nearinfrared
- TEM
transmission electron microscopy
- rhTNF
recombinant human tumor necrosis factor alpha
- kV
kilovoltage
- MV
megavoltage
- HER-2
human epidermal growth factor receptor 2
- siRNA
small interfering RNA
- HAuNSs
hollow gold nanoshells
- kVp
peak kilovoltage
- LHRH
luteinizing hormone releasing hormone
- PAR-1
protease-activated receptor-1
- TPIP
two-photon-induced photoluminescence
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.canlet.2014.02.006.
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA: Cancer J. Clinicians. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Howlader NA, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA KA, editors. SEER Cancer Statistics Review, 1975–2009. Bethesda, MD: National Cancer Institute; (Vintage 2009 Populations) [Google Scholar]
- 3.Yezhelyev MV, Gao X, Xing Y, Al-Hajj A, Nie S, O’Regan RM. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006;7:657–667. doi: 10.1016/S1470-2045(06)70793-8. [DOI] [PubMed] [Google Scholar]
- 4.Sau TK, Murphy CJ. Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir: ACS J. Surf. Colloids. 2004;20:6414–6420. doi: 10.1021/la049463z. [DOI] [PubMed] [Google Scholar]
- 5.Huff TB, Hansen MN, Zhao Y, Cheng JX, Wei A. Controlling the cellular uptake of gold nanorods. Langmuir: ACS J. Surf. Colloids. 2007;23:1596–1599. doi: 10.1021/la062642r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauck TS, Ghazani AA, Chan WC. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small. 2008;4:153–159. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- 7.Kah JC, Wong KY, Neoh KG, Song JH, Fu JW, Mhaisalkar S, Olivo M, Sheppard CJ. Critical parameters in the pegylation of gold nanoshells for biomedical applications: an in vitro macrophage study. J. Drug Target. 2009;17:181–193. doi: 10.1080/10611860802582442. [DOI] [PubMed] [Google Scholar]
- 8.Hainfeld JF, Smilowitz HM, O’Connor MJ, Dilmanian FA, Slatkin DN. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomed. (Lond.) 2013;8:1601–1609. doi: 10.2217/nnm.12.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prades R, Guerrero S, Araya E, Molina C, Salas E, Zurita E, Selva J, Egea G, Lopez-Iglesias C, Teixido M, Kogan MJ, Giralt E. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials. 2012;33:7194–7205. doi: 10.1016/j.biomaterials.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 10.Weissleder R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 11.Day ES, Morton JG, West JL. Nanoparticles for thermal cancer therapy. J. Biomech. Eng. 2009;131:074001. doi: 10.1115/1.3156800. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomed. (Lond.) 2007;2:681–693. doi: 10.2217/17435889.2.5.681. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008;23:217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 14.O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc. Chem. Res. 2008;41:1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 16.Roeske JC, Nunez L, Hoggarth M, Labay E, Weichselbaum RR. Characterization of the theoretical radiation dose enhancement from nanoparticles. Technol. Cancer Res. Treat. 2007;6:395–401. doi: 10.1177/153303460700600504. [DOI] [PubMed] [Google Scholar]
- 17.Zhang SX, Gao J, Buchholz TA, Wang Z, Salehpour MR, Drezek RA, Yu TK. Quantifying tumor-selective radiation dose enhancements using gold nanoparticles: a monte carlo simulation study. Biomed. Microdev. 2009;11:925–933. doi: 10.1007/s10544-009-9309-5. [DOI] [PubMed] [Google Scholar]
- 18.Herold DM, Das IJ, Stobbe CC, Iyer RV, Chapman JD. Gold microspheres: a selective technique for producing biologically effective dose enhancement. Int. J. Radiat. Biol. 2000;76:1357–1364. doi: 10.1080/09553000050151637. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, Hunting DJ, Ayotte P, Sanche L. Radiosensitization of DNA by gold nanoparticles irradiated with high-energy electrons. Radiat. Res. 2008;169:19–27. doi: 10.1667/RR1080.1. [DOI] [PubMed] [Google Scholar]
- 20.Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6:520–528. doi: 10.1016/S1470-2045(05)70246-1. [DOI] [PubMed] [Google Scholar]
- 21.Huang K, Ma H, Liu J, Huo S, Kumar A, Wei T, Zhang X, Jin S, Gan Y, Wang PC, He S, Liang XJ. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6:4483–4493. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Ji Y, Wu X, Xu H. Trafficking of gold nanorods in breast cancer cells: uptake, lysosome maturation, and elimination. ACS Appl. Mater. Interfaces. 2013;5:9856–9865. doi: 10.1021/am4033857. [DOI] [PubMed] [Google Scholar]
- 23.Hillyer JF, Albrecht RM. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharmaceut. Sci. 2001;90:1927–1936. doi: 10.1002/jps.1143. [DOI] [PubMed] [Google Scholar]
- 24.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alric C, Miladi I, Kryza D, Taleb J, Lux F, Bazzi R, Billotey C, Janier M, Perriat P, Roux S, Tillement O. The biodistribution of gold nanoparticles designed for renal clearance. Nanoscale. 2013;5:5930–5939. doi: 10.1039/c3nr00012e. [DOI] [PubMed] [Google Scholar]
- 26.Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Jr, Gannon WE, Walker M, Seidel GD, Yuldasheva N, Tamarkin L. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clinical Cancer Res.: An Off. J. Am. Assoc. Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, Olmedo I, Clos A, Sadagopa Ramanujam VM, Urayama A, Vergara L, Kogan MJ, Soto C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010;393:649–655. doi: 10.1016/j.bbrc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 29.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 30.Love SA, Thompson JW, Haynes CL. Development of screening assays for nanoparticle toxicity assessment in human blood: preliminary studies with charged Au nanoparticles. Nanomedi. (Lond.) 2012 doi: 10.2217/nnm.12.17. [DOI] [PubMed] [Google Scholar]
- 31.Glazer ES, Zhu C, Hamir AN, Borne A, Thompson CS, Curley SA. Biodistribution and acute toxicity of naked gold nanoparticles in a rabbit hepatic tumor model. Nanotoxicology. 2011;5:459–468. doi: 10.3109/17435390.2010.516026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asharani PV, Lianwu Y, Gong Z, Valiyaveettil S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology. 2011;5:43–54. doi: 10.3109/17435390.2010.489207. [DOI] [PubMed] [Google Scholar]
- 33.Gad SC, Sharp KL, Montgomery C, Payne JD, Goodrich GP. Evaluation of the toxicity of intravenous delivery of auroshell particles (gold-silica nanoshells) Int. J. Toxicol. 2012;31:584–594. doi: 10.1177/1091581812465969. [DOI] [PubMed] [Google Scholar]
- 34.Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004;49:N309–N315. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 35.Jain S, Coulter JA, Hounsell AR, Butterworth KT, McMahon SJ, Hyland WB, Muir MF, Dickson GR, Prise KM, Currell FJ, O’Sullivan JM, Hirst DG. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int. J. Radiat. Oncol., Biol., Phys. 2011;79:531–539. doi: 10.1016/j.ijrobp.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butterworth KT, McMahon SJ, Currell FJ, Prise KM. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale. 2012;4:4830–4838. doi: 10.1039/c2nr31227a. [DOI] [PubMed] [Google Scholar]
- 37.Kauer-Dorner D, Potter R, Resch A, Handl-Zeller L, Kirchheiner K, Meyer-Schell K, Dorr W. Partial breast irradiation for locally recurrent breast cancer within a second breast conserving treatment: alternative to mastectomy? Results from a prospective trial. Radiother. Oncol.: J. Eur. Soc. Therap. Radiol. Oncol. 2012;102:96–101. doi: 10.1016/j.radonc.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loo C, Lowery A, Halas N, West J, Drezek R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5:709–711. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 40.Lowery AR, Gobin AM, Day ES, Halas NJ, West JL. Immunonanoshells for targeted photothermal ablation of tumor cells. Int. J. Nanomed. 2006;1:149–154. doi: 10.2147/nano.2006.1.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loo C, Lin A, Hirsch L, Lee MH, Barton J, Halas N, West J, Drezek R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol. Cancer Res. Treat. 2004;3:33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- 42.Zharov VP, Galitovskaya EN, Johnson C, Kelly T. Synergistic enhancement of selective nanophotothermolysis with gold nanoclusters: potential for cancer therapy. Lasers Surg. Med. 2005;37:219–226. doi: 10.1002/lsm.20223. [DOI] [PubMed] [Google Scholar]
- 43.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol./Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 44.Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2005;23:3079–3085. doi: 10.1200/JCO.2005.05.520. [DOI] [PubMed] [Google Scholar]
- 45.Zagar TM, Oleson JR, Vujaskovic Z, Dewhirst MW, Craciunescu OI, Blackwell KL, Prosnitz LR, Jones EL. Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: a review of the randomised data. Int. J. Hyperthermia: Off. J. Eur. Soc. Hyperthermic Oncol., North American Hyperthermia Group. 2010;26:612–617. doi: 10.3109/02656736.2010.487194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atkinson RL, Zhang M, Diagaradjane P, Peddibhotla S, Contreras A, Hilsenbeck SG, Woodward WA, Krishnan S, Chang JC, Rosen JM. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci. Transl. Med. 2010;2:55ra79. doi: 10.1126/scitranslmed.3001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11:169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 48.Huschka R, Zuloaga J, Knight MW, Brown LV, Nordlander P, Halas NJ. Light-induced release of DNA from gold nanoparticles: nanoshells and nanorods. J. Am. Chem. Soc. 2011;133:12247–12255. doi: 10.1021/ja204578e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibson JD, Khanal BP, Zubarev ER. Paclitaxel-functionalized gold nanoparticles. J. Am. Chem. Soc. 2007;129:11653–11661. doi: 10.1021/ja075181k. [DOI] [PubMed] [Google Scholar]
- 50.Liu H, Chen D, Li L, Liu T, Tan L, Wu X, Tang F. Multifunctional gold nanoshells on silica nanorattles: a platform for the combination of photothermal therapy and chemotherapy with low systemic toxicity. Angew. Chem. Int. Ed. Engl. 2011;50:891–895. doi: 10.1002/anie.201002820. [DOI] [PubMed] [Google Scholar]
- 51.Kim ST, Chompoosor A, Yeh YC, Agasti SS, Solfiell DJ, Rotello VM. Dendronized gold nanoparticles for siRNA delivery. Small. 2012 doi: 10.1002/smll.201201141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu C, Wang B, Sun S. Dumbbell-like Au-Fe3O4 nanoparticles for target-specific platin delivery. J. Am. Chem. Soc. 2009;131:4216–4217. doi: 10.1021/ja900790v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joshi P, Chakraborti S, Ramirez-Vick JE, Ansari ZA, Shanker V, Chakrabarti P, Singh SP. The anticancer activity of chloroquine-gold nanoparticles against MCF-7 breast cancer cells. Colloid Surface B. 2012;95:195–200. doi: 10.1016/j.colsurfb.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 54.Kuo TR, Hovhannisyan VA, Chao YC, Chao SL, Chiang SJ, Lin SJ, Dong CY, Chen CC. Multiple release kinetics of targeted drug from gold nanorod embedded polyelectrolyte conjugates induced by near-infrared laser irradiation. J. Am. Chem. Soc. 2010;132:14163–14171. doi: 10.1021/ja105360z. [DOI] [PubMed] [Google Scholar]
- 55.You J, Zhang R, Zhang G, Zhong M, Liu Y, Van Pelt CS, Liang D, Wei W, Sood AK, Li C. Photothermal-chemotherapy with doxorubicin-loaded hollow gold nanospheres: a platform for near-infrared light-trigged drug release. J. Control Release: Off. J. Control. Release Soc. 2012;158:319–328. doi: 10.1016/j.jconrel.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chattopadhyay N, Cai Z, Pignol JP, Keller B, Lechtman E, Bendayan R, Reilly RM. Design and characterization of HER-2-targeted gold nanoparticles for enhanced X-radiation treatment of locally advanced breast cancer. Mol. Pharmaceut. 2010;7:2194–2206. doi: 10.1021/mp100207t. [DOI] [PubMed] [Google Scholar]
- 57.Chen J, Irudayaraj J. Quantitative investigation of compartmentalized dynamics of ErbB2 targeting gold nanorods in live cells by single molecule spectroscopy. ACS Nano. 2009;3:4071–4079. doi: 10.1021/nn900743v. [DOI] [PubMed] [Google Scholar]
- 58.Eghtedari M, Liopo AV, Copland JA, Oraevsky AA, Motamedi M. Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett. 2009;9:287–291. doi: 10.1021/nl802915q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res.: BCR. 2006;8:215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carpin LB, Bickford LR, Agollah G, Yu TK, Schiff R, Li Y, Drezek RA. Immunoconjugated gold nanoshell-mediated photothermal ablation of trastuzumab-resistant breast cancer cells. Breast Cancer Res. Treat. 2011;125:27–34. doi: 10.1007/s10549-010-0811-5. [DOI] [PubMed] [Google Scholar]
- 61.Li JL, Wang L, Liu XY, Zhang ZP, Guo HC, Liu WM, Tang SH. In vitro cancer cell imaging and therapy using transferrin-conjugated gold nanoparticles. Cancer Lett. 2009;274:319–326. doi: 10.1016/j.canlet.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 62.Jin H, Hong B, Kakar SS, Kang KA. Tumor-specific nano-entities for optical detection and hyperthermic treatment of breast cancer. Adv. Exp. Med. Biol. 2008;614:275–284. doi: 10.1007/978-0-387-74911-2_31. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W, Meng J, Ji Y, Li X, Kong H, Wu X, Xu H. Inhibiting metastasis of breast cancer cells in vitro using gold nanorod-siRNA delivery system. Nanoscale. 2011;3:3923–3932. doi: 10.1039/c1nr10573f. [DOI] [PubMed] [Google Scholar]
- 64.Park J, Estrada A, Sharp K, Sang K, Schwartz JA, Smith DK, Coleman C, Payne JD, Korgel BA, Dunn AK, Tunnell JW. Two-photon-induced photoluminescence imaging of tumors using near-infrared excited gold nanoshells. Optics Express. 2008;16:1590–1599. doi: 10.1364/oe.16.001590. [DOI] [PubMed] [Google Scholar]
- 65.Bickford L, Sun J, Fu K, Lewinski N, Nammalvar V, Chang J, Drezek R. Enhanced multi-spectral imaging of live breast cancer cells using immunotargeted gold nanoshells and two-photon excitation microscopy. Nanotechnology. 2008;19:315102. doi: 10.1088/0957-4484/19/31/315102. [DOI] [PubMed] [Google Scholar]
- 66.Bickford LR, Agollah G, Drezek R, Yu TK. Silica-gold nanoshells as potential intraoperative molecular probes for HER2-overexpression in ex vivo breast tissue using near-infrared reflectance confocal microscopy. Breast Cancer Res. Treat. 2010;120:547–555. doi: 10.1007/s10549-009-0408-z. [DOI] [PubMed] [Google Scholar]
- 67.Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Gold nanoparticles: a new X-ray contrast agent. Brit. J. Radiol. 2006;79:248–253. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 68.Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 69.Su CH, Sheu HS, Lin CY, Huang CC, Lo YW, Pu YC, Weng JC, Shieh DB, Chen JH, Yeh CS. Nanoshell magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2007;129:2139–2146. doi: 10.1021/ja0672066. [DOI] [PubMed] [Google Scholar]
- 70.Copland JA, Eghtedari M, Popov VL, Kotov N, Mamedova N, Motamedi M, Oraevsky AA. Bioconjugated gold nanoparticles as a molecular based contrast agent: implications for imaging of deep tumors using optoacoustic tomography. Mol. Imag. Biol.: MIB: The Off. Publ. Acad. Mol. Imag. 2004;6:341–349. doi: 10.1016/j.mibio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 71.Song KH, Kim C, Cobley CM, Xia Y, Wang LV. Near-infrared gold nanocages as a new class of tracers for photoacoustic sentinel lymph node mapping on a rat model. Nano Lett. 2009;9:183–188. doi: 10.1021/nl802746w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng L, Yang K, Li Y, Zeng X, Shao M, Lee ST, Liu Z. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials. 2012;33:2215–2222. doi: 10.1016/j.biomaterials.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 73.Fales AM, Yuan H, Vo-Dinh T. Silica-coated gold nanostars for combined surface-enhanced Raman scattering (SERS) detection and singlet-oxygen generation: a potential nanoplatform for theranostics. Langmuir: ACS J. Surf. Colloids. 2011;27:12186–12190. doi: 10.1021/la202602q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, West JL, Drezek RA. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7:169–183. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]
- 75.Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269:57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA: J. Am. Med. Assoc. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.