Abstract
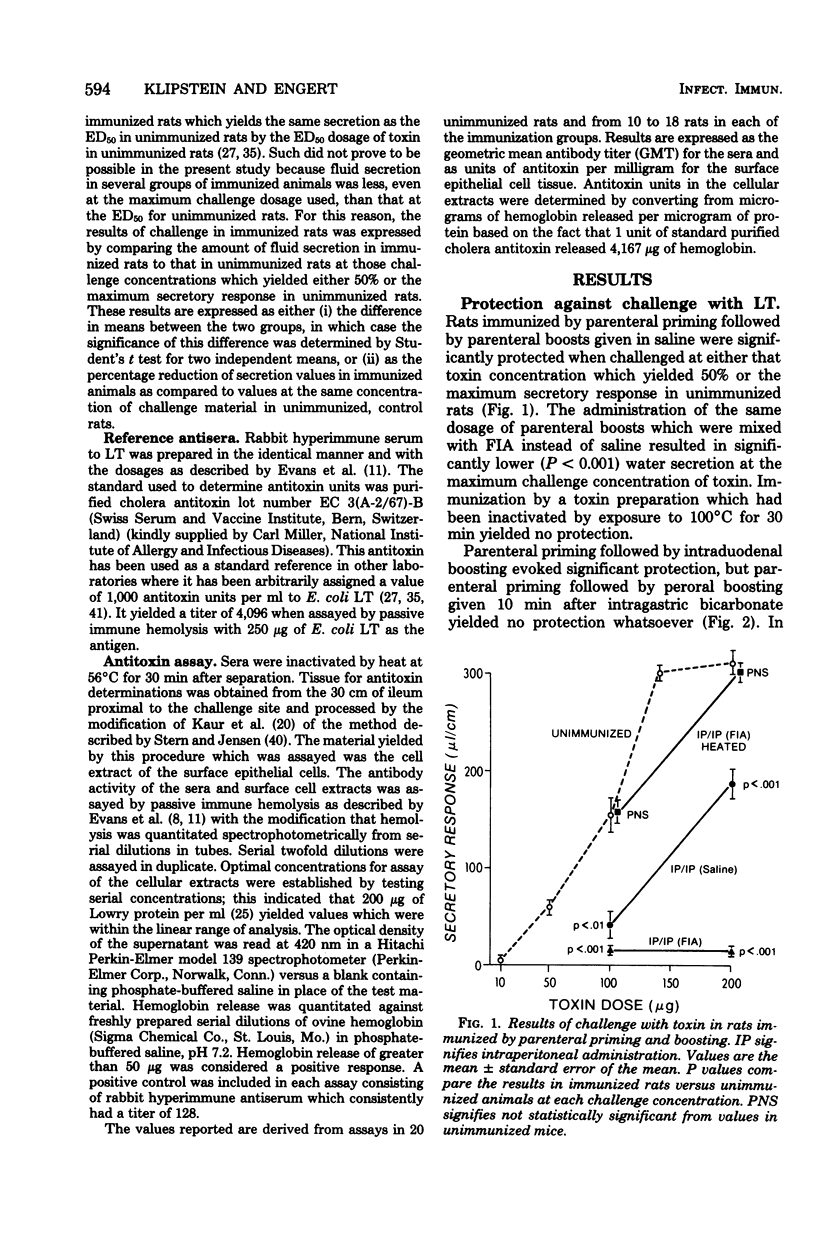
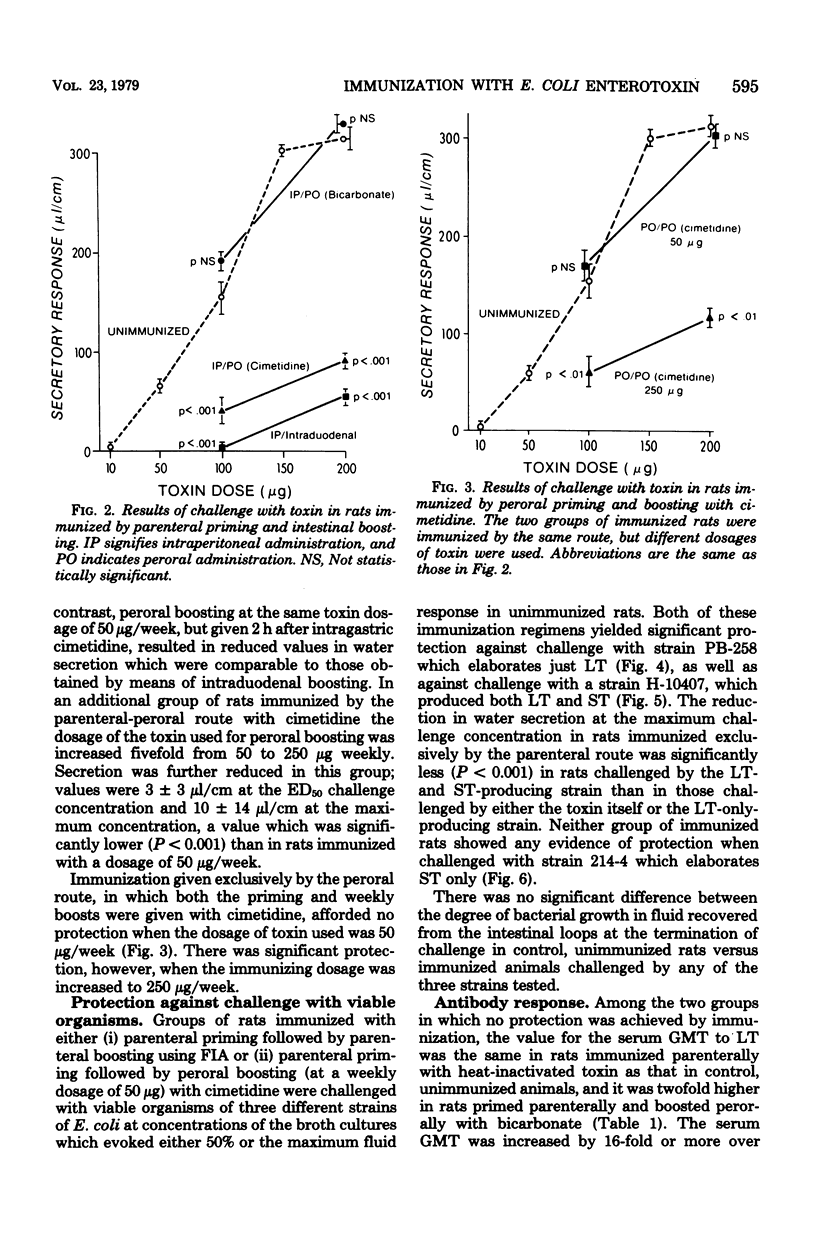
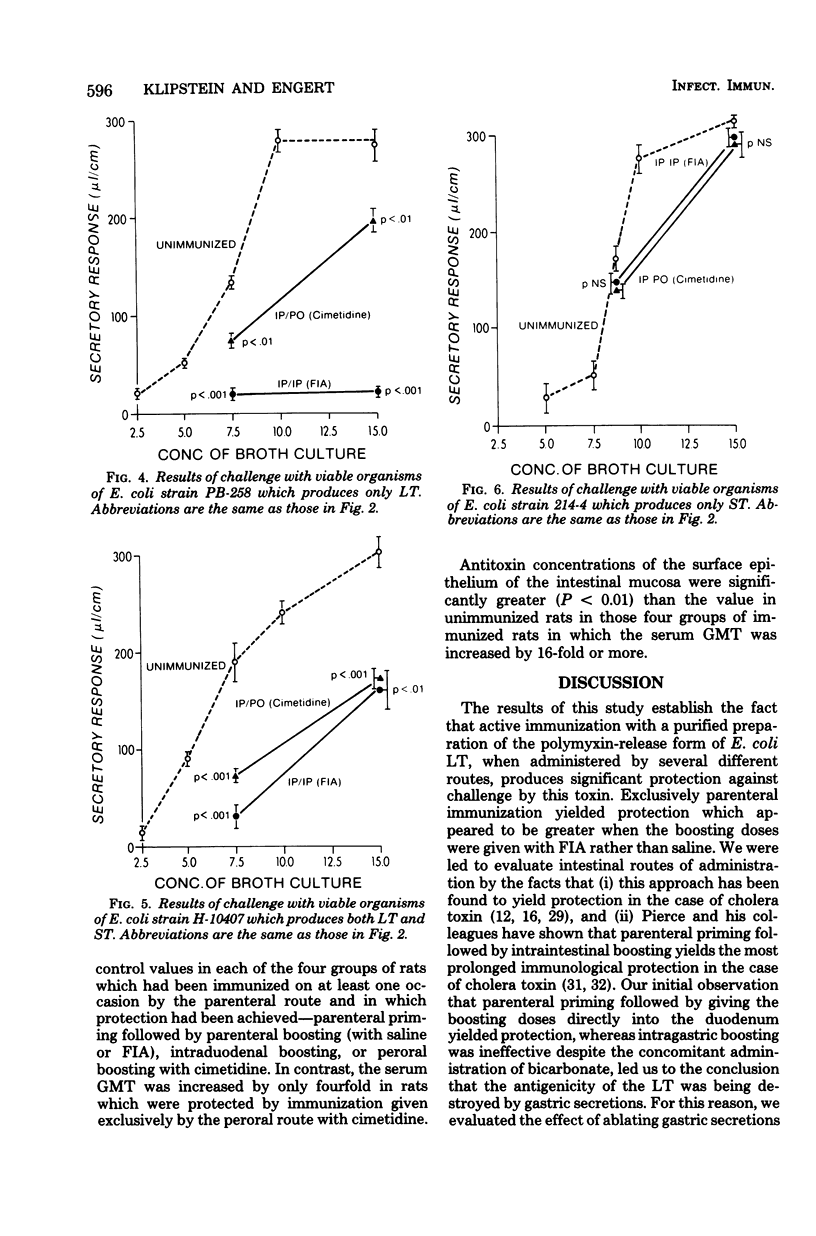
The protective effect of active immunization by different routes with a purified preparation of the polymyxin-release form of Escherichia coli heat-labile toxin was evaluated in rats. Immunized animals were challenged by placing toxin into ligated ileal loops at dosages which produced either 50% or the maximum secretory response in unimmunized rats. Immunization exclusively by the parenteral route yielded significant protection. Rats were also protected when parenteral priming was followed by boosting given either directly into the duodenum or perorally 2 h after intragastric cimetidine, but not when the peroral boosts were given with bicarbonate. Immunization administered entirely by the peroral route with cimetidine yielded protection but only when the immunizing dosage was fivefold greater than that found effective in the parenteral-peroral approach. Rats immunized exclusively by the parenteral route and those boosted perorally with cimetidine were also tested, and found to be protected, against challenge with viable organisms of strains that produce either heat-labile toxin alone or both heat-labile and heat-stable toxin, but they were not protected against a strain which produces just heat-stable toxin. Geometric mean serum antibody titers were increased by 16-fold or more over control values in those groups of rats in which protection was achieved, with the exception of those immunized exclusively by the peroral route. These observations demonstrate that (i) active immunization with purified E. coli heat-labile toxin results in significant protection against both this toxin as well as viable organisms which produce it, but not against viable strains which produce heat-stable toxin only, and (ii) concomitant ablation of gastric secretion by the use of cimetidine renders the peroral route of immunization effective. They suggest that prophylactic immunization against diarrheal disease caused by heat-labile toxin-producing strains of E. coli may be feasible in humans.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Robertson D. C. Purification and chemical characterization of the heat-stable enterotoxin produced by porcine strains of enterotoxigenic Escherichia coli. Infect Immun. 1978 Mar;19(3):1021–1030. doi: 10.1128/iai.19.3.1021-1030.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benenson A. S., Mosley W. H., Fahimuddin M., Oseasohn R. O. Cholera vaccine field trials in east Pakistan. 2. Effectiveness in the field. Bull World Health Organ. 1968;38(3):359–372. [PMC free article] [PubMed] [Google Scholar]
- Brimblecombe R. W., Duncan W. A., Durant G. J., Emmett J. C., Ganellin C. R., Leslie G. B., Parsons M. E. Characterization and development of cimetidine as a histamine H2-receptor antagonist. Gastroenterology. 1978 Feb;74(2 Pt 2):339–347. [PubMed] [Google Scholar]
- Etkin S., Gorbach S. L. Studies on enterotoxin from Escherichia coli associated with acute diarrhea in man. J Lab Clin Med. 1971 Jul;78(1):81–87. [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Pierce N. F. Differences in the response of rabbit small intestine to heat-labile and heat-stable enterotoxins of Escherichia coli. Infect Immun. 1973 Jun;7(6):873–880. doi: 10.1128/iai.7.6.873-880.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Evans D. G., Gorbach S. L. Polymyxin B-Induced Release of Low-Molecular-Weight, Heat-Labile Enterotoxin from Escherichia coli. Infect Immun. 1974 Nov;10(5):1010–1017. doi: 10.1128/iai.10.5.1010-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G. Direct serological assay for the heat-labile enterotoxin of Escherichia coli, using passive immune hemolysis. Infect Immun. 1977 May;16(2):604–609. doi: 10.1128/iai.16.2.604-609.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Ruiz-Palacios G., Evans D. E., DuPont H. L., Pickering L. K., Olarte J. Humoral immune response to the heat-labile enterotoxin of Escherichia coli in naturally acquired diarrhea and antitoxin determination by passive immune hemolysis. Infect Immun. 1977 Jun;16(3):781–788. doi: 10.1128/iai.16.3.781-788.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Finkelstein R. A. Antitoxic immunity in experimental cholera: comparison of immunity induced perorally and parenterally in mice. J Infect Dis. 1972 Jun;125(6):647–655. doi: 10.1093/infdis/125.6.647. [DOI] [PubMed] [Google Scholar]
- Gyles C. L., Barnum D. A. A heat-labile enterotoxin from strains of Eschericha coli enteropathogenic for pigs. J Infect Dis. 1969 Oct;120(4):419–426. doi: 10.1093/infdis/120.4.419. [DOI] [PubMed] [Google Scholar]
- Gyles C. L. Immunological study of the heat-labile enterotoxins of Escherichia coli and Vibrio cholerae. Infect Immun. 1974 Mar;9(3):564–570. doi: 10.1128/iai.9.3.564-570.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Andersson A., Wallerstrom G., Ouchterlony O. Experimental studies on cholera immunization. II. Evidence for protective antitoxic immunity mediated by serum antibodies as well as local antibodies. Infect Immun. 1972 May;5(5):662–667. doi: 10.1128/iai.5.5.662-667.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Lönnroth I., Fall-Persson M., Markman B., Lundbeck H. Development of improved cholera vaccine based on subunit toxoid. Nature. 1977 Oct 13;269(5629):602–604. doi: 10.1038/269602a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Ouchterlony O., Anderson A., Walletström G., Westerberg-Berndtsson U. Antitoxic immunity in experimental cholera: protection, and serum and local antibody responses in rabbits after enteral and parenteral immunization. Infect Immun. 1975 Dec;12(6):1331–1340. doi: 10.1128/iai.12.6.1331-1340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Söderlind O., Wadström T. Cross-reactivity between heat labile enterotoxins of Vibrio cholerae and Escherichia coli in neutralization tests in rabbit ileum and skin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Dec;81(6):757–762. doi: 10.1111/j.1699-0463.1973.tb02272.x. [DOI] [PubMed] [Google Scholar]
- Kaur J., Burrows W., Furlong M. A. Immunity to cholera: antibody response in the lower ileum of the rabbit. J Infect Dis. 1971 Oct;124(4):359–366. doi: 10.1093/infdis/124.4.359. [DOI] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Immunological interrelationships between cholera toxin and the heat-labile and heat-stable enterotoxins of coliform bacteria. Infect Immun. 1977 Oct;18(1):110–117. doi: 10.1128/iai.18.1.110-117.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Immunological relationship of different preparations of coliform enterotoxins. Infect Immun. 1978 Sep;21(3):771–778. doi: 10.1128/iai.21.3.771-778.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler E. M., Cross R. F. Feeding bacteria-free whole cell lysates of Escherichia coli to gnotobiotic pigs and the effects of giving antiserums. Am J Vet Res. 1971 May;32(5):739–748. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine M. M., Caplan E. S., Waterman D., Cash R. A., Hornick R. B., Snyder M. J. Diarrhea caused by Escherichia coli that produce only heat-stable enterotoxin. Infect Immun. 1977 Jul;17(1):78–82. doi: 10.1128/iai.17.1.78-82.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merson M. H., Morris G. K., Sack D. A., Wells J. G., Feeley J. C., Sack R. B., Creech W. B., Kapikian A. Z., Gangarosa E. J. Travelers' diarrhea in Mexico. A prospective study of physicians and family members attending a congress. N Engl J Med. 1976 Jun 10;294(24):1299–1305. doi: 10.1056/NEJM197606102942401. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaniecki E. A., Northrup R. S. Protection against experimental cholera by antitoxin. J Infect Dis. 1972 Dec;126(6):606–616. doi: 10.1093/infdis/126.6.606. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. Protection against challenge with Escherichia coli heat-labile enterotoxin by immunization of rats with cholera toxin/toxoid. Infect Immun. 1977 Nov;18(2):338–341. doi: 10.1128/iai.18.2.338-341.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Reynolds H. Y. Immunity to experimental cholera. II. Secretory and humoral antitoxin response to local and systemic toxoid administration. J Infect Dis. 1975 Apr;131(4):383–389. doi: 10.1093/infdis/131.4.383. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B. Immune response of the intestinal mucosa to cholera toxoid. J Infect Dis. 1977 Aug;136 (Suppl):S113–S117. doi: 10.1093/infdis/136.supplement.s113. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Sircar B. K. Immunity to experimental cholera. III. Enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977 Jun;135(6):888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- Ryder R. W., Wachsmuth I. K., Buxton A. E., Evans D. G., DuPont H. L., Mason E., Barrett F. F. Infantile diarrhea produced by heat-stable enterotoxigenic Escherichia coli. N Engl J Med. 1976 Oct 14;295(16):849–853. doi: 10.1056/NEJM197610142951601. [DOI] [PubMed] [Google Scholar]
- Sack D. A., Merson M. H., Wells J. G., Sack R. B., Morris G. K. Diarrhoea associated with heat-stable enterotoxin-producing strains of Escherichia coli. Lancet. 1975 Aug 9;2(7928):239–241. doi: 10.1016/s0140-6736(75)90958-7. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Froehlich J. L. Antigenic similarity of heat-labile enterotoxins from diverse strains of Escherichia coli. J Clin Microbiol. 1977 Jun;5(6):570–572. doi: 10.1128/jcm.5.6.570-572.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B. Immunization with Escherichia coli enterotoxin protects against homologous enterotoxin challenge. Infect Immun. 1973 Oct;8(4):641–644. doi: 10.1128/iai.8.4.641-644.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B., Johnson J., Pierce N. F., Keren D. F., Yardley J. H. Challenge of dogs with live enterotoxigenic Escherichia coli and effects of repeated challenges on fluid secretion in jejunal Thiry-Vella loops. J Infect Dis. 1976 Jul;134(1):15–24. doi: 10.1093/infdis/134.1.15. [DOI] [PubMed] [Google Scholar]
- Smith H. W. The production of diarrhoea in baby rabbits by the oral administration of cell-free preparations of enteropathogenic Escherichia coli and Vibrio cholerae: the effect of antisera. J Med Microbiol. 1972 Aug;5(3):299–303. doi: 10.1099/00222615-5-3-299. [DOI] [PubMed] [Google Scholar]
- Smith N. W., Sack R. B. Immunologic cross-reactions of enterotoxins from Escherichia coli and Vibrio cholerae. J Infect Dis. 1973 Feb;127(2):164–170. doi: 10.1093/infdis/127.2.164. [DOI] [PubMed] [Google Scholar]
- Stern B. K., Jensen W. E. Active transport of glucose by suspensions of isolated rat intestinal epithelial cells. Nature. 1966 Feb 19;209(5025):789–790. doi: 10.1038/209789a0. [DOI] [PubMed] [Google Scholar]
- Wachsmuth I. K., Wells J. G., Ryder R. W. Escherichia coli heat-labile enterotoxin: comparison of antitoxin assays and serum antitoxin levels. Infect Immun. 1977 Nov;18(2):348–351. doi: 10.1128/iai.18.2.348-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



