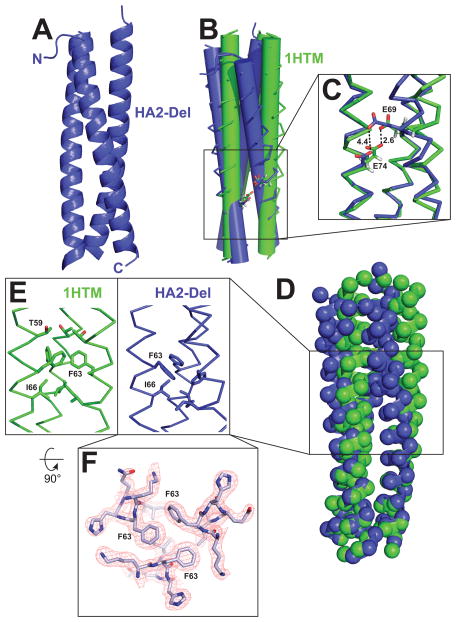
Figure 3. Crystal structure of the HA2-Del trimer and comparison to WT HA2 (PDB ID 1HTM).
(A) Overall structure of the HA2-coiled-coil. (B) Overlay of α-helical bundles for WT HA2 and HA2-Del; the α-helices are represented as cylinders to illustrate differences in α-helical packing geometry. (C) Potential hydrogen bonding interaction between E69 and E74. In HA2-Del, the residues are separated by 4.5 Å. (D) Locations of Cα carbons by spheres, demonstrating that although there are some differences in overall positioning of residues, there are no gross deviations between the two structures. (E) Differences in packing arrangement of F63 and I66 in the trimer cores. (F) Top-down view of the F63 interaction at the α-helical interface, with sample electron density.