Abstract
BACKGROUND
Mouse embryonic stem cells null for Rad9 are sensitive to deleterious effects of ionizing radiation exposure. Likewise, integrin β1 is a known radioprotective factor. Previously, we showed that RAD9 downregulation in human prostate cancer cells reduces integrin β1 protein levels and ectopic expression of Mrad9 restores inherent high levels.
METHODS
We used RNA interference to knockdown Rad9 expression in PC3 and DU145 prostate cancer cells. These cells were then subjected to ionizing radiation, and integrin β1 protein levels were measured by immunoblotting. Survival of irradiated cells was measured by clonogenicity, cell cycle analysis, PARP-1 cleavage, and trypan blue exclusion.
RESULTS
The function of RAD9 in controlling integrin β1 expression is unique and not shared by the other members of the 9-1-1 complex, HUS1 and RAD1. RAD9 or integrin β1 silencing sensitizes DU145 and PC3 cells to ionizing radiation. Irradiation of DU145 cells with low levels of RAD9 induces cleavage of PARP-1 protein. High levels of ionizing radiation have no effect on integrin β1 protein levels. However, when RAD9 downregulation is combined with 10 Gy of ionizing radiation in DU145 or PC3 cells, there is an additional 50% downregulation of integrin β1 compared with levels in unirradiated RAD9 knockdown cells. Finally, PC3 cells growing on fibronectin display increased radioresistance. However, PC3 cells with RAD9 knockdown are no longer protected by fibronectin after treatment with ionizing radiation.
CONCLUSIONS
Downregulation of RAD9 when combined with ionizing radiation results in reduction of ITGB1 protein levels in prostate cancer cells, and increased lethality.
Keywords: RAD9, integrin β1, ionizing radiation, prostate cancer
INTRODUCTION
Prostate cancer is the second leading cause of cancer-related death among men in the United States [1]. Radiation therapy is one of the mainstays of treatment for localized prostate tumors. Initially, tumors respond well to ionizing radiation (IR); however, often radiation resistant tumors arise. For this reason much effort has been devoted to the development of new interventions that will sensitize tumors to IR. Several factors contribute to intrinsic tumor radioresistance, including DNA damage response and repair pathways, as well as cell-cell and cell-extracellular matrix interactions, and are thus targets for promoting radiosensitization [2].
RAD9 is best known for its role in the DNA damage response and DNA repair. As part of the RAD9-HUS1-RAD1 complex, it acts as a sensor of DNA damage that enables ATR kinase, independently recruited to the site of damage, to phosphorylate and activate its downstream effector CHK1 [3]. Moreover, RAD9, either alone or as part of the 9-1-1 complex, can potentiate the action of a number of DNA repair pathways, including base excision repair, nucleotide excision repair, homologous recombination repair, and mismatch repair (reviewed in [4]). However, RAD9 can interact with several other proteins outside the context of the 9-1-1 complex and checkpoint functions [4]. Interestingly, RAD9 can act independently of its partners HUS1 and RAD1 to transactivate a number of genes, including p21waf1/cip1 [6]. Aberrant RAD9 expression has been associated with prostate, breast, lung, skin, thyroid, and gastric cancers [4]. RAD9 is aberrantly overexpressed in human prostate cancer specimens as well as prostate cancer cell lines [7]. Downregulation of RAD9 in PC3 and DU145 human tumor cell line xenografts impairs growth in nude mice. Furthermore, immunohistochemical analysis of normal and tumor prostate specimens showed that RAD9 protein abundance increased along with the advancement of cancer stages, suggesting a role for RAD9 in prostate malignant progression [7].
Previously, we have shown that RAD9 downregulation hampers migration and invasion as well as anchorage-independent growth of prostate cancer cells [5], whereas ectopic expression of Mrad9, the mouse homolog, restores these traits. Importantly, RAD9 controls expression of integrin β1, but not other integrins, such as β3, α2, or α5, in DU145 and LNCaP cells, although the mechanism is not known [5].
Integrins form a large family of αβ heterodimeric receptors that mediate cell adhesion to components of the extracellular matrix or heterotypic cell-cell interactions such as between tumor cells and endothelial cells. Moreover, integrin-mediated interactions activate signals that regulate a number of processes, including cell migration and invasion, proliferation, and differentiation. Tumor cells rely on adhesion to extracellular matrix in order to promote survival and proliferation. In addition, cell adhesion to an extracellular matrix protein, such as fibronectin, can have profound effects on the radioresistance of tumors. Integrins play a central role in facilitating these interactions and not surprisingly they also induce radioresistance in numerous human cell types. This phenomenon is called cell adhesion mediated radioresistance [8].
Integrin β1 (ITGB1) protein levels and activity are often modulated by IR in a great number of human tumors and cancer cell lines [9, 10], and abundance of integrin β1 has been associated with radioresistance in many human tumors such as breast, and head and neck carcinoma [11, 12]. The ability of ITGB1 to confer radioresistance to human prostate cancer has only recently been explored. Goel and colleagues found that integrin β1 downregulation sensitized prostate cancer cell lines to IR [13], whereas in another study, fractionated irradiation of prostate cancer cell lines DU145 and PC3 results in downregulation of ITGB1 protein accompanied by decreased adhesion of cells to fibronectin and increased apoptotic index [14].
In this report, we show that ionizing radiation exposure alone causes modest levels of DU145 or PC3 cell death. On the other hand, we demonstrate that reduction of RAD9 or ITGB1 protein levels by RNA interference in these cells enhances radiation-induced lethality, as determined by sub-G1 content and trypan blue exclusion. Furthermore, irradiation of DU145 cells with RAD9 knockdown causes production of two poly [ADP-ribose] polymerase 1 (PARP-1) cleavage fragments, one at 89kDa, which is associated with apoptosis, and a second fragment at approximately 65kDa, which is indicative of programmed necrotic death [15]. Clonogenic survival assays show that the degree of PC3/shRad9 or DU145/shRad9 cell killing is comparable to that of PC3/shRad9/siITGB1 or DU145/shRad9/siITGB1 cells, respectively, suggesting that RAD9 knockdown may radiosensitize PC3 and DU145 cells via reduction of ITGB1 protein levels. Finally, irradiated PC3 cells with RAD9 knockdown, and therefore diminished levels of ITGB1, are no longer radioprotected when attached to fibronectin, relative to PC3 cells with normal, inherent levels of RAD9.
MATERIALS AND METHODS
Cell culture
Prostate cancer cells DU145 and PC3 were grown at 37°C, 5% CO2 in RPMI 1640 (Invitrogen), supplemented with 8% fetal bovine serum (FBS; Atlanta Biologicals), 100 units/ml penicillin, 100 µg/ml streptomycin, and 2.5 µg/ml fungizone (Invitrogen).
Irradiation
Subconfluent cell cultures were exposed to γ-rays at room temperature with the indicated doses by an Atomic Energy of Canada Gammacell 40 Cesium-137 Unit, providing a dose rate of 0.8 Gy/min.
Clonogenic Survival Assay
To assess clonogenic survival, DU145 cells stably expressing shControl or shRad9 were transiently transfected with either Luciferase or Itgb1 siRNA. Forty-eight hours later, cells were trypsinized, counted and added at 200 cells/well (DU145) or 400 cells/well (PC3) into 12-well plates in triplicate, whereas the experiment in Figure 5A was carried out by plating 1000 cells/well in 6-well plates in triplicate. Four to six hours later (or next day for Figure 5A), cells were irradiated with 0, 2, 4, 6, or 8 Gy (DU145) or 0, 1, 2, 4, or 6 Gy (PC3) and incubated for 8–11 days. At the end of the incubation period, cells were fixed with 100% cold (−20°C) methanol for 20 min, washed once with PBS and stained with 0.5% crystal violet diluted in 20% methanol for 20 min. Colonies with more than 50 cells were counted under a microscope. The surviving fraction was calculated as number of colonies formed after irradiation relative to unirradiated control.
FIG. 5.
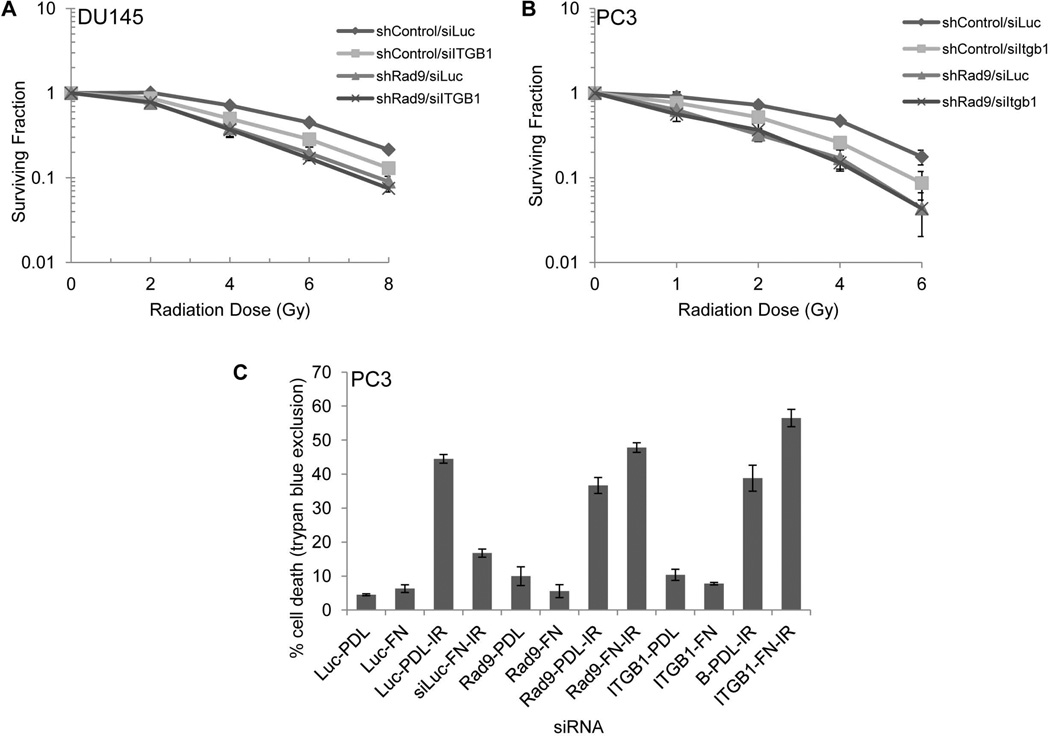
Fibronectin confers protection against ionizing radiation, which is abrogated in PC3/siRad9 cells. A: Clonogenic survival. DU145 cells stably expressing shRad9 or insertless vector (shControl) were transiently transfected with 30 nM Itgb1 siRNA. Three days post-transfection, cells were plated in triplicate and irradiated with indicated doses. Colonies were allowed to form for 11 days, then fixed and stained with crystal violet. B: Clonogenic survival. PC3 cells stably expressing shRad9 or insertless vector (shControl) were transiently transfected with 30 nM Itgb1 siRNA. Three days post-transfection, cells were plated in triplicate and irradiated with indicated doses. Colonies were allowed to form for 8 days, then fixed and stained with crystal violet. Shown, mean ± SD (n=3). C: PC3 cells transiently expressing Luciferase, Rad9, or Itgb1 siRNA were grown on plates treated with either poly-D-lysine (PDL; 100 µg/ml) or fibronectin (FN; 10 µg/ml). Cells were irradiated with 10 Gy or left unirradiated and 48h later cell death was assessed by trypan blue exclusion. Shown, mean ± SD (n=3). Representative experiment of two independent trials performed in triplicate. Shown, mean ± SD.
RNA interference and plasmid construction
The pSUPER.retro.puro Rad9 shRNA expression vector (Oligoengine, Inc.) and viral production have been described [7]. The establishment of stable DU145 clones with reduced levels of RAD9 has been reported [5]. Additionally, we isolated a clone of PC3 cells that stably downregulates RAD9 (Supplemental Fig. 1B). Down-regulation of RAD9 protein was assessed by Western blotting with RAD9 antibody (BD Biosciences).
RNA interference experiments were carried out with siRNA against Rad9 (two non-overlapping siRNAs denoted 1 and 2), Itgb1, and Luciferase (siLuc) that served as control. The sequences and concentrations of these siRNA have been reported [5]. In addition, Hus1 and Rad1 specific siRNAs were purchased from Ambion and used at 20 and 30 nM concentration, respectively. For transient transfection, cells at approximately 30% confluency were transfected with the indicated siRNA using Lipofectamine 2000 (Invitrogen), and protein abundance was examined 48–72h later by western blot analysis.
Integrin β1 cDNA was generated as described [5]. However, mRNA was extracted from PC3 cells instead of DU145 cells.
Cell cycle analysis
Cells were harvested by trypsinization, washed in PBS and fixed in 70% ethanol overnight at −20°C. Fixed cells were subsequently washed in PBS once and incubated with propidium iodide/RNase staining buffer (BD Biosciences) for 30 min at 37°C. Flow cytometry was performed by a FACScalibur in conjunction with CellQuest software (BD Biosciences).
Cell adhesion to fibronectin and irradiation
PC3 cells were transiently transfected with siLuc, siRad9, or siItgb1. Two days post-transfection, cells were serum-starved for 24h. Subsequently, cells were detached from the plate by incubating with 5 mM EDTA/Dulbecco’s PBS, washed in DPBS, counted with a hemocytometer, and added at equal numbers (1×105) to either poly-D-lysine (100 µg/ml; Sigma) or fibronectin (10 µg/ml; BD Biosciences) coated 6-well plates. Cells were allowed to attach for 1h and then were exposed to 10 Gy of ionizing radiation or left untreated. Twenty-four hours later, cell viability was assessed by a trypan blue exclusion assay.
Western blot analysis and subcellular fractionation
Cells were lysed in radioimmune precipitation assay (RIPA) buffer as described before [5]. Protein was subjected to either 8% Tris-glycine or 4–12% Bis/Tris (Invitrogen) SDS-PAGE. Quantitation of band intensity was performed with ImageJ software v1.47f (http://rsb.info.nih.gov/ij/). The following antibodies were used in this study: RAD9, integrin β1, integrin α2, integrin α5, PARP-1 monoclonal antibodies (BD Biosciences), β-actin, and α-tubulin monoclonal antibodies (Sigma).
To isolate the nuclear fraction, cells from three 100-mm culture dishes were fractionated using a NE-PER nuclear and cytoplasmic extraction kit (Thermo Scientific), following the manufacturer’s instructions. PARP-1 and α-tubulin were used as nuclear and cytosolic markers respectively.
Quantitative Real-time PCR
Trizol reagent (Invitrogen) was used to isolate mRNA. One microgram of mRNA was reverse-transcribed to cDNA using the Superscript III transcriptase First Strand Synthesis System (Invitrogen). Real-time PCR was carried out in triplicate using SYBR Green PCR Master Mix on the Vii7 Real-Time PCR instrument. The primers for ITGB1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) have been published [5]. Expression levels were normalized to GAPDH levels. Analysis of relative gene expression was performed using the 2−ΔΔCT method [40].
Statistical Analysis
Data were represented as mean ± standard deviation (S.D.). p values were calculated by a paired two-sided Student’s t-test, and values ≤ 0.05 were considered statistically significant.
RESULTS
Downregulation of RAD9, but not HUS1 or RAD1, reduces ITGB1 levels in prostate cancer cell lines
We showed previously that RAD9 controls expression of integrin β1 in DU145 and LNCaP human prostate cancer cells [5]. Silencing of Rad9 downregulates specifically the abundance of ITGB1 protein, whereas other integrins such as β3, α2, or α5 were unaffected [5]. In contrast, ectopic expression of Mrad9, the mouse homolog, in DU145 cells with low endogenous RAD9 mediated by shRNA increased ITGB1 levels. In addition to DU145 and LNCaP, PC3 cells show reduced amounts of ITGB1 when RAD9 is either transiently or stably downregulated (Supplemental Fig. 1A, B).
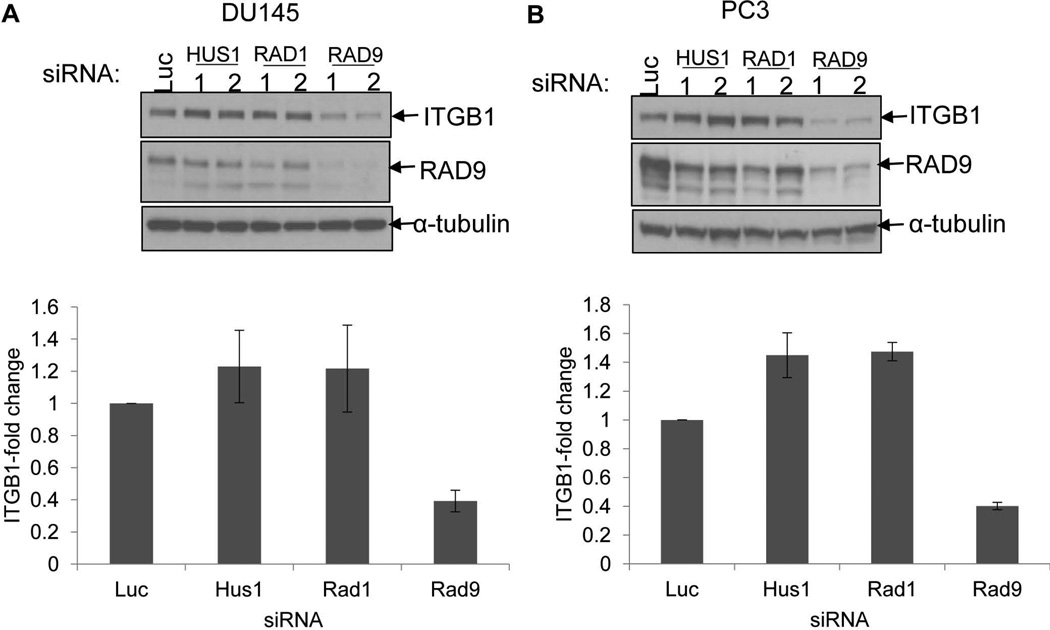
RAD9 functions in cell cycle checkpoint control and DNA repair as part of the RAD9-HUS1-RAD1 (9-1-1) heterotrimeric complex. However, RAD9 can act independently of this complex in various physiological contexts [4]. To find out whether the other partners of RAD9 in 9-1-1 are able to affect expression of ITGB1, we transiently downregulated Hus1 and Rad1 with two specific siRNAs for each gene in DU145 and PC3 cells, and achieved 80–90% and 35–55% reduction in endogenous HUS1 and RAD1 protein levels, respectively (Supplemental Fig. 2). We also silenced Rad9 with two different siRNAs, or used a control Luciferase (Luc) siRNA, and two (DU145 cells) or three (PC3 cells) days post-transfection ITGB1 protein levels were analyzed by Western blot. As expected, knockdown of Rad9 in DU145 cells by two different siRNAs (1, 2) caused a reduction in ITGB1 protein levels to 39.3 ± 6.7% compared to cells transfected with control Luciferase siRNA (Fig. 1A). Likewise, silencing of Rad9 in PC3 diminished ITGB1 protein levels to 40.2 ± 2.5%, when compared to Luciferase control siRNA cells (Fig. 1B). On the other hand, neither HUS1 nor RAD1 downregulation had any appreciable effect on ITGB1 protein abundance. Therefore, we conclude that RAD9 uniquely affects ITGB1 expression and, most likely, acts, in this regard, independently of the 9-1-1 complex.
FIG. 1.
RAD9, but not HUS1 nor RAD1, controls expression levels of ITGB1. A: Top panel, DU145 cells were transiently transfected with siRNA against Luciferase (control), two of each Hus1, Rad1, and Rad9 siRNAs and two days later cell lysates were examined for ITGB1 protein abundance. Bottom panel, relative levels of ITGB1 protein normalized against α-tubulin. Shown, mean ± S.D. (n=4). B: Top panel, PC3 cells were transiently transfected with siRNA against Luciferase (control), or two different siRNAs for each of Hus1, Rad1, and Rad9, and three days later cell lysates were examined for ITGB1 protein levels. Bottom panel, quantitation of ITGB1 relative to α-tubulin. Shown, mean ± S.D. (n=3). Each experiment in this Figure was conducted with two different siRNAs for Hus1, Rad1, and Rad9, and ITGB1 protein levels were averaged.
Targeting RAD9 or ITGB1 sensitizes DU145 prostate cancer cells to ionizing radiation
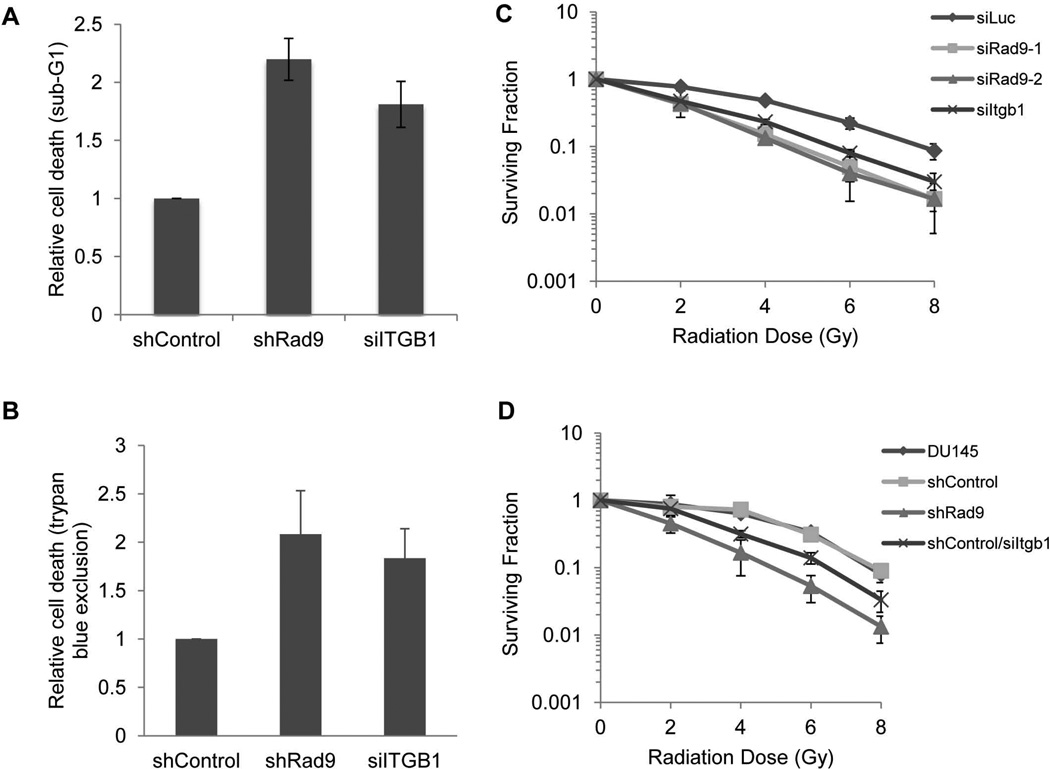
Rad9 confers radioresistance to mouse embryonic stem cells [16, 17]. However, the role of Rad9 in radioresistance of human prostate cancer cell lines has not been defined. To demonstrate this, we depleted RAD9 from DU145 cells and examined their survival in response to ionizing radiation. DU145 is known to be a highly radioresistant cell line [18]. When DU145 cells expressing shControl (insertless vector) were irradiated with 10 Gy and cell death was examined three days later by either flow cytometry (sub-G1 content) (Fig. 2A) or the trypan blue exclusion assay (Fig. 2B) no increased cell death above control was measured in irradiated cells. In contrast, when the DU145 population stably expressing shRad9 was irradiated with 10 Gy, the cells became sensitized to IR and cell death assayed three days later was increased two-fold compared with DU145/shControl cells.
FIG. 2.
RAD9 or ITGB1 downregulation sensitizes DU145 cells to ionizing radiation. A: DU145 cells were irradiated with 10 Gy and 48h later cell death (sub-G1) was quantified by flow cytometry analysis. Induced cell death was normalized against values for unirradiated (UI) cells. Control values were set at 1 and results for cells with shRad9 or siItgb1 were expressed as fold change compared with the shControl. Data represent the average from two independent experiments. B: DU145 cells were unirradiated or irradiated with 10 Gy, and 48h later cell death was measured by trypan blue exclusion. Quantitation was performed as in (A). Shown, mean ± SD (n=3). C: Clonogenic survival. DU145 cells transiently expressing two different siRad9, siItgb1, or control siLuc. Two days post-transfection, cells were plated in triplicate and irradiated with indicated doses. Shown, mean ± SD (n=3). D: Clonogenic survival. Parental DU145 cells or cells stably expressing insertless shRNA vector (shControl), shRad9, or control cells (shControl) transiently transfected with siItgb1 were irradiated with indicated doses. Shown, mean ± SD (n=3).
Integrin β1 confers protection from the deleterious effects of ionizing radiation in many cancer cell types, including those of breast and prostate origin [11, 13]. Reduced expression of endogenous ITGB1 by RNA interference does not affect cell survival [5, 19]. As mentioned above, irradiating DU145 cells with 10 Gy has a very limited impact on survival. In contrast, exposing siITGB1-depleted DU145 cells to 10 Gy of IR induced significant cell death as judged by sub-G1 content and the trypan blue cell viability assay (Fig. 2A, B). In addition to the short term survival assays, clonogenic survival assays confirmed that RAD9 or ITGB1 confer radioresistance to DU145 cells. DU145 cells transiently transfected with two different Rad9 siRNAs (Fig. 2C), as well as DU145 cells stably downregulating Rad9 (Fig. 2D), were significantly more radiosensitive than cells transfected with Luc siRNA or an insertless vector. Silencing Itgb1 radiosensitized cells as well. The conclusion that RAD9 and ITGB1 confer radioresistance in human prostate cancer cells was further strengthened by repeating these experiments using PC3 cells (Supplemental Fig. 3A and B). In all cases, levels of RAD9 and ITGB1 proteins were confirmed by western blot analysis (Supplemental Fig. 3C and D).
Downregulation of RAD9 in DU145 cells synergizes with ionizing radiation to induce PARP-1 cleavage
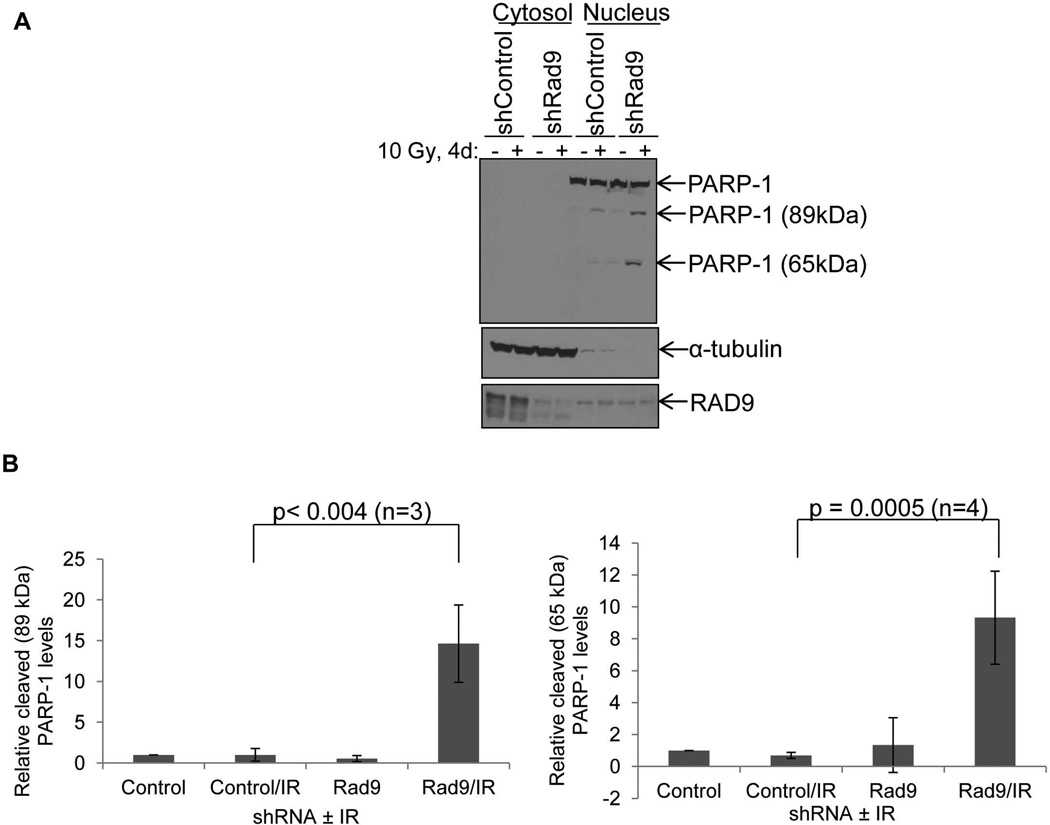
PARP-1 cleavage and the generation of a fragment 89 kDa in size is a hallmark of apoptotic cell death [20]. Initial dose response experiments with increasing amounts of IR showed that PARP-1 cleavage was increased in DU145/shRad9 after exposure to 10 Gy of γ-rays, whereas DU145/shControl cells showed no PARP-1 cleavage even after 15 Gy (Supplemental Fig. 4). To determine whether the increased cell death in DU145 cells observed after combining Rad9 knockdown with IR can be attributed, at least in part, to apoptosis, DU145/shControl or DU145/shRad9 cells were irradiated with 10 Gy of γ-rays and four days later PARP-1 cleavage was measured. To better visualize the generation of PARP-1 cleavage fragments, we fractionated the cells into nuclear and non-nuclear components, and PARP-1 was assayed in the nuclear fraction. As shown in Figure 3, irradiated DU145/shControl cells displayed only a small amount of PARP-1 fragment at 89 kDa. However, irradiated DU145/shRad9 cells generated significant PARP-1 fragmentation compared with the levels of PARP-1 cleavage in irradiated DU145/shControl cells (Fig. 3A). In addition to the 89 kDa fragment, a second cleavage product of PARP-1 at around 65 kDa was prominent in the irradiated DU145/shRad9 cells, but nearly absent in irradiated DU145/shControl cells or unirradiated DU145/shRad9 cells. (Fig. 3A). This latter PARP-1 fragment has been associated with programmed necrotic cell death [21]. Quantitative analysis of PARP-1 cleavage in whole cell extracts from irradiated DU145 cells with reduced levels of RAD9 revealed a 16.6 ± 4.7-fold and a 9.8 ± 3.4-fold increase in the 89 kDa and 65 kDa fragments, respectively (Fig. 3B). In summary, RAD9 knockdown combined with irradiation sensitizes DU145 cells to killing that can be attributed to apoptosis, necrosis, and possibly other forms of demise.
FIG. 3.
Downregulation of RAD9 synergizes with ionizing radiation to induce PARP-1 cleavage in DU145 cells. A: DU145/shControl and DU145/shRad9 cells were irradiated with 10 Gy IR and 4 days later cells were fractionated into nuclear and non-nuclear components. Cell lysates were immunoblotted for intact or fragmented forms of PARP-1. α-tubulin and total PARP-1 served as cytosolic and nuclear markers, respectively. RAD9 immunoblotting confirmed downregulation of the protein in shRad9 expressing cells. B: Relative levels of PARP-1 cleavage fragments 89 kDa (right panel) and 65 kDa (left panel). Shown are mean ± S.D. (n=3 for 89 kDa or n=4 for 65 kDa fragments) with p-values.
Ionizing radiation reduces ITGB1 protein levels in RAD9-depleted cells
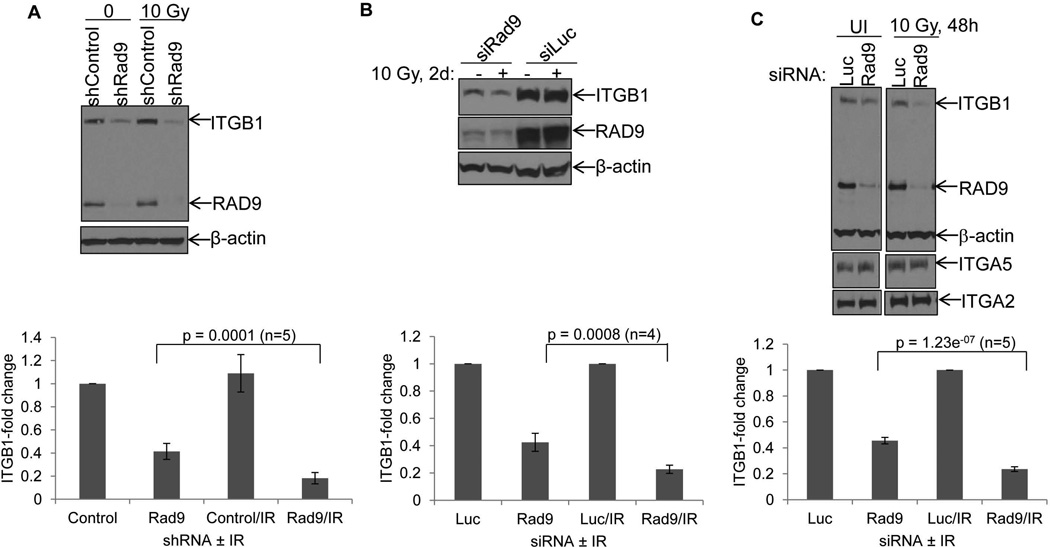
We have shown previously that RAD9 depletion reduces levels of ITGB1 protein [5]. Furthermore, it has been reported before that ITGB1 protein levels are diminished when cells are exposed to high doses of radiation [14]. In that study, a fractionated protocol of 25 Gy resulted in severe reduction of ITGB1 protein in DU145 cells. To assess whether a combination of RAD9 reduction and irradiation has a more dramatic effect on ITGB1 protein levels compared with the single conditions alone, DU145 cells with Rad9 expression knocked down, either stably (shRad9) or transiently (siRad9), were exposed to different doses of gamma rays. Forty-eight hours later, ITGB1 protein levels were measured by western blotting. ITGB1 protein abundance did not change in DU145/shControl cells even after 15 Gy of γ-rays. On the other hand, levels of this protein were reduced in DU145/shRad9 cells after 6 Gy and reached a minimum at 10 Gy (Supplemental Fig. 5). Therefore, we used 10 Gy of ionizing radiation to study ITGB1 protein changes in DU145 and PC3 cells. DU145/shRad9 showed only a 41.4 ± 6.9% expression of ITGB1 levels when compared with DU145/shControl cells. These levels were further reduced to 18.2 ± 4.9% after irradiation, again when compared with DU145/shControl cells (Fig. 4A). Student’s t-test analysis showed that the difference in ITGB1 protein in irradiated vs. unirradiated cells was highly significant (p = 0.0001, n=5). DU145/shControl cells expressing normal levels of RAD9 showed no such reduction in ITGB1 levels after irradiation. When PC3 cells were transiently transfected with Rad9 siRNA they displayed only 42.5 ± 6.6% expression of ITGB1 protein levels compared with PC3 transfected with control Luciferase siRNA, whereas combining RAD9 knockdown with a single dose of 10 Gy of IR, ITGB1 levels were even further reduced to 22.5 ± 3.0%, relative to the levels of PC3 cells with Luciferase siRNA (Fig. 4B). The p-value was 0.0008 (n=4). In agreement with the DU145 results, ITGB1 levels did not change after irradiation of cells with Luciferase siRNA (Fig. 4B). Likewise, transient downregulation of Rad9 in DU145 reduced relative ITGB1 levels to 45.6 ± 2.5%, whereas the combination of siRad9 with γ-rays reduced ITGB1 levels even further to 23.6 ± 3.0% (Fig. 4C) with a p-value at 1.23e−07 (n=5). On the other hand, neither ITGA2 nor ITGA5 levels were affected by RAD9 knockdown in DU145 cells, and irradiation did not change the levels of these integrins compared with unirradiated cells (Fig. 4C). We conclude that combining Rad9 knockdown with moderate levels of ionizing radiation specifically reduces ITGB1 protein levels, but not ITGA2 or ITGA5 in prostate cancer cell lines. Quantitative real-time PCR showed a small relative decrease in Itgb1 mRNA levels between Rad9 silencing alone and Rad9 silencing combined with γ-radiation in both PC3 cells (transient and stable Rad9 downregulation) and DU145 (stable Rad9 downregulation) (Supplemental Fig. 6). However, these differences did not attain statistical significance. Therefore, regulation of ITGB1 by RAD9 or RAD9 combined with ionizing radiation occurs post-translationally, most likely through control of ITGB1 protein stability [5].
FIG. 4.
RAD9 downregulation synergizes with ionizing radiation to reduce ITGB1 protein levels. A: Top panel, DU145 cells stably bearing shRad9 or insertless vector (shControl) were left unirradiated or irradiated with 10 Gy, and two days later they were subjected to western blot analysis with ITGB1, RAD9 and β-actin (loading control) antibodies. Bottom panel, mean ± S.D. (n=5) of ITGB1 levels relative to β-actin. B: Top panel, PC3 cells were transiently transfected with either Luciferase control or Rad9 siRNA and two days post-transfection cells were irradiated with 10 Gy or left unirradiated. Two days post-IR cell lysates were analyzed for ITGB1 and RAD9 protein by immunoblotting. Bottom panel, mean ± S.D. (n=4; each experiment conducted with two different siRad9 and values were averaged) of ITGB1 levels relative to β-actin. C: Top panel, DU145 cells transiently transfected with siLuciferase (Luc) or siRad9 (Rad9-2) were irradiated or left unirradiated, and 48h later cell lysates were subjected to immunoblotting with the indicated antibodies. Both irradiated and unirradiated samples were run in the same gel. Bottom panel, mean ± S.D. (n=5; each experiment conducted with two different siRad9 and values were averaged) of ITGB1 levels relative to β-actin. For all experiments, p values were calculated based on Student’s t-test statistical analysis.
Co-depletion of RAD9 and ITGB1 proteins does not increase cell death above that seen when RAD9 abundance alone is reduced
To examine the contribution of ITGB1 to RAD9-induced radioresistance, we attempted to ectopically express ITGB1 cDNA in DU145/shRad9 cells. However, overexpression of unligated ITGB1 resulted in enhanced cell death even when the transfection was carried out with cells attached to fibronectin (up to 10 µg/cm2). Therefore, we depleted ITGB1 in shRad9 cells and measured radioresistance by clonogenic survival. We reasoned that if ITGB1 lies downstream of a RAD9-related radioresistance pathway, then depleting ITGB1 while levels of the upstream effector (RAD9) is reduced should have only marginal effect on radiosensitization; otherwise, an additive or synergistic effect would point to RAD9 and ITGB1 affecting radioresistance by two different mechanisms. We carried out clonogenic survival assays with DU145 and PC3 cells stably expressing shControl or shRad9 that had been transiently transfected with either siLuc or siItgb1. In agreement with the short-term cell death assays, downmodulation of either RAD9 or ITGB1 sensitized both DU145 and PC3 cells to the effects of ionizing radiation (Fig. 5A and B). However, we observed no significant difference in radioresistance when RAD9 inhibition alone or with additional ITGB1 silencing is tested, suggesting that RAD9 and ITGB1 act in a common pathway that sensitizes prostate cancer cells to ionizing radiation (Fig. 5A and B).
Fibronectin does not confer radioresistance to PC3 cells when RAD9 abundance is reduced
Fibronectin is an extracellular matrix protein involved in tumor migration and invasion [22]. Cancer cells attached to fibronectin are also protected from the lethal effects of chemotherapeutic drugs, as well as ionizing radiation [23, 24]. Integrin β1 is the major receptor for fibronectin binding. As we have shown so far, the combination of RAD9 downregulation and ionizing radiation exposure results in severely diminished levels of ITGB1 (Fig. 4). We sought to address the question whether this reduction in ITGB1 levels impairs the protection fibronectin confers to PC3 cells in response to IR. In this experiment, we used PC3 cells because they are more sensitive to ionizing radiation than DU145 cells, express higher levels of ITGB1, and seem to be more dependent on fibronectin adhesion for their survival in response to ionizing radiation (unpublished observations). For this purpose, PC3 cells expressing either Luciferase or Rad9 siRNA were seeded onto plates coated with poly-D-lysine (a medium that does not engage the integrins) or fibronectin (attachment is integrin-mediated).
PC3 with ITGB1 down regulated by a specific siRNA was also used as a positive control. The cells were subsequently irradiated with 10 Gy and incubated for an additional 48h. At the end of incubation, cell death was assessed by trypan blue exclusion. When PC3 cells expressing siLuciferase were grown on poly-D-lysine and irradiated, percent cell death increased from approximately 5% to 45%. The same increase in cell death was also measured in PC3/siRad9 and PC3/siItgb1 cells. However, PC3/siLuc cells grown on fibronectin (10 µg/ml) were more radioresistant compared with those grown on poly-D-lysine, and showed only 15% cell death (Fig. 5C). In contrast, PC3/siRad9 and PC3/siItgb1 with reduced levels of endogenous ITGB1 did not benefit from attachment on fibronectin, and displayed approximately 50% and 55% cell death, respectively (Fig. 5C). We thus conclude that combining Rad9 silencing with ionizing radiation exposure, which results in severe downregulation of ITGB1, abrogates the pro-survival effect fibronectin confers to PC3 cells.
DISCUSSION
RAD9 is involved in cell cycle checkpoint control and multiple DNA repair pathways [5]. The protein participates in these pathways as part of the RAD9-HUS1-RAD1 (9-1-1) complex. From a clinical point of view, RAD9 is upregulated in a high percentage of human prostate tumors and its expression correlates with metastatic progression. Therefore, it is of interest to understand the role of RAD9 in promoting radioresistance in the control of this type of cancer.
RAD9 depletion sensitizes mouse embryonic stem cells to ionizing radiation [16]. In HeLa cells, Rad9 knockdown reduces the phosphorylation of CHK1 in response to DNA replication stress and irradiation [25]. However, the long-term effect on clonogenic survival of RAD9 depletion combined with ionizing radiation had not been shown in any human cancer cell line so far. In the present report, we demonstrate for the first time that Rad9 acts as a radioresistance gene in human prostate cancer cell lines. Combining RAD9 downregulation with ionizing radiation resulted in increased cell death as judged by a number of assays, including sub-G1 content and clonogenic survival, as well as PARP-1 cleavage. However, besides appearance of the 89 kDa PARP-1 fragment, consistent with a caspase-dependent apoptotic cell death, a second cleavage product ~65 kDa in size was also detected. This latter atypical PARP-1 cleavage fragment is consistent with μ-calpain-dependent, but caspase-independent programmed necrotic cell death [26]. Similar atypical PARP-1 cleavage has been observed in cells treated with a combination of ionizing radiation and β-lapachone, a drug that promotes death through NAD(P)H:quinone oxidoreductase 1-induced reactive oxygen species in PC3 and DU145 cells [15]. As RAD9 plays important roles in DNA repair and specifically in base excision repair that mends DNA damage caused by reactive oxygen species, it is reasonable to hypothesize that downregulation of RAD9 sensitizes cells to the lethal effect of reactive oxygen species generated by ionizing radiation.
Accumulating experimental evidence has revealed that RAD9 has numerous additional physiological roles besides DNA damage response and repair, many of which are not shared by its 9-1-1 complex partners HUS1 and RAD1. It can act as a sequence specific transcription factor to modulate a number of genes, including the cyclin-dependent kinase inhibitor p21waf/Cip1, and some are not related to DNA damage/repair [6]. Adding a layer of complexity to the functions of RAD9, more recent studies have provided evidence that the protein can influence expression of integrin β1. We have shown that RAD9 depletion in human prostate cancer cell lines reduces specifically ITGB1 protein levels, but not other integrins [5]. As predicted, ectopic expression of Rad9 restored the levels of ITGB1 protein to normal. RAD9 did not control ITGB1 protein abundance at the transcriptional level as quantitative PCR analysis failed to show any Itgb1 mRNA changes. However, RAD9 knockdown shortened the half-life of ITGB1. This effect was not reversed by the proteosomal inhibitor MG132, so the mechanism was 26S proteosome-independent [5].
One goal of the present study was to examine the role of HUS1 and RAD1 in the regulation of ITGB1 protein levels in DU145 and PC3 cells. Downregulation of either HUS1 or RAD1 in DU145 and PC3 cells did not influence ITGB1 expression levels. Thus, control of ITGB1 protein abundance is unique to RAD9 and is HUS1 and RAD1 independent, indicating that RAD9 must act outside the 9-1-1 complex for this function.
Changes in tumor microenvironment can influence the response of cells to radiation. Additionally, cell to cell contacts and cell to extracellular matrix adhesion also modulate the response to DNA damaging drugs and ionizing radiation [10, 27]. Cell adhesion-mediated chemo- and radio-resistance is a common reason for anticancer therapy failures [8]. Integrins play a pivotal role in adhesion of cells to proteins of the extracellular matrix and are major pro-survival contributors after cells incur radiation-induced DNA damage [28]. Studies have shown that cellular adhesion by means of integrin β1 reduces DNA double-strand breaks in cancer cells and inhibits cell death induced by DNA damaging agents [29]. Likewise, integrin β1 activation is required for small-cell lung cancer cells to escape apoptosis after etoposide or radiation treatment [30]. Ionizing radiation exposure of tumor cells results in the modulation of integrin expression levels. For example, ionizing radiation induced integrin β1 in human lung tumor cell lines growing on plastic, but not on fibronectin, and promoted radioresistance and cell survival under those conditions [10]. In addition, cell surface expression of ITGB1 in a colon carcinoma cell line was increased after exposure to as little as 1 Gy of ionizing radiation [9]. Irradiation induced a dose-dependent increase in functional β1- and β3 integrins in four glioma cell lines [31], whereas β1- and β3- integrins modulated basal and radiation-altered gelatinolytic activity of matrix-metalloproteinase (MMP)-2 [31]. Elevated levels of integrin expression correlated with more advanced and aggressive forms of cancer in several studies involving prostate, bladder, breast, brain, and lung tumors [10, 32–34]. The effect of ionizing radiation is not restricted to tumor cells, and it can affect normal cells in the tumor microenvironment as well. Previous studies showed that integrin activation was able to suppress etoposide-induced DNA strand breaks in tumor-derived endothelial cells [35]. Furthermore, radiation up-regulates αvβ3 expression in endothelial cells with a concomitant activation of Akt, which helps tumor cells escape from death induced by irradiation [36]. How integrins mediate radioresistance is not entirely clear. Downstream effector kinases such as Src, focal adhesion kinase (FAK) and Akt become phosphorylated in response to ionizing radiation exposure and contribute to radiation resistance [8, 37]. It is therefore conceivable that integrin-mediated radioresistance may be due to activation of these kinases. In line with published reports [13], neither FAK nor Src kinase activation, nor their downstream effector Akt, was affected in PC3 cells with reduced levels of integrin β1 (data not shown). Therefore, the downstream effectors of this integrin β1-related pathway still remain unknown.
The contribution of integrin β1 to prostate tumor cell radioresistance has only become apparent recently [2]. It has been reported that levels of integrin β1 protein in prostate cancer cell lines do not change significantly after ionizing radiation exposure, and only at very high doses of IR ITGB1 levels diminish [14]. In that study, however, a causal relationship between ITGB1 downregulation and radiosensitization was not proven experimentally and neither was the cause of ITGB1 downregulation revealed. On the other hand, in a recent report, DU145 cells with silenced ITGB1 exposed to 5 or 10 Gy of γ-rays displayed JNK1 activation and caspase-3-dependent apoptosis [13], whereas ITGB1 expression can suppress JNK1 activation and apoptosis in response to IR and confer radioresistance through cross-talk with the insulin-like growth factor receptor [13]. Finally, LNCaP cells stimulated with androgen and exposed to high doses of IR (10 and 15 Gy) showed an increased cell surface production of integrins av and β1 [38], again illustrating the link between integrins and radiation exposure.
RAD9 and ITGB1 confer radioresistance in multiple cell systems, and we have extended these findings by showing that the two proteins, when downregulated, sensitize prostate cancer cells to the deleterious effects of ionizing radiation. As RAD9 controls ITGB1 levels in these cells as well, it is reasonable to hypothesize that RAD9-induced radioresistance is dependent, at least partially, on ITGB1. However, it is difficult to quantitate the contribution of ITGB1 to RAD9-dependent radioresistance. A major obstacle is that ectopic expression of ITGB1 in RAD9-depleted cells can induce cell death [5].
Targeting integrins while treating tumors with ionizing radiation is a viable strategy to counteract cancer cell radioresistance. Integrin β1 inhibition has been used as a means to sensitize cells to the deleterious effects of IR in human breast cancer xenografts [11] and human head and neck cancer [39]. High levels of ionizing radiation cause a reduction of ITGB1 protein abundance in prostate cancer cell lines and increase cellular radiosensitivity [14]. Although RAD9 can either promote or inhibit tumorigenesis depending on the cellular context, the protein is oncogenic in prostate cancer [7]. Therefore, depleting RAD9 can compromise survival and metastatic potential of these cells [5]. Moreover, depletion of RAD9 results in reduction of ITGB1 and thus, further increased radiosensitivity. Thus, combining RAD9 inhibition with radiotherapy may be a feasible strategy to selectively target tumor cells, as normal prostate cells harbor very little RAD9 protein.
CONCLUSIONS
RAD9 controls signaling pathways associated with increased radioresistance. Likewise, high levels of ITGB1, as well as increased abundance of certain extracellular matrix proteins, most notably fibronectin, have also been associated with increased radioresistance. In prostate cancer, clinically relevant doses of IR have no effect on ITGB1 protein levels, whereas very high doses of IR reduce the abundance of this protein. In this report, we demonstrate that downregulating Rad9 expression will lower ITGB1 level, and thus sensitize cells to radiation. We show that when Rad9 silencing is combined with ionizing radiation, ITGB1 protein levels diminish dramatically. As a result, cells attached to fibronectin are no longer protected from the deleterious effects of ionizing radiation. These findings suggest that it would be beneficial to combine radiation with RAD9 inhibition as a novel anti-cancer therapy to treat individuals with prostate cancer.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by National Institutes of Health Grants R01CA130536, R01GM079107, and P01CA49062 (to H. B. L).
REFERENCES
- 1.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer. New prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang T, Languino LR, Lian J, Stein G, Blute M, Fitzgerald TJ. Molecular targets for radiation oncology in prostate cancer. Front Oncol. 2011;13:1–17. doi: 10.3389/fonc.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieberman HB, Bernstock JD, Broustas CG, Hopkins KM, Leloup C, Zhu A. The role of RAD9 in tumorigenesis. J Mol Cell Biol. 2011;3(1):39–43. doi: 10.1093/jmcb/mjq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broustas CG, Lieberman HB. Contributions of Rad9 to tumorigenesis. J Cell Biochem. 2012;113(3):742–751. doi: 10.1002/jcb.23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broustas CG, Zhu A, Lieberman HB. Rad9 protein contributes to prostate tumor progression by promoting cell migration and anoikis resistance. J Biol Chem. 2012;287(49):41324–41333. doi: 10.1074/jbc.M112.402784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin Y, Zhu A, Jin YJ, Liu YX, Zhang X, Hopkins KM, Lieberman HB. Human RAD9 checkpoint control/proapoptotic protein can activate transcription of p21. Proc Natl Acad Sci U S A. 2004;101(24):8864–8869. doi: 10.1073/pnas.0403130101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu A, Zhang CX, Lieberman HB. Rad9 has a functional role in human prostate carcinogenesis. Cancer Res. 2008;68(5):1267–1274. doi: 10.1158/0008-5472.CAN-07-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775(1):163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Meineke V, Gilbertz KP, Schilperoort K, Cordes N, Sendler A, Moede T, van Beuningen D. Ionizing radiation modulates cell surface integrin expression and adhesion of COLO-320 cells to collagen and fibronectin in vitro. Strahlenther Onkol. 2002;178(12):709–714. doi: 10.1007/s00066-002-0993-9. [DOI] [PubMed] [Google Scholar]
- 10.Cordes N, Blaese MA, Meineke V, Van Beuningen D. Ionizing radiation induces up-regulation of functional beta1-integrin in human lung tumour cell lines in vitro. Int J Radiat Biol. 2002;78(5):347–357. doi: 10.1080/09553000110117340. [DOI] [PubMed] [Google Scholar]
- 11.Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68(11):4398–4405. doi: 10.1158/0008-5472.CAN-07-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eke I, Deuse Y, Hehlgans S, Gurtner K, Krause M, Baumann M, Shevchenko A, Sandfort V, Cordes N. β1Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J Clin Invest. 2012;122(4):1529–1540. doi: 10.1172/JCI61350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goel HL, Sayeed A, Breen M, Zarif MJ, Garlick DS, Leav I, Davis RJ, Fitzgerald TJ, Morrione A, Hsieh CC, Liu Q, Dicker AP, Altieri DC, Languino LR. β1 integrins mediate resistance to ionizing radiation in vivo by inhibiting c-Jun amino terminal kinase 1. J Cell Physiol. 2013;228(7):1601–1609. doi: 10.1002/jcp.24323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon EL, Goel HL, Teider N, Wang T, Languino LR, Fitzgerald TJ. High dose fractionated ionizing radiation inhibits prostate cancer cell adhesion and beta(1) integrin expression. Prostate. 2005;64(1):83–91. doi: 10.1002/pros.20227. [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Bey EA, Li LS, Kabbani W, Yan J, Xie XJ, Hsieh JT, Gao J, Boothman DA. Prostate cancer radiosensitization through poly(ADP-Ribose) polymerase-1 hyperactivation. Cancer Res. 2010;70(20):8088–8096. doi: 10.1158/0008-5472.CAN-10-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins KM, Auerbach W, Wang XY, Hande MP, Hang H, Wolgemuth DJ, Joyner AL, Lieberman HB. Deletion of mouse rad9 causes abnormal cellular responses to DNA damage, genomic instability, and embryonic lethality. Mol Cell Biol. 2004;24(16):7235–7248. doi: 10.1128/MCB.24.16.7235-7248.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu A, Zhou H, Leloup C, Marino SA, Geard CR, Hei TK, Lieberman HB. Differential impact of mouse Rad9 deletion on ionizing radiation-induced bystander effects. Radiat Res. 2005;164(5):655–661. doi: 10.1667/rr3458.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowen C, Birrer M, Gelmann EP. Retinoblastoma protein-mediated apoptosis after gamma-irradiation. J Biol Chem. 2002;277(47):44969–44979. doi: 10.1074/jbc.M202000200. [DOI] [PubMed] [Google Scholar]
- 19.Goel HL, Breen M, Zhang J, Das I, Aznavoorian-Cheshire S, Greenberg NM, Elgavish A, Languino LR. beta1A integrin expression is required for type 1 insulin-like growth factor receptor mitogenic and transforming activities and localization to focal contacts. Cancer Res. 2005;65(15):6692–6700. doi: 10.1158/0008-5472.CAN-04-4315. [DOI] [PubMed] [Google Scholar]
- 20.Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7(4):321–328. doi: 10.1023/a:1016119328968. [DOI] [PubMed] [Google Scholar]
- 21.Chaitanya GV, Steven AJ, Babu PP. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal. 2010;8:31. doi: 10.1186/1478-811X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akiyama SK, Olden K, Yamada KM. Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 1995;14(3):173–189. doi: 10.1007/BF00690290. [DOI] [PubMed] [Google Scholar]
- 23.Cordes N, Beinke C. Fibronectin alters cell survival and intracellular signaling of confluent A549 cultures after irradiation. Cancer Biol Ther. 2004;3(1):47–53. [PubMed] [Google Scholar]
- 24.Eke I, Sandfort V, Mischkus A, Baumann M, Cordes N. Antiproliferative effects of EGFR tyrosine kinase inhibition and radiation-induced genotoxic injury are attenuated by adhesion to fibronectin. Radiother Oncol. 2006;80(2):178–184. doi: 10.1016/j.radonc.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Dang T, Bao S, Wang XF. Human Rad9 is required for the activation of S-phase checkpoint and the maintenance of chromosomal stability. Genes Cells. 2005;10(4):287–295. doi: 10.1111/j.1365-2443.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 26.Tagliarino C, Pink JJ, Reinicke KE, Simmers SM, Wuerzberger-Davis SM, Boothman DA. Mu-calpain activation in beta-lapachone-mediated apoptosis. Cancer Biol Ther. 2003;2(2):141–152. doi: 10.4161/cbt.2.2.237. [DOI] [PubMed] [Google Scholar]
- 27.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93(5):1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 28.Cordes N, Seidler J, Durzok R, Geinitz H, Brakebusch C. beta1-integrin-mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene. 2006;25(9):1378–1390. doi: 10.1038/sj.onc.1209164. [DOI] [PubMed] [Google Scholar]
- 29.Hazlehurst LA, Valkov N, Wisner L, Storey JA, Boulware D, Sullivan DM, Dalton WS. Reduction in drug-induced DNA double-strand breaks associated with beta1 integrin-mediated adhesion correlates with drug resistance in U937 cells. Blood. 2001;98(6):1897–1903. doi: 10.1182/blood.v98.6.1897. [DOI] [PubMed] [Google Scholar]
- 30.Hodkinson PS, Elliott T, Wong WS, Rintoul RC, Mackinnon AC, Haslett C, Sethi T. ECM overrides DNA damage-induced cell cycle arrest and apoptosis in small-cell lung cancer cells through beta1 integrin-dependent activation of PI3-kinase. Cell Death Differ. 2006;13(10):1776–1788. doi: 10.1038/sj.cdd.4401849. [DOI] [PubMed] [Google Scholar]
- 31.Cordes N, Hansmeier B, Beinke C, Meineke V, van Beuningen D. Irradiation differentially affects substratum-dependent survival, adhesion, and invasion of glioblastoma cell lines. Br J Cancer. 2003;89(11):2122–2132. doi: 10.1038/sj.bjc.6601429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murant SJ, Handley J, Stower M, Reid N, Cussenot O, Maitland NJ. Co-ordinated changes in expression of cell adhesion molecules in prostate cancer. Eur J Cancer. 1997;33(2):263–271. doi: 10.1016/s0959-8049(96)00418-2. [DOI] [PubMed] [Google Scholar]
- 33.Chakraborty A, White SM, Lerner SP. Granulocyte colony-stimulating factor receptor signals for beta1-integrin expression and adhesion in bladder cancer. Urology. 2004;63(1):177–183. doi: 10.1016/s0090-4295(03)00786-6. [DOI] [PubMed] [Google Scholar]
- 34.Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, Noonan DM, Natali PG, Albini A. The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int J Cancer. 2000;87(3):336–342. [PubMed] [Google Scholar]
- 35.Hoyt DG, Rusnak JM, Mannix RJ, Modzelewski RA, Johnson CS, Lazo JS. Integrin activation suppresses etoposide-induced DNA strand breakage in cultured murine tumor-derived endothelial cells. Cancer Res. 1996;56(18):4146–4149. [PubMed] [Google Scholar]
- 36.Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, Saffrich R, Gröne HJ, Hallahan DE, Reisfeld RA, Debus J, Niethammer AG, Huber PE. Inhibition of alpha(v)beta3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res. 2005;11(17):6270–6279. doi: 10.1158/1078-0432.CCR-04-1223. [DOI] [PubMed] [Google Scholar]
- 37.Seidler J, Durzok R, Brakebusch C, Cordes N. Interactions of the integrin subunit beta1A with protein kinase B/Akt, p130Cas and paxillin contribute to regulation of radiation survival. Radiother Oncol. 2005;76(2):129–134. doi: 10.1016/j.radonc.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 38.Wang T, Alavian MR, Goel HL, Languino LR, Fitzgerald TJ. Bicalutamide inhibits androgen-mediated adhesion of prostate cancer cells exposed to ionizing radiation. Prostate. 2008;68(16):1734–1742. doi: 10.1002/pros.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eke I, Dickreuter E, Cordes N. Enhanced radiosensitivity of head and neck squamous cell carcinoma cells by β1 integrin inhibition. Radiother Oncol. 2012;104(2):235–242. doi: 10.1016/j.radonc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.