Abstract
Vaginal drug administration can improve prophylaxis and treatment of many conditions affecting the female reproductive tract, including sexually transmitted diseases, fungal and bacterial infections, and cancer. However, achieving sustained local drug concentrations in the vagina can be challenging, due to the high permeability of the vaginal epithelium and expulsion of conventional soluble drug dosage forms. Nanoparticle-based drug delivery platforms have received considerable attention for vaginal drug delivery, as nanoparticles can provide sustained release, cellular targeting, and even intrinsic antimicrobial or adjuvant properties that can improve the potency and/or efficacy of prophylactic and therapeutic modalities. Here, we review the use of polymeric nanoparticles, liposomes, dendrimers, and inorganic nanoparticles for vaginal drug delivery. Although most of the work toward nanoparticle-based drug delivery in the vagina has been focused on HIV prevention, strategies for treatment and prevention of other sexually transmitted infections, treatment for reproductive tract cancer, and treatment of fungal and bacterial infections are also highlighted.
Keywords: Microbicides, HIV PrEP, cervical cancer, sexually transmitted infections, mucosal vaccines
Introduction
In the nearly 100 years since the early descriptions of drug absorption occurring after topical vaginal administration, research on vaginal drug delivery has recently intensified. Vaginal drug administration has many advantages over conventional oral administration, such as avoidance of the harsh gastrointestinal (GI) environment and the hepatic first-pass effect. Absorption from the GI tract can be affected by fed state, drug-drug interactions, microbiota, and GI disturbances. Further, the fraction of drug that is absorbed from the GI tract can then be metabolized and eliminated by first passage through the liver. Thus, vaginal drug administration allows for smaller drug doses and the potential for reduced side effects. Additionally, for drugs that act locally in the female reproductive tract, topical vaginal application results in much higher drug concentrations and improved efficacy.
Typical vaginal dosage forms include rings, films, creams, and gels, each of which has advantages for particular drugs and particular indications, as reviewed elsewhere [1]. More recently, nanoparticle-based platforms for drug delivery to the vagina have received increasing attention. The development of nanoparticle-based vaginal drug delivery formulations has largely been focused on HIV pre-exposure prophylaxis (PrEP). Nanoparticles can provide sustained release of microbicide drugs, which is necessary for maintaining protective drug concentrations between the time of dosing and the time of intercourse. Drug release from nanoparticles can result in more controlled vaginal absorption compared to a drug depot like a vaginal gel, thereby potentially requiring reduced amounts of drug. Nanoparticles can be designed to contain multiple modalities, to target specific cells, and to have intrinsic antimicrobial activity. Achieving adequate vaginal drug distribution can be challenging; appropriately designed nanoparticles can provide improved drug distribution to target cells and tissues, improving efficacy. However, the advantages and unique characteristics of nanoparticle-based vaginal formulations need not be limited to PrEP, as evidenced by demonstrations of nanoparticles applied to the vagina of experimental animals to achieve vaccination, or treatment of cervical cancer and other female reproductive tract infections. The goal of this review is to discuss: (i) factors that influence vaginal drug delivery that should be considered when designing and testing nanoparticle-based platforms, (ii) nanoparticle-based formulations designed for vaginal drug delivery, and (iii) other opportunities for nanoparticle-based vaginal drug delivery formulations to improve prophylactic and therapeutic outcomes.
Considerations for the rational design of nanoparticle platforms for vaginal drug delivery
Physiology of the vagina and implications for drug delivery
Often cited drawbacks of oral drug administration include the lack of predictability in drug absorption due to physiological variations and the hepatic first-pass effect. Drugs absorbed from the vagina enter the peripheral circulation through a venous plexus that empties into the internal iliac veins and the hemorrhoidal veins, thus avoiding the liver [2]. However, similar to oral drug absorption, vaginal drug absorption is affected by numerous physiological factors. First, the vagina is not simply a straight tube as often depicted in anterior drawings of the female reproductive tract. Imaging of the lateral view has demonstrated that the lower portion of the vagina is tilted at approximately 45° leading up to the wider upper portion that is almost horizontal when a woman is standing [3, 4]. From a cross-sectional view, the vagina is collapsed with the anterior and posterior walls in contact with each other, forming what is often referred to as an “H” shape [5]. The distended shape of the vagina varies widely between women [6]. The vaginal walls are lined with stratified squamous epithelium containing numerous folds, or rugae, which allow for this distension and increased surface area for absorption [4]. Due to the intra-abdominal pressure that collapses the rugae, high internal surface area, and tortuosity of the vaginal canal, achieving adequate distribution of a vaginal product can be challenging, and is often dependent on the influence of numerous factors [7]. Unfortunately, animal models for vaginal drug delivery fail to recapitulate many of these uniquely human features, including the acidic pH (~3.5–4.0) observed in the human vagina in women with healthy, Lactobacilli-dominated microbiota [8, 9]. Under certain conditions, such as during a yeast infection or when semen is introduced into the vagina, the pH can be temporarily elevated [10, 11], which can affect the ionization and absorption of certain drugs [3, 7]. Thus, the basic physiology of the vagina must be considered when designing vaginal dosage forms, and care must be taken in interpreting experimental observations obtained using animal models.
The mucus barrier and implications for drug delivery
In addition to the epithelium itself, cervicovaginal mucus (CVM) serves as a physical barrier to protect the vagina against infection. CVM can have a significant impact on the penetration, distribution, and residence time of nanoparticle-based systems for vaginal drug delivery applications. Mucus produced at the cervix bathes and coats the vaginal walls, mixing with vaginal epithelial cells and vaginal transudate. The composition of CVM is similar outside of the period of ovulation, and is composed mostly of water (~90–95%) with gel-forming glycoproteins, lipids, soluble proteins, enzymes, and various immune factors [12, 13]. During ovulation, cervical mucus becomes watery and mucin proteins align to allow sperm to pass more readily through the cervix into the uterus. However, ovulatory mucus is produced in more copious amounts, thus facilitating clearance and impeding drug absorption [14]. Mucins in CVM from non-ovulatory women and women on hormonal contraceptives form a tight meshwork, acting as a barrier to protect the epithelium [13]. Non-ovulatory human CVM was recently found to have pores in the range of 50 – 1800 nm, with an average of 340 ± 70 nm [15]. In addition to sterically trapping pathogens and particulates, CVM also traps particles by adhesive interactions [12]. Vaginal dosage forms, such as gels, are often designed to be mucoadhesive to increase residence time in the vagina [16]. It was also found that conventional nanoparticle formulations are inherently mucoadhesive due to interactions between mucus and the hydrophobic polymer nanoparticles [17, 18], which was expected to increase residence time [19]. However, it was recently demonstrated that nanoparticles engineered with non-adhesive surface coatings of densely packed polyethylene glycol (mucus penetrating particles, or MPP) can rapidly penetrate human and mouse mucus, reaching deep into the more slowly cleared mucus layers, including those in the collapsed vaginal folds, thereby increasing epithelial coverage and vaginal retention compared to mucoadhesive conventional nanoparticles (CP) (see Polymeric nanoparticles) [17, 20, 21]. A microbicide drug formulated as an MPP was also found to provide increased protection in a mouse model of vaginal herpes infection, compared to even 10-times the amount of unformulated drug [20]. Thus, it appears as though the barrier properties of CVM to pathogens and nanoparticles must also be considered when designing an effective nanoparticle-based formulation for vaginal drug delivery.
Immunology of the vaginal mucosa and implications for drug delivery
The immune system of the female reproductive tract must protect against infectious pathogens while tolerating commensal bacteria and the presence of foreign materials, such as sperm. Whether nanoparticle systems are designed to interact directly with the immune system, such as with vaccine delivery and immune cell-targeted delivery, or to be unrecognized by the immune system, a thorough understanding of vaginal immunity is necessary. Although not as highly studied as the immune systems in the respiratory and gastrointestinal (GI) tracts, understanding of how the vaginal immune system strikes a balance between tolerance and pathogen recognition, and how it differs from other mucosae, is progressing [22].
The respiratory and GI tracts are type I mucosae, which is coated with simple columnar epithelia linked by tight junctions and containing organized lymphoid structures (MALT). In contrast, the vaginal and ectocervical epithelia are type II mucosa, covered with squamous epithelia and lacking MALT [23]. Thus, rather than allowing access to underlying MALT for immune surveillance like type I mucosa, the multiple layers of nonkeratinized squamous epithelium (as well as the overlying mucus layer) in the human vagina acts as a physical barrier to pathogens [24]. Vaginal epithelial cells are not only a barrier to pathogens, but also an active component of the vaginal immune system that expresses toll-like receptors for pathogen recognition and produces antimicrobials, chemokines, and cytokines [25, 26]. Thus, the vaginal epithelium itself plays an important protective role, such that disruption or irritation of the vaginal epithelium can have serious consequences. For example, products that are toxic to the vaginal epithelium can increase susceptibility to infection and other diseases [27, 28].
The lack of MALT in the vagina also means that dendritic cells within and beneath the stratified epithelium must migrate to the surrounding lymph nodes to present antigens for T cell priming. Compared to the respiratory and GI tract mucosa, the vaginal mucosa appears to be resistant to recruitment of antigen-specific T and B cells [22]. In addition, the regulation of immune cells and immune responses is sensitive to variations in estradiol and progesterone levels that occur throughout the menstrual cycle [24, 29]. For these and other reasons, the vagina is not often a target for vaccine development, even though local mucosal immune responses are more important than systemic immunity for mucosal protection [22]. It was recently demonstrated that IgG antibodies in vaginal mucus facilitate trapping of HSV-1 in mucus, which would restrict viral access to target cells [30]. Interestingly, various investigators have found in animal models that nasal immunization can be beneficial for vaginal antibody production [31, 32]. However, for long-term protection by vaccines, induction of T cell-mediated immunity, and particularly antigen-specific CD4+ T cells, is likely required [22]. As understanding of the vaginal immune system progresses, it is likely that more effective nanoparticle-based vaccine strategies for inducing vaginal immunity will be developed [23]. In the meantime, it is clear that vaginal administration of various drugs, such as microbicides for protection against HIV infection, is more effective than oral delivery for providing vaginal protection [33, 34]. However, reliable use of drugs is frequently limited by poor user adherence. Nanoparticle-based systems can provide sustained release, targeted delivery, and other benefits that can also improve upon current vaginal drug delivery strategies [35, 36].
As murine models are often used prior to studies with non-human primates or clinical studies, it is important to understand how immunology of the vaginal mucosa differs between mice and humans. For example, the changes in immune cell populations and epithelial characteristics during the mouse estrous cycle are quite significant compared to what occurs during the human menstrual cycle (for more about the estrous cycle, see The impact of hormones on the vagina). The human female reproductive tract contains a substantial population of immune cells distributed in the stromal layer and the epithelium, and only subtle changes in the migration of macrophages, B cells, and neutrophils into the lower tract and dendritic cells entering the squamous epithelium are observed throughout the menstrual cycle [24]. In contrast, phases of the mouse estrous cycle are identified by significant populations of leukocytes in cervicovaginal lavage fluid (metestrus, diestrus), or the near lack thereof (proestrus, estrus), and these changes occur on the order of hours-to-days [37]. In addition to the neutrophil invasion that occurs during the metestrus and diestrus phases, these phases are also associated with a thinned vaginal epithelium and an abundance of Langerhan’s cells (LC) and dendritic cells (DC) near and throughout the epithelial layer [38]. In contrast, during the estrus and proestrus phases, when the epithelium is thick and cornified, providing a markedly enhanced physical barrier to pathogen invasion, there are essentially no LC and DC near the epithelium (Fig 1) [38]. Mice used for experimental models are commonly treated with a synthetic progestin birth control (Depo-Provera, or DP) to synchronize the estrous cycle, which produces a prolonged diestrus-like state and increased susceptibility to vaginal HSV-2 infection [39]. Thus, studies involving DP-treated mice that investigate the interactions between nanoparticles and the immune system, such as vaccine products or lymph trafficking, must be interpreted with care, as the interplay with the immune system may be over-emphasized in a way that would not occur in the human vagina. In some cases, such as studies that seek to determine whether vaginal products may cause vaginal toxicity or inflammation, the heightened vaginal immune reactivity of DP-treated mice can provide a sensitive screen for effects that may only become evident after repeated doses in humans [28]. Of note, numerous commercially available vaginal products, particularly ones that were developed decades ago, were not subjected to these kinds of rigorous tests for impact on the vaginal mucosa and the implications for vaginal infection. Many investigators have recently been uncovering the unintended consequences of lubricants, gels, and douches intended for vaginal use [27, 40, 41]. Thus, the composition of the vehicle and its potential effects in the vagina are just as important to consider when designing nanoparticle-based formulations for vaginal drug delivery.
Figure 1.
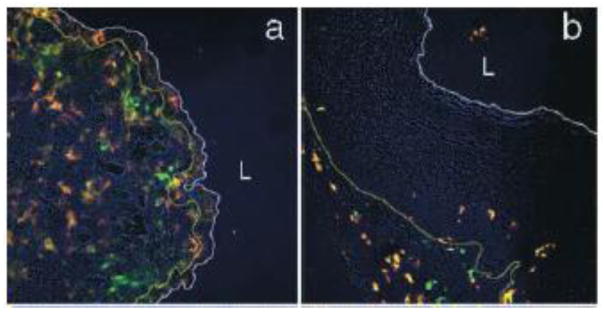
Distribution of Langerhan’s cells (LC) in the vaginal epithelium in (a) diestrus and (b) estrus phase mouse vaginal tissue. Antibodies against MHC class II (red) and CD11c (green) denote LC. The white line indicates the luminal edge of the epithelium, whereas the yellow line indicates the basement membrane. The images were obtained at 20x magnification. L denotes the lumen. Note the thick tissue barrier between the LC and the lumen space in the estrus phase mouse vagina. Reprinted with permission from [38].
The role of microbiota in the vagina and implications for drug delivery
Vaginal microbiota play a critical role in female reproductive tract health. As such, nanoparticle-based drug delivery systems intended for vaginal applications must be designed with the preservation of healthy bacterial colonization in mind. The stratified squamous epithelium of the human vagina sheds continuously, thus enhancing the physical barrier to infection and providing a source of glycogen to promote growth of Lactobacilli. Lactobacilli compete with other microbes for nutrients, and secrete factors, such as lactic acid, that make the environment inhospitable to other bacteria and pathogens [42].
Bacterial vaginosis (BV) is a condition in which the vagina is dominated by a polymicrobial overgrowth of mostly anaerobic bacteria and few if any lactobacilli, resulting in elevated pH (> 4.5), increased susceptibility to numerous sexually transmitted pathogens, and increased risk of inflammatory conditions such as endometritis and pelvic inflammatory disease (PID) [43]. Thus, products designed for vaginal use must not negatively impact the vaginal environment and survival of lactobacilli, as it is critical that the products do not increase the incidence of BV. Broad-spectrum antimicrobial properties of certain types of nanoparticles (for more details, see Dendrimers and Inorganic nanoparticles) may have unintended consequences on the vaginal microbiota. Unfortunately, lactobacillus dominance and vaginal acidity are uniquely human, and there are no suitable animal models for testing the impact of products on lactobacillus colonization [8, 9]. The most common approach is to incubate nanoparticles with lactobacillus cultures in vitro, which is a useful way to test acute toxic effects, though the only way to test the impact of repeated dosing of a product on human vaginal microbiota is with clinical testing.
Approximately one-third of all women have BV at any given time, a fraction that is increased in certain populations of women at high risk of acquiring sexually transmitted infections [44]. Thus, changes in the vaginal environment caused by BV may be particularly important to consider when designing microbicide products. Vaginal secretions from women with BV often have increased enzymatic activity [45–47], and biofilms can form on the epithelial surface of the vagina that may impact drug absorption [48]. Although pathogenic bacteria are generally associated with local inflammation, BV bacteria appear to have immune suppressive mechanisms that lead to with the high prevalence of asymptomatic BV [48]. The effect of these immune suppressive mechanisms on vaccines designed for vaginal administration is unknown, but should be considered. As described later, nanoparticle-based platforms with promise for treating BV exist; these may overcome antibiotic resistance and increase the time between BV episodes.
The impact of hormones on the vagina and implications for drug delivery
Estradiol and progesterone have tremendous impact on the physiology and immunology of the female reproductive tract, as reviewed extensively elsewhere [24, 26]. Thus, the menstrual cycle, hormonal contraceptive use, and reproductive status all have significant impact on the potential effectiveness of vaginal dosing strategies. Estradiol drives thickening of the epithelium [49], glycogen production and epithelial cell shedding that supports survival of Lactobacilli [10], and production of copious amounts of more watery midcycle mucus [13]. Cyclical variations in vaginal thickness and vascularity have also been shown to impact absorption of drugs, such as steroids and the anti-herpes drug Vidarabine [50]. Estradiol also regulates innate immunity by suppressing the secretion and/or expression of pro-inflammatory mediators by epithelial cells, which may reduce HIV infection by inhibiting target cell migration into the epithelium [24]. It has been demonstrated that sustained estradiol treatment via subcutaneous implants inhibited topical vaginal SIV infection in macaques, whereas vaginal infections were transmitted by injections directly into the vaginal submucosa [51]. Subcutaneous estradiol injection to ovariectomized rats also provided complete protection against intrauterine Chlamydia trachomatis infection in rats, whereas progesterone-treatment led to significant infectious burden and inflammation [39]. Similarly, subcutaneous estradiol injection to ovariectomized mice led to complete protection against vaginal HSV-2 infection, whereas progesterone treatment rendered mice 100% susceptible to lethal HSV-2 infection [52]. Progesterone, including synthetic forms of progesterone in hormonal contraceptives, appears to increase the immune cell presence in the vagina in humans and mice [53]. For this reason, hormonal contraceptive use may impact susceptibility to HIV infection and disease progression [29].
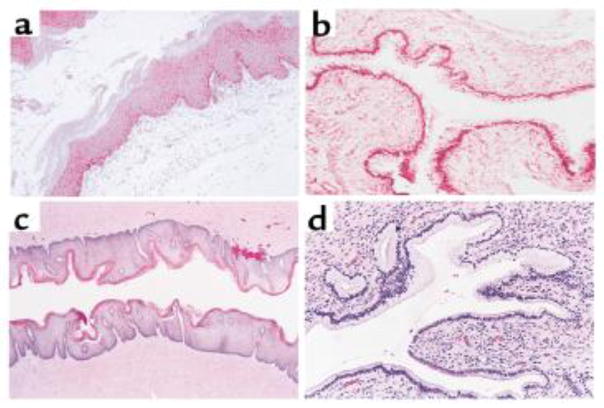
The effects of hormones on the vagina are particularly pronounced in animal models, including mice. In the four distinct phases of the 4–5 day murine estrous cycle, the vaginal epithelium first doubles in thickness (proestrus), becomes keratinized (estrus), and then sheds (metestrus) to only 7–8 cell layers in thickness (diestrus) [54]. When the epithelium is thickest, and more similar to the human vaginal epithelium [55, 56], immune cells are absent from the epithelial and luminal spaces. We also demonstrated that the vaginal mucus barrier in estrus-phase mice is most similar to human cervicovaginal mucus [20]. In contrast, progesterone-driven epithelial thinning is associated with an increase in immune cells and their proximity to the lumen (Fig 1) [38], as well a tightening of the mucus mesh, limiting penetration of nanoparticles [57]. Treating mice with a high concentration of synthetic progestin, a common procedure in studies in murine and non-human primate vaginal models, induces a diestrus-like state with even more significant thinning of the epithelium and a transition to the columnar type epithelia typically seen in the human endocervix and uterus, but not in the vagina (Fig 2) [58].
Figure 2.
Hemotoxylin and eosin stained tissue sections from (a) an untreated mouse in the estrus phase (x120), (b) a progestin-treated mouse (x120), (c) normal human vaginal epithelium (x50), and (d) normal human cervix (x160). Note the similarities between the thin, columnar, progestin-treated murine vaginal epithelium and the columnar, human ectocervical epithelium. In contrast, the murine estrus phase vaginal epithelium and the human vaginal epithelium are thick, squamous cell layers. Reprinted with permission from (58).
Epithelial thinning in the diestrus phase was found to allow increased drug absorption compared to the estrus phase in guinea pigs [59]. The diestrus phase epithelium in mice was also demonstrated to be more conducive to transfection with plasmid DNA than the proestrus and estrus phase vaginal epithelium [60]. Wu et al. demonstrated that, in contrast to the extensive transfection observed in the vaginas of DP-treated mice [61], the outer layer of cornified epithelial cells in the estrus phase mouse vagina, that is more similar to the human vagina, created a physical barrier to liposome-mediated oligonucleotide delivery, and that disruption of the estrus epithelium with 5% citric acid still resulted in only minimal oligonucleotide uptake [62].
The rapid and significant changes that occur in the vagina during the mouse estrous cycle make it difficult to avoid the use of hormone treatments to “synchronize” the vaginal environment, but such treatments induce physiological changes that may not fully recapitulate the human vagina. However, this does not negate the usefulness of animal models for preclinical studies of nanoparticle-based formulations for vaginal drug delivery. As previously described, the thinning of the epithelium and the increased immune cell presence in the vaginal epithelium of DP-treated mice can provide a more sensitive screen for vaginal products and potential toxic effects [63, 64]. Also, the cervix is thought to be a potential site of infection for sexually transmitted diseases such as HIV, and the vaginal epithelium of the DP-treated mice has similar structure to the human ectocervix. As previously described, it was demonstrated that the thickness and infolding of the vaginal epithelium and the pore structure of vaginal mucus in estradiol-treated mice was more similar to that of humans, making this model more appropriate for investigating the vaginal distribution and retention of nanoparticle-based systems [20]. However, as described, estradiol-treated animals resist infection with otherwise lethal titres of infectious pathogens, making preclinical assessment of protection against infection difficult. Thus, hormone treated animals are certainly useful for studying vaginal drug delivery, but care must be taken when selecting appropriate animal models.
Nanoparticle platforms for vaginal drug delivery
Polymeric nanoparticles
The development of polymeric nanoparticles for vaginal drug delivery applications has included a significant focus on vaginal mucus as a barrier and a clearance mechanism that can limit vaginal retention. Olmsted and coworkers first demonstrated that 59 nm polystyrene nanoparticles (PS) were trapped in midcycle human cervical mucus, causing the mucin proteins to bundle together into cables [18]. However, the capsids of Norwalk virus and human papilloma virus (HPV) (55 nm) diffused freely in the mucus, indicating that the larger PS particles were trapped by adhesive interactions with mucins. Dawson et al. then demonstrated that coating poly(lactic-co-glycolic) acid (PLGA) nanoparticles with DNA to make a more hydrophilic surface increased particle transport through gastric mucin gels 10-fold compared to PS nanoparticles, and increased in vitro transfection rates 50-fold compared to naked DNA [65]. Lai et al. later demonstrated that PS particles 100 nm – 1 um in diameter were adhesively trapped in fresh, undiluted human cervicovaginal mucus (CVM), whereas PS particles densely coated with polyethylene glycol (PEG-PS) diffused through CVM at rates only a few-fold slower than their theoretical diffusion in pure water and several hundred-fold faster than their uncoated counterparts [17]. It was hypothesized that by penetrating through vaginal mucus, mucus-penetrating particles (MPP) could provide improved vaginal drug distribution and retention, rather than being trapped and more rapidly cleared in the outer, luminal layers of mucus (for more details, see The mucus barrier).
The importance of achieving high PEG density on the surface of the nanoparticle in order to effectively shield against adhesive interactions with mucus and to make mucus-penetrating nanoparticles was highlighted by Wang et al. High density 2 kDa PEG surface coatings allowed 200 nm PEG-PS nanoparticles to rapidly penetrate human CVM, whereas PEG-coated nanoparticles with as little as a 40% reduction in PEG density were adhesively immobilized in CVM [66]. The effects of PEG density were also highlighted in the work by Lai et al., which reported that 100 nm PEG-PS were slowed ~2000-fold in CVM [17]. Lai and coworkers later demonstrated that improved PEG conjugation methods could produce 100 nm particles with dense enough PEG coatings to rapidly penetrate CVM [67].
The importance of PEG surface density for achieving efficient mucus penetration was further highlighted in works involving biodegradable nanoparticles. Cu et al. demonstrated that PLGA with a low-density surface coating of PEG (PEG-NP), formed by adsorbing avidin-palmitic acid to the surface of the PLGA nanoparticles and subsequently attaching biotin functionalized PEG, exhibited 2- to 10-fold improvement in nanoparticle penetration through ovulatory (midcycle) cervical mucus (OCM) [68]. OCM is watery and can be penetrated by sperm [13], which is likely why uncoated PLGA nanoparticles that would be adhesively immobilized by non-ovulatory CVM were only slowed 8- to 13- fold by OCM [68]. It is likely that passive absorption of PEG onto the nanoparticle surface would not produce sufficiently dense coatings to resist adhesion to non-ovulatory CVM, the requirement highlighted by Wang and coworkers [66]. Tang et al. then went on to demonstrate that biodegradable, densely PEG-coated poly(sebacic acid) nanoparticles (PEG-PSA) rapidly penetrate non-ovulatory CVM at rates 400-fold faster than uncoated PSA nanoparticles that adhere to CVM [69]. They estimated that these PEG-PSA nanoparticles had at least 100-fold more PEG on the nanoparticle surface compared to the PEG-NP, further suggesting that dense PEG coatings are critical for rapid nanoparticle penetration through highly viscoelastic mucus secretions, such as non-ovulatory CVM [69].
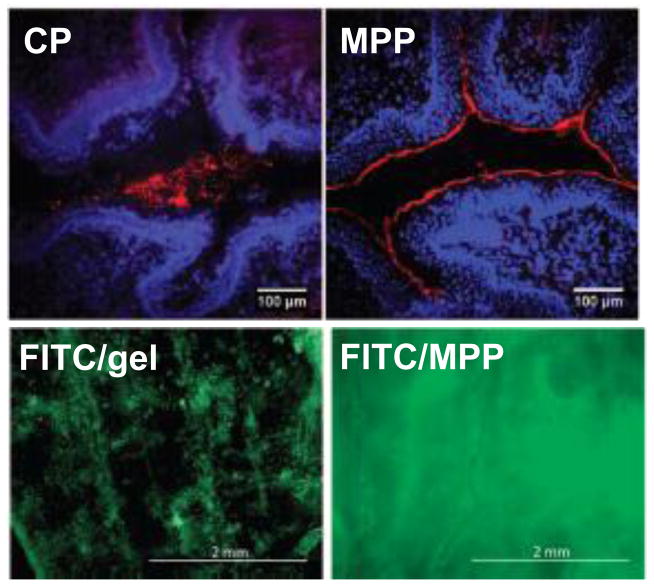
The importance of mucus-penetration and PEG surface density was then demonstrated in vivo. Cu et al. demonstrated in vivo that even a low density PEG coating on the surface of PLGA nanoparticles (PEG-NP) 150–170 nm in size provided increased retention in the vaginal tract of Depo-Provera (DP)-treated mice [70]. More of the uncoated NP leaked from the vagina and were removed (associated) with the vaginal mucus by vaginal lavage, whereas a larger amount of PEG-NP were found to have penetrated through the mucus barrier and were associated with the vaginal tissue (~29 μg compared to 15 μg of uncoated NP), representing ~4% of the original dose 30 min after administration [70]. However, as described previously, the mucus barrier in the vagina of DP-treated mice may not be representative of the mucosal environment in the human vagina; nanoparticles ~120 nm that readily penetrate human CVM were markedly hindered by CVM in the DP-treated mouse CVM [57]. Perhaps increased PEG density on the nanoparticle surface may further improve distribution and retention in the DP-treated mouse. In contrast, Ensign et al. demonstrated that mouse CVM in the estrus phase mouse vagina (the phase in which the vaginal epithelium is most similar to that of the human vagina) has similar barrier properties to nanoparticles as human CVM [20]. Thus, they were able to demonstrate a striking difference in vaginal and cervical nanoparticle distribution between CP and MPP (Fig 3), composed of either non-biodegradable (PS) and biodegradable (PLGA) polymers. Similar penetration rates and cervicovaginal distribution of both types of polymeric nanoparticles highlighted the importance of the surface properties and not the core polymer material in determining how the nanoparticles behave in the vagina. Uptake of nanoparticles into the estrus phase vaginal mucosa was not observed, as expected, but the goal was to deliver the nanoparticles in close proximity to the cervicovaginal epithelia to provide sustained drug delivery to target cells. Ensign and coworkers demonstrated that administering MPP in a hypotonic vehicle that induces fluid absorption by the epithelia and advection of non-adhesive drugs and nanoparticles to the epithelial surface [71] led to a near complete MPP coating on the epithelial surface, including lining the vaginal folds [20]. As previously hypothesized, MPP penetration into the more slowly cleared mucus layers lining the vaginal folds led to increased retention, as 60% of the original MPP dose was retained compared to 10% for CP after 6 h.
Figure 3.
Distribution in the estrus phase mouse vagina of red fluorescent CP and MPP 10 min after administration (top panel), and green fluorescent fluorescein isothiocyanate (FITC) 24 h after administration in a conventional vaginal gel (FITC/gel) or encapsulated within biodegradable MPP (FITC/MPP). Modified with permission from (20).
Biodegradable PLGA MPP coated with a mucoinert Pluronic coating [72] and loaded with a model fluorescent drug (fluorescein isothiocyanate, FITC) provided a nearly uniform vaginal drug coating 24 h after administration, whereas FITC administered in conventional vaginal gel was poorly distributed over the vaginal surface (Fig 3) [20]. To demonstrate in vivo the efficacy of densely PEG coated MPP, the DP-treated mouse model of vaginal HSV-2 infection was used. To penetrate DP-treated mouse CVM, which has a reduced pore size compared to estrus phase mouse vaginal mucus [57], MPP nanocrystals composed of acyclovir monophosphate with the same mucoinert Pluronic coating with sufficiently small diameter (~65 nm) were formulated. MPP were shown to provide increased protection against vaginal HSV-2 infection compared to 10-fold higher concentration of soluble drug [20]. MPP nanocrystals are an exciting option for vaginal drug delivery, as the nanoparticles are composed of nearly pure drug, as opposed to >90% lipids or polymers like many other formulations. The combination of mucus-penetrating ability and sustained drug release has promise for improving vaginal drug delivery for a variety of applications, including prevention of sexually transmitted infections.
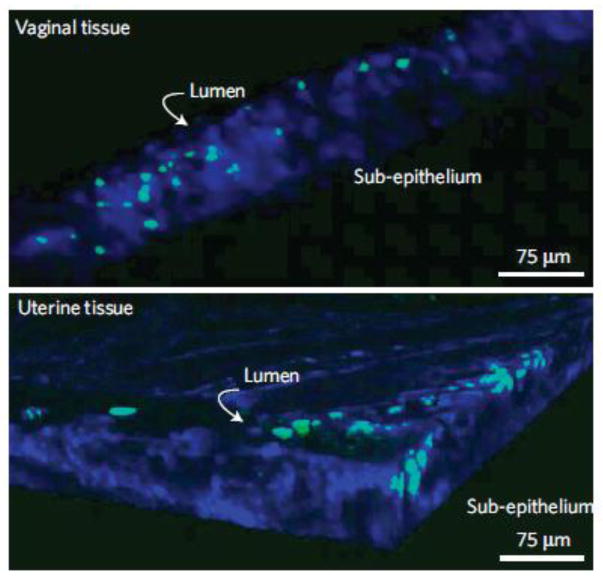
Polymeric nanoparticles have also been used as vehicles for vaginal siRNA delivery (for more background on vaginal siRNA delivery, see the next section, Liposomes). It was demonstrated that conventional transfection reagents for siRNA delivery were toxic in the vagina [73], leading Woodrow et al. to use encapsulation within a polymeric nanoparticle system to improve the safety of siRNA complexes [74]. They were able to encapsulate over one-thousand molecules of siRNA into a PLGA nanoparticle <200 nm in diameter, and achieved similar in vitro gene knockdown as compared to a commercial transfection reagent, Lipofectamine RNAiMax, in cultured HEK293T cells stably expressing luciferase. In vivo, they observed fluorescently labeled nanoparticle penetration into the tissue in the lower and upper mouse reproductive tract (Fig 4), leading to in vivo EGFP knockdown in the lower and upper tracts of GFP transgenic mice 10 d after topical administration of siRNA-loaded PLGA nanoparticles. The effect of the mucus barrier on the nanoparticle distribution and tissue penetration was not evaluated, as the vagina was washed and swabbed to remove mucus prior to nanoparticle instillation. Also, the mice were Depo-Provera treated, which thins the vagina and causes a transition to a columnar epithelium with reduced barrier properties (see above, The impact of hormones on the vagina for more details). However, these results provided important proof of principle that siRNA complexes could be successfully loaded into and released by PLGA nanoparticles, and that sustained gene silencing could occur in the female reproductive tract after successful intracellular delivery of siRNA [74].
Figure 4.
Penetration of siRNA-loaded PLGA nanoparticles (green) into the vagina and uterine tissue of mice 24 h after vaginal administration. Multiphoton microscopy was used to obtain deep tissue images. Image dimensions are 400 μm x 400 μm x 75 μm. Tissue is stained blue with Hoescht dye. Modified with permission from (74).
In another study, Steinbach et al. demonstrated that siRNA delivery via PLGA nanoparticles could provide protection against vaginal infection [75]. PLGA nanoparticles were loaded with siRNA complexes targeting a host cell receptor integral to vaginal HSV-2 infection in mice (nectin-1). Mice were treated with three doses of all formulations: 24 h and 2 h prior to infection, as well as 4 h post-infection. siRNA-loaded nanoparticles led to 20% (2 mg dose) and 60% (4 mg dose) survival against a lethal HSV-2 dose for 28 days, compared to 100% lethality observed by 14 days for scramble siRNA, cholesterol-conjugated siRNA, and 1 mg dose of siRNA-loaded nanoparticles. Interestingly, cholesterol-conjugated siRNAs that were previously associated with some protective effects (Wu) had no effect on survival in this work, and the 28 day survival observed for siRNA-loaded nanoparticles at 2 mg and 4 mg doses was a full 2 weeks longer than prior reports of siRNA-mediated improvement in survival [75]. Similar to prior work with siRNA-loaded PLGA nanoparticles, the mice were DP-treated and the vaginas were swabbed prior to nanoparticle instillation [74]. However, these results were an exciting further proof of principle of the potential for PLGA nanoparticles as a delivery vehicle for siRNA to provide vaginal protection from sexually transmitted infections with improved safety compared to conventional siRNA delivery vehicles.
The focus on polymeric nanoparticles as a microbicide delivery vehicle is relatively recent. Drug-loaded, biodegradable nanoparticles for vaginal antiretroviral drug delivery was reported by Ham et al. in 2009 [76]. They used PSC-RANTES, which is a CCR5 chemokine inhibitor that required orders of magnitude greater concentrations when administered as a free drug to inhibit HIV infection in vivo compared to in vitro. They demonstrated that PSC-RANTES encapsulation within PLGA nanoparticles provided sustained release and increased uptake into ex vivo cervical tissue compared to soluble PSC-RANTES, which they attributed to increased cell uptake of nanoparticles and protection of PSC-RANTES from degradation [76].
Soon after the pioneering work by Ham and coworkers, the success in a human clinical trial of 1% tenofovir (TFV) gel at reducing risk of vaginal HIV infection (ref) led to a series of papers detailing biodegradable TFV-loaded nanoparticle formulations for vaginal use. Zhang et al. formulated TFV and tenofovir disproxil fumarate (TDF, oral prodrug version) nanoparticles composed of a blend of PLGA and pH-responsive Eudragit ® S-100 polymer that was intended to release tenofovir upon contact with semen in the vagina. Despite the high water solubility of TFV/TDF and the low drug loading (0.5%/1.2%), only ~50% of the drug was released in vitro in 24 h. However, for the application of semen-triggered release of microbicide drugs, an initial burst release of drug would likely be advantageous for inactivating the viral load. For a drug like TFV that must be taken up into cells and phosphorylated twice before becoming active, triggering release at the same time as introduction of the viral load may be too late to prevent infection. They demonstrated that the nanoparticles were tolerated in vitro by vaginal epithelial cells and Lactobacillus crispatus vaginal bacteria, indicating that the formulation would have potential for future development with other drugs [77].
Yoo et al. reported the development of pH-responsive nanoparticles composed entirely of Eudragit ® S-100, and similarly demonstrated that the encapsulation efficiency (the overall loading was not reported) for hydrophilic compounds was low (26%) compared to hydrophobic compounds (71%) [78]. Since this formulation was composed entirely of pH sensitive polymer, burst release occured at a pH (7.4) in the range expected when semen contacts vaginal mucus [78]. In another study, Meng et al. developed TFV-loaded nanoparticles made from chitosan to maximize mucoadhesion. The nanoparticles were well-tolerated in vitro by vaginal epithelial cells and Lactobacillus crispatus [79]. However, as described previously (see The mucus barrier), mucoadhesive nanoparticles likely do not provide widespread distribution to all of the surfaces of the vagina, and suffer from short vaginal residence time due to the more rapid clearance by the luminal mucus layers [20]. Belletti et al. also developed TFV-loaded PLGA/chitosan blend nanoparticles with drug loading <1% and drug release occurred on the order of hours [80]. It may warrant evaluating whether TFV is an ideal microbicide candidate for loading into hydrophobic polymeric nanoparticles, as the high water solubility limits the encapsulation efficiency and potential for sustained release. Also, the active form of tenofovir (tenofovir diphosphate) already has a long intracellular half-life, so further improvements in delivery would likely require several days of sustained release. However, formulating tenofovir to achieve adequate distribution throughout the cervicovaginal tract is still necessary for providing reliable protection against infection [81].
Due to the success of combination drug strategies for suppressing active HIV infections, it is thought that combination approaches may also provide improved protection against the establishment of infection. However, combining multiple drugs with different physiochemical properties into the same dosage form can be challenging. Chaowanachan et al. investigated the possibility of creating drug synergy by loading microbicides with low water solubility into nanoparticles, and combining the drug-loaded nanoparticles with soluble TFV [82]. Efavirenz (EFV) and saquinavir (SQV) were individually loaded into PLGA nanoparticles at overall loadings of 6.7% for EFV nanoparticles (NP-EFV) and 7.2% for SQV nanoparticles (NP-SQV). The in vitro drug release from NP-EFV and NP-SQV was “biphasic”, with an initial burst release of 10–20% of the drug within 1 h followed by sustained release over 24 h. However, only ~34% of EFV and ~42% of the SQV was released in 24 h, and no additional release was observed up to 144 h, possibly indicating that sink conditions were not met in the release study. Nonetheless, drug released from the nanoparticles was effective at inhibiting HIV-1 BaL replication in TZM-bl cells in vitro; the NP-EFV provided up to a 50-fold reduction in the IC50 compared to free EFV, and the NP-SQV provided similar IC50 to free SQV. NP-EFV and free TFV mixed at the molar ratio that provided equal potency of the free drugs (1:11 molar ratio) provided a 3-fold reduction in the IC50 value compared to the combination of free EFV and TFV. Interestingly, a synergistic effect was not observed when the NP-EFV and free TFV was mixed at the molar ratio that provided equal potency of the NP-EFV and free TFV (1:600 molar ratio). In contrast NP-SQV did demonstrate synergistic effects (20-fold reduction in IC50 value) when mixed with free TFV at the molar ratio that provided equal potency of the NP-SQV and free TFV (1:3 molar ratio) [82]. A potential limitation of the NP-EFV and the NP-SQV formulations is the overall negative ζ-potential (~ −24 mV), likely indicating that portions of the surface would be exposed to adhesive interactions with mucus in the vagina, limiting vaginal distribution and retention [66]. However, this study highlights the potential for nanoparticles to improve vaginal delivery of drugs with low water solubility, as well as a method for combining drugs with significantly different water solubility in a single dosage form.
Dapivirine, another microbicide candidate with low water solubility, was loaded into poly(epsilon-caprolactone) (PCL) nanoparticles in a series of papers by Alonso and coworkers, including evaluating antiretroviral activity in vitro [83], in vitro interactions of the nanoparticles with simulated vaginal fluid [84], ex vivo penetration of pig vaginal and rectal tissue [85], and in vivo biodistribution in mice [86]. It was demonstrated throughout that PEG was a preferable nanoparticle coating to sodium lauryl sulfate (SLS) and cetyl trimethylammonium bromide (CTAB), particularly due to cell toxicity associated with SLS and CTAB. In contrast to hydrophilic TFV, loading of dapivirine was ~13%, though >60% of the drug was released from the nanoparticles within 1 h [83]. Using confocal imaging of tissue slices, fluorescently labeled nanoparticles appeared to penetrate into ex vivo pig vaginal and rectal tissue, as well as DP-treated mouse vaginal tissue to a lesser extent in vivo, after 2 h of incubation [85, 86]. Despite various reports of ex vivo tissue penetration and in vivo tissue penetration in DP-treated mice, it remains to be determined whether polymeric nanoparticle uptake occurs in the intact squamous epithelium of the human vagina.
Date et al. loaded both an NNRTI (efavirenz) and an HIV-1 integrase inhibitor (raltegravir) into PLGA nanoparticles (RAL-EFV-NPs) to potentially gain an additive effect from co-encapsulating two compounds with two different mechanisms of action [87]. They found that the RAL-EFV-NPs had a slightly lower EC90 value (~90 ng/mL) compared to drug solutions (~144 ng/mL) in vitro, supporting the notion that formulation within a nanoparticle can augment the efficacy of drugs. Rather than incorporation into a conventional vaginal gel vehicle, they formulated the RAL-EFV-NPs in a thermosensitive liquid vehicle that gels at body temperature [87]. Although this strategy has yet to be tested in vivo, nanoparticle-based drug combination platforms and thermosensitive gel vehicles are promising new strategies for vaginal microbicide delivery.
Peng et al. also employed the use of a thermosensitive gel to selectively target delivery of nanoparticles to the surface of the cervix [88]. They attempted to design a DNA construct encoding HPV16-regulated diphtheria toxin that could be selectively expressed in HPV16-infected cells, resulting in cell death. The construct was used to formulate poly(β-amino ester) nanoparticles for use as a nanotherapy for pre-neoplastic cervical lesions. Although they did not demonstrate that the construct was selectively expressed in HPV16-infected cells, it was selective for epithelial cells. The thermosensitive gel mixed with a luciferase reporter gene/nanoparticle construct induced luciferase expression in human cervical tissue with high grade precancerous lesions after ex vivo exposure, and in mouse cervical tissue 24 h after intravaginal injection [88]. Although the DNA construct will need further development before testing the nanoparticle/formulation for treatment of cervical lesions, this work highlighted the potential for using polymeric carriers for vaginal DNA delivery.
One limitation to developing local vaginal treatments for reproductive tract cancer is a lack of orthotopic mouse models. Blum et al. described the development of an inducible model of vaginal squamous cell carcinoma via activation of oncogenic K-ras and inactivation of tumor suppressor Pten [89]. Although vaginal cancer (as opposed to cervical cancer) is relatively rare, the animal model provides an opportunity for testing topical chemotherapy delivery in the vagina. Camptotecin (CPT)-loaded PLGA nanoparticles were prepared with 6.2% loading drug loading and release that lasted over 28 days in vitro. Five weekly treatments with the CPT nanoparticles prophylactically prevented tumor growth in 6 of 6 mice, whereas tumors developed in 3 of 4 mice treated with free CPT [89]. One limitation of this study is that it was a demonstration of prevention of tumor establishment, rather than treatment of an existing tumor. Also, the nanoparticles were lavaged into the vagina, which could artificially disrupt the protective mucus barrier. Nonetheless, this study supported the promise that nanoparticle-based therapies hold for treating cancers of the female reproductive tract.
Recently, Yang et al. tested topical nanoparticle administration for treating established cervical cancer in a mouse model established by locally implanting TC-1 tumor cells in the mouse vagina [21]. The tumor cells express luciferase enzyme, such that tumor growth can be monitored over time by live animal bioluminescence imaging. As described earlier, the mucus barrier in the female reproductive tract precludes effective nanoparticle distribution and retention. Yang et al. sought to demonstrate that non-adhesive, biodegradable mucus-penetrating particles (MPP) could improve the delivery of chemotherapy to cervical tumors [72]. They demonstrated that F127-coated PLGA nanoparticles (MPP) were able to distribute throughout vaginal mucus and contact the epithelium, coming in close proximity to deliver drug directly to the tumor. In contrast, PLGA nanoparticles without the PEG coatings provided by F127 (CP) aggregated in the luminal mucus, and were sequestered away from the tumor (Fig 5). Vaginally administered paclitaxel-loaded MPP (PTX/MPP) were more effective than paclitaxel-loaded CP (PTX/CP) at suppressing cervical tumor growth, nearly doubling the median survival time in an aggressive cervical tumor model [21]. The MPP platform has promise for improving vaginal drug delivery for a variety of applications, including treatment of female reproductive tract cancers.
Figure 5.
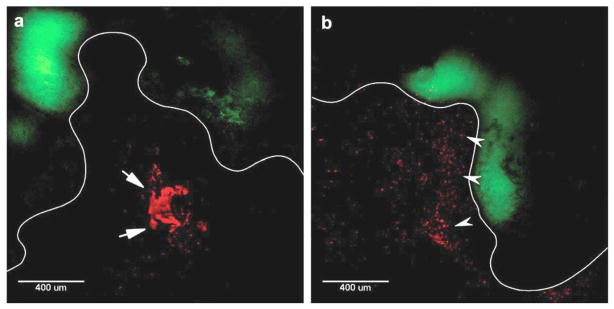
Transverse sections of the mouse reproductive tract with TC-1 cervical tumors expressing green fluorescent protein (GFP, green). The epithelium is outlined in white to help distinguish the tissue surface. (a) Arrows indicate large mucoadhesive particle (CP) aggregates. (b) Arrows indicate well-dispersed mucus-penetrating particles (MPP) in close proximity to the tumor at the epithelial surface. Reprinted with permission from (21).
Liposomes
Bangham and coworkers described liposomal drug carriers in 1965 [90], making liposome formulations the nanocarriers with the longest history of research and development. Liposomes are essentially model cell membrane systems that can generally be described as enclosed phospholipid bilayer structures, although the literature is full of descriptions of modifications for improved intracellular delivery, targeting, circulation time, and drug loading and release [91]. Although the first liposomal product, Doxil®, received FDA approval for intravenous treatment of chemotherapy refractory acquired immune deficiency syndrome-related Kaposis’s sarcoma in 1995 [92], development of liposomal formulations for vaginal administration has been a more recent focus. As with other nanoparticle-based formulations, HIV prevention has been a major focus in liposome formulation.
For microbicides with limited water solubility, such as the non-nucleoside reverse transcriptase inhibitor (NNRTI) MC1220, liposome formulations can improve drug loading and stability in vaginal products. Caron et al. tested MC1220-loaded phosphatidylcholine/cholesterol liposomes suspended in a Carbopol/hydroxypropylcellulose/glycerol gel for vaginal protection against RT-SHIV, a form of the chimeric simian immunodeficiency virus expressing HIV-1 envelope protein engineered with sensitivity to NNRTI, in rhesus macaques [93]. Dosing strategies tested included single application of liposome gels containing 0.5% or 1.5% MC1220, and treatment with the 0.5% gel daily for 4 days. In all cases, partial protection was achieved, with 2 out of 4 and 3 out of 5 animals infected after a single dose of 0.5% and 1.5% gel, respectively compared to 5 out of 5 control animals infected. The unexpected lack of increased protection by the higher concentration MC1220 gel was attributed to the low aqueous solubility of MC1220, which the authors plan to address by identifying alternative vehicles. Three out of 5 animals were protected after 4 daily doses of 0.5% gel. RNA viral load at necropsy was significantly lower in MC1220-treated infected animals compared to controls, and the authors hypothesize that improved protection could be achieved in combination with other synergistic microbicides [93]. It is likely, though, that additional optimization to increase vaginal protection by MC1220 must be done before it is considered as promising of a candidate as microbicides such as tenofovir and dapivirine.
Wang et al. formulated another novel candidate microbicide with low water solubility, octylglycerol, in phosphatidylcholine liposomes for vaginal application [94]. In comparison to water-based Carobopol or poloxamer hydrogel formulations with glycerin as a solubilizing agent for octylglycerol, after 2 months of storage, octylglycerol liposomes had better in vitro activity against HSV-1, HSV-2, and HIV-1, presumably due to increased product stability. Formulation of octylglycerol into liposomes increased the minimum cidal concentration (MCC) of octylglycerol to lactobacillus, but the lipid/octylglycerol ratio had to be carefully controlled to avoid significant killing, measured as >4-log reduction in Lactobacilli. No signs of toxicity were observed after 2 h exposure of octylgelycerol liposomes to ex vivo human cervical tissue [94].
Malavia et al. investigated liposomes themselves as an antiviral agent, as certain lipid compositions can bind to HIV and modulate its activity [95]. They varied the composition of naturally-occurring and synthetic lipids to balance the antiviral properties with the observed cytotoxicity, and found that increasing the cardiolipin content and the degree of unsaturation increased the balance between decreasing HIV infection and causing cytotoxicity (referred to by the authors as the therapeutic index) of the liposomes. This work brought to attention that lipid-based formulations can have inherent toxicity that must be assessed to ensure that prophylactic treatment does not result in increased risk of vaginal infection. The optimized liposome formulation found by Malavia did not appear to cause toxicity 3 and 7 days after vaginal administration to mice, but it would be important to study potential acute toxic effects and vaginal protection in vivo [96].
Hood et al. also developed lipid-based nanoparticles that could interact directly with HIV [97]. Liquid perfluorocarbon core nanoparticles were coated with a phospholipid monolayer membrane that was infused with melittin, a peptide component of bee venom. Melittin is thought to have pore-forming cytolytic activity, and the uptake of melittin by HIV-1 infected cells was shown to decrease HIV-1 gene expression and replication [98]. The lipid/melittin coated nanoparticles had activity against HIV in vitro, while appearing to have minimal effects on cells, despite the evidence that melittin facilitates cell apoptosis and necrosis [99]. The authors postulate that normal cells have a larger lipid membrane surface area and can rapidly repair membrane defects induced by melittin, whereas enveloped viruses would be more sensitive to rupture [97]. The potential toxic effects should be tested extensively in appropriate models prior to clinical testing.
Alukda et al. also prepared lipid based nanoparticles with a functionalized surface [100]. The lipid core was coated in layers of poly-L-lysine to increase cell uptake and heparin to target natural killer cells, though these properties were not experimentally tested. The solid lipid nanoparticles were formulated for vaginal delivery of tenofovir, although the loading was ~0.1%, which is likely due to the high water solubility of tenofovir. However, the formulation at concentrations up to 0.9 mg/ml appeared to be well-tolerated by cells in vitro, so further studies toward developing formulations encapsulating drugs with more lipophilic or hydrophobic character may be of interest [100].
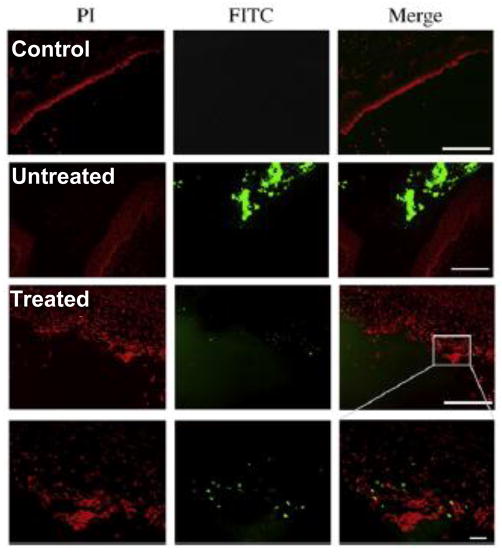
Numerous investigators have also been designing nanoparticle systems for vaginal delivery of small interfering RNA (siRNA) to silence viral gene expression and prevent the establishment of active infection [101]. Palliser et al. demonstrated in 2006 that siRNA complexed with a transfection reagent (oligofectamine) could be delivered into cells in the mouse vagina and ectocervix, and that vaginal administration of siRNA targeting three essential HSV-2 genes could provide protection against lethal (high titre) HSV-2 infection [61]. Later work involving Palliser and others demonstrated vaginal toxicity associated with the transfection reagent that was not discerned in the prior proof of principle study, necessitating the design of non-toxic vehicles for siRNA delivery [73]. The focus to date for formulating siRNA for vaginal adminstration has been on non-viral vehicles, such as liposomes, rather than viral vectors [101]. However, Wu et al. demonstrated that the mucus barrier may limit efficient delivery of liposomes in the vagina, and that the mouse estrous cycle impacts in vivo vaginal oligonucleotide delivery [62]. Liposome-oligonucleotide complexes formed from a common commercially available lipid (dioleoyl trimethylammonium propane, DOTAP) did not transfect the mouse vaginal epithelium, regardless of estrous cycle state, unless the vagina was pretreated with a toxic agent (5% citric acid) (Fig 6). However, even with citric acid treatment, the authors indicated that the minimal oligonucleotide delivery via conventional liposomes they observed would not mediate clinically significant siRNA responses intravaginally. Thus, they designed liposomes with PEG on their surface to facilitate penetration through the mucus barrier, as well as a scaffold system for increasing residence time in the vagina. Their PEGylated lipoplex-entrapped alginate scaffold system (PLAS) provided a 6-fold increased uptake of oligonucleotides in vaginal tissues compared to the conventional liposomes, as well as enhanced siRNA-mediated knockdown of Lamin in the vagina of mice. Although this work is limited by the fact that the mice were pretreated with 5% citric acid prior to vaginal administration of any liposomes, they made a point that prior studies involving vaginal administration of siRNA must also be viewed critically since the mice were pretreated with Depo-Provera (DP), which artificially thins the vaginal epithelium amongst other effects, as described earlier. DP treatment (causing diestrus phase synchronization) is also necessary for the vaginal HSV-2 infection mouse model [39, 102], so it is difficult to evaluate the efficacy of siRNA delivery against HSV-2 infection when the structure of the mouse vagina (estrus phase) is most similar to that of the human vagina.
Figure 6.
Vaginal delivery of lipoplexes in the estrus phase mouse vagina (Untreated), which is more reminiscent of the structure of the human vagina, was minimal and restricted to tissue debris in the lumen, unless the epithelium was pretreated with 5% citric acid (Treated). The scale bars present 200 μm in the top three panels and 20 μm in the bottom panel. Propidium idodide (PI) was used to stain the epithelium. Adapted with permission from (62).
In addition to prevention of sexually transmitted infections, liposomes have been formulated for vaginal treatment of bacterial and fungal infections. Pavelic and coworkers have published several papers detailing the design of various liposome formulations for vaginal drug delivery. The first described liposomes made of phosphatidylcholine (PC) or distearylphosphatidylcholine (DSPC) made by two different formulation methods. Liposomes were formulated containing clotrimazole (used to treat yeast infections), metronidazole (used to treat bacterial vaginosis), and chloramphenicol (antibiotic not currently in clinical use vaginally). The entrapment efficiency ranged from 5–94% depending on the drug and method, and the drug loading ranged from ~2–9%. Drug release occurred over at least 24 h, both using standard in vitro techniques and when incubated in vitro with cow vaginal mucosa to test the stability of the liposomes in the presence of vaginal tissue, though ~50% of the drug was released within the first hour of incubation with the tissue [103]. The burst release may make it difficult to deliver sustained therapeutic amounts of drug in the vagina. They next described a general (9:1) PC:phosphatidylglycerol-sodium (PG) liposome formulation for vaginal delivery of hydrophilic drugs. The liposomes were prepared encapsulating calcein dye using five different formulation techniques, and then suspended within Carbopol gels. The encapsulation efficiency varied from <10% to >60% depending on the method, but the overall loading was not discussed. Dispersing the liposomes within Carbopol gel prolonged the in vitro calcein release (only ~40% over 24 h), but the viscosity of the gel was significantly reduced after addition of liposomes, which would likely impact its vaginal spread and retention [104]. They then went on to demonstrate formulation of a hydrophilic anti-herpes drug (acyclovir) using the (9:1) PC:PG formulation, as well as a PC-only formulation and a (9:3) PC:stearylamine (SA) formulation. The loading efficiency ranged from 8% (PC) to ~27% (PC:PG), but the overall loading ranged from ~1–3%. Incorporation of the liposomes into Carbopol gel provided prolonged release over at least 24 h in vitro, and the rate of release in the presence of mucin in the simulated vaginal fluid release media was increased for PC:PG liposomes (−56.2 mV surface charge), decreased for PC:SA liposomes (+8.3 mV surface charge), and unaffected for PC liposomes (−20 mV surface charge). The authors hypothesized that the negatively charged mucins coated the positively charged liposomes, increasing stability in the simulated vaginal fluid [105]. However, interactions with mucins would also negatively impact the vaginal distribution and retention of the liposomes. The potential for viral suppression was not evaluated for these liposomes, and it is unclear whether the low drug:lipid ratio would impact the ability to deliver therapeutically relevant concentrations to target tissues and cells in the vagina without toxicity.
Next to BV, yeast infections are the second most common vaginal infection. Karimunnisa and Atmaran sought to develop a mucoadhesive liposomal gel for sustained release of the antifungal drug ciclopirox olamine (CPO) with the goal of reducing frequency and volume of dosing [106]. It is worth noting that the volume and frequency of dosing appeared to be based on guidelines in India, whereas CPO gels are not indicated for vaginal use in the US. Liposomes were formulated with Phospholipon 90H (a hydrogenated phosphatidylcholine), cholesterol, and diacetyl phosphate, and formulated in a Carbopol gel. In this work, the liposome-loaded gel (L-gel) demonstrated increased viscosity compared to the blank Carbopol gel (B-gel). The L-gel also demonstrated increased maximum detachment forces (MDF) in vitro compared to the B-gel and reported values for other commercial vaginal gel preparations, as determined by pulling from sheep vaginal mucosa [106]. Although hypothesized to increase the vaginal residence time, the increased viscosity and “stickiness” of the gel may make achieving adequate vaginal distribution even more difficult. Karimunnisa then confirmed the anti-fungal activity of the CPO liposomes in vitro. Pure CPO killed all Candida colonies within 3 h, whereas a significant reduction in colonies was seen after 3 h exposure to the CPO liposomes. Complete killing of the Candida colonies by the CPO liposomes was observed after 6 h [106]. Further testing will be required to ensure that the formulation is not toxic to the vaginal epithelium.
As previously discussed, bacterial vaginosis (BV) is a major worldwide women’s health problem. Various investigators have designed liposome gel formulations for sustained release of metronidazole to treat BV, and to improve upon the leakage, messiness, and short residence time of conventional gel formulations. Patel and Patel formulated liposomes containing metronidazole using a thin film hydration technique using soy lecithin and cholesterol, and loaded the liposomes into a Carbopol gel [107]. Unfortunately, formulation details are not specific enough to determine the drug content, but the entrapment efficiency ranged from 12–36% depending on the formulation conditions. In contrast to many other studies, the much larger liposomes in this work (11–15 μm in diameter), released ~60% of the drug in vitro within 12 h. Similar to other studies, formulation of the liposomes in the carbopol gel slowed the release in vitro; ~50% of the drug was released within 12 h, in comparison to ~80% of drug released from a control metronidazole gel [107]. However, if the control gel and the liposome gel have similar rheological properties, it is yet to be determined whether this modest increase in the time of drug release would have benefits that outweigh the potential toxicity of the lipid components in vivo. Vanic et al. formulated “deformable” liposomes containing metronidazole. These deformable liposomes were developed for applications in the skin, where conventional liposomes remained confined to the upper layer of the stratum corneum. The deformable, or flexible, liposomes are thought to penetrate intact through skin. The lipid bilayers of the liposomes contain a single chain surfactant that is thought to destabilize the liposomes, allowing them to “squeeze” though pores in the stratum corneum [108]. Vanic et al. hypothesized that this system may also be useful for mucosal applications, such as in the vagina. The liposomes were made from PC and PG, containing sodium deoxycholate, Tween 80, or Span 90 as surfactants. Using Caco-2 cell monolayers, they demonstrated that both the conventional and the deformable liposomes decreased the transepithelial resistance by ~50%, which the authors attributed to tight junction opening [109], though it is unclear how this relates to vaginal delivery of metronidazole since the vaginal epithelium is stratified squamous, not a columnar epithelium with tight junctions. The surfactants used to produce these flexible liposomes are also likely to be toxic to the vaginal epithelium, an effect that must be avoided for vaginal drug delivery systems.
Basnet et al. evaluated a phosphatidylcholine liposome formulation containing curcumin for treating local vaginal inflammation. The liposomes were formed by the film method, resulting in highly polydisperse liposomes nearly 1 μm in diameter, followed by a sonication step that reduced the liposome size to ~100–200 nm. The in vitro antioxidant activity of the curcumin liposomes, as measured by quenching of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, was comparable to Vitamin C. Production of superoxide dismutase (SOD), nitric oxide (NO), IL-1β, and TNF-α was measured from lipopolysaccharide-activated murine macrophages. SOD, an antioxidant enzyme, was increased ~45% by soluble curcumin and ~65% by curcumin liposomes. NO is produced by macrophages as part of a pro-inflammatory cascade, and curcumin liposomes were more potent at decreasing NO production in vitro compared to curcumin. Liposomal curcumin also reduced IL-1β (75% reduction) and TNF-α (80% reduction) production by LPS-induced murine macrophages in vitro compared to control (soluble curcumin provided 60% and 44% reductions, respectively) [110]. Although the liposomal curcumin formulation appeared to increase the potency of curcumin at suppressing inflammation in vitro, which the authors attribute to improved curcumin solubility, it is not clear whether treatment of inflammatory signaling would be preferable to directly treating the cause of inflammation (e.g. fungal or bacterial infection). Also, in certain cases, such as BV, overt inflammation may not be present, so the impact of delivering anti-inflammatory compounds would be less clear.
Kang et al. combined an anti-fungal liposome formulation with a thermosensitive gel vehicle [111]. Thermosensitive gels are liquids at room temperature and solidify to form a hydrogel once applied in vivo, and have been explored for numerous applications (ref). In the vagina, thermosensitive gels may be advantageous for achieving improved distribution throughout the highly folded vaginal surface compared to conventional hydrogels. Amphotericin B (amB), an antifungal drug that is not indicated for topical vaginal use due to its toxic side effects, was formulated in 1,2-dioleoyl-sn-glycerol-3-phosphoethanolamine (DOPE), 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), cholesterol (4:5:1) liposomes. Distearoyl phosphatidyl ethanolamine-polyethylene glycol was used to increase the solubility of AmB prior to encapsulation within liposomes. Due to the properties of the lipids, these liposomes were positively charged, meaning that they would likely stick to mucus and are potentially cytotoxic. AmB liposomes were formulated in Pluronic gels with varying composition of P407/P188 to find a formulation with an appropriate gelation temperature, though the liposomes did not have a significant effect on the gelation temperature of the Pluronic mixtures. Using HEK293 cells, Kang et al. observed significant toxicity of free AmB, as expected, though similar toxicity was observed from the AmB liposome gel (~50% reduction in cell viability). If the AmB liposome gel was diluted 1:1 or 1:3, there was not an apparent reduction in cell viability, but even free AmB in a 1:3 diluted gel did not appear toxic to cells. Thus, although the authors state that AmB encapsulated in cationic liposome gels was less toxic than AmB loaded in cationic liposomes, the free drug appeared to lose its toxicity when diluted in gel as well. It is not clear whether AmB could be formulated in a way to reduce the toxicity enough to have clinical applicability for vaginal treatment.
Dendrimers
Dendrimers are a class of branched polymeric nano-structures that are typically much smaller (~5–10 nm) than standard nanoparticles (often 100 nm or larger). Processes for dendrimer synthesis allow for precise control of structure and composition, including tailoring the number of branching units and the surface functional groups [112]. The surface functional groups can be used to directly interact with target cells and/or pathogens, conjugate drugs, or complex with other therapeutic moieties like oligodeoxynucleotides and siRNA. Dendrimers have been used extensively for HIV PrEP, and in fact, the only nanotechnology that has advanced to human clinical trials for HIV PrEP is the SPL7013 dendrimer-based VivaGel® [113]. SPL7013 is a polylysine dendrimer with anionic sulfate surface functional groups demonstrating antiviral activity for both HIV and HSV, similar to polyanionic polymers that were used in the first generation of vaginal microbicides [114]. Tyssen et al. found that SPL7013 binds to HIV gp120, the HIV envelope protein that binds to CD4 receptors on T cells, inhibiting viral attachment [115]. Early in vitro studies demonstrated that concentrations ~10,000-fold higher than the EC50 were non-toxic to Vero cells [114], and phase 1 trials demonstrated general safety and tolerability in healthy, sexually abstinent women [116]. However, expanded safety studies indicated that several mucosal immune parameters (including cytokines IL-2, IL-5, IL-10, and IFNγ; CD8+/CD69+ and CD4+/CCR5+T-cell subsets) were increased with VivaGel compared to placebo after 7–14 days of twice daily vaginal use [117]. As epithelial toxicity and the presence of HIV target cells are believed to increase risk of mucosal HIV transmission, Moscicki et al. concluded that these findings and the observed increase in adverse events, such as grade 1 and 2 genitourinary events and superficial colposcopic findings, reported in two phase 1 trials should be taken into consideration in planning future clinical trials with VivaGel [117]. Potential toxicity to the healthy vaginal epithelium was also a limitation of sulfated anionic polymers as vaginal microbicides [118]. Starpharma is not currently testing VivaGel in clinical trials as a vaginal microbicide, but is currently in Phase 2 trials as a treatment for bacterial vaginosis (BV) that may prevent BV recurrence. It is important to keep in mind, too, that the clinically demonstrated antibacterial properties of dendrimers must be considered when designing dendrimer-based formulations for HIV prophylaxis in women with healthy vaginal microbiota. There is a potential for dendrimers to negatively impact lactobacillus, which may have other unintended consequences (see The role of microbiota in the vagina).
Numerous other formulations that take advantage of the innate antiviral properties of dendrimers are in development as vaginal microbicides. Similar to SPL7013, various other dendrimers with simple anionic surface functionalization have been shown to bind to gp120 in vitro [119, 120]. However, binding of gp120 to CD4 receptors is not the only mechanism in which HIV infects target cells; HIV is also known to interact with carbohydrate receptors on immune cells and epithelial cells. This knowledge led to the development of various types of glycodendrimers [35, 120]. For example, HIV binds to glycosphingolipids on non-CD4-expressing cells to gain entrance [121, 122]. Functionalizing dendrimers with various sulfated carbohydrates and/or multivalent carbohydrates [123–125] resulted in potent HIV inhibition in vitro. Kensinger et al. demonstrated that sulfated galactose terminated dendrimers were more potent at inactivating HIV in vitro than galactose terminated dendrimers [124]. Also, it has been shown that HIV and other pathogens traffic to the lymph by binding to a carbohydrate recognition domain in DC-SIGN, a transmembrane protein present on dendritic cells that reside in the vaginal mucosa [126]. Tabarani et al. demonstrated that mannose hyperbranched dendritic polymers inhibited in vitro interactions between HIV gp120 and DC-SIGN [127]. Ciobanu et al. went on to show that dendrimers functionalized with a synthetic mannose-based oligomer selected for increased binding to DC-SIGN prevent HIV gp120 binding to dendritic cells more efficiently than a natural polymer of mannose [128]. Other investigators have explored varying dendrimer core material, including using inorganic backbones [129], as well as functionalizing dendrimers with peptides as a potential vaccine [130]. However, using dendrimers themselves as the active ingredient is far more common in vaginal microbicide development. Although not the focus here, dendrimers functionalized with various ligands for targeting infected cells, antiretroviral drugs, and siRNA [131–133] have been developed for treating existing HIV infections. For more details on the use of dendrimers as microbicides and treatments for HIV, the reader is directed toward other more comprehensive reviews [120, 134].
In addition to being developed for HIV prevention, the antimicrobial properties of dendrimers have been explored for other vaginal applications. It is particularly important to treat vaginal infections during pregnancy to prevent ascension into the uterus, and vaginal application of microbicides is also under evaluation in pregnant women [135]. It is important to ensure that medications applied vaginally do not have a negative impact on the developing fetus. For this reason, Menjoge et al. determined whether topical vaginal application of polyamidoamine (PAMAM) dendrimers could result in transport across the human fetal membranes that separate the extra-amniotic space and the fetus. Using human chorioamniotic membranes obtained from women with uncomplicated pregnancies, it was demonstrated that less than 3% of dendrimers were able to cross the intact chorioamniotic membrane during 5 h of incubation, in contrast to 20% permeation by unconjugated fluorophore (fluorescein isothiocyanate, FITC). Largely, the dendrimer was found in the interstitial regions of stromal and trophoblast cells, indicating that the transport mechanism was likely passive diffusion. These results indicate that dendrimers may find use as topical vaginal agents in pregnant women, potentially without affecting the fetus, and that conjugation of drugs to macromolecules could reduce drug exposure to the fetus after vaginal application [135].
The antimicrobial properties of PAMAM dendrimers were also investigated for preventing bacterial infections that lead to chorioamnionitis, or inflammation of the chorioamnionic membrane [136]. Wang et al. demonstrated that treating E. coli with PAMAM dendrimers in vitro resulted in damage to the bacterial cell wall leading to bacterial death (Fig 7). Of note, hydroxyl-terminated and amine-terminated dendrimers both inhibited bacterial growth, but amine-terminated dendrimers were cytotoxic above 10 μg/ml, whereas hydroxyl-terminated dendrimers were non-cytotoxic up to 1 mg/ml concentration. In a guinea pig model of E. coli-induced chorioamnionitis, hydroxyl-terminated dendrimer treatment 5 min after bacterial inoculation led to complete inhibition of bacterial infection in the amniotic fluid, whereas 57% of control fetuses had amniotic fluid infections. Bacterial inhibition in dendrimer treated animals was accompanied by cytokine levels similar to uninfected controls, whereas E. coli infected controls had increased TNF-α, IL-6, and IL-1β in their placental tissue [136].
Figure 7.
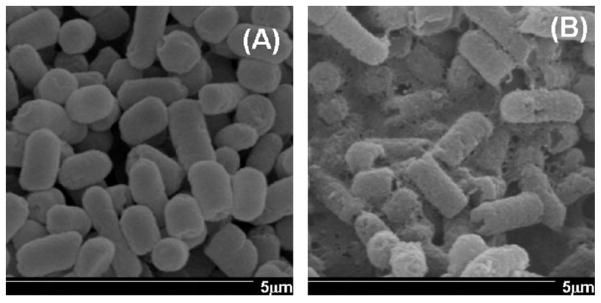
SEM images of E. Coli either (A) untreated, or (B) after 8 h exposure to carboxyl-terminated PAMAM dendrimers. Magnification 20,000x. The treatment with dendrimers shows damage to the bacterial cell wall. Adapted with permission from (135).
Rather than exploiting the antimicrobial properties of dendrimers themselves, Navath et al. investigated the use of dendrimers as a component of an in situ forming hydrogel for vaginal delivery of amoxicillin during pregnancy [137]. Topical delivery of therapeutic agents is favorable to treat ascending genital infections in pregnant women, though the delivery form must selectively treat the infection without affecting the fetus. A biodegradable gel was formed in situ by creating disulfide bridges between thiopyridine functionalized dendrimers and thiol-terminated 8-arm PEG. When physically loaded with amoxicillin, the gel provided sustained release in vitro for over 10 days. Gel containing dendrimers labeled with fluorescein isothiocyanate (FITC-dendrimer) was found to reside in the vagina of pregnant guinea pigs for at least 72 h, though the physical appearance was more plug-like than gel-like. Hemotoxylin and eosin staining of the reproductive tract of the guinea pigs did not show any sign of inflammation and edema 24 and 72 h after vaginal administration. Importantly, it appeared that although the FITC-dendrimer labeled gel was present on the outside of the fetal membrane of a pup close to the cervix, upon visual inspection of the pup, the gel did not appear to cross the fetal membrane and enter into the gestational cavity. Navath et al. also demonstrated by confocal imaging of tissue slices that the gel remained in the cervicovaginal lumen, apparently restricted from the tissue surface by the mucus barrier, rather than penetrating or degrading into the subepithelial or submucosal layers (Fig 8). The fetal membrane and uterus of pregnant guinea pigs did not show any evidence of gel penetrating across the tissue, as well [137].
Figure 8.
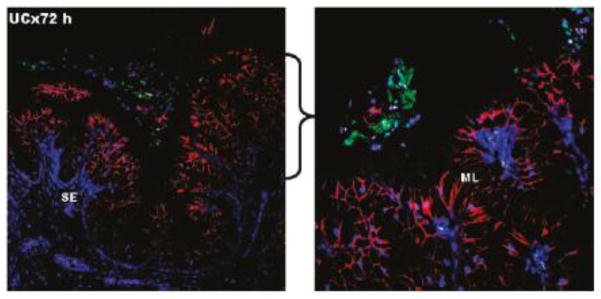
Confocal images of the cervical region of pregnant guinea pigs treated with hydrogels for 72 h. The hydrogel (green) is seen on the surface of the mucosal layer (red), separated by the mucus coating the epithelium (unlabeled). The nuclei are stained blue with DAPI. SE = subepithelial layer, ML = mucified epithelial layer. The right panel is a higher magnification image of the indicated region of the left panel to further illustrate the mucus layer separating the gel from the epithelium. Adapted with permission from [136].
Inorganic nanoparticles
The antimicrobial properties of inorganic metals, such as silver, have been exploited for thousands of years [138]. Recently, Elechiguerra et al. demonstrated that, similar to dendrimers, silver nanoparticles have intrinsic anti-HIV activity [139]. Mechanistically, it was demonstrated that polyvinyl pyrollidone coated silver nanoparticles bind to the disulfide bonds in the CD4 binding domain of gp120 and inhibit viral fusion to cells, and this antiviral activity was apparent against various HIV strains, including drug-resistant strains [140]. Lara et al. found that silver nanoparticles prevented infection of cervical explants by both cell-free and cell-associated virus [141]. The nanoparticle concentration that prevented infection (0.15 mg/ml) was effective after only 1 minute of pretreatment, and was well-tolerated by the cervical explants [141]. Fayaz et al. recently reported on the design of a silver nanoparticle-coated condom for inhibiting HIV and HSV transmission [142]. The coated condoms also demonstrated antibacterial and antifungal activity [142]. However, although condom failure does represent a potential source of disease transmission, resistance to using condom is the most significant issue in the context of disease transmission. The intrinsic antibacterial properties of silver nanoparticles also bring about concern regarding their potential effects on healthy vaginal microbiota. However, similar to dendrimer formulations, the antibacterial properties of silver nanoparticles may present an opportunity for developing antibiotic-free treatments for bacterial vaginosis.
Rather than intrinsic antimicrobial properties, as found with silver nanoparticles, the advantage of using gold nanoparticles is that free thiols on their surfaces are easily functionalized with various biomolecules. Similar to the inhibition of interactions between HIV and DC-SIGN demonstrated with carbohydrate-functionalized dendrimers, Martinez-Avila et al. demonstrated that gold nanoparticles coated with multivalent oligomannosides were active against R5 and X4 tropic HIV at nM concentrations [143] due to their competitive binding with DC-SIGN [144]. Similarly, multivalent presentation of an HIV fusion inhibitor (SDC-1721) on the surface of gold nanoparticles exhibited anti-HIV activity at nM concentrations, whereas soluble SDC-1721 did not exhibit anti-HIV activity, supporting the notion that biomolecules can have increased activity when displayed using a nanoparticle system [145]. Also bearing similarities to dendrimer design, the anti-HIV activity of gold nanoparticles with anionic surface groups was evaluated [146]. Similar to what has been demonstrated previously, sulfate-functionalized gold nanoparticles inhibited HIV in vitro, whereas phosphate functionalized gold nanoparticles did not have anti-HIV activity [146]. Although gold is generally considered biocompatible, gold nanoparticles are not biodegradable and can potentially accumulate and disrupt cell function [147]. Therefore, it would be necessary to investigate the potential for cell uptake and accumulation in the vagina if gold nanoparticles are developed for repetitive vaginal administration for HIV prophylaxis.
Various accounts have demonstrated that nanoparticle formulations can have intrinsic adjuvant properties for vaccination [148]. For example, He et al. demonstrated that calcium phosphate nanoparticles (CAP) were a superior alternative to conventional alum adjuvants [149]. They demonstrated that CAP enhanced systemic and mucosal immunity to HSV-2 antigens following intravaginal immunization [150]. Increased levels of IgG and IgA were found in vaginal fluid after intravaginal immunization compared to intranasal immunization, which resulted in increased resistance to vaginal HSV-2 infection [150]. Ballou et al. investigated the transport of cadmium quantum dots (QD) (diameter not specified) from the vagina to adjacent lymph nodes [151]. They used quantum dots with various surface functionalization, including streptavidin QD (Sav), streptavidin QD functionalized with biotin-polyarginine (Sav-polyarg), carboylated QD (Carboxyl) and PEGylated QD. Significantly increased amounts of Sav, Sav-polyarg, and Carboxyl quantum dots were observed in the lymph nodes 36 h after vaginal instillation when the mouse vagina was pretreated 12 h before instillation with nonoxynol-9 (N9), a compound known to cause vaginal inflammation and toxicity. Using various mixtures of QD with different sizes and different emission spectra, the authors concluded that QD uptake in the lymph nodes after pretreatment with N9 was not significantly different for Sav, Sav-polyarg, Carboxyl, or PEGylated QD, though there was significant error associated with the method of quantification. The authors note that they observed QD aggregation in the presence of mucus, even for the PEGylated QD. However, there is little description of the methods for PEGylation or characterization of the PEG surface density, and it has been demonstrated that only nanoparticles densely coated with PEG resisted adhesion to mucus [66]. The authors conclude that the transport of the QD to the lymph nodes was the result of quantum dots penetrating the vaginal epithelium at localized foci, though the contributions of immune cell uptake and trafficking were not ruled out. The authors conclude that transport of immunogens from the vagina to the local lymph organs is feasible, and that quantum dot transport to the lymph could be used to assess vaginal toxicity of compounds intended for vaginal use [151]. Treatment of the mice with synthetic progestin (medroxyprogesterone acetate) is a limitation in both of these studies, as it is known that progestin treatment of mice significantly impacts both vaginal physiology and the vaginal immune system in ways that are not representative of the human vagina (for more details, see The impact of hormones on the vagina). It would be necessary to determine whether similar phenomena occur after intravaginal administration in humans, where the mucosal immune cells are less accessible, making the vagina potentially less reactive, and the stratified epithelium poses a more significant barrier to uptake or translocation.
Conclusion
Nanoparticle-based drug delivery strategies have demonstrated great promise for improving vaginal drug delivery. In addition to delivering small molecule drugs, nanoparticles have been used to deliver siRNA and peptides, and have also demonstrated intrinsic antimicrobial as well as adjuvant properties in the vagina. However, limitations of animal models leave certain questions that can only be answered through clinical testing, such as whether various nanoparticle formulations are tolerated by healthy vaginal microbiota, whether repeated administration will be non-toxic to the vaginal epithelium, and whether nanoparticle uptake will occur in the human vagina. Lastly, it has yet to be determined whether vaccine and other immune cell targeting strategies that are effective in animals will be effective in humans. Certainly, these questions are all worth answering, and will hopefully be addressed with increased translation of novel nanomedicine-based strategies into the clinic. Although the focus for nanoparticle-based vaginal drug delivery has largely been on delivering microbicides for prevention of sexually transmitted infections, it is likely that additional applications for nanoparticle-based strategies will be forthcoming, including hormone replacement therapy, non-hormonal contraception, and labor induction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ndesendo VM, Pillay V, Choonara YE, Buchmann E, Bayever DN, Meyer LC. A review of current intravaginal drug delivery approaches employed for the prophylaxis of HIV/AIDS and prevention of sexually transmitted infections. AAPS PharmSciTech. 2008;9:505–520. doi: 10.1208/s12249-008-9073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashok V, Kumar RM, Murali D, Chatterjee A. A review on vaginal route as a systemic drug delivery. Critical Review in Pharmaceutical Sciences. 2012;1:1–19. [Google Scholar]
- 3.Alexander NJ, Baker E, Kaptein M, Karck U, Miller L, Zampaglione E. Why consider vaginal drug administration? Fertility and sterility. 2004;82:1–12. doi: 10.1016/j.fertnstert.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Garg S. Vaginal Microbicide Formulations Workshop. Pharmaceutical Science & Technology Today. 1998;1:369. [Google Scholar]
- 5.Odeblad E. Intracavitary Circulation of Aqueous Material in the Human Vagina. Acta obstetricia et gynecologica Scandinavica. 1964;43:360–368. doi: 10.3109/00016346409162686. [DOI] [PubMed] [Google Scholar]
- 6.Pendergrass PB, Belovicz MW, Reeves CA. Surface area of the human vagina as measured from vinyl polysiloxane casts. Gynecologic and obstetric investigation. 2003;55:110–113. doi: 10.1159/000070184. [DOI] [PubMed] [Google Scholar]
- 7.Barnhart KT, Pretorius ES, Timbers K, Shera D, Shabbout M, Malamud D. In vivo distribution of a vaginal gel: MRI evaluation of the effects of gel volume, time and simulated intercourse. Contraception. 2004;70:498–505. doi: 10.1016/j.contraception.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Mirmonsef P, Gilbert D, Veazey RS, Wang J, Kendrick SR, Spear GT. A comparison of lower genital tract glycogen and lactic acid levels in women and macaques: implications for HIV and SIV susceptibility. AIDS research and human retroviruses. 2012;28:76–81. doi: 10.1089/aid.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sande MA, Zak O. Handbook of Animal Models of Infection: Experimental Models in Antimicrobial Chemotherapy. Elsevier Science; 1999. [Google Scholar]
- 10.Boskey ER, Telsch KM, Whaley KJ, Moench TR, Cone RA. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infection and immunity. 1999;67:5170–5175. doi: 10.1128/iai.67.10.5170-5175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox CA, Meldrum SJ, Watson BW. Continuous measurement by radio-telemetry of vaginal pH during human coitus. Journal of reproduction and fertility. 1973;33:69–75. doi: 10.1530/jrf.0.0330069. [DOI] [PubMed] [Google Scholar]
- 12.Cone RA. Barrier properties of mucus. Advanced drug delivery reviews. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Odeblad E. The functional structure of human cervical mucus. Acta obstetricia et gynecologica Scandinavica. 1968;47:57–79. doi: 10.3109/00016346809156845. [DOI] [PubMed] [Google Scholar]
- 14.Katz DF, Dunmire EN. Cervical-Mucus - Problems and Opportunities for Drug-Delivery Via the Vagina and Cervix. Advanced drug delivery reviews. 1993;11:385–401. [Google Scholar]
- 15.Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acarturk F. Mucoadhesive vaginal drug delivery systems. Recent patents on drug delivery & formulation. 2009;3:193–205. doi: 10.2174/187221109789105658. [DOI] [PubMed] [Google Scholar]
- 17.Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophysical journal. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.das Neves J, Amiji M, Sarmento B. Mucoadhesive nanosystems for vaginal microbicide development: friend or foe? Wiley interdisciplinary reviews Nanomedicine and nanobiotechnology. 2011;3:389–399. doi: 10.1002/wnan.144. [DOI] [PubMed] [Google Scholar]
- 20.Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, Hanes J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Science translational medicine. 2012;4:138ra179. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang M, Yu T, Wang YY, Lai SK, Zeng Q, Miao B, Tang BC, Simons BW, Ensign LM, Liu G, Chan KW, Juang CY, Mert O, Wood J, Fu J, McMahon MT, Wu TC, Hung CF, Hanes J. Vaginal Delivery of Paclitaxel via Nanoparticles with Non-Mucoadhesive Surfaces Suppresses Cervical Tumor Growth. Advanced healthcare materials. 2013 doi: 10.1002/adhm.201300519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumamoto Y, Iwasaki A. Unique features of antiviral immune system of the vaginal mucosa. Current opinion in immunology. 2012;24:411–416. doi: 10.1016/j.coi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery. Annual review of biomedical engineering. 2012;14:17–46. doi: 10.1146/annurev-bioeng-071811-150054. [DOI] [PubMed] [Google Scholar]
- 24.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. American journal of reproductive immunology. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunological reviews. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 26.Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. American journal of reproductive immunology. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dezzutti CS, Brown ER, Moncla B, Russo J, Cost M, Wang L, Uranker K, Kunjara Na Ayudhya RP, Pryke K, Pickett J, Leblanc MA, Rohan LC. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PloS one. 2012;7:e48328. doi: 10.1371/journal.pone.0048328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moench TR, Mumper RJ, Hoen TE, Sun M, Cone RA. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC infectious diseases. 2010;10:331. doi: 10.1186/1471-2334-10-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocrine reviews. 2010;31:79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang YY, Kannan A, Nunn KL, Murphy MA, Subramani DB, Moench T, Cone R, Lai SK. IgG in cervicovaginal mucus traps HSV and prevents vaginal Herpes infections. Mucosal immunology. 2014 doi: 10.1038/mi.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arias MA, Loxley A, Eatmon C, Van Roey G, Fairhurst D, Mitchnick M, Dash P, Cole T, Wegmann F, Sattentau Q, Shattock R. Carnauba wax nanoparticles enhance strong systemic and mucosal cellular and humoral immune responses to HIV-gp140 antigen. Vaccine. 2011;29:1258–1269. doi: 10.1016/j.vaccine.2010.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pattani A, McKay PF, Garland MJ, Curran RM, Migalska K, Cassidy CM, Malcolm RK, Shattock RJ, McCarthy HO, Donnelly RF. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. Journal of controlled release : official journal of the Controlled Release Society. 2012;162:529–537. doi: 10.1016/j.jconrel.2012.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anton PA, Cranston RD, Kashuba A, Hendrix CW, Bumpus NN, Richardson-Harman N, Elliott J, Janocko L, Khanukhova E, Dennis R, Cumberland WG, Ju C, Carballo-Dieguez A, Mauck C, McGowan I. RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS research and human retroviruses. 2012;28:1412–1421. doi: 10.1089/aid.2012.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuttall J, Kashuba A, Wang R, White N, Allen P, Roberts J, Romano J. Pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques. Antimicrobial agents and chemotherapy. 2012;56:103–109. doi: 10.1128/AAC.00597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Date AA, Destache CJ. A review of nanotechnological approaches for the prophylaxis of HIV/AIDS. Biomaterials. 2013;34:6202–6228. doi: 10.1016/j.biomaterials.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mallipeddi R, Rohan LC. Nanoparticle-based vaginal drug delivery systems for HIV prevention. Expert opinion on drug delivery. 2010;7:37–48. doi: 10.1517/17425240903338055. [DOI] [PubMed] [Google Scholar]
- 37.Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem. 2005;80:79–87. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- 38.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. The Journal of experimental medicine. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. Journal of virology. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brotman RM, Klebanoff MA, Nansel TR, Andrews WW, Schwebke JR, Zhang J, Yu KF, Zenilman JM, Scharfstein DO. A longitudinal study of vaginal douching and bacterial vaginosis--a marginal structural modeling analysis. American journal of epidemiology. 2008;168:188–196. doi: 10.1093/aje/kwn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorbach PM, Weiss RE, Fuchs E, Jeffries RA, Hezerah M, Brown S, Voskanian A, Robbie E, Anton P, Cranston RD. The slippery slope: lubricant use and rectal sexually transmitted infections: a newly identified risk. Sexually transmitted diseases. 2012;39:59–64. doi: 10.1097/OLQ.0b013e318235502b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PloS one. 2013;8:e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherpes TL, Marrazzo JM, Cosentino LA, Meyn LA, Murray PJ, Hillier SL. Hormonal contraceptive use modulates the local inflammatory response to bacterial vaginosis. Sexually transmitted infections. 2008;84:57–61. doi: 10.1136/sti.2007.026625. [DOI] [PubMed] [Google Scholar]
- 44.Hemmerling A, Harrison W, Schroeder A, Park J, Korn A, Shiboski S, Cohen CR. Phase 1 dose-ranging safety trial of Lactobacillus crispatus CTV-05 for the prevention of bacterial vaginosis. Sexually transmitted diseases. 2009;36:564–569. doi: 10.1097/OLQ.0b013e3181a74924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Briselden AM, Moncla BJ, Stevens CE, Hillier SL. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. Journal of clinical microbiology. 1992;30:663–666. doi: 10.1128/jcm.30.3.663-666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cauci S, Culhane JF, Di Santolo M, McCollum K. Among pregnant women with bacterial vaginosis, the hydrolytic enzymes sialidase and prolidase are positively associated with interleukin-1 beta. American journal of obstetrics and gynecology. 2008;198 doi: 10.1016/j.ajog.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 47.Culhane JF, Nyirjesy P, McCollum K, Goldenberg RL, Gelber SE, Cauci S. Variation in vaginal immune parameters and microbial hydrolytic enzymes in bacterial vaginosis positive pregnant women with and without Mobiluncus species. American journal of obstetrics and gynecology. 2006;195:516–521. doi: 10.1016/j.ajog.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 48.Verstraelen H, Swidsinski A. The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis and treatment. Current opinion in infectious diseases. 2013;26:86–89. doi: 10.1097/QCO.0b013e32835c20cd. [DOI] [PubMed] [Google Scholar]
- 49.Patton DL, Thwin SS, Meier A, Hooton TM, Stapleton AE, Eschenbach DA. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. American journal of obstetrics and gynecology. 2000;183:967–973. doi: 10.1067/mob.2000.108857. [DOI] [PubMed] [Google Scholar]
- 50.Hussain A, Ahsan F. The vagina as a route for systemic drug delivery. Journal of Controlled Release. 2005;103:301–313. doi: 10.1016/j.jconrel.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 51.Smith SM, Baskin GB, Marx PA. Estrogen protects against vaginal transmission of simian immunodeficiency virus. The Journal of infectious diseases. 2000;182:708–715. doi: 10.1086/315776. [DOI] [PubMed] [Google Scholar]
- 52.Gillgrass AE, Fernandez SA, Rosenthal KL, Kaushic C. Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation. Journal of virology. 2005;79:3107–3116. doi: 10.1128/JVI.79.5.3107-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandra N, Thurman AR, Anderson S, Cunningham TD, Yousefieh N, Mauck C, Doncel GF. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS research and human retroviruses. 2013;29:592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans GS, Gibson DFC, Roberts SA, Hind TM, Potten CS. Proliferative Changes in the Genital Tissue of Female Mice during the Estrous-Cycle. Cell Tissue Kinet. 1990;23:619–635. doi: 10.1111/j.1365-2184.1990.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 55.Asscher AW, De Boer CH, Turner CJ. Cornification of the human vaginal epithelium. Journal of anatomy. 1956;90:547–552. [PMC free article] [PubMed] [Google Scholar]
- 56.Smith BG, Brunner EK. The structure of the human vaginal mucosa in relation to the menstrual cycle and to pregnancy. Am J Anat. 1934;54:27–85. [Google Scholar]
- 57.Ensign LM, Henning A, Schneider CS, Maisel K, Wang YY, Porosoff MD, Cone R, Hanes J. Ex vivo characterization of particle transport in mucus secretions coating freshly excised mucosal tissues. Molecular pharmaceutics. 2013;10:2176–2182. doi: 10.1021/mp400087y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khanna KV, Whaley KJ, Zeitlin L, Moench TR, Mehrazar K, Cone RA, Liao Z, Hildreth JE, Hoen TE, Shultz L, Markham RB. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. The Journal of clinical investigation. 2002;109:205–211. doi: 10.1172/JCI13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durrani MJ, Kusai A, Ho NFH, Fox JL, Higuchi WI. Topical Vaginal Drug Delivery in the Guinea-Pig .1. Effect of Estrous-Cycle on the Vaginal Membrane-Permeability of Vidarabine. Int J Pharm. 1985;24:209–218. [Google Scholar]
- 60.Kanazawa T, Takashima Y, Hirayama S, Okada H. Effects of menstrual cycle on gene transfection through mouse vagina for DNA vaccine. Int J Pharm. 2008;360:164–170. doi: 10.1016/j.ijpharm.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 61.Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- 62.Wu SY, Chang HI, Burgess M, McMillan NA. Vaginal delivery of siRNA using a novel PEGylated lipoplex-entrapped alginate scaffold system. Journal of controlled release : official journal of the Controlled Release Society. 2011;155:418–426. doi: 10.1016/j.jconrel.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Cone RA, Hoen T, Wong X, Abusuwwa R, Anderson DJ, Moench TR. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC infectious diseases. 2006;6:90. doi: 10.1186/1471-2334-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson SS, Cheshenko N, Fakioglu E, Mesquita PM, Keller MJ, Herold BC. Susceptibility to genital herpes as a biomarker predictive of increased HIV risk: expansion of a murine model of microbicide safety. Antiviral therapy. 2009;14:1113–1124. doi: 10.3851/IMP1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dawson M, Krauland E, Wirtz D, Hanes J. Transport of polymeric nanoparticle gene carriers in gastric mucus. Biotechnology progress. 2004;20:851–857. doi: 10.1021/bp0342553. [DOI] [PubMed] [Google Scholar]
- 66.Wang YY, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angewandte Chemie. 2008;47:9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lai SK, Wang YY, Cone R, Wirtz D, Hanes J. Altering mucus rheology to “solidify” human mucus at the nanoscale. PloS one. 2009;4:e4294. doi: 10.1371/journal.pone.0004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cu Y, Saltzman WM. Controlled surface modification with poly(ethylene)glycol enhances diffusion of PLGA nanoparticles in human cervical mucus. Molecular pharmaceutics. 2009;6:173–181. doi: 10.1021/mp8001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, Zeitlin P, Boyle MP, Fu J, Hanes J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cu Y, Booth CJ, Saltzman WM. In vivo distribution of surface-modified PLGA nanoparticles following intravaginal delivery. Journal of controlled release : official journal of the Controlled Release Society. 2011;156:258–264. doi: 10.1016/j.jconrel.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ensign LM, Hoen TE, Maisel K, Cone RA, Hanes JS. Enhanced vaginal drug delivery through the use of hypotonic formulations that induce fluid uptake. Biomaterials. 2013;34:6922–6929. doi: 10.1016/j.biomaterials.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang M, Lai SK, Wang YY, Zhong W, Happe C, Zhang M, Fu J, Hanes J. Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus. Angewandte Chemie. 2011;50:2597–2600. doi: 10.1002/anie.201006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Y, Navarro F, Lal A, Basar E, Pandey RK, Manoharan M, Feng Y, Lee SJ, Lieberman J, Palliser D. Durable protection from Herpes Simplex Virus-2 transmission following intravaginal application of siRNAs targeting both a viral and host gene. Cell host & microbe. 2009;5:84–94. doi: 10.1016/j.chom.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nature materials. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinbach JM, Weller CE, Booth CJ, Saltzman WM. Polymer nanoparticles encapsulating siRNA for treatment of HSV-2 genital infection. Journal of controlled release : official journal of the Controlled Release Society. 2012;162:102–110. doi: 10.1016/j.jconrel.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ham AS, Cost MR, Sassi AB, Dezzutti CS, Rohan LC. Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharmaceutical research. 2009;26:502–511. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang T, Sturgis TF, Youan BB. pH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmission. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e V. 2011;79:526–536. doi: 10.1016/j.ejpb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoo JW, Giri N, Lee CH. pH-sensitive Eudragit nanoparticles for mucosal drug delivery. Int J Pharm. 2011;403:262–267. doi: 10.1016/j.ijpharm.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 79.Meng J, Sturgis TF, Youan BB. Engineering tenofovir loaded chitosan nanoparticles to maximize microbicide mucoadhesion. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2011;44:57–67. doi: 10.1016/j.ejps.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Belletti D, Tosi G, Forni F, Gamberini MC, Baraldi C, Vandelli MA, Ruozi B. Chemico-physical investigation of tenofovir loaded polymeric nanoparticles. Int J Pharm. 2012;436:753–763. doi: 10.1016/j.ijpharm.2012.07.070. [DOI] [PubMed] [Google Scholar]
- 81.Achilles SL, Shete PB, Whaley KJ, Moench TR, Cone RA. Microbicide efficacy and toxicity tests in a mouse model for vaginal transmission of Chlamydia trachomatis. Sexually transmitted diseases. 2002;29:655–664. doi: 10.1097/00007435-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 82.Chaowanachan T, Krogstad E, Ball C, Woodrow KA. Drug synergy of tenofovir and nanoparticle-based antiretrovirals for HIV prophylaxis. PloS one. 2013;8:e61416. doi: 10.1371/journal.pone.0061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.das Neves J, Michiels J, Arien KK, Vanham G, Amiji M, Bahia MF, Sarmento B. Polymeric nanoparticles affect the intracellular delivery, antiretroviral activity and cytotoxicity of the microbicide drug candidate dapivirine. Pharmaceutical research. 2012;29:1468–1484. doi: 10.1007/s11095-011-0622-3. [DOI] [PubMed] [Google Scholar]
- 84.das Neves J, Amiji M, Bahia MF, Sarmento B. Assessing the physical-chemical properties and stability of dapivirine-loaded polymeric nanoparticles. Int J Pharm. 2013;456:307–314. doi: 10.1016/j.ijpharm.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 85.das Neves J, Araujo F, Andrade F, Michiels J, Arien KK, Vanham G, Amiji M, Bahia MF, Sarmento B. In Vitro and Ex Vivo Evaluation of Polymeric Nanoparticles for Vaginal and Rectal Delivery of the Anti-HIV Drug Dapivirine. Molecular pharmaceutics. 2013;10:2793–2807. doi: 10.1021/mp4002365. [DOI] [PubMed] [Google Scholar]
- 86.das Neves J, Araujo F, Andrade F, Amiji M, Bahia MF, Sarmento B. Biodistribution and Pharmacokinetics of Dapivirine-Loaded Nanoparticles after Vaginal Delivery in Mice. Pharmaceutical research. 2014 doi: 10.1007/s11095-013-1287-x. [DOI] [PubMed] [Google Scholar]
- 87.Date AA, Shibata A, Goede M, Sanford B, La Bruzzo K, Belshan M, Destache CJ. Development and evaluation of a thermosensitive vaginal gel containing raltegravir+efavirenz loaded nanoparticles for HIV prophylaxis. Antiviral research. 2012;96:430–436. doi: 10.1016/j.antiviral.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peng W, Dunton C, Holtz D, Parva M, Stampler K, Forwood M, Gogoi R, Lace MJ, Anderson DG, Sawicki JA. DNA nanotherapy for pre-neoplastic cervical lesions. Gynecologic oncology. 2013;128:101–106. doi: 10.1016/j.ygyno.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 89.Blum J, Weller CE, Booth CJ, Babar I, Liang X, Slack F, Saltzman WM. Prevention of K-Ras- and Pten-mediated intravaginal tumors by treatment with camptothecin-loaded PLGA nanoparticles. Drug delivery and translational research. 2011;1:383–394. doi: 10.1007/s13346-011-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. Journal of molecular biology. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 91.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Advanced drug delivery reviews. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 92.Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. International journal of nanomedicine. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caron M, Besson G, Etenna SL, Mintsa-Ndong A, Mourtas S, Radaelli A, de Morghen CG, Loddo R, La Colla P, Antimisiaris SG, Kazanji M. Protective properties of non-nucleoside reverse transcriptase inhibitor (MC1220) incorporated into liposome against intravaginal challenge of Rhesus macaques with RT-SHIV. Virology. 2010;405:225–233. doi: 10.1016/j.virol.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 94.Wang L, Sassi AB, Patton D, Isaacs C, Moncla BJ, Gupta P, Rohan LC. Development of a liposome microbicide formulation for vaginal delivery of octylglycerol for HIV prevention. Drug development and industrial pharmacy. 2012;38:995–1007. doi: 10.3109/03639045.2011.637048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Konopka K, Davis BR, Larsen CE, Alford DR, Debs RJ, Duzgunes N. Liposomes modulate human immunodeficiency virus infectivity. The Journal of general virology. 1990;71(Pt 12):2899–2907. doi: 10.1099/0022-1317-71-12-2899. [DOI] [PubMed] [Google Scholar]
- 96.Malavia NK, Zurakowski D, Schroeder A, Princiotto AM, Laury AR, Barash HE, Sodroski J, Langer R, Madani N, Kohane DS. Liposomes for HIV prophylaxis. Biomaterials. 2011;32:8663–8668. doi: 10.1016/j.biomaterials.2011.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hood JL, Jallouk AP, Campbell N, Ratner L, Wickline SA. Cytolytic nanoparticles attenuate HIV-1 infectivity. Antiviral therapy. 2013;18:95–103. doi: 10.3851/IMP2346. [DOI] [PubMed] [Google Scholar]
- 98.Wachinger M, Kleinschmidt A, Winder D, von Pechmann N, Ludvigsen A, Neumann M, Holle R, Salmons B, Erfle V, Brack-Werner R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. The Journal of general virology. 1998;79(Pt 4):731–740. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- 99.Maher S, McClean S. Melittin exhibits necrotic cytotoxicity in gastrointestinal cells which is attenuated by cholesterol. Biochemical pharmacology. 2008;75:1104–1114. doi: 10.1016/j.bcp.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 100.Alukda D, Sturgis T, Youan BB. Formulation of tenofovir-loaded functionalized solid lipid nanoparticles intended for HIV prevention. Journal of pharmaceutical sciences. 2011;100:3345–3356. doi: 10.1002/jps.22529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang S, Chen Y, Ahmadie R, Ho EA. Advancements in the field of intravaginal siRNA delivery. Journal of controlled release : official journal of the Controlled Release Society. 2013;167:29–39. doi: 10.1016/j.jconrel.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 102.Zeitlin L, Whaley KJ, Hegarty TA, Moench TR, Cone RA. Tests of vaginal microbicides in the mouse genital herpes model. Contraception. 1997;56:329–335. doi: 10.1016/s0010-7824(97)00154-6. [DOI] [PubMed] [Google Scholar]
- 103.Pavelic Z, Skalko-Basnet N, Jalsenjak I. Liposomes containing drugs for treatment of vaginal infections. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 1999;8:345–351. doi: 10.1016/s0928-0987(99)00033-0. [DOI] [PubMed] [Google Scholar]
- 104.Pavelic Z, Skalko-Basnet N, Schubert R. Liposomal gels for vaginal drug delivery. Int J Pharm. 2001;219:139–149. doi: 10.1016/s0378-5173(01)00637-8. [DOI] [PubMed] [Google Scholar]
- 105.Pavelic Z, Skalko-Basnet N, Filipovic-Grcic J, Martinac A, Jalsenjak I. Development and in vitro evaluation of a liposomal vaginal delivery system for acyclovir. Journal of controlled release : official journal of the Controlled Release Society. 2005;106:34–43. doi: 10.1016/j.jconrel.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 106.Karimunnisa S, Atmaram P. Mucoadhesive nanoliposomal formulation for vaginal delivery of an antifungal. Drug development and industrial pharmacy. 2013;39:1328–1337. doi: 10.3109/03639045.2012.707204. [DOI] [PubMed] [Google Scholar]
- 107.Patel DB, Patel JK. LIposomal drug delivery of metronidazole for the local treatment of vaginitis. International Journal of Pharmaceutical Sciences and Nanotechnology. 2009;2:421–427. [Google Scholar]
- 108.Vanic Z, Hurler J, Ferderber K, Golja Gasparovic P, Skalko-Basnet N, Filipovic-Grcic J. Novel vaginal drug delivery system: deformable propylene glycol liposomes-in-hydrogel. Journal of liposome research. 2014;24:27–36. doi: 10.3109/08982104.2013.826242. [DOI] [PubMed] [Google Scholar]
- 109.Vanic Z, Hafner A, Bego M, Skalko-Basnet N. Characterization of various deformable liposomes with metronidazole. Drug development and industrial pharmacy. 2013;39:481–488. doi: 10.3109/03639045.2012.670247. [DOI] [PubMed] [Google Scholar]
- 110.Basnet P, Hussain H, Tho I, Skalko-Basnet N. Liposomal delivery system enhances anti-inflammatory properties of curcumin. Journal of pharmaceutical sciences. 2012;101:598–609. doi: 10.1002/jps.22785. [DOI] [PubMed] [Google Scholar]
- 111.Kang JW, Davaa E, Kim YT, Park JS. A new vaginal delivery system of amphotericin B: a dispersion of cationic liposomes in a thermosensitive gel. Journal of drug targeting. 2010;18:637–644. doi: 10.3109/10611861003649712. [DOI] [PubMed] [Google Scholar]
- 112.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug discovery today. 2010;15:171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 113.du Toit LC, Pillay V, Choonara YE. Nano-microbicides: challenges in drug delivery, patient ethics and intellectual property in the war against HIV/AIDS. Advanced drug delivery reviews. 2010;62:532–546. doi: 10.1016/j.addr.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 114.Rupp R, Rosenthal SL, Stanberry LR. VivaGel (SPL7013 Gel): a candidate dendrimer--microbicide for the prevention of HIV and HSV infection. International journal of nanomedicine. 2007;2:561–566. [PMC free article] [PubMed] [Google Scholar]
- 115.Tyssen D, Henderson SA, Johnson A, Sterjovski J, Moore K, La J, Zanin M, Sonza S, Karellas P, Giannis MP, Krippner G, Wesselingh S, McCarthy T, Gorry PR, Ramsland PA, Cone R, Paull JR, Lewis GR, Tachedjian G. Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PloS one. 2010;5:e12309. doi: 10.1371/journal.pone.0012309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.O’Loughlin J, Millwood IY, McDonald HM, Price CF, Kaldor JM, Paull JR. Safety, tolerability, and pharmacokinetics of SPL7013 gel (VivaGel): a dose ranging, phase I study. Sexually transmitted diseases. 2010;37:100–104. doi: 10.1097/OLQ.0b013e3181bc0aac. [DOI] [PubMed] [Google Scholar]
- 117.Moscicki AB, Kaul R, Ma Y, Scott ME, Daud, Bukusi EA, Shiboski S, Rebbapragada A, Huibner S, Cohen CR. Measurement of mucosal biomarkers in a phase 1 trial of intravaginal 3% StarPharma LTD 7013 gel (VivaGel) to assess expanded safety. Journal of acquired immune deficiency syndromes. 2012;59:134–140. doi: 10.1097/QAI.0b013e31823f2aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ramjee G, Kamali A, McCormack S. The last decade of microbicide clinical trials in Africa: from hypothesis to facts. Aids. 2010;24(Suppl 4):S40–49. doi: 10.1097/01.aids.0000390706.81383.f3. [DOI] [PubMed] [Google Scholar]
- 119.McCarthy TD, Karellas P, Henderson SA, Giannis M, O’Keefe DF, Heery G, Paull JR, Matthews BR, Holan G. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Molecular pharmaceutics. 2005;2:312–318. doi: 10.1021/mp050023q. [DOI] [PubMed] [Google Scholar]
- 120.Peng J, Wu Z, Qi X, Chen Y, Li X. Dendrimers as Potential Therapeutic Tools in HIV Inhibition. Molecules. 2013;18:7912–7929. doi: 10.3390/molecules18077912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harouse JM, Collman RG, Gonzalez-Scarano F. Human immunodeficiency virus type 1 infection of SK-N-MC cells: domains of gp120 involved in entry into a CD4-negative, galactosyl ceramide/3′ sulfo-galactosyl ceramide-positive cell line. Journal of virology. 1995;69:7383–7390. doi: 10.1128/jvi.69.12.7383-7390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yahi N, Baghdiguian S, Moreau H, Fantini J. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. Journal of virology. 1992;66:4848–4854. doi: 10.1128/jvi.66.8.4848-4854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han S, Kanamoto T, Nakashima H, Yoshida T. Synthesis of a new amphiphilic glycodendrimer with antiviral functionality. Carbohydrate polymers. 2012;90:1061–1068. doi: 10.1016/j.carbpol.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 124.Kensinger RD, Yowler BC, Benesi AJ, Schengrund CL. Synthesis of novel, multivalent glycodendrimers as ligands for HIV-1 gp120. Bioconjugate Chem. 2004;15:349–358. doi: 10.1021/bc034156a. [DOI] [PubMed] [Google Scholar]
- 125.Rosa Borges A, Wieczorek L, Johnson B, Benesi AJ, Brown BK, Kensinger RD, Krebs FC, Wigdahl B, Blumenthal R, Puri A, McCutchan FE, Birx DL, Polonis VR, Schengrund CL. Multivalent dendrimeric compounds containing carbohydrates expressed on immune cells inhibit infection by primary isolates of HIV-1. Virology. 2010;408:80–88. doi: 10.1016/j.virol.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rojo J, Delgado R. Glycodendritic structures: promising new antiviral drugs. The Journal of antimicrobial chemotherapy. 2004;54:579–581. doi: 10.1093/jac/dkh399. [DOI] [PubMed] [Google Scholar]
- 127.Tabarani G, Reina JJ, Ebel C, Vives C, Lortat-Jacob H, Rojo J, Fieschi F. Mannose hyperbranched dendritic polymers interact with clustered organization of DC-SIGN and inhibit gp120 binding. FEBS letters. 2006;580:2402–2408. doi: 10.1016/j.febslet.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 128.Ciobanu M, Huang KT, Daguer JP, Barluenga S, Chaloin O, Schaeffer E, Mueller CG, Mitchell DA, Winssinger N. Selection of a synthetic glycan oligomer from a library of DNA-templated fragments against DC-SIGN and inhibition of HIV gp120 binding to dendritic cells. Chemical communications. 2011;47:9321–9323. doi: 10.1039/c1cc13213j. [DOI] [PubMed] [Google Scholar]
- 129.Chonco L, Pion M, Vacas E, Rasines B, Maly M, Serramia MJ, Lopez-Fernandez L, De la Mata J, Alvarez S, Gomez R, Munoz-Fernandez MA. Carbosilane dendrimer nanotechnology outlines of the broad HIV blocker profile. Journal of controlled release : official journal of the Controlled Release Society. 2012;161:949–958. doi: 10.1016/j.jconrel.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 130.Ionov M, Ciepluch K, Klajnert B, Glinska S, Gomez-Ramirez R, de la Mata FJ, Munoz-Fernandez MA, Bryszewska M. Complexation of HIV derived peptides with carbosilane dendrimers, Colloids and surfaces. B. Biointerfaces. 2013;101:236–242. doi: 10.1016/j.colsurfb.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 131.Dutta T, Garg M, Jain NK. Targeting of efavirenz loaded tuftsin conjugated poly(propyleneimine) dendrimers to HIV infected macrophages in vitro. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2008;34:181–189. doi: 10.1016/j.ejps.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 132.Dutta T, Jain NK. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochimica et biophysica acta. 2007;1770:681–686. doi: 10.1016/j.bbagen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 133.Weber N, Ortega P, Clemente MI, Shcharbin D, Bryszewska M, de la Mata FJ, Gomez R, Munoz-Fernandez MA. Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. Journal of Controlled Release. 2008;132:55–64. doi: 10.1016/j.jconrel.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 134.Dzmitruk V, Shcharbin D, Pedziwiatr-Werbicka E, Bryszewska M. Dendrimers in Anti-HIV Therapy. 2011. [Google Scholar]
- 135.Menjoge AR, Navath RS, Asad A, Kannan S, Kim CJ, Romero R, Kannan RM. Transport and biodistribution of dendrimers across human fetal membranes: implications for intravaginal administration of dendrimer-drug conjugates. Biomaterials. 2010;31:5007–5021. doi: 10.1016/j.biomaterials.2010.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang B, Navath RS, Menjoge AR, Balakrishnan B, Bellair R, Dai H, Romero R, Kannan S, Kannan RM. Inhibition of bacterial growth and intramniotic infection in a guinea pig model of chorioamnionitis using PAMAM dendrimers. Int J Pharm. 2010;395:298–308. doi: 10.1016/j.ijpharm.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Navath RS, Menjoge AR, Dai H, Romero R, Kannan S, Kannan RM. Injectable PAMAM dendrimer-PEG hydrogels for the treatment of genital infections: formulation and in vitro and in vivo evaluation. Molecular pharmaceutics. 2011;8:1209–1223. doi: 10.1021/mp200027z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nature reviews Microbiology. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 139.Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, Yacaman MJ. Interaction of silver nanoparticles with HIV-1. Journal of nanobiotechnology. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lara HH, Ayala-Nunez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. Journal of nanobiotechnology. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lara HH, Ixtepan-Turrent L, Garza-Trevino EN, Rodriguez-Padilla C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. Journal of nanobiotechnology. 2010;8:15. doi: 10.1186/1477-3155-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mohammed Fayaz A, Ao Z, Girilal M, Chen L, Xiao X, Kalaichelvan P, Yao X. Inactivation of microbial infectiousness by silver nanoparticles-coated condom: a new approach to inhibit HIV- and HSV-transmitted infection. International journal of nanomedicine. 2012;7:5007–5018. doi: 10.2147/IJN.S34973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Martinez-Avila O, Bedoya LM, Marradi M, Clavel C, Alcami J, Penades S. Multivalent manno-glyconanoparticles inhibit DC-SIGN-mediated HIV-1 trans-infection of human T cells. Chembiochem : a European journal of chemical biology. 2009;10:1806–1809. doi: 10.1002/cbic.200900294. [DOI] [PubMed] [Google Scholar]
- 144.Martinez-Avila O, Hijazi K, Marradi M, Clavel C, Campion C, Kelly C, Penades S. Gold manno-glyconanoparticles: multivalent systems to block HIV-1 gp120 binding to the lectin DC-SIGN. Chemistry. 2009;15:9874–9888. doi: 10.1002/chem.200900923. [DOI] [PubMed] [Google Scholar]
- 145.Bowman MC, Ballard TE, Ackerson CJ, Feldheim DL, Margolis DM, Melander C. Inhibition of HIV fusion with multivalent gold nanoparticles. Journal of the American Chemical Society. 2008;130:6896–6897. doi: 10.1021/ja710321g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Di Gianvincenzo P, Marradi M, Martinez-Avila OM, Bedoya LM, Alcami J, Penades S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorganic & medicinal chemistry letters. 2010;20:2718–2721. doi: 10.1016/j.bmcl.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 147.Ma X, Wu Y, Jin S, Tian Y, Zhang X, Zhao Y, Yu L, Liang XJ. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS nano. 2011;5:8629–8639. doi: 10.1021/nn202155y. [DOI] [PubMed] [Google Scholar]
- 148.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nature materials. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.He Q, Mitchell AR, Johnson SL, Wagner-Bartak C, Morcol T, Bell SJ. Calcium phosphate nanoparticle adjuvant. Clinical and diagnostic laboratory immunology. 2000;7:899–903. doi: 10.1128/cdli.7.6.899-903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.He Q, Mitchell A, Morcol T, Bell SJ. Calcium phosphate nanoparticles induce mucosal immunity and protection against herpes simplex virus type 2. Clinical and diagnostic laboratory immunology. 2002;9:1021–1024. doi: 10.1128/CDLI.9.5.1021-1024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ballou B, Andreko SK, Osuna-Highley E, McRaven M, Catalone T, Bruchez MP, Hope TJ, Labib ME. Nanoparticle transport from mouse vagina to adjacent lymph nodes. PloS one. 2012;7:e51995. doi: 10.1371/journal.pone.0051995. [DOI] [PMC free article] [PubMed] [Google Scholar]