Abstract
While the cis-acyltransferase modular polyketide synthase assembly lines have largely been structurally dissected, enzymes from within the recently discovered trans-acyltransferase polyketide synthase assembly lines are just starting to be observed crystallographically. Here we examine the ketoreductase from the first polyketide synthase module of the bacillaene nonribosomal peptide synthetase/polyketide synthase at 2.35-Å resolution. This ketoreductase naturally reduces both α- and β-keto groups and is the only ketoreductase known to do so during the biosynthesis of a polyketide. The isolated ketoreductase not only reduced an N-acetylcysteamine-bound β-keto substrate to a D-β-hydroxy product, but also an N-acetylcysteamine- bound α-keto substrate to an L-α-hydroxy product. That the substrates must enter the active site from opposite directions to generate these stereochemistries suggests that the acyl-phosphopantetheine moiety is capable of accessing very different conformations despite being anchored to a serine residue of a docked acyl carrier protein. The features enabling stereocontrolled α-ketoreduction may not be extensive since a β-ketoreductase from a cis-acyltransferase polyketide synthase was identified that performs a completely stereoselective reduction of the same α-keto substrate to generate the D-α-hydroxy product. A sequence analysis of trans-acyltransferase ketoreductases reveals that a single residue, rather than a three-residue motif found in cis-acyltransferase ketoreductases, is predictive of the orientation of the resulting β-hydroxyl group.
Keywords: polyketide synthase, crystallography, enzymology, α-ketoreduction, biocatalysis
INTRODUCTION
Modular polyketide synthases (PKSs) synthesize diverse secondary metabolites often possessing bioactivities that enable them to be employed as insecticides, anticholesterol agents, antibiotics, and anticancer medicines. These complex molecules are produced by conceptually-simple assembly lines containing sets of enzymes known as modules, each responsible for one round of chain elongation and processing.1,2 Constituent domains include an acyl carrier protein (ACP) that acquires an extender unit such as a malonyl group from an acyltransferase (AT) and a ketosynthase (KS) that catalyzes the condensation of this extender unit with the growing polyketide chain. The ATs of cis-AT PKSs are integrated into the modules of the assembly lines, whereas the ATs of the recently discovered trans-AT PKSs exist as separate polypeptides.1,3 Tethered to the ~18-Å phosphopantetheinyl arm of ACP, a newly extended polyketide is shuttled between processing domains such as a ketoreductase (KR) that stereoselectively reduces its β-keto group to a β-hydroxy group, a dehydratase (DH) that removes the β-hydroxy group to yield an α,β-double bond, and an enoylreductase (ER) that reduces the double bond to generate a β-methylene.4–6 A thioesterase (TE) at the C-terminal end of the synthase commonly catalyzes the release of the mature polyketide through cyclization or hydrolysis.
The bacillaene nonribosomal peptide synthetase/polyketide synthase (NRPS/PKS) is a prototypical trans-AT PKS found in both Bacillus subtilis and Bacillus amyloliquefaciens FZB42 (pksX and baeX gene clusters, respectively) (Figure 1a).1,7–12 Through deleting the TE of the bacillaene synthase, the structures of thirteen intermediates in the biosynthesis of bacillaene were revealed.13 The substrate of the first adenylation (A) domain was found to be α-ketoisocaproate (α-KIC), not α-hydroxyisocaproate (α-HIC) as previously thought.11 The intermediates implicated PksKR1, the KR from the first PKS module, in the reduction of the α-KIC moiety (Figure 1a).7 Thus, in addition to PksKR1 acting as a β-ketoreductase, it was hypothesized to act as an α-ketoreductase.
Figure 1. α-Ketoreduction.
(A) The KR from the first PKS module within the bacillaene synthase (PksKR1) performs α-ketoreduction. Whether this occurs before or after it performs β-ketoreduction is unknown. (B) A few other KRs are known to catalyze α-ketoreduction. α-Ketoisocaproyl and α-ketoisovaleroyl groups are reduced by KRs in the cereulide nonribosomal peptide synthetase, and an α-ketopropionyl moiety is reduced by a KR in the bryostatin synthase.
Few KRs are known to catalyze α-ketoreduction. One such KR, embedded within an A domain of the cereulide NRPS, reduces an α-KIC group to a D-α-HIC group, while another in the cereulide NRPS reduces an α-ketoisovaleroyl (α-KIV) group to L-α-hydroxyisovaleroyl (L-α-HIV) group.14 A KR in the bryostatin loading module stereoselectively reduces a pyruvoyl group to a D-lactoyl group (Figure 1b).1,15 While PksKR1 may be in a class of its own in that it performs both α-and β-ketoreduction, other KRs are known to produce hydroxyl groups of opposite stereochemistries. The KR from the hypothemycin iterative PKS introduces different β-hydroxy orientations in different intermediates.16 Attempts have been made to biocatalytically employ KRs in the reduction of α-keto groups: 1,2-cyclohexanedione was reduced, but α-keto acids were not.17,18
From sequence analysis of KRs from cis-AT PKSs that generate known hydroxyl group orientations, fingerprints have been identified that are predictive of the stereoisomer produced by those KRs.19–21 A tryptophan eight residues N-terminal to the catalytic tyrosine indicates an A-type KR that generates L-β-hydroxyl groups, and a Leu-Asp-Asp motif indicates a B-type KR that generates D-β-hydroxyl groups. While this analysis has been applied in predicting the orientations of hydroxyl groups generated by the KRs of trans-AT PKSs, these KRs do not possess fingerprints as robust as those of KRs from cis-AT PKSs.22–26 The structure of a KR from a trans-AT PKS could help explain the relative absence of fingerprint residues and help reveal how stereocontrol is enforced by these KRs.
Here, we report the crystal structure of PksKR1 bound to NADP+ at 2.35-Å resolution. This first KR from within a trans-AT PKS assembly line to be structurally elucidated shows many similarities to the KRs of cis-AT PKSs. PksKR1 stereoselectively reduced a N-acetylcysteamine-bound β-ketoacyl substrate to a D-β-hydroxy product as well as an N-acetylcysteamine-bound α-ketoacyl substrate to an L-α-hydroxy product. Binding modes are proposed to describe the dual function of PksKR1.
MATERIALS AND METHODS
Cloning, expression, and purification
The DNA encoding PksKR1 (from pksJ, accession number NP_389598) was amplified from B. subtilis str. 168 gDNA with primers 5-ATCGTAATCcatatgGAACGCTTAATGCTTGAACCGGTGT-3 and 5-TGATTCGATgaattcATCCTTGATCCTGATCCGCCTTTCTC-3, resulting in restriction sites NdeI and EcoRI (shown in lowercase). These sites were used to clone the fragment into pET28b plasmid, which was subsequently transformed into BL21(DE3) Escherichia coli cells. The cells were grown to an OD600 of 0.5 in Luria broth containing 50 mg/L kanamycin at 37 °C. The temperature was then dropped to 15 °C prior to inducing protein expression with 0.5 mM IPTG and grown for an additional 16 h. Cells were collected via centrifugation at 4,000 × g for 30 min, resuspended in lysis buffer containing 500 mM NaCl and 30 mM HEPES pH 7.5, and lysed by sonication. Cell debris was removed by centrifugation at 30,000 × g for 30 min and the cell lysate was then poured over Ni-NTA resin (Qiagen) which was equilibrated in lysis buffer. Bound protein was washed with lysis buffer containing 15 mM imidazole, and eluted with lysis buffer containing 150 mM imidazole. A Superdex 200 gel filtration column equilibrated with 150 mM NaCl and 10 mM HEPES pH 7.5 was used to polish prior to crystallization trials. Buffer exchange into 25 mM NaCl, 10 mM HEPES pH 7.5, and 1 mM DTT was performed using protein concentrators to a final concentration of ~50 mg/mL. Selenomethionine-labeled protein was obtained similarly but through expression in minimal media with 50 mg/L kanamycin supplemented with selenomethionine and amino acids for methionine pathway inhibition.27
Crystallization and structure determination
Crystals of PksKR1 grew over one week by sitting drop vapor diffusion at 22 °C. Selenomethionine-labeled crystals were formed by mixing 2 μL protein solution (23.5 mg/mL PksKR1 in 25 mM NaCl, 1 mM DTT, and 10 mM HEPES pH 7.5) with 1 μL crystallization buffer (30% Jeffamine ED-2001 and 0.1 M HEPES pH 7.0). Native crystals were formed by mixing equal volumes of protein solution and crystallization buffer (25% w/v PEG 1000 and 0.1 M Tris pH 8.5). A final concentration of 5 mM NADP+ was added to the native protein solution to obtain crystals of the PksKR1/NADP+ complex, which were formed by mixing equal volumes of protein solution and crystallization buffer (30% w/v PEG 400 and 0.1 M Tris pH 8.5) at 22 °C. Harvested crystals were directly frozen in liquid nitrogen. The selenomethionine-labeled data sets and the native data sets were collected at ALS Beamlines 5.0.3 and 5.0.2, respectively. All data sets were processed in HKL2000.28 The structure was solved by single wavelength anomalous dispersion phasing using the program Phenix.29 All models were iteratively built and refined by using the programs Coot and Refmac5.30,31 The high R-factors in the unliganded structure primarily reflect the low resolution of the collected data.
Synthesis of compounds for ketoreduction assays (also see Figure S2)
α-Ketoisocaproyl-N-acetylcysteamine thioester (α-KIC-S-NAC) (1)
To a stirred solution of 4-methyl-2-oxopentanoic acid (260 mg, 2.0 mmol, 1.0 eq.) in DMF (8 mL) at 23 °C was added N,N-diisopropylethylamine (0.89 mL, 5.0 mmol, 2.5 eq.) followed by HBTU (830 mg, 2.2 mmol, 1.1 eq.). After 5 min N-acetylcysteamine (0.23 mL, 2.2 mmol, 1.1 eq.) was added in one portion. At 12 hr, the reaction was diluted with 1:1 hexanes:EtOAc (50 mL) and washed with sat. aqueous LiCl (3 × 50 mL). The organic layer was dried over sodium sulfate and concentrated in vacuo to furnish 1 (38 mg, 8%) as a pale oil.
1H NMR: 400 MHz (CDCl3) δ = 5.75 (bs, 1H), 3.47 (q, J = 6.2 Hz, 2H), 3.10 (t, J = 6.7 Hz, 2H), 2.70 (d, J = 6.9 Hz, 2H), 2.14–2.23 (m, 1H), 1.98 (s, 3H), 0.97 (d, J = 6.7 Hz, 6H). ESI-MS expected mass: 232.3; observed mass: 232.2.
Methyl-4-(4-methyl-2-oxopentanamido)butanoate (2a)
To a stirred solution of 4-methyl-2-oxopentanoic acid (150 mg, 1.15 mmol, 1.0 eq.) in DMF (5.8 mL) at 23 °C was added N,N-diisopropylethylamine (0.51 mL, 4.0 mmol, 3.5 eq.) followed by HBTU (493 mg, 1.30 mmol, 1.1 eq.). The solution was stirred for 5 min and γ-aminobutyric acid methylester hydrochloride (199 mg, 1.30 mmol, 1.1 eq.) was added in one portion. After 12 h, the reaction was diluted with 1:1 hexanes:EtOAc (100 mL) and washed with sat. aqueous LiCl (3 × 50 mL). The organic layer was dried over sodium sulfate and concentrated in vacuo to furnish 2a (72 mg, 27%) as a pale oil, which did not require chromatographic purification. For analytical purposes, the material was chromatographed on silica gel eluting with 3:1 hexanes:EtOAc.
1H NMR: 400 MHz (CDCl3) δ = 7.08 (bs, 1H), 3.68 (s, 3H), 3.34 (q, J = 6.8 Hz, 2H), 2.79 (d, J = 6.8 Hz, 2H), 2.37 (t, J = 7.2 Hz, 2H), 2.16 (m, 1H), 1.89 (p, J = 7.2 Hz, 2H), 0.95 (d, J = 6.5 Hz, 6H).
4-(4-Methyl-2-oxopentanamido)butanoic acid (2b)
To a stirred solution of 2a (72 mg, 0.31 mmol, 1.0 eq.) in THF (1.6 mL) at 0 °C was slowly added a solution of 1 M aqueous NaOH (0.47 mL, 0.47 mmol, 1.5 eq.). The reaction was allowed to warm to 23 °C and at 5 h the reaction was cooled to 0 °C and a solution of 1 M aqueous HCl (0.5 mL) was added. The reaction was diluted with EtOAc (50 mL) and the aqueous layer was separated. The organic layer was dried over sodium sulfate and concentrated in vacuo. Crude 2b was used immediately without further purification.
α-Ketoisocaproyl-γ-aminobutyryl-N-acetylcysteamine thioester (α-KIC-GABA-S-NAC) (2)
To a stirred solution of crude 2b (160 mg, 0.74 mmol, 1.0 eq.) in DMF (3.7 mL) at 23 °C was added N,N-diisopropylethylamine (0.33 mL, 1.85 mmol, 2.5 eq.) followed by HBTU (340 mg, 0.90 mmol, 1.2 eq.). After 5 min N-acetylcysteamine (0.09 mL, 0.90 mmol, 1.2 eq.) was added in one portion. After 12 h the reaction was diluted with 1:1 hexanes:EtOAc (50 mL) and was washed with sat. aqueous LiCl (3 × 50 mL). The organic layer was dried over sodium sulfate and concentrated in vacuo. The crude material was chromatographed on silica gel eluting with 1:2 hexanes:EtOAc to yield 2 (58 mg, 24%) as a pale yellow oil.
1H NMR 400 MHz (CDCl3) δ = 6.10 (bs, 1H), 5.79 (bs, 1H), 3.45 (q, J = 12 Hz, J = 5.8 Hz, 2H), 3.33 (qd, J = 6.8 Hz, J = 2.7 Hz, 2H) 3.03 (m, 2H), 2.63 (t, J = 6.8 Hz, 2H), 1.97 (s, 3H), 1.92 (dt, J = 6.8 Hz, J = 2.7 Hz, 1H) 1.84 (m, 1H), 1.66 (m, 2H), 1.53 (m, 2H), 0.97 (d, J = 6.6 Hz, 3H), 0.96 (d, J = 6.6 Hz, 3H). ESI-MS expected mass: 317.4; observed mass: 317.3.
3-Oxopentanoyl-N-acetylcysteamine thioester (3)
Synthesis followed as previously described.32
(2RS)-Methyl-3-oxopentanoyl-N-acetylcysteamine thioester (4)
Synthesis followed as previously described.32
(S)-2-((Tert-butyldimethylsilyl)oxy)-4-methylpentanoic acid (5a)
To a stirred solution of (2S)-hydroxyisocaproic acid (500 mg, 3.8 mmol, 1.0 eq.) in DMF (2.0 mL) at 23 °C was added imidazole (1.24 g, 18.2 mmol, 4.8 eq.) followed by TBS-Cl (1.37 g, 9.1 mmol, 2.4 eq.). The pale yellow solution was stirred for 18 h and diluted with 100 mL of 1:1 hexanes:EtOAc. The solution was washed with 10% aqueous citric acid (30 mL), saturated NaHCO3 (50 mL), water (50 mL), and then brine (50 mL). The organic layer was dried over sodium sulfate and concentrated in vacuo. The residue was dissolved in MeOH (40 mL), cooled to 0 °C, and K2CO3 (1.3 g, 9.5 mmol, 2.5 eq.) and water (12 mL) were added. The resulting mixture was stirred for 4 h at 23 °C and the solvent was removed in vacuo. The residue was dissolved in water (20 mL), cooled to 0 °C, and acidified to pH = 4 by addition of 10% aqueous citric acid solution. The aqueous solution was washed with EtOAc (50 mL), and the organic layer was separated. The aqueous layer was washed with EtOAc (50 mL), and the combined organic layers were dried over sodium sulfate and concentrated in vacuo. The crude product was chromatographed on silica gel, eluting with 2:1 EtOAc:hexanes to afford 5a (68%, 640 mg) as a pale, clear oil.
1H NMR: 400 MHz (CDCl3) δ = 4.27 (dd, J = 7.2 Hz, J = 5.1 Hz, 1H), 1.82 (m, 1H), 1.67–1.56 (m, 2H), 0.95 (d, J = 6.6 Hz, 3H), 0.94 (s, 9H), 0.94 (d, J = 6.6 Hz, 3H), 0.14 (s, 3H), 0.13 (s, 3H).
Methyl-(S)-4-(2-((tert-butyldimethylsilyl)oxy)-4-methylpentanamido)butanoate (5b)
To a stirred solution of 5a (140 mg, 0.57 mmol, 1.0 eq.) in DMF (2.9 mL) at 23 °C was added N,N-diisopropylethylamine (0.36 mL, 2 mmol, 3.5 eq.) followed by HBTU (259 mg, 0.68 mmol, 1.2 eq.). After 5 min γ-aminobutyric acid methylester hydrochloride (107 mg, 0.68 mol, 1.2 eq.) was added in one portion. After 12 h, the reaction was diluted with 1:1 hexanes:EtOAc (100 mL) and washed with sat. aqueous LiCl solution (3 × 50 mL). The organic layer was dried over sodium sulfate and concentrated in vacuo to furnish 5b (150 mg, 76%) as a tan oil that did not require further purification. For analytical purposes, the material was chromatographed on silica gel, eluting with 1:1 hexanes:EtOAc. Rf = 0.5 (2:1 hexanes:EtOAc).
1H NMR: 400 MHz (CDCl3) δ = 6.64 (bs, 1H), 4.12 (dd, J = 7.2 Hz, J = 4.8 Hz, 1H), 3.68 (s, 3H), 3.29 (qd, J = 7.2 Hz, J = 3.1 Hz, 2H) 2.36 (t, J = 7.5 Hz, J = 7.2 Hz, 2H), 1.88-1.74 (m, 3H), 1.56 (m, 2H), 0.93 (s, 9H), 0.92 (d, J = 6.5 Hz, 3H), 0.91 (d, J = 6.8 Hz, 3H), 0.09 (s, 3H), 0.06 (s, 3H).
(S)-4-(2-((Tert-butyldimethylsilyl)oxy)-4-methylpentanamido)butanoic acid (5c)
To a stirred solution of 5b (150 mg, 0.43 mmol, 1.0 eq.) in THF (2.1 mL) at 0 °C was added a solution of aqueous NaOH (1.0 M, 1.29 mL, 1.29 mmol, 2.0 eq.) and upon complete addition the reaction was allowed to warm to 23 °C. At 5 h, the reaction was cooled to 0 °C and was treated with 1.0 M HCl (aqueous, 1.4 mL). The reaction was diluted with EtOAc (100 mL), and the aqueous layer was separated. The organic layer was dried over sodium sulfate and concentrated in vacuo to furnish 5c (125 mg, 88%) as a white, soft solid. The crude material was used without further purification.
1H NMR: 400 MHz (CDCl3) δ = 6.74 (bs, 1H), 4.15 (dd, J = 7.2 Hz, J = 4.8 Hz, 1H), 3.35 (m, 2H) 2.39 (t, J = 7.2 Hz, 2H), 1.91-1.74 (m, 3H), 1.56 (m, 2H), 0.93 (s, 9H), 0.93 (d, J = 6.5 Hz, 3H), 0.91 (d, J = 6.8 Hz, 3H), 0.11 (s, 3H), 0.07 (s, 3H).
S-(2-Acetamidoethyl)-(S)-4-(2-((tert-butyldimethylsilyl)oxy)-4-methylpentanamido)butanethioate (5d)
To a stirred solution of 5c (162 mg, 0.49 mmol, 1.0 eq.) in DMF (2.5 mL) at 23 °C was added N,N-diisopropylethylamine (0.36 mL, 2.0 mmol, 4.0 eq.) followed by HBTU (224 mg, 0.59 mmol, 1.2 eq.). After 5 min, N-acetylcysteamine (0.06 mL, 0.59 mmol, 1.2 eq.) was added in one portion. After 12 h, the reaction was diluted with 1:1 hexanes:EtOAc and washed with sat. aqueous LiCl (3 × 50 mL). The organic layer was dried over sodium sulfate and concentrated in vacuo. The crude material was chromatographed on silica gel eluting with 1:2 hexanes:EtOAc to afford 5d (128 mg, 60%) as a pale oil. Rf = 0.19 (1:2 hexanes:EtOAc)
1H NMR: 400 MHz (CDCl3) δ = 6.63 (bs, 1H), 6.33 (bs, 1H) 4.13 (dd, J = 6.8 Hz, J = 4.8 Hz, 1H), 3.44 (q, J = 12.3 Hz, J = 6.2 Hz, 2H), 3.29 (qd, J = 6.8 Hz, J = 2.1 Hz, 2H) 3.04 (t, J = 6.2 Hz, J = 5.8 Hz, 2H), 2.61 (t, J = 7.2 Hz, 2H), 1.97 (s, 3H), 1.87 (p, J = 7.2 Hz, 2H), 1.78 (m, 1H), 1.56 (m, 1H), 0.94 (s, 9H), 0.92 (d, J = 6.5 Hz, 3H), 0.91 (d, J = 6.8 Hz, 3H), 0.11 (s, 3H), 0.08 (s, 3H).
(4S)-α-Hydroxyisocaproyl-γ-aminobutyryl-N-acetylcysteamine thioester ((4S)-α-HIC-GABA-S-NAC) (5)
To a stirred solution of 5d (153 mg, 0.37 mmol, 1.0 eq.) in DCM (3.7 mL) at 23 °C was added neat TFA (84 μL, 1.1 mmol, 3.0 eq.) in one portion. At 6 h, the reaction was concentrated in vacuo. The crude material was chromatographed on silica gel, eluting with 3% MeOH in DCM to 4:1 DCM:MeOH to afford 5 (93 mg, 82%) as a clear oil. Rf = 0.25 (3% MeOH in DCM)
1H NMR: 400 MHz (CDCl3) δ = 6.67 (bs, 1H), 6.20 (bs, 1H), 4.14 (dd, J = 9.9 Hz, J = 3.1 Hz, 1H), 3.45 (q, J = 12 Hz, J = 5.8 Hz, 2H), 3.33 (qd, J = 6.8 Hz, J = 2.7 Hz, 2H) 3.03 (m, 2H), 2.63 (t, J = 6.8 Hz, 2H), 1.97 (s, 3H), 1.92 (dt, J = 6.8 Hz, J = 2.7 Hz, 1H) 1.84 (m, 1H), 1.66 (m, 2H), 1.53 (m, 2H), 0.97 (d, J = 6.5 Hz, 3H), 0.96 (d, J = 6.5 Hz, 3H).
13C NMR: 100 MHz (CDCl3) δ = 199.2, 175.2, 170.9, 70.6, 43.7, 40.8, 39.1, 37.7, 28.8, 25.2, 24.4, 23.5, 23.0, 21.3. ESI-MS expected mass: 319.2; observed mass: 341.2 [M+Na]+.
Stereocontrol assays
Ketoreduction assays were set up as previously described32 with 1.5 mM substrate dissolved in 10% v/v DMSO, 200 mM HEPES pH 7.5, 100 mM NaCl, 200 mM D-glucose, 10% v/v glycerol, 100 μM NADP+, 1 μM GDH (from B. subtilis), and 5 μM KR to a total volume of 500 μL and were left at 22 °C overnight. Reactions were extracted with ethyl acetate (3 × 500 μL) and dried in a speedvac. All samples were resuspended in ethanol prior to chiral chromatographic analysis.
Chiral chromatography
A ChiralCel OC-H column (250 × 4.6 mm) was used to separate products of ketoreduction on a Beckman Coulter System Gold 126 pump and System Gold 166 PDA detector with a 20 μL loop. Absorbance was tracked at 235 nm. The solvent system and flow rate was optimized to be 14% ethanol in hexanes at 1 mL/min for 1, 2 and 5, and 7% ethanol in hexanes at 0.8 mL/min for 3 and 4.
RESULTS AND DISCUSSION
Protein Purification and Crystallography
The DNA encoding PksKR1 was amplified from B. subtilis sp. 168 genomic DNA and inserted into a pET28b expression vector. Both native and selenomethionine-labeled PksKR1 were overexpressed in E. coli BL21(DE3) and subsequently purified by nickel affinity and gel filtration chromatography. Phases were determined by single-wavelength anomalous dispersion (SAD) from crystals of selenomethionine-labeled protein. Native PksKR1 was observed both in the presence and absence of NADP+ at 2.35-Å and 3.00-Å resolution, respectively (Table I).
Table I.
Data Collection and Refinement Statistics
| Data Collection | |||
|---|---|---|---|
| Dataset | PksKR1 | PksKR1-NADP+ | Seleno-PksKR1 |
| Wavelength (Å) | 0.9765 | 0.9795 | 0.9765 |
| Space group | P61 | P61 | P61 |
| Cell dimensions (Å) | |||
| a, b, c (Å) | 59.6, 59.6, 221.1 | 59.5, 59.5, 220.8 | 59.5, 59.5, 221.5 |
| Resolution (Å) | 50-3.0 (3.05-3.00) | 50-2.35 (2.39-2.35) | 50-2.6 (2.64-2.60) |
| Rmerge | 0.134 (0.502) | 0.069 (0.464) | 0.140 (0.890) |
| I/σ(I) | 18.4 (4.6) | 25.8 (5.2) | 24 (2.8) |
| Completeness (%) | 99.9 (100) | 99.4 (100) | 100 (100) |
| Redundancy | 9.5 (9.6) | 10.9 (11.1) | 22.5 (19.7) |
| Refinement | |||
| Resolution (Å) | 50-3.0 | 50-2.35 | |
| No. reflections | 8353 | 17260 | |
| Rwork/Rfree | 0.258/0.290 | 0.232/0.253 | |
| No. atoms | |||
| Protein | 3330 | 3330 | |
| NADP+ | 48 | ||
| B-factors (Å2) | |||
| Protein | 67.4 | 73.2 | |
| NADP+ | 31.8 | ||
| Ramachandran plot | |||
| Favored/outliers (%) | 90.2/4.6 | ||
| R.m.s. deviations | |||
| Bond lengths (Å) | 0.011 | 0.008 | |
| Bond angles (°) | 0.865 | 1.091 |
PksKR1 is one of the first enzymes from within a trans-AT PKS assembly line to be structurally characterized. The other enzymes to be elucidated from a trans-AT PKS are an AT from the disorazole PKS (PDB Codes: 3RGI and 3SBM)33 and a didomain that includes an unusual KS and beta-branching enzyme from the rhizoxin PKS (PDB Code: 4KC5).34 Structural characterization of the bacillaene AT PksC is ongoing.35
Overall Structure
The ~425-aa PksKR1 consists of two subdomains, one referred to as “structural” and the other as “catalytic”.36 The folds of both subdomains of PksKR1 do not significantly deviate from those of KRs from cis-AT PKSs (Cα r.m.s.d. with AmpKR2: 1.0 Å, AmpKR11: 0.9 Å, TylKR1: 1.9 Å, EryKR1: 2.8 Å) (Figure S1).37,38,21,39 The catalytic subdomain contains the catalytic residues (Y330, K293, S317; numbering based on the first well-defined residue in the electron density map, equivalent to residue 2699 in PksJ) and NADPH binding motif (TGGAGSLG) expected for KRs from cis-AT PKSs. The Fo−Fc omit map for NADP+ in the NADP+-complexed structure encompasses all of the cofactor except for the nicotinamide ring (Figure 2c). The position of NADP+ is equivalent to that observed in structures of nicotinamide coenzyme-bound cis-AT KRs, and the binding of the coenzyme does not result in any major conformational change (Figures 2a and 2b). As with the KRs from cis-AT PKSs, the catalytic residues Tyr, Lys, and Ser are essentially invariant in KRs from trans-AT PKSs. A conserved Gln is also usually three residues N-terminal to the catalytic tyrosine. One difference between the active sites of KRs from trans-AT PKSs compared to those from cis-AT PKSs is that approximately one-third of these KRs possess a Ser or Cys four residues C-terminal to the catalytic Tyr in place of an Asn that is essentially invariant in reductase-competent KRs from cis-AT PKSs.
Figure 2. PksKR1 Bound to its Nicotinamide Coenzyme.
(A) A stereodiagram of the PksKR1 active site shows NADP+ and residues that bind it. (B) A LIGPLOT representation illustrates the interactions between the nicotinamide coenzyme and PksKR1 residues. (C) The Fo−Fc omit map of NADP+ (contoured at 3.0 r.m.s.d.) shows that only the nicotinamide ring is disordered.
Stereoselective α- and β-Ketoreduction of N-Acetylcysteamine-bound Substrates
Experiments employing a fragment of the bacillaene PKS containing PksKR1 and the two subsequent ACPs have demonstrated that PksKR1 performs α- and β-ketoreduction stereoselectively.7 We sought to determine whether binding interactions between PksKR1 and the phosphopantetheinyl-ACP portion of the substrate are necessary to enforce stereocontrolled α- and β-ketoreduction. Thus, reduction assays were performed using substrates linked to the short phosphopantetheinyl arm mimic, N-acetylcysteamine (NAC) (Figure 3a and Figure S2). α-Ketoisocaproyl-S-NAC (1), α-ketoisocaproyl-γ-aminobutyryl-S-NAC (2), 3-oxopentanoyl-S-NAC (3), and (2RS)-methyl-3-oxopentanoyl-S-NAC (4) were incubated with PksKR1 in the presence of an NADPH regeneration system (B. subtilis glucose dehydrogenase and glucose), and the products were analyzed by chiral chromatography.32 PksKR1 reduced 2 and 3, but not 1 or 4.
Figure 3. Stereoselective α- and β-Ketoreduction of Small Molecule Analogues.
(A) Compounds 1–4 were assayed as potential substrates for PksKR1. 1 and 4 were not reduced. (B) α-Keto substrate 2 was reduced by PksKR1 to the anticipated L-α-hydroxy product. TylKR1 (the first KR of the tylosin PKS) did not reduce 2; however, EryKR1 (the first KR of the erythromycin PKS) generated a product with properties consistent with the D-α-hydroxy product. The traces are from chiral HPLC analysis. (C) PksKR1, TylKR1, EryKR1, MycKRA (the A-type KR of the mycolactone PKS), and AmpKR2 (the second KR of the amphotericin PKS) each reduced 4. PksKR1 produces the D-β-hydroxy product expected of a B-type KR. The traces are from chiral HPLC analysis.
PksKR1 stereoselectively reduced the natural substrate analogue 2 to yield the anticipated L-α-hydroxy product (100%, by comparison with the synthetic (4S)-α-hydroxyisocaproyl-γ-aminobutyryl-S-NAC standard 5). To appreciate the extent to which PksKR1 is specialized in performing its role as a stereospecific α-ketoreductase, we incubated the α-keto substrate 2 with the biocatalytically robust cis-AT KR enzymes TylKR1 and EryKR1 (Figure 3b).32,40 EryKR1 was able to perform the stereospecific reduction of 2 to yield a product that has a mass consistent with the D-α-hydroxy reduction product. This reaction, like that catalyzed by PksKR1, is completely stereoselective even though EryKR1 is not under selective pressure to perform a stereoselective α-ketoreduction, suggesting that a KR does not need to be very specialized to catalyze stereoselective α-reduction. As with characterized B-type KRs, PksKR1 stereoselectively reduced 3 to yield the anticipated D-β-hydroxy product (92%; 8% L-β-hydroxy product) (Figure 3c).32 Thus, phosphopantetheine-ACP interactions with PksKR1 are not required for stereoselective α- and β-ketoreduction.
Since polyketides produced by trans-AT PKSs are generally extended by malonyl units, α-substituted, β-keto intermediates are less common in trans-AT PKSs than in cis-AT PKSs. That PksKR1 does not reduce the α-substituted β-ketothioester 4 was therefore not unexpected. This observation and the less extensive fingerprints in KRs from trans-AT PKSs suggest that KRs from trans-AT PKSs require fewer residues to stereoselectively bind and reduce α-unsubstituted substrates. None of the KRs employed in this study (PksKR1, TylKR1, and EryKR1) reduced α-ketoacyl thioester 1; however, as previously noted, KRs from the cereulide and bryostatin synthases stereoselectively reduce ketones adjacent to a thioester.1,14,15
Predicting Hydroxyl Group Stereochemistry from Sequence
To predict β-hydroxyl group stereochemistries generated by the KRs of trans-AT PKSs the fingerprints of these KRs must be elucidated. Towards this goal, we sought to identify the fingerprints that distinguish KRs producing L-β-hydroxy groups from KRs producing D-β-hydroxy groups (i.e., A-type from B-type).19,20 Fingerprints are present in cis-AT KRs (A-type KRs possess a tryptophan eight residues N-terminal to the catalytic tyrosine and B-type KRs possess a Leu-Asp-Asp motif); however, the same motifs are not apparent in the KRs of trans- AT PKS.
During the construction of a polyketide, trans- double bonds are usually formed from the DH-mediated dehydration of intermediates possessing a β-hydroxyl group of D-orientation while cis- double bonds are hypothesized to be formed from the DH-mediated dehydration of intermediates possessing a β-hydroxyl group of L-orientation.41,42 Thus, KRs from modules that generate trans- double bonds are usually B-type (an exception from the terminal module of the rifamycin PKS has been reported) and the KRs from modules that generate cis- double bonds are hypothesized to be A-type.2,19,20,41–47 Compared to polyketides produced by cis-AT PKSs, polyketides produced by trans-AT PKSs contain more cis- double bonds. Thus, KRs from the DH-containing modules of well-characterized trans-AT PKSs were categorized as either A-type or B-type depending on the cis- or trans- double bond intermediate their module is hypothesized to generate.1,48 These KRs were aligned, and residues corresponding to known motifs in the KRs from cis-AT PKSs were highlighted (Figure 4). The tryptophan anticipated for the A-type KRs of cis-AT PKSs is not present, and the Leu-Asp-Asp motif anticipated for the B-type KRs of cis- AT PKSs is rarely observed in this alignment. The only fingerprint distinguishing the A-type and B-type KRs of trans-AT PKSs appears to be an aspartate that is absent in A-type KRs but present in B-type KRs. This residue is equivalent to the second aspartate of the Leu-Asp-Asp motif of the B-type KRs of cis-AT PKSs as well as an aspartate in the high-molecular-weight FabG observed to help bind the terminal portion of the phosphopantetheinyl arm.49 Some have speculated that while the first two residues of the Leu-Asp-Asp motif are generally absent in KRs from trans-AT PKSs, the final aspartate might still be predictive and aid in assigning the orientations of hydroxyl groups of polyketides produced by trans-AT PKSs.22–26 PksKR1 possesses this aspartate and would thus be correctly hypothesized to be a B-type KR with respect to β-ketoreduction. Out of the 49 KR sequences analyzed, only two did not follow the pattern: MmpKR7 from the mupirocin PKS and OzmKR4 from the oxazolomycin PKS.1,48 MmpKR7 contains the aspartate and is thus predicted to be a B-type KR, yet the module containing it is predicted to form a cis- double bond; since many tailoring enzymes in this pathway modify the polyketide after its synthesis by the PKS, the assignment of the cis- double bond may not be accurate. OzmKR4 also contains the aspartate and is predicted to be a B-type KR, yet it is hypothesized to help generate a cis- double bond; the stereoselectivity of this KR deserves further study. An equivalent analysis of KRs from modules that do not contain a DH should be possible as more hydroxyl group orientations produced by these KRs become known. Identifying fingerprints that predict the orientation of α-substituents after β-reduction or of α-hydroxyl groups after α-ketoreduction will require many more examples of trans-AT KRs that mediate these reactions.
Figure 4. An Aspartate is Predictive of β-Hydroxyl Group Stereochemistry.
A sequence alignment was constructed of KRs from trans-AT PKSs. KR sequences were obtained from DH-containing modules and categorized as either A-type or B-type, depending on whether the containing module generates a cis- or trans- double-bonded intermediate, respectively.1 PksKR1 is indicated with an arrow. Catalytic residues are marked with asterisks. The tryptophan conserved in A-type KRs from cis-AT PKSs is absent in the putative A-type KRs of trans-AT PKSs. The second aspartate of the LDD motif that is anticipated from B-type KRs of cis-AT PKSs is present in B-type KRs of trans-AT PKSs and is predictive of both KR-type and β-hydroxyl stereochemistry. The conservation of this aspartate fingerprint is illustrated with a WebLogo diagram.51
The Dual Function of PksKR1
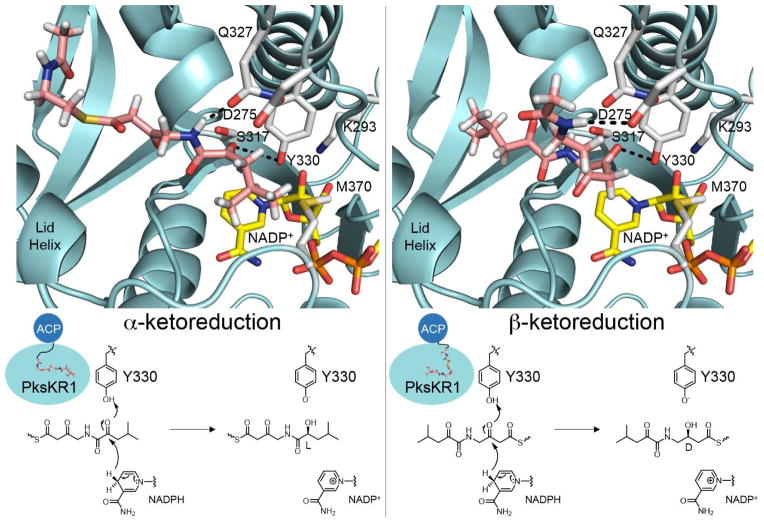
The structure of coenzyme-bound PksKR1 was used to model how substrates enter the PksKR1 active site for α- and β-ketoreduction (Figure 5). It is unknown what reduction level the β-carbon is when α-ketoreduction occurs. We presume here that α-ketoreduction can occur before reduction of the β-carbon. Thus the substrate containing both α- and β-keto moieties was modeled into the active site using the program Coot, placing the keto group undergoing reduction adjacent to both the tyrosine hydroxyl and the pro-4S hydrogen of NADPH. The substrates must enter the active site from opposite directions so that a D-β-hydroxy group is generated when the substrate enters from the side closest the nicotinamide coenzyme and an L-α-hydroxy group is generated when the substrate enters from the side opposite the nicotinamide coenzyme. We hypothesize that the B-type aspartate helps guide the substrate through a hydrogen bond with the terminal NH of the phosphopantetheinyl arm as observed in the complex structure of high-molecular weight FabG with hexanoyl-CoA.49 The opposite orientation may not be accessible due to a clash between the terminal gem-methyl moiety and Met370. The features that enable stereoselective α-ketoreduction are more mysterious, but in this binding mode favorable hydrophobic interactions could form between the terminal gem-dimethyl moiety and Met370.
Figure 5. Potential Binding Modes for PksKR1-mediated α- and β-Ketoreduction.
(A) In α-ketoreduction the substrate enters the active site such that the re- side of the α-keto group faces the NADPH pro-4S hydride to yield an L-α-hydroxyl group. (B) In β-ketoreduction the substrate enters the active site from opposite side such that the si- side of the β-keto group faces the NADPH pro-4S hydride to yield a D-β-hydroxyl group. ACP may dock to the same location on PksKR1 for both reactions.
Acyl-ACPs are hypothesized to dock to KRs during the reduction reaction since ketoreduction is more catalytically efficient with acyl-ACP substrates than with acyl-S-NAC substrates.50 Whether ACPs dock to different KR types through distinct docking sites is unknown. Since PksKR1 performs two reduction reactions on a tethered intermediate that must enter the active site from opposite directions, the ACP may either 1) dock to two separate sites on PksKR1 or 2) dock to one site on PksKR1 from which both conformations required for stereoselective reduction can be accessed. The ~18-Å phosphopantetheinyl arm is long enough that both conformations are accessible if the phosphopantetheinylated ACP serine is within ~15 Å of the catalytic residues.
CONCLUSION
We report here the first structure of a KR from a trans-AT PKS assembly line. PksKR1 is an unusual KR since, in addition to catalyzing the expected stereoselective reduction of the β-keto group, it catalyzes the stereoselective reduction of an α-keto group. This stereoselectivity does not require the phosphopantetheinyl-ACP portion of the natural substrate as PksKR1 was able to stereoselectively reduce NAC-bound small molecule analogues as anticipated from the stereoselectivity enforced by the bacillaene synthase. A sequence analysis of KRs from trans-AT PKSs reveals that the presence or absence of an aspartate is predictive of whether a KR from a trans-AT PKS will produce a D- or L-hydroxy group, respectively. The equivalent aspartate in a related short-chain dehydrogenase/reductase enzyme has been observed to make a hydrogen bond with the terminal NH of the phosphopantetheinyl arm to steer the acyl group into the active site from the side closest the nicotinamide coenzyme. ACP may only need to dock to one site for both α- and β-ketoreduction owing to the flexibility of the phosphopantetheinyl arm. Together, these studies of an unusual KR from a trans-AT PKS significantly contribute to our general understanding of how KRs function within modular PKSs.
Supplementary Material
Acknowledgments
Instrumentation and technical assistance for crystallographic work were provided by Dr. Art Monzingo and the Macromolecular Crystallography Facility, with financial support from the College of Natural Sciences, the Office of the Executive Vice President and Provost, and the Institute for Cellular and Molecular Biology at the University of Texas at Austin. The Berkeley Center for Structural Biology is supported in part by the National Institutes of Health, National Institute of General Medical Sciences, and the Howard Hughes Medical Institute. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. We thank the National Institutes of Health (GM106112) and the Welch Foundation (F-1712) for supporting this research (A.T.K.).
Footnotes
Accession Numbers
Structure coordinates were deposited in the Protein Data Bank under accession codes 4J1S (apo) and 4J1Q (with NADP+).
Supplemental information can be found online.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1.Piel J. Biosynthesis of polyketides by trans-AT polyketide synthases. Nat Prod Rep. 2010;27:996–1047. doi: 10.1039/b816430b. [DOI] [PubMed] [Google Scholar]
- 2.Keatinge-Clay AT. The structures of type I polyketide synthases. Nat Prod Rep. 2012;29:1050–1073. doi: 10.1039/c2np20019h. [DOI] [PubMed] [Google Scholar]
- 3.Cheng YQ, Coughlin JM, Lim SK, Shen B. Type I polyketide synthases that require discrete acyltransferases. Methods Enzymol. 2009;459:165–186. doi: 10.1016/S0076-6879(09)04608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staunton J, Weissman KJ. Polyketide biosynthesis: a millennium review. Nat Prod Rep. 2001;18:380–416. doi: 10.1039/a909079g. [DOI] [PubMed] [Google Scholar]
- 5.Khosla C, Tang Y, Chen AY, Schnarr NA, Cane DE. Structure and mechanism of the 6-deoxyerythronolide B synthase. Annu Rev Biochem. 2007;76:195–221. doi: 10.1146/annurev.biochem.76.053105.093515. [DOI] [PubMed] [Google Scholar]
- 6.Sattely ES, Fischbach MA, Walsh CT. Total biosynthesis: in vitro reconstitution of polyketide and nonribosomal peptide pathways. Nat Prod Rep. 2008;25:757–793. doi: 10.1039/b801747f. [DOI] [PubMed] [Google Scholar]
- 7.Calderone CT, Bumpus SB, Kelleher NL, Walsh CT, Magarvey NA. A ketoreductase domain in the PksJ protein of the bacillaene assembly line carries out both α- and β-ketone reduction during chain growth. Proc Natl Acad Sci USA. 2008;105:12809–12814. doi: 10.1073/pnas.0806305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddick JJ, Antolak SS, Raner GM. PksS from Bacillus subtilis is a cytochrome P450 involved in bacillaene metabolism. Biochem Biophys Res Commun. 2007;358:363–367. doi: 10.1016/j.bbrc.2007.04.151. [DOI] [PubMed] [Google Scholar]
- 9.Patel PS, Huang S, Fisher S, Pirnik D, Aklonis C, Dean L, Meyers E, Fernandes P, Mayerl F. Bacillaene, a novel inhibitor of procaryotic protein synthesis produced by bacillus subtilis: production, taxonomy, isolation, physico-chemical characterization and biological activity. J Antibiot. 1995;48:997–1003. doi: 10.7164/antibiotics.48.997. [DOI] [PubMed] [Google Scholar]
- 10.Chen XH, Vater J, Piel J, Franke P, Scholz R, Schneider K, Koumoutsi A, Hitzeroth G, Grammel N, Strittmatter AW, Gottschalk G, Süssmuth RD, Borriss R. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB 42. J Bacteriol. 2006;188:4024–4036. doi: 10.1128/JB.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butcher RA, Schroeder FC, Fischbach MA, Straight PD, Kolter R, Walsh CT, Clardy J. The identification of bacillaene, the product of the PksX megacomplex in Bacillus subtilis. Proc Natl Acad Sci USA. 2007;104:1506–1509. doi: 10.1073/pnas.0610503104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straight PD, Fischback MA, Walsh CT, Rudner DZ, Kolter R. A singular enzymatic megacomplex from Bacillus subtilis. Proc Natl Acad Sci USA. 2007;104:305–310. doi: 10.1073/pnas.0609073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moldenhauer J, Chen XH, Borriss R, Piel J. Biosynthesis of the antibiotic bacillaene, the product of a giant polyketide synthase complex of the trans-AT family. Angew Chem. 2007;46:8195–8197. doi: 10.1002/anie.200703386. [DOI] [PubMed] [Google Scholar]
- 14.Magarvey NA, Ehling-Schulz M, Walsh CT. Characterization of the cereulide NRPS α-hydroxy acid specifying modules: Activation of α-keto acids and chiral reduction on the assembly line. J Am Chem Soc. 2006;128:10698–10699. doi: 10.1021/ja0640187. [DOI] [PubMed] [Google Scholar]
- 15.Hildebrand M, Waggoner LE, Liu H, Sudek S, Allen S, Anderson C, Sherman DH, Haygood M. bryA: An unusual modular polyketide synthase gene from the uncultivated bacterial symbiont of the marine bryozoan Bugula neritina. Chem Biol. 2004;11:1543–1552. doi: 10.1016/j.chembiol.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, Gao Z, Qiao K, Wang J, Vederas JC, Tang Y. A fungal ketoreductase domain that displays substrate-dependent stereospecificity. Nat Chem Biol. 2012;8:331–333. doi: 10.1038/nchembio.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bali S, Weissman KJ. Ketoreduction in mycolactone biosynthesis: Insight into substrate specificity and stereocontrol from studies of discrete ketoreductase domains in vitro. ChemBioChem. 2006;7:1935–1942. doi: 10.1002/cbic.200600285. [DOI] [PubMed] [Google Scholar]
- 18.Bali S, O’Hare HM, Weissman KJ. Broad substrate specificity of ketoreductases derived from modular polyketide synthases. ChemBioChem. 2006;7:478–484. doi: 10.1002/cbic.200500430. [DOI] [PubMed] [Google Scholar]
- 19.Reid R, Piagentini M, Rodriguez E, Ashley G, Viswanathan N, Carney J, Santi DV, Hutchinson CR, McDaniel R. A model of structure and catalysis for ketoreductase domains in modular polyketide synthases. Biochemistry. 2003;42:72–79. doi: 10.1021/bi0268706. [DOI] [PubMed] [Google Scholar]
- 20.Caffrey P. Conserved amino acid residues correlating with ketoreductase stereospecificity in modular polyketide synthases. ChemBioChem. 2003;4:654–657. doi: 10.1002/cbic.200300581. [DOI] [PubMed] [Google Scholar]
- 21.Keatinge-Clay AT. A tylosin ketoreductase reveals how chirality is determined in polyketides. Chem Biol. 2007;14:898–908. doi: 10.1016/j.chembiol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Janssen D, Albert D, Jansen R, Muller R, Kalesse M. Chivosazole A-Elucidation of the absolute and relative configuration. Angew Chem Int Ed. 2007;46:4898–4901. doi: 10.1002/anie.200605198. [DOI] [PubMed] [Google Scholar]
- 23.Bock M, Buntin K, Muller R, Kirschning A. Stereochemical determination of thuggacins A-C, highly active antibiotics from the myxobacterium Sorangium cellulosum. Angew Chem Int Ed. 2008;47:2308–2311. doi: 10.1002/anie.200704897. [DOI] [PubMed] [Google Scholar]
- 24.Menche D, Arikan F, Perlova O, Horstmann N, Ahlbrecht W, Wenzel SC, Jansen R, Irschik H, Muller R. Stereochemical determination and complex biosynthetic assembly of etnangien, a highly potent RNA polymerase inhibitor from the myxobacterium Sorangium cellulosum. J Am Chem Soc. 2008;130:14234–14243. doi: 10.1021/ja804194c. [DOI] [PubMed] [Google Scholar]
- 25.Ishida K, Lincke T, Hertweck C. Assembly and absolute configuration of short-lived polyketides from Burkholderia thailandensis. Angew Chem Int Ed. 2012;51:5470–5474. doi: 10.1002/anie.201200067. [DOI] [PubMed] [Google Scholar]
- 26.Moebius N, Ross C, Scherlach K, Rohm B, Roth M, Hertweck C. Biosynthesis of the respiratory toxin bongkrekic acid in the pathogenic bacterium Burkholderia gladioli. Chem Biol. 2012;19:1164–1174. doi: 10.1016/j.chembiol.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Doublie S. Methods Enzymol. 1997;276:523–530. [PubMed] [Google Scholar]
- 28.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode, in Methods in Enzymology. In: Carter CW Jr, Sweet RM, editors. Macromolecular Crystallography, Part A. Vol. 276. Academic Press; New York: 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 29.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffnew R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Cryst. 2010;D66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr, Sect D: Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 31.Potterton E, Briggs P, Turkenburg M, Dodson E. A graphical user interface to the CCP4 program suite. Acta Crystallogr, Sect D: Biol Crystallogr. 2003;59:1131–1137. doi: 10.1107/s0907444903008126. [DOI] [PubMed] [Google Scholar]
- 32.Piasecki SK, Taylor CA, Detelich JF, Liu J, Zheng J, Komsoukaniants A, Siegel DR, Keatinge-Clay AT. Employing modular polyketide synthase ketoreductases as biocatalysts in the preparative chemoenzymatic syntheses of diketide chiral building blocks. Chem Biol. 2011;18:1331–1340. doi: 10.1016/j.chembiol.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Wong FT, Jin X, Matthews II, Cane DE, Khosla C. Structure and Mechanism of the trans-Acting Acyltransferase from the Disorazole Synthase. Biochemistry. 2011;50:6539–6548. doi: 10.1021/bi200632j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bretschneider T, Heim JB, Heine D, Winkler R, Busch B, Kusebauch B, Stehle T, Zocher G, Hertweck C. Vinylogous chain branching catalysed by a dedicated polyketide synthase module. Nature. 2013;502:124–8. doi: 10.1038/nature12588. [DOI] [PubMed] [Google Scholar]
- 35.Cuskin F, Solovyova AS, Lewis RJ, Race PR. Crystallization and preliminary X-ray analysis of the bacillaene synthase trans-acting acyltransferase PksC. Acta Cryst. 2011;67:464–466. doi: 10.1107/S1744309111003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng J, Keatinge-Clay AT. The status of type I polyketide synthase ketoreductases. Med Chem Commun. 2013;4:34–40. [Google Scholar]
- 37.Zheng J, Taylor CA, Piasecki SK, Keatinge-Clay AT. Structural and functional analysis of A-type ketoreductases from the amphotericin modular polyketide synthase. Structure. 2010;18:913–22. doi: 10.1016/j.str.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Zheng J, Piasecki SK, Keatinge-Clay AT. Structural studies of an A2-type modular polyketide synthase ketoreductase reveal features controlling α-substituent stereochemistry. ACS Chem Biol. 2013;8:1964–71. doi: 10.1021/cb400161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keatinge-Clay AT, Stroud RM. The structure of a ketoreductase determines the organization of the beta-carbon processing enzymes of modular polyketide synthases. Structure. 2006;14:737–48. doi: 10.1016/j.str.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Hackh M, Muller M, Ludeke S. Substrate-dependent stereospecificity of Tyl-KR1: An isolated polyketide synthase ketoreductase domain from Streptomyces fradiae. Chem Eur J. 2013;19:8922–8928. doi: 10.1002/chem.201300554. [DOI] [PubMed] [Google Scholar]
- 41.Keatinge-Clay AT. Crystal structure of the erythromycin polyketide synthase dehydratase. J Mol Biol. 2008;384:941–953. doi: 10.1016/j.jmb.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akey DL, Razelun JR, Tehranisa J, Sherman DH, Gerwick WH, Smith JL. Crystal structures of dehydratase domains from the curacin polyketide biosynthetic pathway. Structure. 2010;18:94–105. doi: 10.1016/j.str.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J, Zaleski TJ, Valenzano C, Khosla C, Cane DE. Polyketide double bond biosynthesis. Mechanistic analysis of the dehydratase-containing module 2 of the picromycin/methymycin polyketide synthase. J Am Chem Soc. 2005;127:17393–17404. doi: 10.1021/ja055672+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo X, Liu T, Valenzano CR, Deng Z, Cane DE. Mechanism and stereospecificity of a fully saturating polyketide synthase module: nanchangmycin synthase module 2 and its dehydratase domain. J Am Chem Soc. 2010;132:14694–14696. doi: 10.1021/ja1073432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valenzano CR, You YO, Garg A, Keatinge-Clay AT, Khosla C, Cane DE. Stereospecificity of the dehydratase domain of the erythromycin polyketide synthase. J Am Chem Soc. 2010;132:14697–14699. doi: 10.1021/ja107344h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonnet SA, Whicher JR, Papireddy K, Florova G, Smith JL, Reynolds KA. Structural and stereochemical analysis of a modular polyketide synthase ketoreductase domain required for the generation of a cis-alkene. Chem Biol. 2013;20:772–783. doi: 10.1016/j.chembiol.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gay DC, You Y-O, Keatinge-Clay AT, Cane DE. Structural and stereospecificity of the dehydratase domain from the terminal module of the rifamycin polyketide synthase. Biochemistry. 2013 doi: 10.1021/bi400988t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen T, Ishida K, Jenke-Kodama H, Dittmann E, Gurgui C, Hochmuth T, Taudien S, Platzer M, Hertweck C, Piel J. Exploiting the mosaic structure of trans-acyltransferase polyketide synthases for natural product discovery and pathway dissection. Nat Biotechnol. 2008;26:225–33. doi: 10.1038/nbt1379. [DOI] [PubMed] [Google Scholar]
- 49.Dutta D, Bhattacharyya S, Roychowdhury A, Biswas R, Das AK. Crystal structure of hexanoyl-CoA bound to β-ketoacyl reductase FabG4 of Mycobacterium tuberculosis. Biochem J. 2013;450:127–39. doi: 10.1042/BJ20121107. [DOI] [PubMed] [Google Scholar]
- 50.Castonguay R, He W, Chen AY, Khosla C, Cane DE. Stereospecificity of ketoreductase domains of the 6-deoxyerythronolide B synthase. J Am Chem Soc. 2007;129:13758–13769. doi: 10.1021/ja0753290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: A sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.