Abstract
Most currently available cationic polymers have significant acute toxicity concerns such as cellular toxicity, aggregation of erythrocytes, and entrapment in the lung capillary bed, largely due to their poor biocompatibility and non-degradability under physiological conditions. To develop more intelligent polymers, disulfide bonds are introduced in the design of biodegradable polymers. Herein, the sustained innovations of biomimetic nanosized constructs with bioreducible poly(disulfide amine)s demonstrate a viable clinical tool for the treatment of cardiovascular disease, anemia, diabetes, and cancer.
I. Introduction
Somatic gene therapy has been developed to express or silence gene products that are therapeutically useful and to correct or modulate genetic defects in diverse diseases [1–3]. The success of gene therapy is largely dependent on the development of gene delivery vectors, especially polymeric carriers [4–7]. Cationic polymers are one of the main categories of nonviral vectors, and have received greater attention recently because of their inherent advantages, including non-mmunogenicity, stability, capacity to carry large nucleic acid loads, and ease of manufacturing [5, 8–10]. The backbone linkages of most polymeric gene carriers consist of a –C–C– bond or amide bond, which are not degraded in physiological solutions [11]. The main drawback for these cationic polymers is their cytotoxicity, which is mostly due to their slow degradability and accumulation within cells or tissues [9, 11]. A family of bioreducible poly(disulfide amine)s are introduced as a promising non-viral vector for gene delivery [9, 12, 13]. This review will describe recent updated advances in the development of bioreducible polymers for plasmid DNA (pDNA), siRNA, cells, and adenovirus (Ad) for treatment of cardiovascular disease, diabetes mellitus, and cancer [14].
II. Structure and Characteristics of Bioreducible Polymers
To facilitate intracellular polymeric gene delivery efficiency, diverse biologic microenvironments such as pH, ionic strength, and redox potentials are considered as key modification factors [6, 13, 15–18]. Bioreducible polymers using disulfide bonds instead of ester linkages take advantage of a high difference in reductive potential (100 fold) between the oxidizing extracellular and the reducing intracellular space in normal and diseased cells [16, 19]. Bioreducible polymers, which contain characteristic disulfide linkages, can be degraded in the cytoplasm specifically in response to redox potential through thiol-disulfide exchange reactions due to the elevated levels of glutathione (GSH, γ-glutamyl-cysteinyl-glycine), which are 50–1,000 times higher than that in extracellular media [12, 20]. A disulfide bond (–S–S–) is a covalent linkage which arises as a result of the oxidation of two sulfhydryl (SH) groups of cysteines or other SH-containing material. In bacterial and eukaryotic cells, they are often found in secretory proteins and exoplasmic domains of membrane proteins that face a harsh extracellular environment. In eukaryotic cells, cysteines are bridged in the endoplasmic reticulum (ER) via the disulfide bond, which functions primarily to fortify the tertiary protein structure. Two distinct characteristics that render this disulfide bond attractive in designing drug delivery systems are its reversibility and its relative stability in plasma [12, 16, 18]. The synthesis of reductively degradable linear polycations enables simple linkage of thiol-containing functional molecules (fluorescent labels, bioactive peptides) during polymerization at the termini of the macromolecule [21]. Also, it allows better control of polymerization, polycation properties, and purity than alternative template polycondensations or random cross-linking strategies [21]. Poly(amido amine)s (PAAs) can be synthesized via Michael type addition of primary and secondary aliphatic amines to bisacrylamide monomers, which are convenient in constructing high-throughput libraries by simple combination of monomers to achieve maximum efficacy of polymeric gene carriers [12, 17]. Many promising advantages of the biodegradable polymer, poly(amido amine)s (PAA) containing reducible disulfide bonds (SS-PAA) were reported. PAAs are more hydrolytically stable than poly(amino ester)s, and have good water solubility and biodegradability [17].
2.1. Poly(amido ethylenimine) (SS-PAEIs)
The design of degradable polycationic gene carriers such as reducible disulfide-containing poly(amidoamine) (SS-PAA) and poly(amido ethylenimines) (SS-PAEIs) as well as hydrolysable poly(β-amino ester) families have demonstrated comparable or enhanced gene transfection and reduced cytotoxicity when compared to PEIs [19, 22, 23].
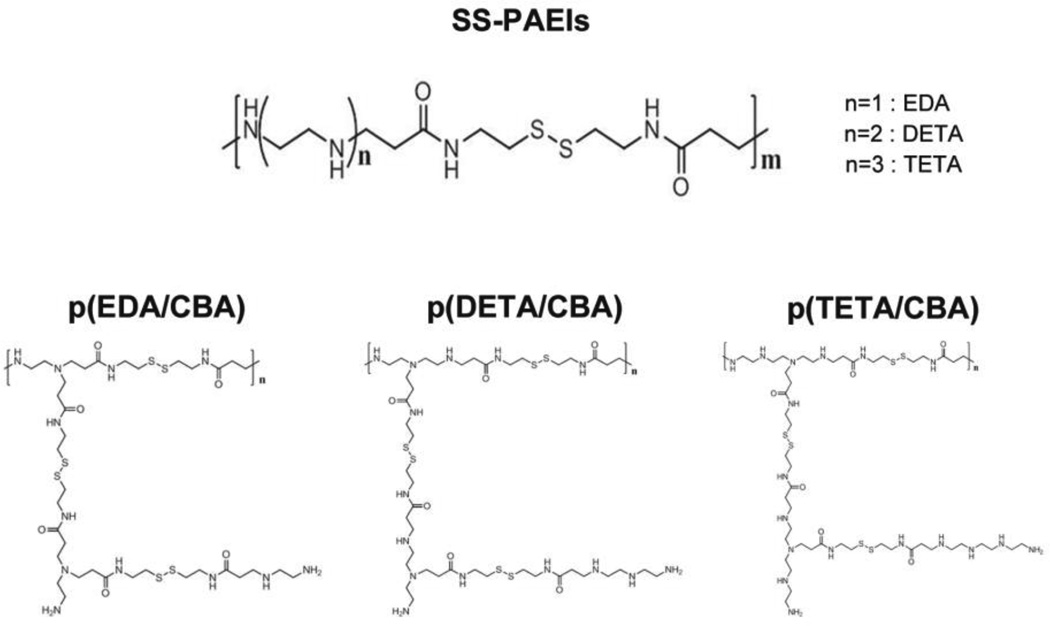
Poly(amido ethylenimine) polymers (SS-PAEIs), a branched-form peptidomimetic polymer, containing multiple disulfide bonds was designed (Fig. 1). Three SS-PAEIs were synthesized using Michael addition reactions between cystamine bisacrylamide (CBA) and three different ethylene amine monomers – ethylenediamine (EDA), diethylenetriamine (DETA), or triethylenetetramine (TETA), making polyplexes with an average size of less than 200 nm and a positive surface charge of ~ 32 mV (Fig. 1) [23]. All three SS-PAEIs exhibited nearly 20 times higher transfection efficiency with greater intracellular distribution of pDNA as compared to bPEI25k [17, 23].
Fig. 1.
Structure of Poly(amido ethylenimine) (SS-PAEIs), branched-form
In serum-containing media, p(TETA/CBA) showed significantly better transgene expression than bPEI25k, whereas p(TETA/CBA) delivery capacity was noticeably lower in the absence of serum. Therefore, to reduce interactions with serum proteins and improve carrier function in the presence of serum, poly(ethylene glycol) (PEG) was conjugated to p(TETA/CBA)5k [22]. Conjugating PEG2K to p(TETA/CBA)5k reduced the polyplex surface charge, however, it adversely affected nucleic acid condensation, corroborating previous other findings [24]. Therefore, increasing the p(TETA/CBA)5k-g-PEG2k amount to 50% and 100% reduced pDNA protection in serum [22]. The p(TETA/CBA)5k alone and 10/90% volumetric mixtures of p(TETA/CBA)5k-g-PEG2k/ p(TETA/CBA)5k sufficiently protected up to 70% of pDNA from serum nuclease degradation over 6 hrs [22]. These results provide evidence that PEG/polycation ratios can be easily altered to evaluate and find the optimal PEG ratios for better gene carrier function. In a biodistribution study following systemic administration in a murine adenocarcinoma model, the 25% p(TETA/CBA/PEG)/p(TETA/CBA) complexes at the w/w of 3:1 with the lowest particle size and surface charge indicated predominantly higher liver deposition and lower spleen accumulation. This suggests relatively low interaction of these complexes with serum proteins, which results in evasion of the retiuloendothelial system (lower accumulation in spleen), and extravasation through liver endothelial fenestrae due to relatively small particle sizes [25].
2.2. Bioreducible polyethylenemines (PEIs)
The biodegradable PEIs were synthesized by crosslinking low molecular weight PEI (0.8 kDa) with either PEG-bis-succinimidyl succinate or disulfide-containing cross-linkers [11, 26]. These crosslinked PEIs had much lower cytotoxicity and improved transfection efficiencies compared to 0.8 kDa PEI [26]. Also, an acid-labile PEI with an acid-labile imine linkage was synthesized by crosslinking low molecular weight PEI (1.8 kDa) with glutardialdehyde [27]. This acid-labile PEI was relatively stable at physiological pH, but half of the imine linkages were degraded within an hour at pH 4.5 [27]. The degraded low molecular weight PEI could be less toxic in the acidic endosomal compartment than its high molecular weight counterpart.
2.3. Poly(cystaminebisacrylamide-diaminohexane) (Poly(CBA-DAH))
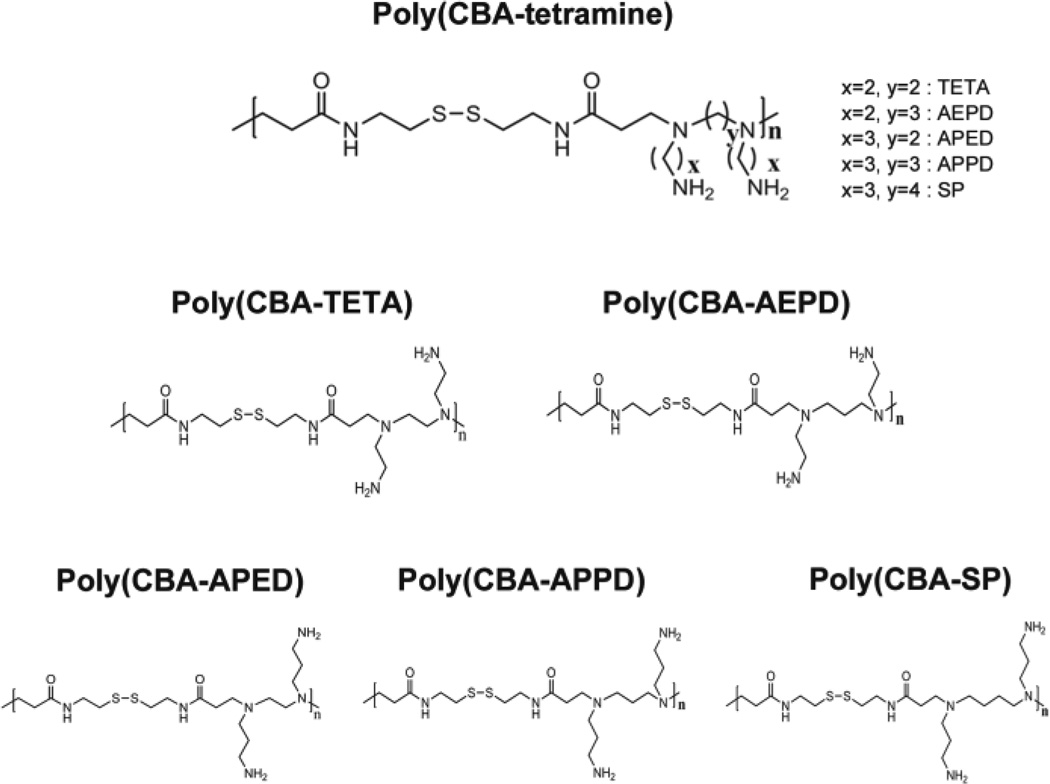
Using different lengths of polymethylene spacer [–(CH2)n–] in the main chain (y ; Fig. 2) and the side chain (x ; Fig. 2), a family of bioreducible poly(disulfide amine)s has been synthesized (Fig. 2) [9]. The Poly(CBA-APED), Poly(CBA-APPD), and Poly(CBA-SP) with the longer propylene [–(CH2)3–] side spacer demonstrated higher transfection efficacy than Poly(CBA-TETA) and Poly(CBA-AEPD) with the shorter ethylene [–(CH2)2–] side spacer [9]. Moreover, the Poly(CBA-APED), Poly(CBA-APPD), and Poly(CBA-SP) with the longer propylene [–(CH2)3–] side spacers showed similar transfection efficiency, indicating that the length of the main chain spacer in these polymers has less influence on transfection efficiency [9]. However, in polymers with the shorter ethylene [–(CH2)2–] side spacers, Poly(CBAAEPD), which contains longer main chain oligomethylene units [–(CH2)3–] showed relatively higher transfection efficiency than Poly(CBA-TETA) with a shorter main chain [9]. In the C2C12 cell line, Poly(CBA-SP) had the highest transfection, while Poly(CBA-APED) had the highest transfection in the HeLa cell line [9]. The longer, more hydrophobic oligomethylene side chains and more flexible backbones of these polymers with [–(CH2)n–, n = 2–4] increased gene transfection efficiency which may be due to the enhanced buffering capacity, protonation degree of tertiary amine groups, basicity and charge density of polymers (Fig. 2).
Fig. 2.
Structure of Poly(CBA-tetramine), linear-form
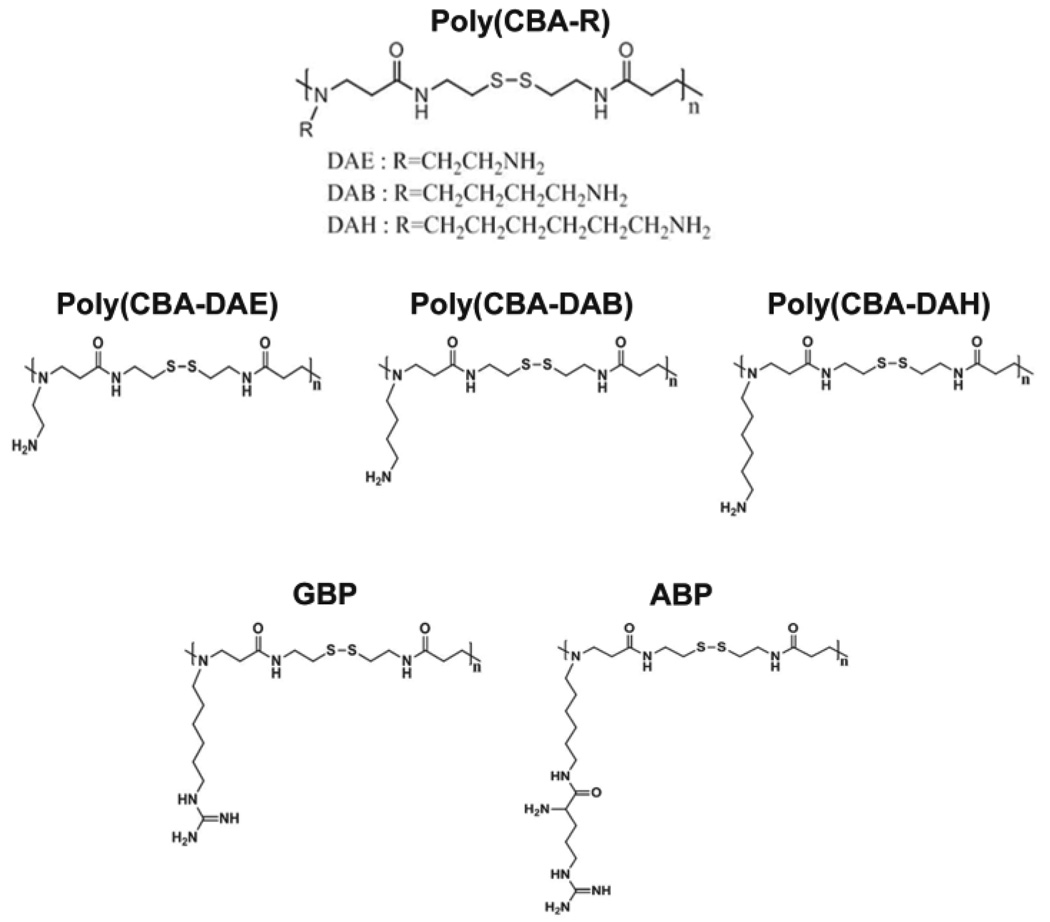
Michael addition between N,N'-Cystaminebisacrylamide (CBA) and three N-Boc protected diamines (N-Boc-1,2-diaminoethane, N-Boc-1,4-diaminobutane, and N-Boc-1,6-diaminohexane) followed by N-Boc deprotection under acidic condition resulted in final cationic polymers with disulfide bonds, tertiary amine groups in main chains, and pendant primary amine groups in side chains (Fig. 3). Genetic transfections mediated by these poly(disulfide amine)s were side-chain spacer length dependent. The poly(disulfide amine) with a hexaethylene spacer, poly(CBA-DAH) showed comparable or higher transfection efficiency in vitro [28]. The molecular weight of poly(CBA-DAH) was 3.52 kDa.
Fig. 3.
Structure of Poly(CBA-R)
2.4. Arginine-grafted bioreducible poly(disulfide amine) (ABP) and guanidinylated bioreducible polymer (GBP)
In several kinds of cell-penetrating peptides (CPP)s, arginine and guanidine groups were reported to possess great cell-penetrating ability [29–33]. Recently, many bioreducible polymers containing disulfide bonds have been developed for gene delivery systems which are degraded by intracellular reducing agents such as glutathione and have lower susceptibility to humidity than hydrolytic polymers [33].
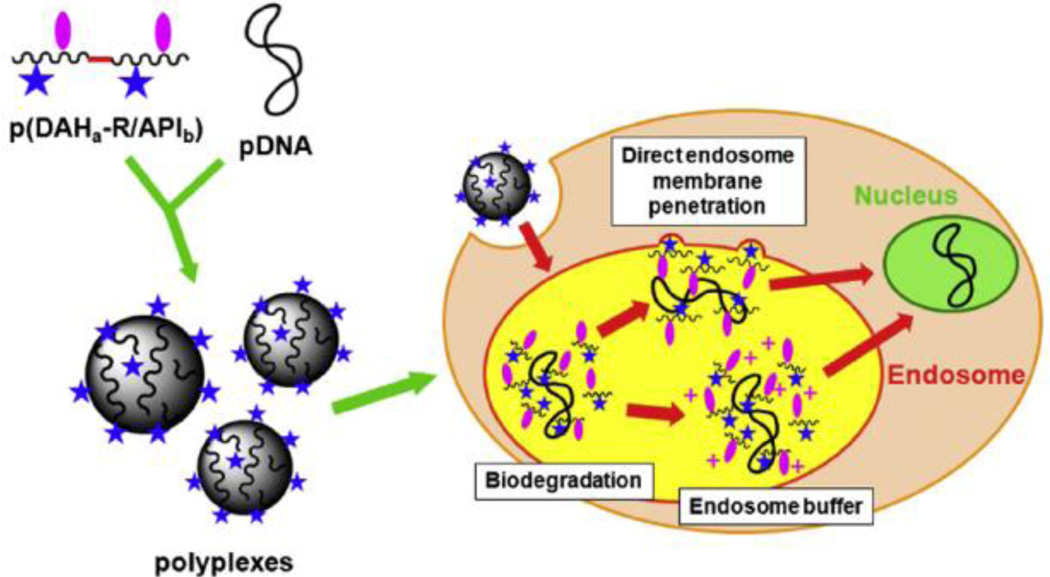
Arginine-grafted bioreducible poly(cystaminebisacrylamide-diaminohexane) polymer (ABP) was synthesized (Fig. 3) [34]. The molecular weight of ABP was estimated to be 4.45×103 Da by an FPLC-SEC. The average size of ABP polyplexes were less than 200 nm at all weight ratios used and reached positive plateau zeta potential values of 30–40 mV at a weight ratio of 10 [34]. A series of transfection experiments to examine the transfection mechanisms of the ABP polyplex suggested that the greatly enhanced transfection efficiency of ABP may not be induced by its high cellular penetrating ability but is instead mediated by other factors such as good nuclear localization ability [34]. To evaluate the effect of endosome buffering moieties on arginine-grafted bioreducible polymeric gene carriers, p(DAH-R/API)s, which are bioreducible cationic polymers containing arginine-grafted diaminohexane (DAH-R) as a cell penetrating moiety and 1-(3-aminopropyl) imidazole (API) as an endosome buffering moiety were synthesized with three different composition ratios, and their structure-activity relationships for gene delivery were examined [29]. This study proposed that p(DAH-R/API) polyplexes may escape from endosomes by direct endosome membrane penetration of arginine moieties as well as the endosome buffering abilities of the polymers after cellular uptake (Fig. 4) [29].
Fig. 4.
Proposed endosome escape mechanisms of p(DAH-R/API) polyplexes. Reprinted from ref [29] with permission from Elsevier.
Despite the widespread use of recombinant human erythropoietin (rHuEPO) in chronic kidney disease patients with anemia, several clinical limitations remain, including frequent injections, limited routes of administration, high medical expenditures, and development of autoimmune pure red cell aplasia [35]. To overcome many of these clinical drawbacks, gene therapy that provides sustained release has been suggested as an attractive alternative to current intermittently administered erythropoietin-stimulating agents. Gene delievery of plasmid human EPO (phEPO) using a bioreducible ABP or ABP-PEG10 (ABP-PEG:ABP = 1:9) can stimulate hematopoietic progenitor cells by colony-forming assay and inhibit cardiomyocytes apoptosis in vitro [36]. Furthermore, a single systemic injection of ABP-complexed phEPO sustained higher hematocrit levels, increased reticulocytosis, raised expression of plasmid hEPO protein, and the time-dependent BM-kidney-spleen distribution of hEPO mRNA. This was accompanied with no significant activation of the innate immune response (IL-6), in contrast to PEI-based gene delivery [37].
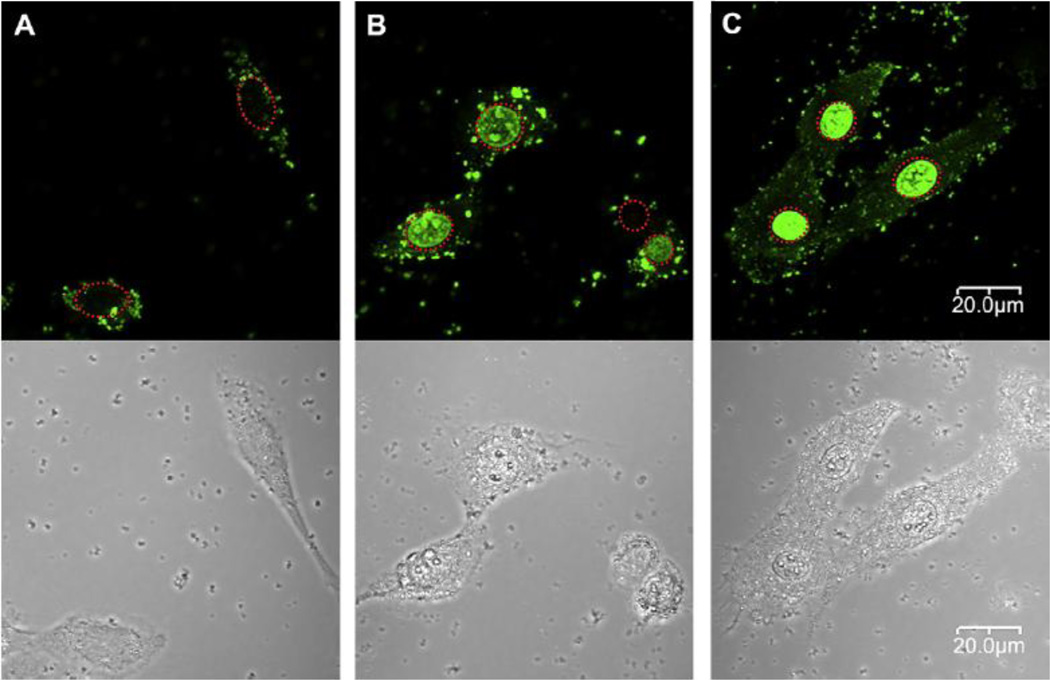
The molecular weight of guanidinylated bioreducible polymer (GBP) was 5.35×103 Da (Fig. 3) [38]. From a weight ratio of 5–40, GBP polyplex particles showed converging properties: 30 mV zeta potential and an almost 200 nm size. In C2C12 cells, the transfection efficiency of GBP polyplexes was almost 40 times higher than bPEI25k polyplexes and about 8 times higher than poly(CBA-DAH) polyplexes [38]. To explain the increased transfection efficiency of GBP polyplexes, the intracellular trafficking of polyplexes were evaluated by confocal microscopy in C2C12 cells. With GBP polyplexes, the strong florescence of YOYO-1 labeled pDNA was observed mostly in the nuclei of cells, which contrasted with bPEI25k polyplexes and poly(CBA-DAH) polyplexes (Fig. 5) [38]. With the structural differences between poly(CBA-DAH) and GBP, this finding supported the stronger nuclear localization ability of guanidine groups and the fast release of pDNA by biodegradation from GBP polyplexes.
Fig. 5.
Intracellular trafficking of polyplexes by confocal microscopy in C2C12 cells. YOYO-1 iodide labeled pDNA was used. Red dashed circles indicate cell nuclei. (A) bPEI25k polyplexes; Almost all bPEI25K polyplexes were observed to accumulate in the cytoplasm as weak green dots. (B) poly(CBA-DAH) polyplexes; poly(CBA-DAH) polyplexes were found in both cytoplasm and nuclei of cells. and (C) GBP polyplexes; high strong fluorescences of YOYO-1 labeled pDNA were observed mostly in nuclei of cells. Reprinted from ref [38] with permission from Elsevier.
2.5. Dendrimer type Bioreducible Polymer (ABP-conjugated PAMAM dendrimer : PAM-ABP)
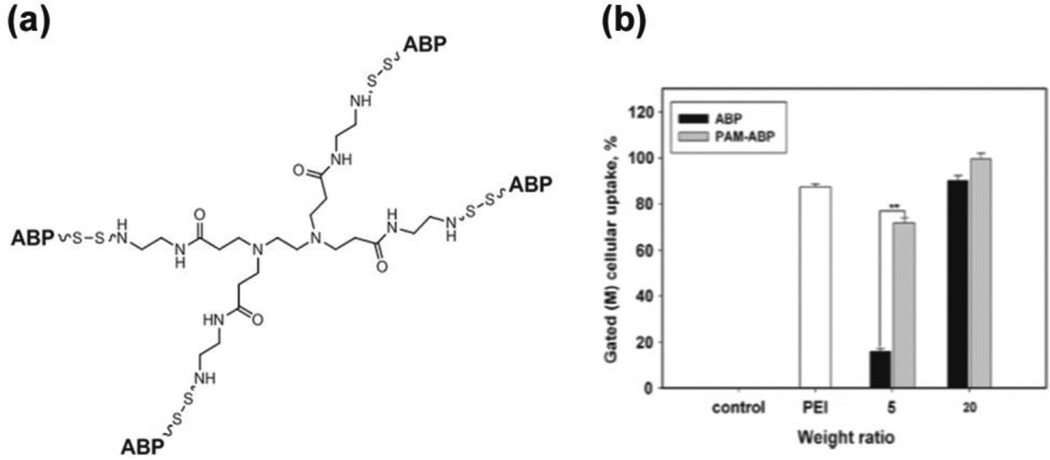
To optimize transfection efficiency of ABP through the formation of compact polyplexes, the weight ratio of ABP polyplex had to be increased to above 20, which limits the incorporation of higher amounts of pDNA in vivo. To overcome this obstacle to the use of ABP in vivo, derivatives of ABP with higher molecular weights were sought. A dendrimer derivative of ABP, PAM-ABP (ABP-conjugated poly(amido amine) (PAMAM) dendrimer) at the molecular weight of 20 kDa was synthesized using a backbone of PAMAM G0 and four ABP residues at the surface (Fig. 6(a)) [39]. The PAM-ABP completely retarded the electrophoretic mobility of the pDNA at a weight ratio of 2 compared to the ABP, which required a weight ratio above 5. The PAM-ABP at a weight ratio of 5 formed polyplexes with a zeta potential of 11.0 mV and an average particle size of 126 nm, making more compact polyplexes than ABP [39]. This PAM-ABP demonstrated superior condensing ability of pDNA through the formation compact nanosized polyplexes, and ultimately enhanced cellular uptake (Fig. 6(b)), was less susceptible to reducing agents, showed negligible cytotoxicity, and exhibited greater transfection efficiency in comparison with ABP polyplexes at the weight ratio of 5[39].
Fig. 6.
(a) Structure of Dendrimer type bioreducible PAM-ABP polymer. (b) Cellular uptake assay of the PAM-ABP polyplexes with YOYO-1 intercalated pDNA by flow cytometry using PEI as a control. Reprinted from ref [39] with permission from Elsevier.
2.6. Bioreducible poly(L-lysine) (PLL) copolymers
Cationic block copolymers with hydrophilic segments, such as poly(ethylene glycol) (PEG), effectively induce a condensation of plasmid pDNA or siRNA upon complexation to form core–shell-type polyplexes with the the hydrophilic outer layer surrounding the polyion complex (PIC) core. These micelles show high colloidal stability under physiological conditions and substantial transfection activity in various cell types even after preincubation with serum proteins [40]. Also, polyplex micelles demonstrate prolonged blood circulation and in vivo gene transfer to the liver and tumor [40]. In 2004, the bioreducible block catiomer polyplex using poly(ethylene glycol)-poly(L-lysine) block copolymer (PEG-PLL), was developed by controlling both the cationic charge and disfulfide cross-linking densities of the backbone polycations [41]. This bioreducible block copolymer, PEG-SS-PLL demonstrated higher stability in the extracellular medium and an efficient release of pDNA in the intracellular compartment, revealing approximately 50 times higher transfection efficiency toward 293T cells than the Traut’s reagent type [41]. Moreover, these crosslinked polyplex micelles (CPMs), PEG-SS-PLL showed excellent stability during freeze-drying and reconstitution processes [42]. The CPM showed uniform gene expression in the liver parenchymal cells after intravenous injection via the orbital vein in mice, suggesting future therapeutic applications for liver diseases [42].
A number of polyion complexes (PICs), formed through electrostatic interaction have been investigated as carriers of therapeutic siRNA as well as pDNA. Especially PEGylated PICs have been prepared through electrostatic interaction between PEG-block-polycation copolymers and nucleic acids, such as pDNA or anti-sense oligo-DNA, and are termed PIC micelles because of a core–shell structure with the core surrounded by a dense PEG corona [20]. These PIC micelles demonstrate remarkable properties as delivery vehicles for DNA: excellent colloidal stability in proteinaceous media, protection of incorporated DNA against enzymatic degradation, and prolonged blood circulation [20]. A core–shell-type PIC micelle with a disulfide cross-linked core was prepared through the assembly of iminothiolanie-modified PEG-block-PLL [PEG-b-(PLL-IM)] and siRNA at a characteristic optimum mixing ratio [20]. These environment-responsive PIC micelles achieved 100-fold higher siRNA transfection efficiency than non-bioreducible PIC micelles [20].
Then, the PEG-SS-P[Asp(DET)] micelles, based on poly(aspartamide) with a flanking N-(2-aminoethl)-2-aminoethyl group, P[Asp(DET)], showed 1–3 orders of magnitude higher gene transfection efficiency and a more rapid onset of gene expression than PEG-P[Asp(DET)] micelles without difulfide linkages, due to much more effective endosomal escape based on the PEG detachment in endosome [40]. P[Asp(DET)] acts as a buffering moiety inducing endosomal escape with minimal cytotoxicity.
2.7. Others
To obtain cancer-specific therapeutic transgene expression, the survivin-inducible plasmid DNA expressing the soluble VEGF receptor, sFlt-1, downstream of the survivin promoter (pSUR-sFlt-1) was constructed [43]. The dual bio-responsive gene delivery system with both reducible poly(amido-butanol) (SS-PABOL) and survivin-inducible plasmid DNA demonstrated the enhanced efficiency compared to traditional carriers [43]. Next, the luciferase expression of pDNA delivered by a bioreducible nonionic triblock copolymer, PEG13-PLGA10-PEG13 was up to three orders of magnitude higher than that of pDNA/bPEI25k, and three times more than that of naked pDNA after intramuscular injections [44]. And this in vivo improved gene delivery effect provided a two-fold higher expression of VEGF, suggesting the triblock copolymer, PEG13-PLGA10-PEG13, is applicable as a carrier for skeletal muscle gene delivery applications [44].
III. Disease Applications of Bioreducible Polymers
3.1. Cardiovascular Disease
Cardiovascular disease is the leading cause of death in the world [17, 19]. After a decade of pre-clinical and early phase 1 clinical investigations, gene therapy has emerged as a genuine possible therapeutic alternative to conventional therapies, especially in coronary artery disease and heart failure [1, 8, 45, 46].
The ECM in the heart, which is filled with negatively charged proteoglycans and glycosaminoglycans such as hyaluronan, has been considered a hurdle in gene therapy. Since these anatomical characteristics of the compact cardiac ventricle muscles lead to very low gene transfection efficiency with the general cationic gene carriers in the myocardium [47], successful cardiac applications of DNA and siRNA therapeutics must be preceded by the development of optimal delivery systems.
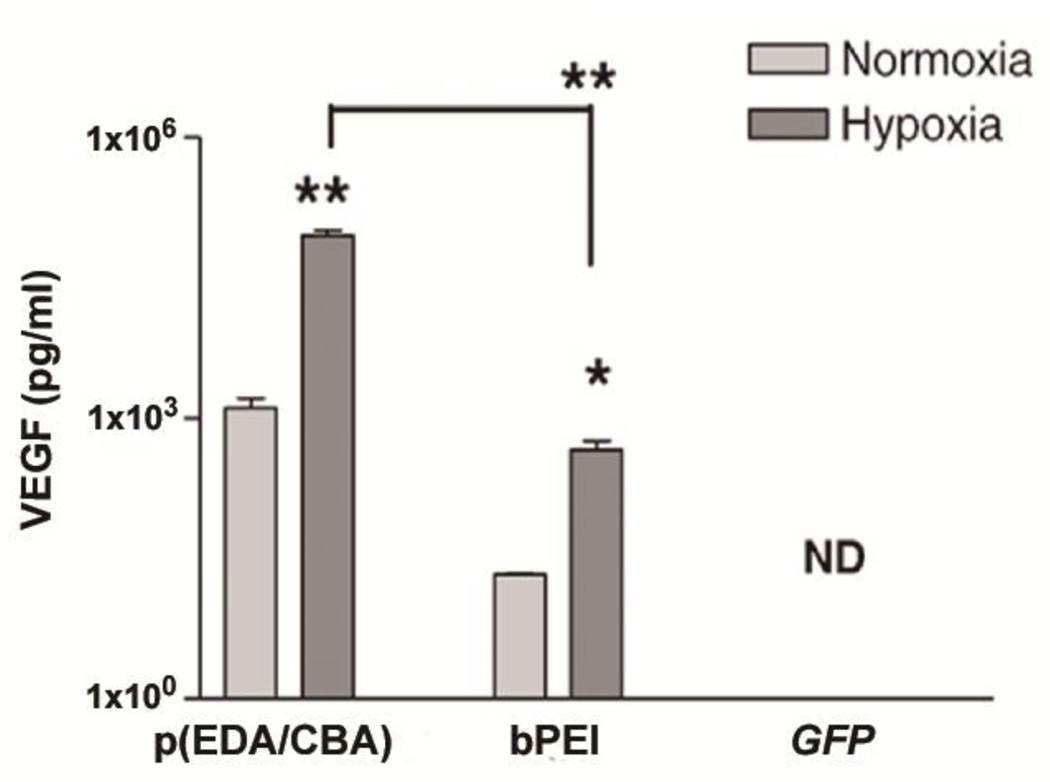
Usually, under hypoxic conditions diverse gene expression is found. The RTP-VEGF/bPEI25k polyplexes showed a 22 fold increase in VEGF protein under hypoxic conditions when compared to normoxia [48]. SS-PAED (Fig. 1; p(EDA/CBA)) mediated delivery of hypoxia-inducible RTP-VEGF plasmid produced a remarkable 67 and 76 fold increase of VEGF expression after 24 and 48 hrs under hypoxic conditions, in comparison with normoxic conditions in H9C2 cells (Fig. 7) [48]. In a rabbit infarct model, RTP-VEGF/p(EDA/CBA) polyplex with a weight ratio of 1:12 showed the highest overall VEGF expression, about 4 fold higher than the RTP-Luc control and 2 fold higher than the water soluble lipopolymer (WSLP) positive control when using half the transfection amount [48]. Other studies supported that VEGF protein expression was attributed to post-translational modifications by the protein kinase C pathway [49, 50].
Fig. 7.
Comparison of VEGF expression of RTPVEGF/ p(EDA/CBA) polyplexes, RTP-VEGF/bPEI polyplexes, and GFP/p(EDA/CBA) polyplexes 24hrs after transfection in H9C2 cells. ND; no detection, all data reported as the mean±SEM. *p<0.05, **p<0.001. Reprinted from ref [48] with permission from Elsevier.
Beyond the traditional erythropoietic effects, erythropoietin (EPO) was recently identified as a pleiotropic and organ-protective cytokine, making it a frontline cardioprotective candidate. Even though the in vitro and in vivo data supporting cardioprotective effects of recombinant human erythropoietin (rHuEPO) protein are numerous, recent randomized clinical trials in acute myocardial infarction (MI) patients have reported conflicting data [51]. The favorable cardioprotective effects of human EPO plasmid DNA (phEPO) delivered by bioreducible ABP are not confined to the LV infarct lesion, but also spread into non-infarcted sites including the interventricular septum, right ventricle, and atria [51]. This intriguing reversal of maladaptive cardiac remodeling by intramyocardial injections of phEPO/ABP polyplexes provided insight into the failure of both phEPO gene and rHuEPO alone in human trials for treating MI [51]. Conclusively, this data suggests that bioreducible delivery vectors can revive the therapeutic potential of phEPO and other cardioprotective genes during post-infarct cardiac remodeling.
After MI, therapeutic angiogenesis induced by angiogenic growth factors, such as vascular endothelial growth factor (VEGF), may restore blood supply to the myocardium, limiting left ventricular dysfunction and adverse cardiac remodeling. However, uncontrolled VEGF expression and secretion may lead to severe side effects, including the development of angiomas that may cause heart failure [52] and bolster the progression of atherosclerosis. Compared to the transcriptionally regulated hypoxia-inducible system pβ-SP-ODD-VEGF, the post-translationally regulated hypoxia-responsive plasmid, was more efficacious at ameliorating the effects of ischemia-reperfusion injury in a rat model [53].
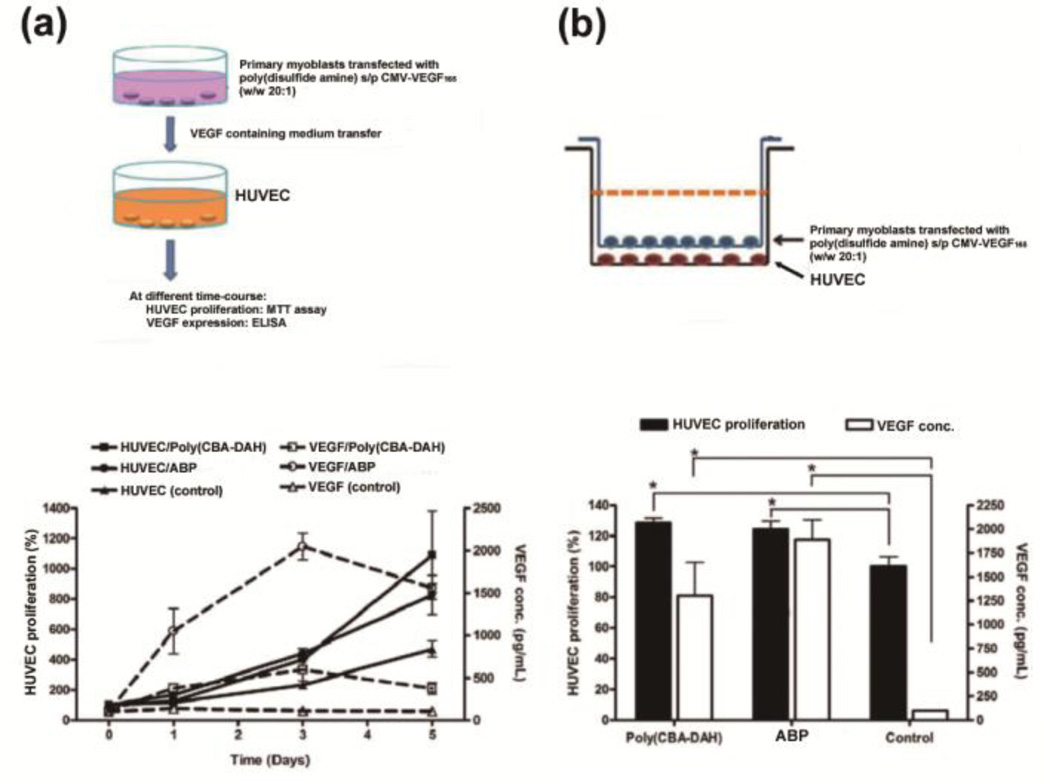
Cell based treatment for this disease is growing in scope and effectiveness [1, 55]. Implantation of cells to the myocardium has long been investigated as a means to regenerate post-infarct myocardium and improve outcomes. The engineered primary skeletal myoblasts were transfected with poly(CBA-DAH) containing a pDNA encoding human vascular endothelial growth factor (VEGF165) [54, 56]. In sequential and co-culture endothelial proliferation assays, VEGF/ABP polyplexes induced higher levels of VEGF expression than VEGF/poly(CBA-DAH), whereas VEGF/poly(CBA-DAH)-transfected primary myoblasts can initiate a higher level of HUVEC proliferation than VEGF/ABP polyplexes, suggesting that an optimal VEGF concentration in the medium is needed for angiogenic proliferation (Fig. 8) [54]. Also, local injection with hVEGF/poly(CBA-DAH) transfected myoblasts after myocardial infarction in male Sprague-Dawley rats significantly decreased cardiac wall thinning, restored left ventricular systolic function, induced neovascularization, conserved viable cardiomyocytes, and reduced cardiac apoptosis more effectively than untransfected myoblasts [56].
Fig. 8.
Polyplex transfected primary myoblasts mediated HUVEC proliferation and VEGF expression levels in vitro. (a) Sequential time-course endothelial proliferation assay. (b) The Co-culture endothelial proliferation assay. Reprinted from ref [54] with permission from Elsevier.
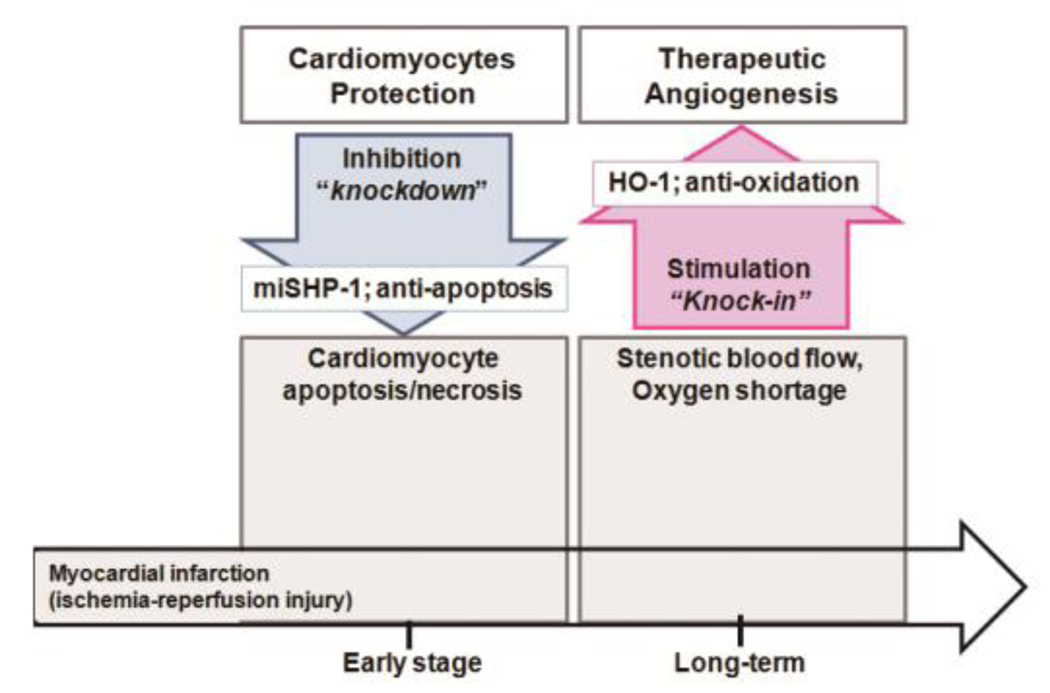
To date, a single gene therapy has failed to prevent the heart failure that is one of potentially fatal complications of ischemic heart disease [57]. In humans, the etiologies are complex and a “one gene fits all” approach seems to not be enough [57]. A combinatorial gene therapy approach that targets specific cardiac cells and signaling pathways may be more clinically relevant. Two molecular pathways that are central to the effective treatment of ischemic heart disease are the inhibition of cardiomyocyte apoptosis/necrosis and the induction of therapeutic angiogenesis [1, 46]. The combinatorial gene therapy of a hypoxiainducible plasmid pEpo-SV-miSHP-HO-1 for the dual expression of heme oxygenase-1 (HO-1 ; knock-in, anti-oxidation) and the Src homology region 2 domain-containing tyrosine phosphatase-1 (SHP-1) microRNA (miSHP-1; knockdown, anti-apoptosis) was constructed for the treatment of ischemic disease (Fig. 9) [58]. Also, the sequential combination of a hypoxia-inducible plasmid expressing both hypoxia-inducible heme oxygenase-1 (HO-1) and miSHP-1, as well as a hypoxia-responsive VEGF plasmid showed synergy in antiapoptotic effects, and exhibited the desired dual gene expression characteristic of knock-in and knockdown (Fig. 9) [59].
Fig. 9.
Overview of myocardial infarction therapy strategies
Currently, RNA interference is a very promising technology; however, several limitations remain: inherent instability of the siRNA, innate immune response to siRNA, poor cellular uptake, and limited/low efficacy with systemic delivery. To overcome these hurdles, delivery of siRNA with polymeric carriers has been proposed as an attractive solution. In our previous studies, we have reported the cardiomyocyte-targeting bioreducible polymer for siRNA delivery to the myocardium [60–63].
Prostaglandin E2 (PGE2) is involved in numerous physiological mechanisms: contraction and relaxation of smooth muscle, control of blood pressure, and modulation of inflammation [64]. The EP4, one of four distinct PGE2 receptors (EP1–4), has been reported to be highly expressed in the myocardium of several species, including humans [65]. To increase the efficiency of siRNA transfer to cardiomyocytes, the use of PGE2 as a specific ligand for cardiovascular-targeted siRNA delivery against Fas, a key regulator of ischemia-induced apoptosis, was considered [60]. The PGE2-Fas siRNA/poly(CBA-DAH) polyplex demonstrated enhanced cellular uptake in H9C2 cells [60]. Also, the PGE2 receptor competition assay in the medium containing free PGE2 showed significantly suppressed cellular uptake of PGE2-Fas siRNA/poly(CBA-DAH) polyplexes, supporting the PGE2 receptor-mediated internalization [60]. The PGE2-Fas siRNA/poly(CBA-DAH) polyplex delivery led to increased Fas gene silencing, and thereby suppressed cardiomyocyte apoptosis without induction of interferon-alpha [60].
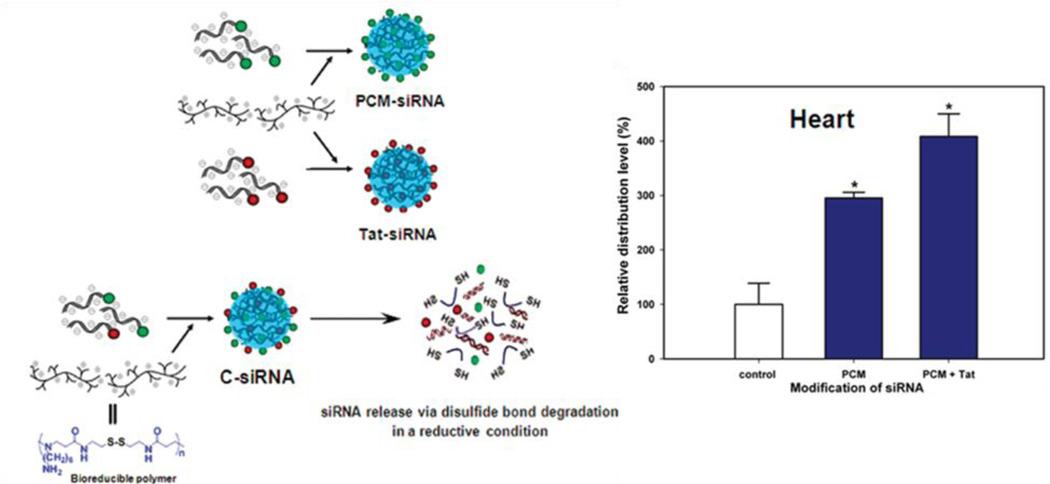
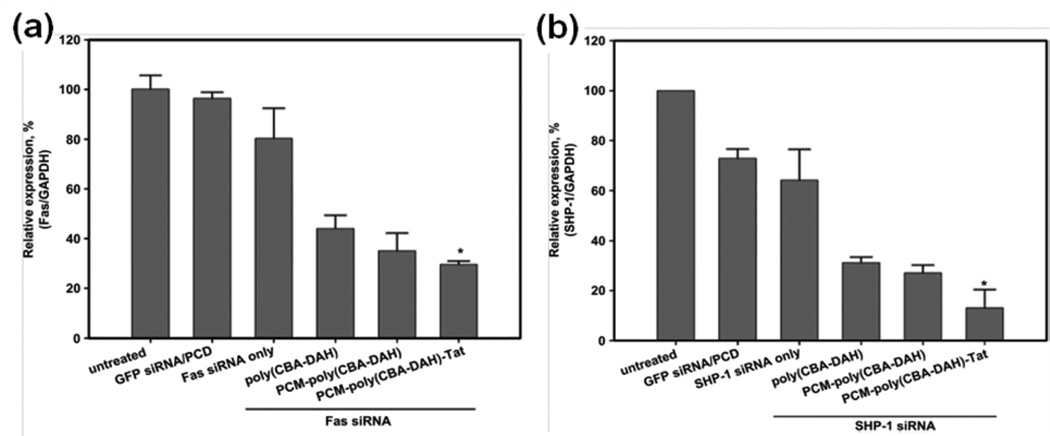
Primary cardiomyocyte (PCM) specific peptide (C-WLSEAGPVVTVRALRGTGSW), a 20 amino acid peptide was isolated by phage display and binds primary cardiomyocytes with 180 times more avidly than control phages from the library [66, 67]. The Fas gene has been identified as an inducer of cardiomyocyte apoptosis [68]. A cardiomyocyte-targeted Fas siRNA delivery system was developed using primary cardiomyocyte (PCM) specific peptide-conjugated to bioreducible poly(CBA-DAH), which can specifically and efficiently bind cardiomyocytes. This construct improved transfection efficiency without significant cytotoxicity [62]. Then, the bioreducible poly(CBA-DAH) polymer conjugated with a PCM peptide, primary cardiomyocyte-targeting peptide, a Tat peptide (C-47YGRKKRRQRRR57), and a cell-penetrating peptide (CPP) was designed (Fig. 10(a)) [31, 32, 61, 69]. Along with Fas, the Src homology domain 2 (SH2) containing tyrosine phosphatase-1 (SHP-1) plays an important role in apoptosis and a negative regulatory role in the phosphrylation of Akt [70]. Akt, a pro-survival signal, is a serine/threonine protein kinase, also known as protein kinase B, which regulates cardiac growth, myocardial angiogenesis, glucose metabolism, and inhibits cardiomyocyte apoptosis through extracellular signal-regulated kinase (ERK)-1/2 [71]. Therefore, siRNA targeting SHP-1 (the Src homology domain 2 (SH2) containing tyrosine phosphatase-1) or Fas was delivered using bioreducible PCM-poly(CBA-DAH)-Tat. These polyplexes showed enhanced gene silencing in cardiomyocytes and inhibited their apoptosis under hypoxic and serum-deprived conditions in vitro (Fig. 11) [61]. In addition, a systemic administration of the combined siRNA polyplexes delivered siRNA to the heart at significantly higher levels than non-targeted carriers (Fig. 10(b)) [69].
Fig. 10.
(a) Schematic diagram of the preparation of peptide-modified siRNA under reductive conditions. (b) Biodistribution of siRNA-pDNA/ poly(CBA-DAH) complexes in mice at 24 hrs post-injection. Reprinted with permission from ref [69], Copyright(2012), American Chemical Society.
Fig. 11.
Quantitative real-time PCR for (a) Fas and (b) SHP-1 siRNA delivered by PCM and Tat-conjugated poly(CBA-DAH) polymer (PCM-poly( CBA-DAH)-Tat) in H9C2 cells under hypoxic conditions. *p < 0.05 for relative expression of Fas or SHP-1 vs all other groups. Reprinted from ref [61] with permission from Elsevier.
Because this primary cardiomyocyte-targeted polymeric gene carrier with a homing peptide, when administered systemically, may deliver the gene to all heart tissue instead of just the ischemic region, a polymeric gene carrier targeting ischemic myocardium was designed. In this design, poly(CBA-DAH) was modified with the ischemic myocardium-targeted peptide (IMTP) and D-9-arginine (9R) for homing to the ischemic myocardium and enhanced transfection efficiency. This system allowed for this minimal use of the polymer [63]. After intravenous injection, the IMTP-Poly(CBA-DAH)-9R specifically targeted the ischemic myocardium and enhanced gene expression in the LV of the I/R rat, providing a new direction of gene therapy for the treatment of myocardial ischemia [63].
3.2. Diabetes, DM
According to data from the 2011 national diabetes fact sheet, diabetes affects 25.8 million children and adults, which amounts to 8.3% of the United States population. Several bioreducible polymers have been used to make advances in diabetes therapeutics by delivering genes [3].
3.2.1. Type 1 Diabetes Mellitus
Type 1 diabetes accounts for 5–10% of all diagnosed cases of diabetes in the United States. Type 1 diabetes develops when autoimmune processes destroy pancreatic β-cells. To survive, patients with type 1 diabetes must have insulin delivered by injection or with a pump. Thus, in type 1 diabetes, there are many challenges to protect insulin-producing β-cells from autoimmune destruction and to target gene expression to pancreatic islets [3, 72–75]. Systemic administration of pCAGGS mIL-10 plasmid delivered by biodegradable poly[α-(4-aminobutyl)-L-glycolic acid] (PAGA) showed reduced severity of insulitis in non-obese diabetic (NOD) mice [18, 72]. The plasmid IL-4 driven by the rat insulin promoter (pRIP-IL4) complexed with water soluble lipopolymer (WSLP) demonstrated increased expression level of IL-4 in a glucose concentration-dependent manner [74]. Next, the combined delivery of pDNA encoding IL-4 and IL-10 using PAGA polymer left 75% of islets intact six weeks after injection, compared to less than 3% in the control group, and prevented the development of autoimmune diabetes up to 32 weeks of age in 75% of the treated NOD mice [73]. Also, to repress glutamic acid decarboxylase (GAD) expression in islet β-cells, an antisense GAD mRNA expression plasmid (pRIP-AS-GAD) or anti- GAD Fab' fragment was delivered using poly(ethylene glycol)-grafted poly-L-lysine (PEG-g-PLL) or PEI via a PEG linker, respectively [75].
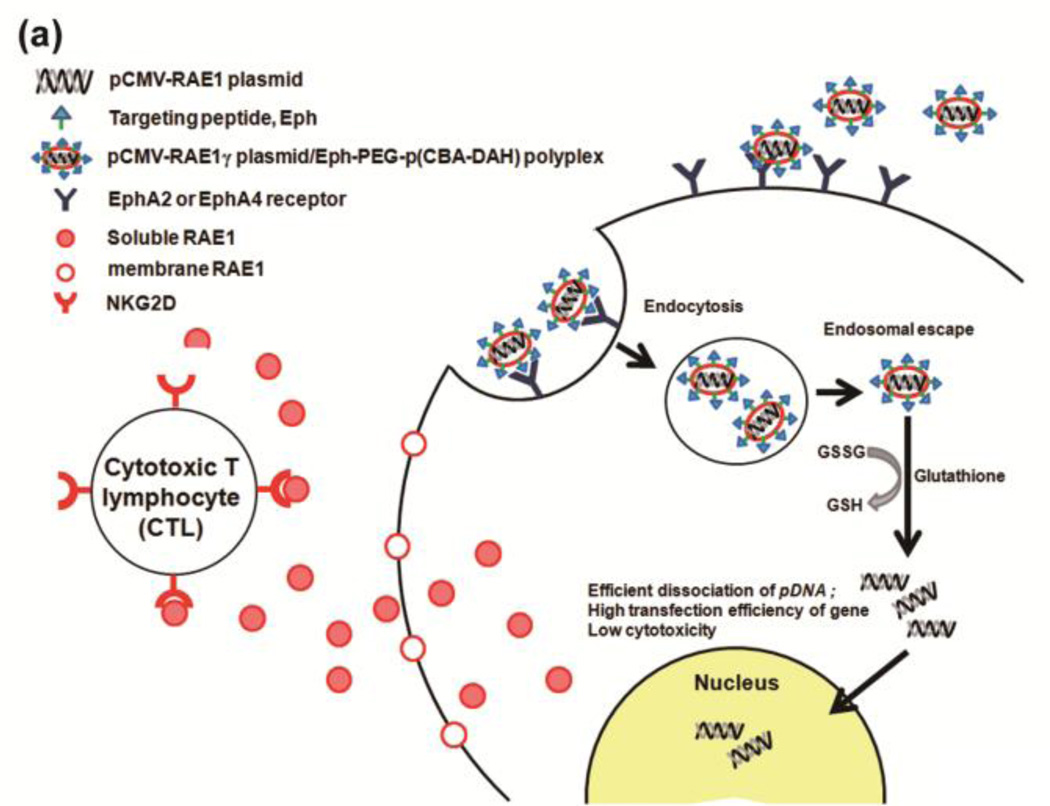
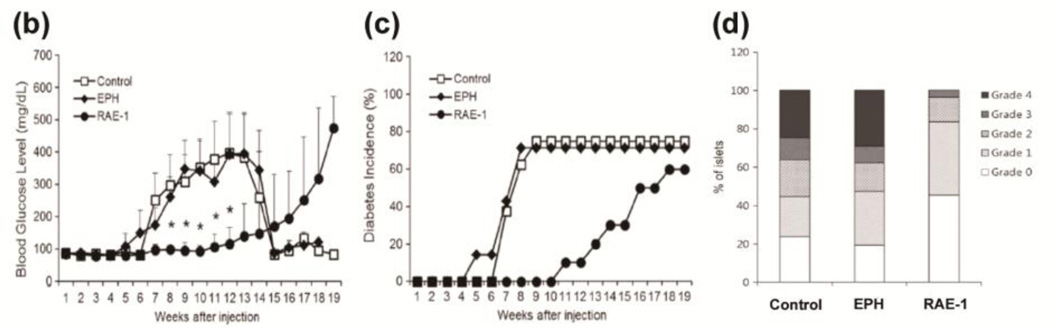
The co-stimulatory stress-inducible receptor natural killer group 2, member D (NKG2D) interacts with ligands on β-cells and is expressed in natural killer (NK) cells, CD8+ T-cells, and γδ T-cells that constantly survey tissues for unhealthy, stressed, foreign, or transformed cells [76, 77]. Retinoic acid early inducible gene-1 (RAE-1) is a tightly regulated high affinity ligand for the NKG2D receptor in mice [77, 78]. The targeting peptide CHVLWSTRC (Eph), which is known to target the EphA2 and EphA4 receptors overexpressed in the pancreatic islet microvasculature, was added to the distal end of a PEG chain and conjugated to pCBADAH forming Eph-PEG-p(CBA-DAH) [79]. The plasmid DNA encoding a soluble ligand to NKG2D (sRAE-1γ) was delivered to the pancreatic islet microvasculature using a targeted bioreducible polymeric carrier, Eph-PEG-p(CBA-DAH) and demonstrated significantly lower autoimmune destruction of β-cells, delayed onset of diabetes, and a prominent survival rate in the non-obese diabetic (NOD) mice model (Fig. 12) [80, 81]. Single systemic injections of the plasmid sRAE-1γ alone and targeting Eph-PEG-p(CBA/DAH) polymer alone showed hyperglycemia (glucose level > 13.9 mmol/L (~ 250 mg/dl)) 6 and 7 weeks after injection respectively, whereas the sRAE-1γ/Eph-PEG-p(CBA/DAH) polyplexes injection group demonstrated hyperglycemia after 16 weeks after injection — a delay in the onset of diabetes of up to 10 weeks (Fig. 12) [80].
Fig. 12.
(a) Conceptual diagram of sRAE-1γ therapy in type 1 diabetes. Delivering a gene coding for soluble RAE-1 locally to the pancreas will result in the binding and internalization of the NKG2D receptor of infiltrating lymphocytes. This will allow the pancreas to undergo “immune evasion,” and will halt the progression of type 1 diabetes. (b) Hyperglycemia, (c) cumulative incidence of diabetes, and (d) insulitis grade in NOD mice after sRAE-1 treatment. Reprinted from ref [80] with permission from Elsevier.
3.2.2 Type 2 Diabetes Mellitus
Type 2 diabetes mellitus constitutes 90–95% of all diabetes cases and the incidence of type 2 diabetes is rapidly increasing worldwide. Type 2 diabetes is characterized by basal hyperglycemia and exaggerated postprandial hyperglycemia induced by a combination of β-cell dysfunction and impaired insulin sensitivity.
In 1930, La Barre introduced the ‘incretin’ effect [82]. The glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) contribute approximately 60–70% of the total postprandial insulin response (incretin effect) in healthy individuals. Interestingly, in type 2 diabetic patients, GLP-1 secretion is significantly impaired in contrast to normal or near-normal meal-induced GIP secretion. Here, current approaches with bioreducible polymers for the delivery of GLP-1 and exendin-4 for higher and longer therapeutic effects will be discussed [3, 83–88].
Glucagon-like peptide-1 (GLP-1) is a gut-derived incretin hormone secreted by L cells in the distal ileum and colon in response to food ingestion [84, 89]. The physiologic actions of GLP-1 on glucose homeostasis are glucose-stimulated insulin secretion from islet β-cells, suppression of glucagon secretion, deceleration of gastric emptying, and reduction in appetite and food intake due to early satiety [84, 89, 90]. GLP-1 can induce euglycemia without hypoglycemic side effects, unlike insulin and other oral hypoglycemic agents. In spite of several promising antidiabetic properties of GLP-1, its therapeutic potential is limited by its short biological half-life (approximately 2 min), which results mainly from rapid enzymatic degradation by dipeptidyl peptidase IV (DPP-IV) and/or renal clearance [84, 91]. In an attempt to extend the plasma half-life and enhance the potency of GLP-1, analogs with modifications of the amino acids or N-terminal groups, agonists resistant to DPP-IV, and fusion proteins conjugating GLP-1 to other peptides have been developed with two, exenatide (Byetta) and liraglutide (Victoza) having been approved for use in the U.S. However, these modified peptides still have a half-life of only a few hours when injected subcutaneously, so multiple injections each day would be required for a therapeutic effect.
Although various GLP-1 pDNA systems using a chicken β-actin promoter (pβ-GLP-1) or a SV40 promoter/enhancer with nuclear factor κB binding sites (pSIGLP-1-NFκB) delivered by PEI25k showed increased insulin secretion and decreased blood glucose levels longer than 2–3 weeks in ZDF rats or diet-induced obese (DIO) mice after a single injection, blood glucose level was not normalized to 250 mg/dl [3, 84, 92]. Therefore, upgraded GLP-1 encoding dual pDNA system, TSTA(SP-GLP-1) (pβ-Gal4-p65 and pUAS-SP-GLP-1) was constructed by combining the secretion signal peptide (SP) to stimulate its secretion in nonendocrine cells or tissues and previous two-step transcription amplification (TSTA) system (pβ-Gal4-p65 + pUAS-GLP-1).[85, 87] The TSTA(SP-GLP-1)/ABP polyplexes exhibited an enhanced GLP-1 secretion level, which resulted in increased insulin secretion in insulinoma cells [84, 85].
A glucagon-like peptide-1 (GLP-1) receptor agonist, exendin-4 exocrine hormone, was isolated from the parotid gland of the Gila monster lizard [84]. Despite the fact that exendin-4 shares diverse functions of GLP-1 as an incretin, several clinical limitations remain, including anti-exendin-4 antibody formation, nausea after administration, and relatively short half-life [84, 90, 93, 94]. Exendin-4 is prescribed as once- or twice-daily injections or combination therapies with oral GLP-1 analogue drugs to maintain the normal glucose level in patients with type 2 diabetes. Likewise, the exendin-4 expression system, TSTA(SP-exendin-4) which is composed of pβ-Gal4-p65 and pUAS-SP-exendin-4 delivered by ABP, showed remarkably decreased blood glucose levels from 3 days to 12 days after a single intravenous injection [83, 84].
3.3. Cancer
In cancer chemotherapy, long circulating carrier systems improve chemotherapeutic drug delivery potency. The clinical applications of paclitaxel, a microtubule-stabilizing and cell division-blocking anti-cancer chemotherapeutic, are limited by disadvantages such as poor water solubility (0.3 µg/ml) and instability in aqueous solution. Polymeric micelles are one promising candidate that can solubilize poorly soluble drugs, protect hydrolytically sensitive drugs from the aqueous environment, change temporal and spatial distribution (pharmacokinetics and biodistribution), and stay in the blood circulation long enough to provide accumulation at the target site, thereby increasing therapeutic efficacy [4, 95].
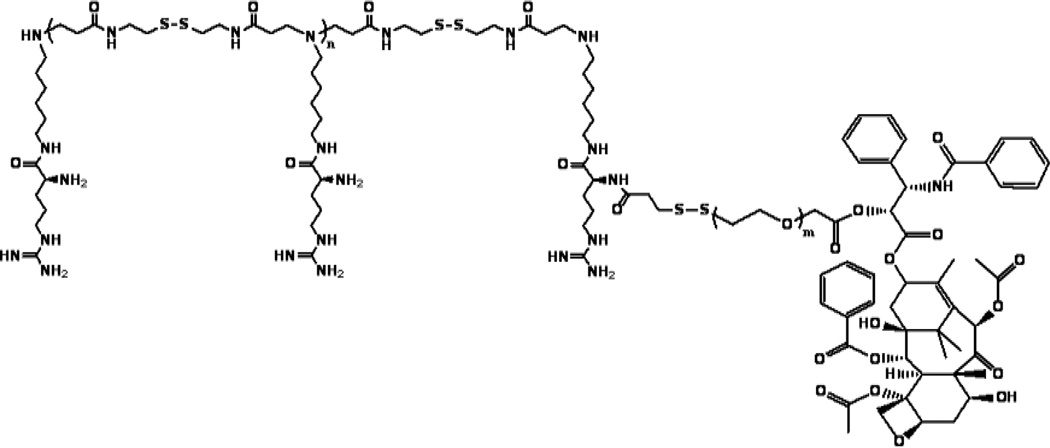
The paclitaxel-conjugated polymeric micelle, ABP-PEG3.5k-Paclitaxel (APP) is designed for the co-delivery of gene and drug (Fig. 13) [96]. The APP micelles had a molecular weight of 9.01×103 estimated by 1H NMR, a critical micelle concentration (CMC) value of 0.062 mg/ml determined by UV absorbance of pyrene, an average particle size of 2.61 ± 0.98 nm, and a surface charge of 13.5 ± 4.1 mV [96]. These APP polyplexes showed increased gene delivery efficiency, cellular uptake, low cytotoxicity, and higher anticancer potency than paclitaxel alone in vitro [96].
Fig. 13.
Structure of paclitaxel-conjugated polymeric micelle, ABP-PEG3.5k-Paclitaxel (APP).
3.3.1. Hybrid and Smart Oncolytic Gene Carrier
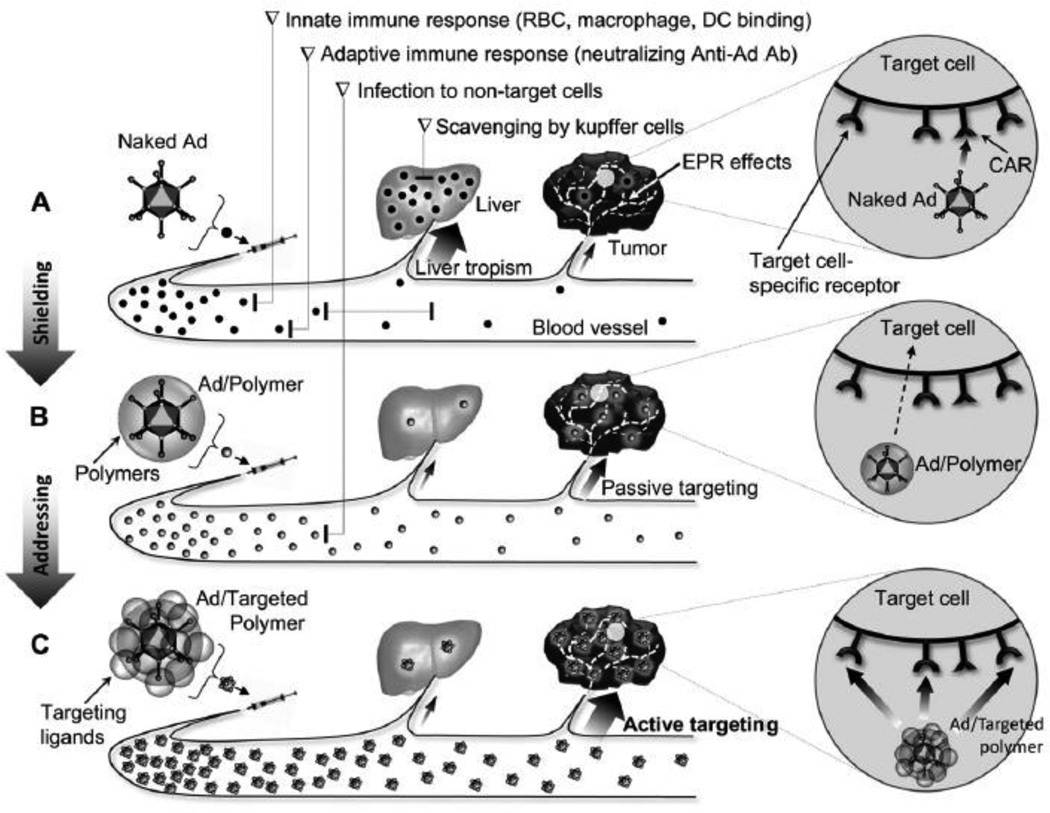
Since adenovirus (Ad) was discovered in 1953, oncolytic Ads have attracted a great deal of interest as cancer therapeutics with many advantages such as high transduction efficiency, self-propagation, lysis of infected cancer cells, and secondary infection of adjacent cells within the tumor [97–101]. However, systemic administration of Ad vectors is complicated by host immune responses, oncogenic effects, and viral accumulation in the liver, resulting in a short circulatory viral half-life, low efficacy, and host side effects, which must be considered before use in clinical trials[18, 101, 102]. Since then, “armed” oncolytic Ads have been developed to deliver therapeutic genes. Recently, the smart hybrid system of oncolytic Ads delivered by passively or actively targeted bioreducible polymers have been developed to exhibit enhanced cancer cell-specific oncolytic effects with fewer side effects (Fig. 14) [100, 103–109]. The fusion of bioengineering and biopharmaceutical technologies can provide solutions to the obstacles encountered during systemic delivery of Ads [109].
Fig. 14.
Scheme of systemically administered naked Ad, shielded Ad with polymer (passive targeting), and targeted polymer-coated Ad (active targeting). Reprinted from ref [100] with permission from Elsevier.
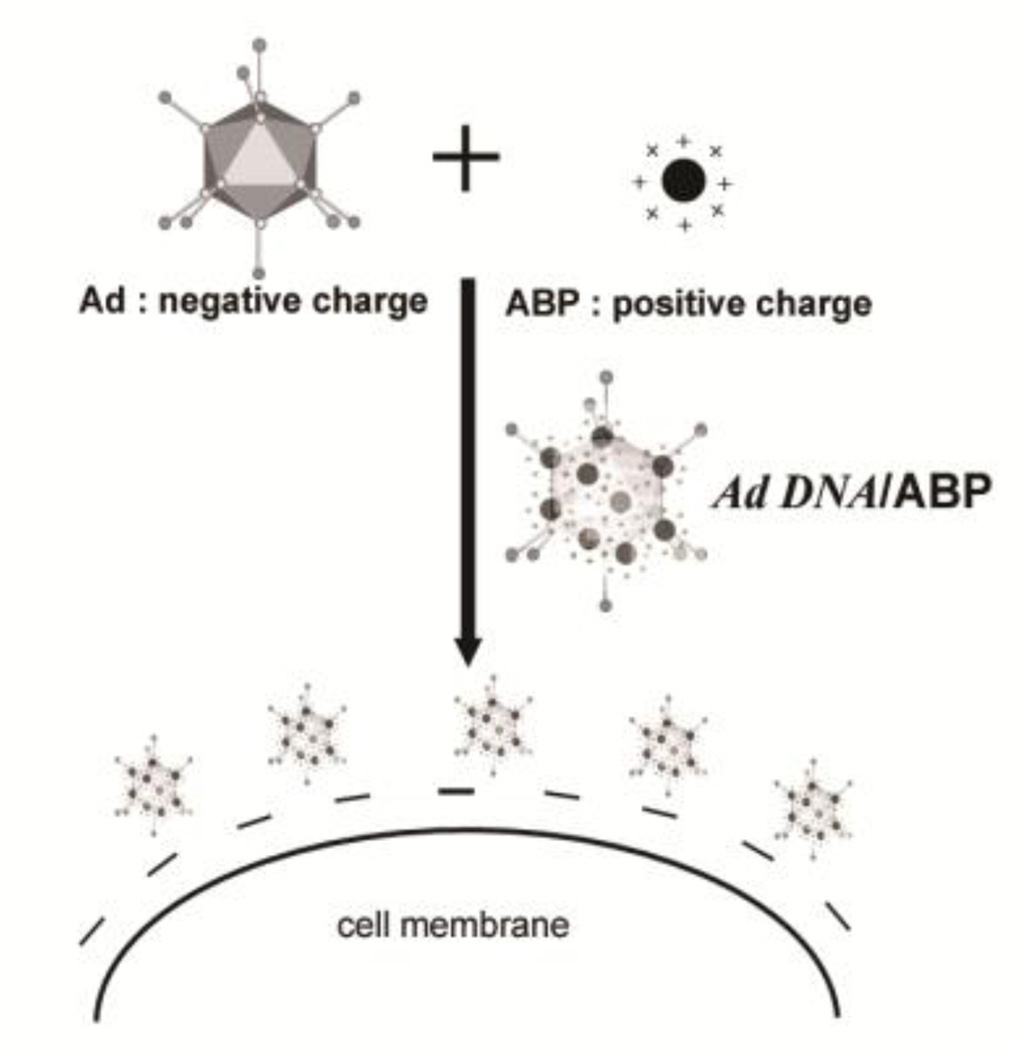
The hybrid adenovirus (Ad) complex coated with bioreducible ABP (Fig. 15) exhitibed enhanced transduction efficiency and transgene expression in both CAR-positive and CAR-negative cells, with minimal cytotoxicity and reduced innate immune response assessed by interleukin-6 cytokine release [106]. In contrast to naked Ad, this ABP-coated Ad complex was significantly stable in serum [106]. Both shielding of the immunogenic surface of the Ad and the reduced potential for attachment to serum proteins may result in extended circulation time and increased antitumor efficacy of the ABP-coated Ad complex.
Fig. 15.
Scheme of ABP-coated Ad complex and cell attachment via electrostatic interaction. Adapted from ref [106].
Another hybrid hepatoma-specific oncolytic Ad (YKL-1001) conjugated with an ABP polymer demonstrated increased hepatoma-specific cell killing when compared to naked YKL-1001 in vitro and in vivo [104]. Also, this ABP-conjugated YKL-1001 showed reduced immune response against Ad, increased blood circulation time, decreased accumulation in liver, strong anti-tumor effects, and minimal liver toxicity after systemic administration, suggesting a potential treatment of disseminated metastatic tumors [104].
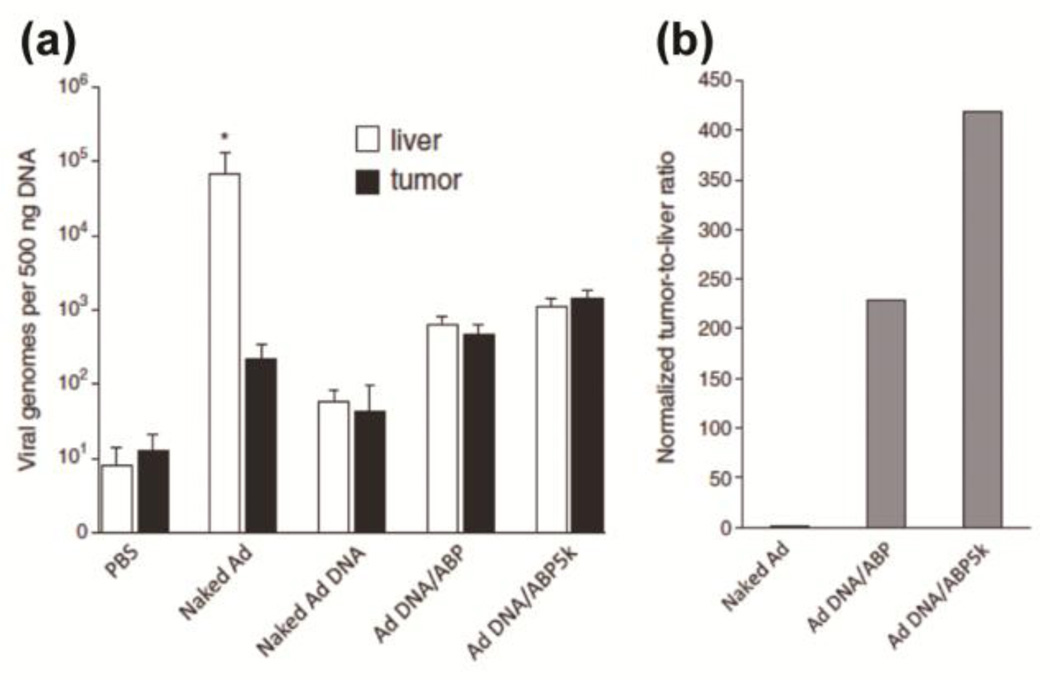
The initiation of Ad replication takes place at both ends of the inverted terminal repeat (ITR) sequence regions in the Ad genome, indicating that circular Ad plasmid DNA is unable to produce infectious Ad particles [107, 110]. Only the linearized oncolytic Ad pDNA polyplexes showed cancer-selective replication and progeny viral production, while sparing normal cells compared with circular oncolytic Ad pDNA polyplexes [107]. In contrast to the well-known accumulation of naked Ad in liver, the Ad genome content in the livers of the mice treated with Ad DNA/ABP or Ad DNA/ABP5K was significantly lower than that of mice treated with naked Ad, showing 99.1% and 98.4% reduced liver accumulation respectively [107]. In addition, the biodistribution of Ad DNA/ABP or Ad DNA/ABP5K within tumors showed a 2.1 and 6.6 fold increase compared with that of naked Ad (Fig. 16) [107]. Conclusively, the tumor-to-liver ratio of the Ad DNA delivered by two bioreducible polymers, ABP or PEG5K-conjugated ABP (ABP5K) was significantly elevated at 229 and 419 fold greater than that of naked Ad, respectively (Fig. 16) [107].
Fig. 16.
(a) Biodistribution of naked Ad, linearized Ad DNA, linearized Ad DNA polyplexed with ABP or ABP5K in tumor-bearing mice after systemic injection. Naked Ad (1×1010 VP), Ad DNA (10 µg), and each polyplex (10µg DNA/100µg polymer). * p < 0.05 vs Ad DNA/ABP or Ad DNA/ABP5K. (b) the tumor-to-liver ratios of viral genomes normalized with amount of injected naked Ad. Reprinted from ref [107] with permission from Elsevier.
The three amino-acid peptide, Arg-Gly-Asp (RGD) has been extensively used as a ligand for targeting the cell-surface integrin receptor αvβ3/αvβ5, which is an important biomarker overexpressed in sprouting tumor vessels and most tumor cells [111, 112]. A cancer cell-specific replicating oncolytic Ad (Ad-DB7-U6shIL8) expressing short hairpin RNA (shRNA) against interleukin-8 (IL-8) mRNA, a potent pro-angiogenic factor, was physically complexed with the poly(CBA-DAH)-PEG-RGD polymers [105]. The selective and dose-dependent cancer cell-killing effect of Ad/poly(CBA-DAH)-PEG500-RGD was followed by strong induction of apoptosis and suppression of IL-8 and VEGF expression [105].
ABP has a relatively low charge density and high steric hindrance caused by the long side chains, resulting in the weak interactions between ABP and Ad and yielding the large size of the Ad/ABP complex. Therefore, to deliver the therapeutic oncolytic Ad, we developed methoxy poly(ethylene glycol)-b-poly{N-[N-(2-aminoethyl)-2-aminoethyl]-l-glutamate (PNLG), a cationic and biodegradable polymer conjugated with polyethylene glycol (PEG) [108]. After systemic administration, tumor-to-liver ratio (therapeutic index) of oncolytic Ad coated with PNLG polymer was increased by 1229-fold compared to naked Ad [108]. Also, circumvention of host immune responses and liver evasion was successfully accomplished by masking the surface of Ad with the polymers [108].
3.3.2. DNA and small interfering RNA (siRNA)
About 27 clinical trials are currently underway evaluating the suitability of RNAi-therapy for human diseases (http://www.ClinicalTrials.gov) [113]. Most clinical trials involve direct local administration of siRNA to target sites such as the eye, the skin and the lung, thereby avoiding the complexity of systemic delivery. Therefore, to improve the reach of siRNA therapy, effective and safe systemic delivery approaches for siRNA need to be developed.
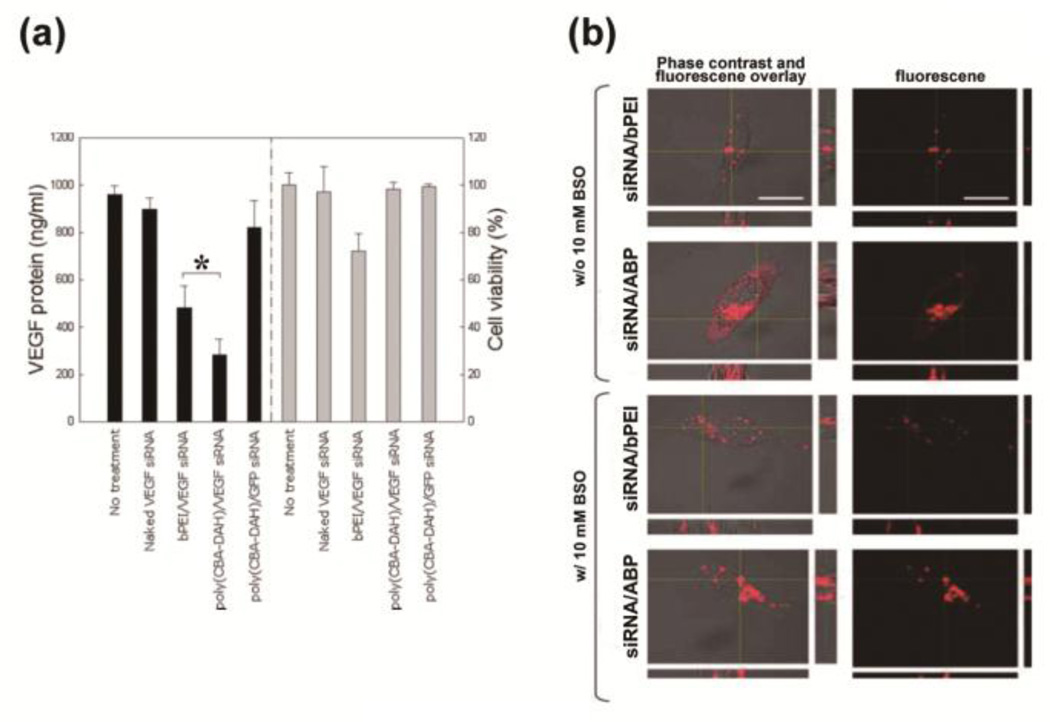
RNA interference (RNAi), which uses short 21–25 nucleotide RNAs (small-interfering RNAs, siRNAs) to recognize and degrade the target mRNA in a sequence-specific manner, has been considered as a potential therapeutic approach for the treatment of human genetic disorders caused by the over-expression of certain genes due to a strong gene silencing effect [114, 115]. Recently, different strategies of siRNA delivery by non-viral polymeric vehicles have been developed in vitro and in vivo [14]. The efficiency of RNAi mainly depends on the cytoplasmic localization of intact siRNAs, since RNAi only occurs in the cytoplasm where the target mRNA is translated [114, 116]. The VEGF siRNA/poly(CBA-DAH) polyplexes exhibited a relatively high efficiency of VEGF suppression (70.4% silencing) in a sequence specific manner without any cytotoxic effect detected, compared to VEGF siRNA/bPEI (49.7% silencing) (Fig. 17(a)) [114].
Fig. 17.
(a) VEGF gene silencing and cell toxicity of VEGF siRNA/poly(CBA-DAH) polyplexes in PC-3 cells. (b) Subcellular confocal images of cytoplasmic Cy3-modified siRNA release of siRNA/ABP polyplexes and siRNA/bPEI polyplexes with or without glutathione-depleting agent BSO. Scale bars = 10 µm. Reprinted with permission from ref [118], Copyright(2009), American Chemical Society.
Systemic administration of siRNA/ABP resulted in preferential and enhanced accumulation into tumor tissue [117]. Additionally, the combinatorial siRNA/ABP complexes targeting the three genes Bcl-2, VEGF, and Myc delivered by ABP exhibited a significant tumor regression of 35–50% by area and weight compared to untreated control mice [117]. Also, a two-time administration protocol yielded superior regression of advanced stage tumors, implying that the synergistic effects of three targets combined with a the delivery system using bioreducible ABP can enhance therapeutic efficacy at minimal dose [117]. These results may be explained by cytoplasmic degradation behavior that induces release of active siRNAs into cytoplasm and produces less toxic monomers. Also, the VEGF siRNA/ABP polyplexes showed greater inhibition of in vitro VEGF expression, due to enhanced intracellular delivery and effective localization to the cytoplasm of the VEGF siRNAs (Fig. 17) [118].
Perfluorocarbon-exposed-sonicated-dextrose-albumin microbubbles (MB) have been used for the last two decades as ultrasound contrast agents for diagnostic imaging purposes. MBs in combination with ultrasound (US) can be used to deliver DNA systemically while releasing DNA through focused US treatment (sonication) at specific target sites. However, DNA delivery with MB shows only moderate DNA uptake and expression due to degradation of the released DNA at the target site by extracellular nucleases and due to poor cellular uptake efficiency. Therefore, a combination of MBs with nonviral polycationic polymeric carriers can overcome the disadvantages of MBs used as a gene delivery system alone [119]. The siRNA-ABP-MB (SAM) complexes in combination with US showed significant VEGF knock down in A2780 human ovarian cancer cell line compared to naked siRNA when incubated for a short time after sonication treatment [119].
Suicide gene therapy is based on the introduction of a gene into tumor cells with pro-drug. In the herpes simplex virus, the thymidine kinase (HSV-TK) gene and ganciclovir (GCV) pro-drug system (TK/GCV), the TK gene is delivered and expressed in tumor cells, converting to the administered GCV pro-drug into its toxic phosphorylated form. The GCV inhibits DNA synthesis, resulting in the killing of dividing cancer cells and kills neighboring cancer cells via the bystander effect. The TK/GCV systems have been used in several clinical trials and promising results were achieved in various different types of cancers [120]. The suicide gene plasmid with hypoxia/hepatoma dual expression, delivered using bioreducible polymer, PAM-ABP was constructed for hepatocellular carcinoma therapy [121].
IV. Conclusions and Perspectives
In this review, a family of bioreducible poly(disulfide amine)s are reviewed as efficient gene vectors. The incorporation of multiple functional groups, such as amine groups and disulfide bonds, gives good DNA condendation ability, protonation ability, charge density, buffering capacity, and intracellular biodegradability. The enhanced gene transfection efficiency with minimal cytotoxicity establishes these bioreducible poly(disulfide amine)s family as a promising vector system for gene delivery. The works described in this review demonstrate current advances in the field of non-viral bioreducible polymers for therapeutic gene delivery to cardiovascular disease, anemia, diabetes, and cancer. The smart and advanced chemical modifications of bioreducible polymers will accelerate their progess toward human clinical application. Addition of targeting peptides will amplify their therapeutic efficacy while lowering their side effects toward non-target organs. PEGylation will be successful in prolonging their blood circulation time and extending therapeutic duration. Antibodies, folate and other disease-specific molecules are currently being developed, and will demonstrate tailored and optimal therapeutic effects. Stimuli sensitive systems, such as light, temperature, redox sensitive molecules will provide adjustable systems that can be turned on and off. Diverse modifications based on the physiology of the human body will make possible human clinical applications of the biodegradable polymers in the near future. Above all, ahead of the human clinical application, the thorough and systematic evaluation of biocompatibility will be important and necessary. Momentous and advanced progress in bioreducible polymer therapeutics will continue to make important contributions in the clinical applications of gene therapy and drug delivery, providing novel treatments for life-threatening and debilitating diseases.
Acknowledgments
These projects were supported by NIH grants HL065477, CA107070, DK07703, and DK085075.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isner JM. Myocardial gene therapy. Nature. 2002;415:234–239. doi: 10.1038/415234a. [DOI] [PubMed] [Google Scholar]
- 2.Nabel GJ. Genetic, cellular and immune approaches to disease therapy: past and future. Nat Med. 2004;10:135–141. doi: 10.1038/nm990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SW. Polymeric gene delivery for diabetic treatment. Diabetes Metab J. 2011;35:317–326. doi: 10.4093/dmj.2011.35.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 5.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 6.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman AS. The origins and evolution of "controlled" drug delivery systems. J Control Release. 2008:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Yla-Herttuala S, Martin JF. Cardiovascular gene therapy. Lancet. 2000;355:213–222. doi: 10.1016/S0140-6736(99)04180-X. [DOI] [PubMed] [Google Scholar]
- 9.Ou M, Xu R, Kim SH, Bull DA, Kim SW. A family of bioreducible poly(disulfide amine)s for gene delivery. Biomaterials. 2009;30:5804–5814. doi: 10.1016/j.biomaterials.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam AP, Dean DA. Progress and prospects: nuclear import of nonviral vectors. Gene Ther. 2010;17:439–447. doi: 10.1038/gt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Kim TI, Kim SW. Bioreducible polymers for gene delivery. React Funct Polym. 2011;71:344–349. doi: 10.1016/j.reactfunctpolym.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable block copolymers as injectable drugdelivery systems. Nature. 1997;388:860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 14.Kim WJ, Kim SW. Efficient siRNA delivery with non-viral polymeric vehicles. Pharm Res. 2009;26:657–666. doi: 10.1007/s11095-008-9774-1. [DOI] [PubMed] [Google Scholar]
- 15.Wagner E. Strategies to improve DNA polyplexes for in vivo gene transfer: will "artificial viruses" be the answer? Pharm Res. 2004;21:8–14. doi: 10.1023/b:pham.0000012146.04068.56. [DOI] [PubMed] [Google Scholar]
- 16.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 17.Yockman JW, Kastenmeier A, Erickson HM, Brumbach JG, Whitten MG, Albanil A, Li DY, Kim SW, Bull DA. Novel polymer carriers and gene constructs for treatment of myocardial ischemia and infarction. J Control Release. 2008;132:260–266. doi: 10.1016/j.jconrel.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 19.Yockman JW, Kim SW, Bull DA. Women and heart disease--physiologic regulation of gene delivery and expression: bioreducible polymers and ischemia-inducible gene therapies for the treatment of ischemic heart disease. Adv Drug Deliv Rev. 2009;61:863–870. doi: 10.1016/j.addr.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto S, Christie RJ, Nishiyama N, Miyata K, Ishii A, Oba M, Koyama H, Yamasaki Y, Kataoka K. Environment-responsive block copolymer micelles with a disulfide cross-linked core for enhanced siRNA delivery. Biomacromolecules. 2009;10:119–127. doi: 10.1021/bm800985e. [DOI] [PubMed] [Google Scholar]
- 21.Oupicky D, Parker AL, Seymour LW. Laterally stabilized complexes of DNA with linear reducible polycations: strategy for triggered intracellular activation of DNA delivery vectors. J Am Chem Soc. 2002;124:8–9. doi: 10.1021/ja016440n. [DOI] [PubMed] [Google Scholar]
- 22.Brumbach JH, Lin C, Yockman J, Kim WJ, Blevins KS, Engbersen JF, Feijen J, Kim SW. Mixtures of poly(triethylenetetramine/cystamine bisacrylamide) and poly(triethylenetetramine/cystamine bisacrylamide)-g-poly(ethylene glycol) for improved gene delivery. Bioconjug Chem. 2010;21:1753–1761. doi: 10.1021/bc900522x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong Z, Lin C, Engbersen JF, Feijen J. Reducible poly(amido ethylenimine)s designed for triggered intracellular gene delivery. Bioconjug Chem. 2006;17:1233–1240. doi: 10.1021/bc0602026. [DOI] [PubMed] [Google Scholar]
- 24.Suh W, Han SO, Yu L, Kim SW. An angiogenic, endothelial-cell-targeted polymeric gene carrier. Mol Ther. 2002;6:664–672. [PubMed] [Google Scholar]
- 25.Brumbach JH, Lee YW, Kim SW, Yockman JW. Functional properties and biodistribution of poly(triethylenetetramine/cystamine bisacrylamide) and poly(triethylenetetramine/cystamine bisacrylamide)- poly(ethylene glycol) mixtures formed with nucleic acid. J Control Release. 2012;159:111–119. doi: 10.1016/j.jconrel.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn CH, Chae SY, Bae YH, Kim SW. Biodegradable poly(ethylenimine) for plasmid DNA delivery. J Control Release. 2002;80:273–282. doi: 10.1016/s0168-3659(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 27.Kim YH, Park JH, Lee M, Park TG, Kim SW. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release. 2005;103:209–219. doi: 10.1016/j.jconrel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Ou M, Wang XL, Xu R, Chang CW, Bull DA, Kim SW. Novel biodegradable poly(disulfide amine)s for gene delivery with high efficiency and low cytotoxicity. Bioconjug Chem. 2008;19:626–633. doi: 10.1021/bc700397x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim TI, Rothmund T, Kissel T, Kim SW. Bioreducible polymers with cell penetrating and endosome buffering functionality for gene delivery systems. J Control Release. 2011;152:110–119. doi: 10.1016/j.jconrel.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wender PA, Galliher WC, Goun EA, Jones LR, Pillow TH. The design of guanidinium-rich transporters and their internalization mechanisms. Adv Drug Deliv Rev. 2008;60:452–472. doi: 10.1016/j.addr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Futaki S. Membrane-permeable arginine-rich peptides and the translocation mechanisms. Adv Drug Deliv Rev. 2005;57:547–558. doi: 10.1016/j.addr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev. 2005;57:529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Nakase I, Takeuchi T, Tanaka G, Futaki S. Methodological and cellular aspects that govern the internalization mechanisms of arginine-rich cell-penetrating peptides. Adv Drug Deliv Rev. 2008;60:598–607. doi: 10.1016/j.addr.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Kim TI, Ou M, Lee M, Kim SW. Arginine-grafted bioreducible poly(disulfide amine) for gene delivery systems. Biomaterials. 2009;30:658–664. doi: 10.1016/j.biomaterials.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis. 2006;47:S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Nam HY, Lee Y, Lee M, Shin SK, Kim TI, Kim SW, Bull DA. Erythropoietin gene delivery using an arginine-grafted bioreducible polymer system. J Control Release. 2012;157:437–444. doi: 10.1016/j.jconrel.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y, Nam HY, Kim J, Lee M, Yockman JW, Shin SK, Kim SW. Human erythropoietin gene delivery using an arginine-grafted bioreducible polymer system. Mol Ther. 2012;20:1360–1366. doi: 10.1038/mt.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim TI, Lee M, Kim SW. A guanidinylated bioreducible polymer with high nuclear localization ability for gene delivery systems. Biomaterials. 2010;31:1798–1804. doi: 10.1016/j.biomaterials.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nam HY, Nam K, Lee M, Kim SW, Bull DA. Dendrimer type bio-reducible polymer for efficient gene delivery. J Control Release. 2012;160:592–600. doi: 10.1016/j.jconrel.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Takae S, Miyata K, Oba M, Ishii T, Nishiyama N, Itaka K, Yamasaki Y, Koyama H, Kataoka K. PEG-detachable polyplex micelles based on disulfide-linked block catiomers as bioresponsive nonviral gene vectors. J Am Chem Soc. 2008;130:6001–6009. doi: 10.1021/ja800336v. [DOI] [PubMed] [Google Scholar]
- 41.Miyata K, Kakizawa Y, Nishiyama N, Harada A, Yamasaki Y, Koyama H, Kataoka K. Block catiomer polyplexes with regulated densities of charge and disulfide cross-linking directed to enhance gene expression. J Am Chem Soc. 2004;126:2355–2361. doi: 10.1021/ja0379666. [DOI] [PubMed] [Google Scholar]
- 42.Miyata K, Kakizawa Y, Nishiyama N, Yamasaki Y, Watanabe T, Kohara M, Kataoka K. Freeze-dried formulations for in vivo gene delivery of PEGylated polyplex micelles with disulfide crosslinked cores to the liver. J Control Release. 2005;109:15–23. doi: 10.1016/j.jconrel.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 43.Namgung R, Brumbach JH, Jeong JH, Yockman JW, Kim SW, Lin C, Zhong Z, Feijen J, Engbersen JF, Kim WJ. Dual bio-responsive gene delivery via reducible poly(amido amine) and survivin-inducible plasmid DNA. Biotechnol Lett. 2010;32:755–764. doi: 10.1007/s10529-010-0219-7. [DOI] [PubMed] [Google Scholar]
- 44.Chang CW, Choi D, Kim WJ, Yockman JW, Christensen LV, Kim YH, Kim SW. Non-ionic amphiphilic biodegradable PEG-PLGA-PEG copolymer enhances gene delivery efficiency in rat skeletal muscle. J Control Release. 2007;118:245–253. doi: 10.1016/j.jconrel.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 45.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rissanen TT, Yla-Herttuala S. Current status of cardiovascular gene therapy. Mol Ther. 2007;15:1233–1247. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- 47.Ruponen M, Ronkko S, Honkakoski P, Pelkonen J, Tammi M, Urtti A. Extracellular glycosaminoglycans modify cellular trafficking of lipoplexes and polyplexes. J Biol Chem. 2001;276:33875–33880. doi: 10.1074/jbc.M011553200. [DOI] [PubMed] [Google Scholar]
- 48.Christensen LV, Chang CW, Yockman JW, Conners R, Jackson H, Zhong Z, Feijen J, Bull DA, Kim SW. Reducible poly(amido ethylenediamine) for hypoxia-inducible VEGF delivery. J Control Release. 2007;118:254–261. doi: 10.1016/j.jconrel.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finkenzeller G, Marme D, Weich HA, Hug H. Platelet-derived growth factor-induced transcription of the vascular endothelial growth factor gene is mediated by protein kinase C. Cancer Res. 1992;52:4821–4823. [PubMed] [Google Scholar]
- 50.Wu G, Mannam AP, Wu J, Kirbis S, Shie JL, Chen C, Laham RJ, Sellke FW, Li J. Hypoxia induces myocyte-dependent COX-2 regulation in endothelial cells: role of VEGF. Am J Physiol Heart Circ Physiol. 2003;285:H2420–H2429. doi: 10.1152/ajpheart.00187.2003. [DOI] [PubMed] [Google Scholar]
- 51.Lee Y, McGinn AN, Olsen CD, Nam K, Lee M, Shin SK, Kim SW. Human erythropoietin gene delivery for cardiac remodeling of myocardial infarction in rats. J Control Release. 2013;171:24–32. doi: 10.1016/j.jconrel.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwarz ER, Speakman MT, Patterson M, Hale SS, Isner JM, Kedes LH, Kloner RA. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat--angiogenesis and angioma formation. J Am Coll Cardiol. 2000;35:1323–1330. doi: 10.1016/s0735-1097(00)00522-2. [DOI] [PubMed] [Google Scholar]
- 53.Won YW, McGinn AN, Lee M, Nam K, Bull DA, Kim SW. Post-translational regulation of a hypoxia-responsive VEGF plasmid for the treatment of myocardial ischemia. Biomaterials. 2013;34:6229–6238. doi: 10.1016/j.biomaterials.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ou M, Kim TI, Yockman JW, Borden BA, Bull DA, Kim SW. Polymer transfected primary myoblasts mediated efficient gene expression and angiogenic proliferation. J Control Release. 2010;142:61–69. doi: 10.1016/j.jconrel.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borden BA, Yockman J, Kim SW. Thermoresponsive hydrogel as a delivery scaffold for transfected rat mesenchymal stem cells. Mol Pharm. 2010;7:963–968. doi: 10.1021/mp100094k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGinn AN, Nam HY, Ou M, Hu N, Straub CM, Yockman JW, Bull DA, Kim SW. Bioreducible polymer-transfected skeletal myoblasts for VEGF delivery to acutely ischemic myocardium. Biomaterials. 2011;32:942–949. doi: 10.1016/j.biomaterials.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vinge LE, Raake PW, Koch WJ. Gene therapy in heart failure. Circ Res. 2008;102:1458–1470. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Won YW, Lee M, Kim HA, Bull DA, Kim SW. Hypoxia-inducible plasmid expressing both miSHP-1 and HO-1 for the treatment of ischemic disease. J Control Release. 2013;165:22–28. doi: 10.1016/j.jconrel.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Won YW, Lee M, Kim HA, Nam K, Bull DA, Kim SW. Synergistically combined gene delivery for enhanced VEGF secretion and antiapoptosis. Mol Pharm. 2013;10:3676–3683. doi: 10.1021/mp400178m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SH, Jeong JH, Ou M, Yockman JW, Kim SW, Bull DA. Cardiomyocyte-targeted siRNA delivery by prostaglandin E(2)-Fas siRNA polyplexes formulated with reducible poly(amido amine) for preventing cardiomyocyte apoptosis. Biomaterials. 2008;29:4439–4446. doi: 10.1016/j.biomaterials.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nam HY, Kim J, Kim S, Yockman JW, Kim SW, Bull DA. Cell penetrating peptide conjugated bioreducible polymer for siRNA delivery. Biomaterials. 2011;32:5213–5222. doi: 10.1016/j.biomaterials.2011.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nam HY, McGinn A, Kim PH, Kim SW, Bull DA. Primary cardiomyocyte-targeted bioreducible polymer for efficient gene delivery to the myocardium. Biomaterials. 2010;31:8081–8087. doi: 10.1016/j.biomaterials.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Won Y-W, McGinn AN, Lee M, Bull DA, Kim SW. Targeted Gene Delivery to Ischemic Myocardium by Homing Peptide-Guided Polymeric Carrier. Mol Pharm. 2012 doi: 10.1021/mp300500y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergstrom S, Carlson LA, Weeks JR. The prostaglandins: a family of biologically active lipids. Pharmacol Rev. 1968;20:1–48. [PubMed] [Google Scholar]
- 65.Xiao CY, Yuhki K, Hara A, Fujino T, Kuriyama S, Yamada T, Takayama K, Takahata O, Karibe H, Taniguchi T, Narumiya S, Ushikubi F. Prostaglandin E2 protects the heart from ischemiareperfusion injury via its receptor subtype EP4. Circulation. 2004;109:2462–2468. doi: 10.1161/01.CIR.0000128046.54681.97. [DOI] [PubMed] [Google Scholar]
- 66.McGuire MJ, Samli KN, Johnston SA, Brown KC. In vitro selection of a peptide with high selectivity for cardiomyocytes in vivo. J Mol Biol. 2004;342:171–182. doi: 10.1016/j.jmb.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 67.Barry MA, Dower WJ, Johnston SA. Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med. 1996;2:299–305. doi: 10.1038/nm0396-299. [DOI] [PubMed] [Google Scholar]
- 68.Yue TL, Ma XL, Wang X, Romanic AM, Liu GL, Louden C, Gu JL, Kumar S, Poste G, Ruffolo RR, Jr, Feuerstein GZ. Possible involvement of stress-activated protein kinase signaling pathway and Fas receptor expression in prevention of ischemia/reperfusion-induced cardiomyocyte apoptosis by carvedilol. Circ Res. 1998;82:166–174. doi: 10.1161/01.res.82.2.166. [DOI] [PubMed] [Google Scholar]
- 69.Nam HY, Kim J, Kim SW, Bull DA. Cell targeting peptide conjugation to siRNA polyplexes for effective gene silencing in cardiomyocytes. Mol Pharm. 2012;9:1302–1309. doi: 10.1021/mp200589z. [DOI] [PubMed] [Google Scholar]
- 70.Sugano M, Tsuchida K, Hata T, Makino N. RNA interference targeting SHP-1 attenuates myocardial infarction in rats. FASEB J. 2005;19:2054–2056. doi: 10.1096/fj.05-4020fje. [DOI] [PubMed] [Google Scholar]
- 71.Chaanine AH, Hajjar RJ. AKT signalling in the failing heart. Eur J Heart Fail. 2011;13:825–829. doi: 10.1093/eurjhf/hfr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koh JJ, Ko KS, Lee M, Han S, Park JS, Kim SW. Degradable polymeric carrier for the delivery of IL-10 plasmid DNA to prevent autoimmune insulitis of NOD mice. Gene Ther. 2000;7:2099–2104. doi: 10.1038/sj.gt.3301334. [DOI] [PubMed] [Google Scholar]
- 73.Ko KS, Lee M, Koh JJ, Kim SW. Combined administration of plasmids encoding IL-4 and IL-10 prevents the development of autoimmune diabetes in nonobese diabetic mice. Mol Ther. 2001;4:313–316. doi: 10.1006/mthe.2001.0459. [DOI] [PubMed] [Google Scholar]
- 74.Lee M, Han S, Ko KS, Kim SW. Cell type specific and glucose responsive expression of interleukin-4 by using insulin promoter and water soluble lipopolymer. J Control Release. 2001;75:421–429. doi: 10.1016/s0168-3659(01)00416-3. [DOI] [PubMed] [Google Scholar]
- 75.Lee M, Han SO, Ko KS, Koh JJ, Park JS, Yoon JW, Kim SW. Repression of GAD autoantigen expression in pancreas beta-Cells by delivery of antisense plasmid/PEG-g-PLL complex. Mol Ther. 2001;4:339–346. doi: 10.1006/mthe.2001.0458. [DOI] [PubMed] [Google Scholar]
- 76.Maasho K, Opoku-Anane J, Marusina AI, Coligan JE, Borrego F. NKG2D is a costimulatory receptor for human naive CD8+ T cells. J Immunol. 2005;174:4480–4484. doi: 10.4049/jimmunol.174.8.4480. [DOI] [PubMed] [Google Scholar]
- 77.Cerwenka A, Lanier LL. Ligands for natural killer cell receptors: redundancy or specificity. Immunol Rev. 2001;181:158–169. doi: 10.1034/j.1600-065x.2001.1810113.x. [DOI] [PubMed] [Google Scholar]
- 78.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 79.Yao VJ, Ozawa MG, Trepel M, Arap W, McDonald DM, Pasqualini R. Targeting pancreatic islets with phage display assisted by laser pressure catapult microdissection. Am J Pathol. 2005;166:625–636. doi: 10.1016/S0002-9440(10)62283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joo WS, Jeong JH, Nam K, Blevins KS, Salama ME, Kim SW. Polymeric delivery of therapeutic RAE-1 plasmid to the pancreatic islets for the prevention of type 1 diabetes. J Control Release. 2012;162:606–611. doi: 10.1016/j.jconrel.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blevins KS, Jeong JH, Ou M, Brumbach JH, Kim SW. EphA2 targeting peptide tethered bioreducible poly(cystamine bisacrylamide-diamino hexane) for the delivery of therapeutic pCMV-RAE-1gamma to pancreatic islets. J Control Release. 2012;158:115–122. doi: 10.1016/j.jconrel.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jean LB, Still EU. STUDIES ON THE PHYSIOLOGY OF SECRETIN: III. Further Studies on the Effects of Secretin on the Blood. Sugar Am J Physiol Heart Circ Physiol. 1930;91:649–653. [Google Scholar]
- 83.Kim PH, Lee M, Kim SW. Delivery of two-step transcription amplification exendin-4 plasmid system with arginine-grafted bioreducible polymer in type 2 diabetes animal model. J Control Release. 2012;162:9–18. doi: 10.1016/j.jconrel.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim PH, Kim SW. Polymer-based delivery of glucagon-like Peptide-1 for the treatment of diabetes. ISRN Endocrinol. 2012 doi: 10.5402/2012/340632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim TI, Lee M, Kim SW. Efficient GLP-1 gene delivery using two-step transcription amplification plasmid system with a secretion signal peptide and arginine-grafted bioreducible polymer. J Control Release. 2012;157:243–248. doi: 10.1016/j.jconrel.2011.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim SW. Triple Combination Therapy Using Metformin, Thiazolidinedione, and a GLP-1 Analog or DPP-IV Inhibitor in Patients with Type 2 Diabetes Mellitus. Korean Diabetes J. 2010;34:331–337. doi: 10.4093/kdj.2010.34.6.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee M, Oh S, Ahn CH, Kim SW, Rhee BD, Ko KS. An efficient GLP-1 expression system using two-step transcription amplification. J Control Release. 2006;115:316–321. doi: 10.1016/j.jconrel.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 88.Oh S, Lee M, Ko KS, Choi S, Kim SW. GLP-1 gene delivery for the treatment of type 2 diabetes. Mol Ther. 2003;7:478–483. doi: 10.1016/s1525-0016(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 89.MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51(Suppl 3):S434–S442. doi: 10.2337/diabetes.51.2007.s434. [DOI] [PubMed] [Google Scholar]
- 90.Deacon CF. Therapeutic strategies based on glucagon-like peptide 1. Diabetes. 2004;53:2181–2189. doi: 10.2337/diabetes.53.9.2181. [DOI] [PubMed] [Google Scholar]
- 91.Lee Y, Kwon MK, Kang ES, Park YM, Choi SH, Ahn CW, Kim KS, Park CW, Cha BS, Kim SW, Sung JK, Lee EJ, Lee HC. Adenoviral vector-mediated glucagon-like peptide 1 gene therapy improves glucose homeostasis in Zucker diabetic fatty rats. J Gene Med. 2008;10:260–268. doi: 10.1002/jgm.1153. [DOI] [PubMed] [Google Scholar]
- 92.Choi S, Oh S, Lee M, Kim SW. Glucagon-like peptide-1 plasmid construction and delivery for the treatment of type 2 diabetes. Mol Ther. 2005;12:885–891. doi: 10.1016/j.ymthe.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 93.Ranganath LR. Incretins: pathophysiological and therapeutic implications of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1. J Clin Pathol. 2008;61:401–409. doi: 10.1136/jcp.2006.043232. [DOI] [PubMed] [Google Scholar]
- 94.Mikhail N. Incretin mimetics and dipeptidyl peptidase 4 inhibitors in clinical trials for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2008;17:845–853. doi: 10.1517/13543784.17.6.845. [DOI] [PubMed] [Google Scholar]
- 95.Lasic DD. Mixed micelles in drug delivery. Nature. 1992;355:279–280. doi: 10.1038/355279a0. [DOI] [PubMed] [Google Scholar]
- 96.Nam K, Nam HY, Kim PH, Kim SW. Paclitaxel-conjugated PEG and arginine-grafted bioreducible poly (disulfide amine) micelles for co-delivery of drug and gene. Biomaterials. 2012;33:8122–8130. doi: 10.1016/j.biomaterials.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953;84:570–573. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- 98.Huebner RJ, Rowe WP, Schatten WE, Smith RR, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9:1211–1218. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 99.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 100.Kim J, Kim PH, Kim SW, Yun CO. Enhancing the therapeutic efficacy of adenovirus in combination with biomaterials. Biomaterials. 2012;33:1838–1850. doi: 10.1016/j.biomaterials.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 102.Ferber D. Gene therapy. Safer and virus-free? Science. 2001;294:1638–1642. doi: 10.1126/science.294.5547.1638. [DOI] [PubMed] [Google Scholar]
- 103.Choi JW, Lee JS, Kim SW, Yun CO. Evolution of oncolytic adenovirus for cancer treatment. Adv Drug Deliv Rev. 2012;64:720–729. doi: 10.1016/j.addr.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 104.Kim PH, Kim J, Kim TI, Nam HY, Yockman JW, Kim M, Kim SW, Yun CO. Bioreducible polymer-conjugated oncolytic adenovirus for hepatoma-specific therapy via systemic administration. Biomaterials. 2011;32:9328–9342. doi: 10.1016/j.biomaterials.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 105.Kim J, Nam HY, Kim TI, Kim PH, Ryu J, Yun CO, Kim SW. Active targeting of RGD-conjugated bioreducible polymer for delivery of oncolytic adenovirus expressing shRNA against IL-8 mRNA. Biomaterials. 2011;32:5158–5166. doi: 10.1016/j.biomaterials.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim PH, Kim TI, Yockman JW, Kim SW, Yun CO. The effect of surface modification of adenovirus with an arginine-grafted bioreducible polymer on transduction efficiency and immunogenicity in cancer gene therapy. Biomaterials. 2010;31:1865–1874. doi: 10.1016/j.biomaterials.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 107.Kim J, Kim PH, Nam HY, Lee JS, Yun CO, Kim SW. Linearized oncolytic adenoviral plasmid DNA delivered by bioreducible polymers. J Control Release. 2012;158:451–460. doi: 10.1016/j.jconrel.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim J, Li Y, Kim SW, Lee DS, Yun CO. Therapeutic efficacy of a systemically delivered oncolytic adenovirus - biodegradable polymer complex. Biomaterials. 2013;34:4622–4631. doi: 10.1016/j.biomaterials.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 109.Kasala D, Choi JW, Kim SW, Yun CO. Utilizing adenovirus vectors for gene delivery in cancer. Expert Opin Drug Deliv. 2014 doi: 10.1517/17425247.2014.874414. [DOI] [PubMed] [Google Scholar]
- 110.Berkner KL, Sharp PA. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983;11:6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mok H, Park TG. Functional polymers for targeted delivery of nucleic acid drugs. Macromol Biosci. 2009;9:731–743. doi: 10.1002/mabi.200900044. [DOI] [PubMed] [Google Scholar]
- 112.Kim J, Kim SW, Kim WJ. PEI-g-PEG-RGD/small interference RNA polyplex-mediated silencing of vascular endothelial growth factor receptor and its potential as an anti-angiogenic tumor therapeutic strategy. Oligonucleotides. 2011;21:101–107. doi: 10.1089/oli.2011.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen SH, Zhaori G. Potential clinical applications of siRNA technique: benefits and limitations. Eur J Clin Invest. 2011;41:221–232. doi: 10.1111/j.1365-2362.2010.02400.x. [DOI] [PubMed] [Google Scholar]
- 114.Kim SH, Ou M, Bull DA, Kim SW. Reductive degradation behavior of bioreducible poly(disulfide amine) for enhancing SiRNA efficiency. Macromol Biosci. 2010;10:898–905. doi: 10.1002/mabi.200900482. [DOI] [PubMed] [Google Scholar]
- 115.Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov. 2004;3:318–329. doi: 10.1038/nrd1345. [DOI] [PubMed] [Google Scholar]
- 116.Lavigne C, Thierry AR. Specific subcellular localization of siRNAs delivered by lipoplex in MCF-7 breast cancer cells. Biochimie. 2007;89:1245–1251. doi: 10.1016/j.biochi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 117.Beloor J, Choi CS, Nam HY, Park M, Kim SH, Jackson A, Lee KY, Kim SW, Kumar P, Lee SK. Arginine-engrafted biodegradable polymer for the systemic delivery of therapeutic siRNA. Biomaterials. 2012;33:1640–1650. doi: 10.1016/j.biomaterials.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 118.Kim SH, Jeong JH, Kim TI, Kim SW, Bull DA. VEGF siRNA delivery system using arginine-grafted bioreducible poly(disulfide amine) Mol Pharm. 2009;6:718–726. doi: 10.1021/mp800161e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Florinas S, Nam HY, Kim SW. Enhanced siRNA delivery using a combination of an arginine-grafted bioreducible polymer, ultrasound, and microbubbles in cancer cells. Mol Pharm. 2013;10:2021–2030. doi: 10.1021/mp400048p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sangro B, Mazzolini G, Ruiz M, Ruiz J, Quiroga J, Herrero I, Qian C, Benito A, Larrache J, Olague C, Boan J, Penuelas I, Sadaba B, Prieto J. A phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther. 2010;17:837–843. doi: 10.1038/cgt.2010.40. [DOI] [PubMed] [Google Scholar]
- 121.Kim HA, Nam K, Lee M, Kim SW. Hypoxia/hepatoma dual specific suicide gene expression plasmid delivery using bio-reducible polymer for hepatocellular carcinoma therapy. J Control Release. 2013;171:1–10. doi: 10.1016/j.jconrel.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]