Abstract
Drug delivery systems are widely researched and developed to improve the delivery of pharmaceutical compounds and molecules. The last few decades have seen a marked growth of the field fueled by increased number of researchers, research funding, venture capital and the number of start-ups. Collectively, the growth has led to novel systems that make use of micro/nano-particles, transdermal patches, inhalers, drug reservoir implants and antibody-drug conjugates. While the increased research activity is clearly an indication of proliferation of the field, clinical and commercial translation of early-stage research ideas is critically important for future growth and interest in the field. Here, we will highlight some of the examples of novel drug delivery systems that have undergone such translation. Specifically, we will discuss the developments, advantages, limitations and lessons learned from: (i) microparticle-based depot formulations, (ii) nanoparticle-based cancer drugs, (iii) transdermal systems, (iv) oral drug delivery systems, (v) pulmonary drug delivery, (vi) implants and (vii) antibody-drug conjugates. These systems have impacted treatment of many prevalent diseases including diabetes, cancer and cardiovascular diseases, among others. At the same time, these systems are integral and enabling components of products that collectively generate annual revenues exceeding US $100 billion. These examples provide strong evidence of the clinical and commercial impact of drug delivery systems.
Keywords: drug delivery, historical, perspective, pharmaceutics, clinical translation, case study, lab to clinic
Introduction
Drug delivery systems (DDS) improve the administration and efficacy of pharmaceutical compounds including antibodies, peptides, vaccines, drugs and enzymes, among others. Oral pills and injections represent the most common mode of administering drugs today. A majority of small molecule drugs are delivered by pills. Tens of billions of pills are annually consumed worldwide for aspirin alone. Injections remain the primary mode of administering proteins and peptides. More than 10 billion injections are performed each year worldwide [1]. Oral pills offer convenience of pre-determined and measured doses, portability, defined dosing times and the overall non-invasive nature of administration. However, they are also limited by the inability to deliver larger therapeutic molecules such as proteins [2]. Injections, on the other hand, are able to deliver macromolecules, but are limited by their invasive nature and inappropriate use [1]. Collectively, simple pills and injections are unable to meet many advanced therapeutic needs including targeting, broad applicability to macromolecules and on-demand activation. While not all pharmaceutical molecules require these abilities, many do. These limitations have given rise to substantial research focused on the development of novel DDS.
Research in drug delivery has focused not only on improving oral and injectable systems, but also on opening additional routes of administration including pulmonary [3], transdermal [4], ocular [5] and nasal routes [6]. Each route has its own advantages and limitations (Table 1). Many novel DDS that make use of these routes are beginning to enter clinical trials and some have already reached the market. To accomplish successful clinical translation, DDS must, at minimum, be safe, perform their therapeutic function, offer convenient administration and have ease of manufacturing. This review highlights some of the successful technologies that have made this transition (Figure 1). Seven categories of DDS including microsphere-depots, tumor-targeting nanoparticles, transdermal patches, advanced oral pills, inhalers, implants and antibody-drug conjugates are highlighted. A search on clinicaltrials.gov for: (i) ‘Depot’, (ii) ‘Transdermal’, (iii) ‘Inhaler’, (iv) ‘Subcutaneous implant’ and ‘Intravitreal implant’ and ‘Birth control implant’, (v) ‘Nanoparticle and cancer’, (vi) ‘Antibody drug conjugates’ and (vii) ‘OROS’® confirms high activity of clinical trials based on these categories of DDS (Figure 2). We discuss their historical perspective, advantages/limitations in the clinic, the inspiration that they provide for follow-up technologies, and current clinical status of new(er) products in the field. This review is not intended to provide a comprehensive list of clinical and commercial status of all DDS given the high volume of activity in the field. Instead, the article discusses select examples and analyzes their features that led to their success.
Table 1. Routes of administration.
Advantages, disadvantages, potential targets and examples of the most commonly used routes of administration for drug delivery. The number of top 100 commercial drugs and their route of administration was determined by counting the best selling drugs in 2013 as determined by Drugs.com [155].
| Route of Administration | Advantages | Disadvantages | Targets | Examples | Number of Top 100 Commerical Drugs |
|---|---|---|---|---|---|
| Injections: IV, IM and SQ |
|
|
|
|
42 |
| Oral |
|
Poor bioavailability
|
|
|
54 |
| Inhalation |
|
|
|
|
7 |
| Transdermal |
|
|
|
|
4 |
Figure 1.
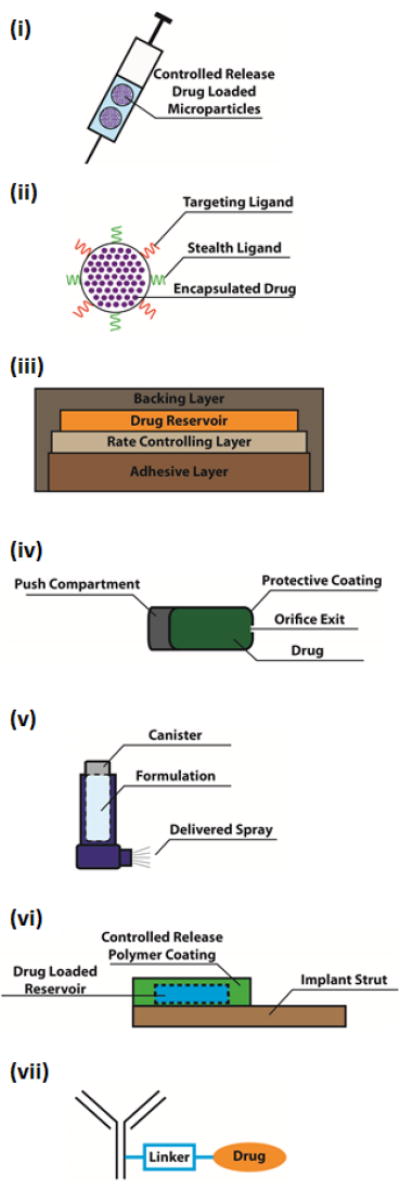
Schematics and brief descriptions of the 7 highlighted DDS: (i) microparticle-based depot formulations, (ii) nanoparticle-based cancer drugs, (iii) transdermal systems (patches highlighted here), (iv) oral drug delivery systems (OROS® highlighted here), (v) pulmonary drug delivery systems (inhalers highlighted here), (vi) implants and (vii) antibody-drug conjugates.
Microparticle-based depot systems comprise formulations of drugs, peptides, or proteins encapsulated in biodegradable polymeric particles. These systems allow for the sustained and controlled release of therapeutics over a long period of time, allowing for a reduced number of treatments.
Nanoparticles are drug carriers that are capable of encapsulating and protecting drugs from rapid degradation in vivo, improving both targeting and circulation profiles via surface modification with application-specific ligands, and controlling the rate of drug release from the particle.
Transdermal patches contain a backing layer that prevents drug leakage, a reservoir to store the drug, a rate controlling layer that controls drug release and an adhesive layer that attaches to the skin. Transdermal patches allow for a painless, patient-compliant interface to facilitate systemic administration of drugs.
OROS® technology is an osmotically driven system that controls the rate of drug release via the design of the osmotic pump and the osmotic properties of the drug. OROS® allows for the controlled release of therapeutics, via the oral route, which decreases dosing frequencey and side effects.
Inhalers are compact devices that are used to store drug formulations which can be delivered as inhalable aerosolized sprays. Inhalers permit rapid absorption of drugs through the lungs, control over drug delivery via fixed doses, and the convenience of self-administration.
Implants are devices that either passively, through material properties, or actively, through various actuation methods, control drug release rates. Implants allow for long-term delivery of therapeutics, often reducing the number of invasive procedures required to maintain similar therapeutic effect.
Antibody drug conjugates are chemical conjugates of monoclonal antibodies and cytotoxic agents. Antibodies allow targeted delivery of highly potent cytotoxic drugs, thereby reducing systemic toxicity.
Figure 2.
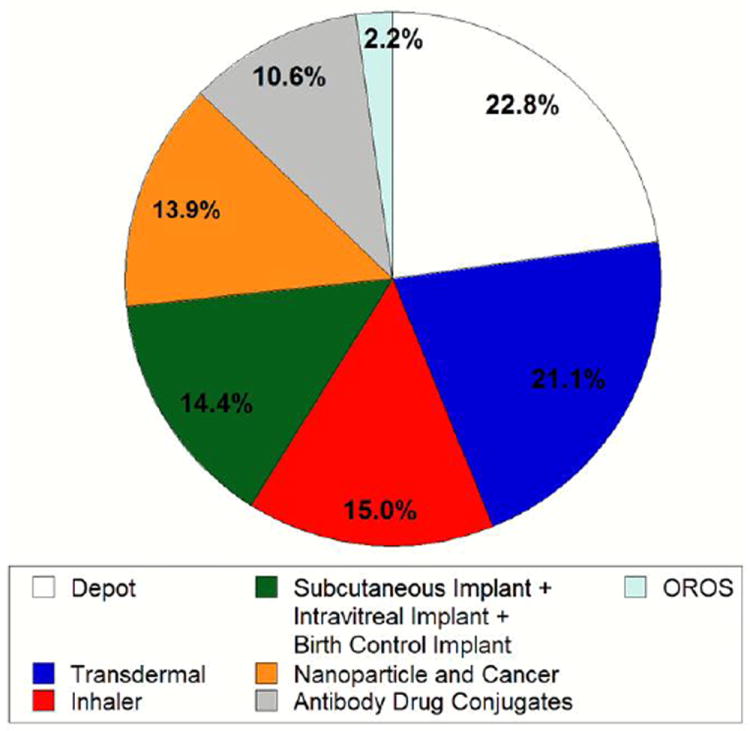
Normalized pie chart for clinical trial search. Search on ClinicalTrials.gov that counted the hits for clinical trials that are active and currently ongoing (but not recruiting). Thus, the data present trials that are actually in process. Data has been normalized to the sum of the total hits (180) for the following search keywords: (i) ‘Depot’ (41), (ii) ‘Transdermal’ (38), (iii) ‘Inhaler’ (27), (iv) ‘Subcutaneous implant’ (7) and ‘Intravitreal implant’ (9) and ‘Birth control implant’ (10), (v) ‘Nanoparticle and cancer’ (25), (vi) ‘Antibody drug conjugates’ (19) and (vii) ‘OROS’ (4). (Search conducted in Feb. 2014)
1. Microparticle-based Sustained Release Formulations
Microparticle-based sustained release formulations have been developed to facilitate the controlled delivery of therapeutics. By sustaining drug release over longer periods, these systems aim to improve the delivery of peptides or proteins by reducing injection frequency [7]. Microparticle-based depots include a polymeric material (often biodegradable) that allows for protection of the drug cargo and control over drug release. A number of polymer choices exist, each with their own advantages and limitations. Poly(lactic-co-glycolic) acid (PLGA), poly(lactic acid) (PLA) and polyglycolic acid (PGA) are perhaps the most commonly studied polymers due to their versatility in tuning biodegradation time and high biocompatibility arising from their natural by-products, lactic acid and glycolic acid. Here, we will highlight one of the first Food and Drug Administration (FDA)-approved, microparticle-based depot DDS, Lupron Depot®.
Development of Lupron Depot®
Lupron Depot® consists of leuprolide encapsulated in PLGA microspheres. Leuprolide was originally approved in 1985 as an injectable; however, constant injections spurred interest in a more patient compliant formulation. It was long thought that controlled release of proteins, and even smaller peptides, from microspheres was impossible [8]. However, research in the mid 70’s showed that this was indeed possible [9], and thereby paved the way for a new class of peptide/protein encapsulated polymeric DDS. Lupron Depot® was one of the first examples of this new class of controlled release polymeric DDS and was originally developed by Takeda-Abbott Products, a joint venture formed in 1977 between Abbott Laboratories and Takeda, and approved by the FDA in 1989 for the treatment of advanced prostate cancer [10]. Since then, Lupron Depot® has been approved for management of endometriosis and also for the treatment of central precocious puberty. Lupron Depot® has been commercially successful, reaching annual sales of near $1 billion [11].
Advantages and Limitations of Lupron Depot®
The main advantage of Lupron Depot® was that it significantly lowered the number of injections required for the treatment. Leuprolide alone required daily injections; however, the depot formulation requires injections every 1 to 6 months depending on the dose, thereby dramatically reducing the number of injections and increasing both patient compliance and convenience. Reduced injection frequency leads to improved patient comfort and compliance, which are requisites for successful self-administered DDS. In terms of the delivery technology, the individual components of Lupron Depot® offer several advantages. Specifically, PLGA polymer provides tunable degradation kinetics along with controlled release and well established safety and biocompatibility. Synthesis methods for Lupron Depot® microparticles must be highly reproducible and consistent in order to maintain efficacy across patients. Indeed, the encapsulation of proteins and peptides in PLGA particles has proven challenging in general, as is maintaining protein stability in microparticles [12]. Lupron Depot® and, in general, all microparticle protein formulations face these same challenges. Further, the production process of PLGA polymer determines product performance and different suppliers may not have identical procedures which ties down drug companies to specific supplier(s). A variety of reasons, ranging from supplier shut down to the high material costs, may pose a manufacturing challenge for microparticles.
Lessons Learned from Lupron Depot®--Current Academic Research
As one of the first clinically and commercially successful peptide delivery microparticle depot DDS in the US, Lupron Depot® inspired not only polymeric depot DDS, but also nanoparticle DDS in general. Lupron Depot® is a perfect example of a polymeric controlled delivery system that improves patient compliance by offering long-acting and long-lasting alternatives to highly invasive (i.e. daily injections) therapies. Since the introduction of Lupron Depot®, researchers have advanced the technology of sustained protein-release microparticles in various ways, including new methods for improving the stability and protection of encapsulated proteins [13, 14].
Current Clinical Landscape and Future Outlook
Many other microparticle depot systems are in clinical use and have been approved by the FDA (Table 2). One example is Nutropin Depot® developed by Genentech and Alkermes, the first long-acting formulation for recombinant growth hormone. Nutropin Depot® is a biodegradable microparticle depot formulation that was approved by the FDA in 1999 for pediatric growth hormone deficiency. Nutropin Depot® performed well in preclinical studies, showing reliable delivery for over one month in monkeys [15]. In clinical trials, Nutropin Depot® showed increase in the growth rate in children with pediatric growth hormone deficiency while requiring only one to two doses a month, compared to the standard of care which requires multiple injections per week. Nutropin Depot® delivered a much larger molecule, ~22,000 Da, than the previously FDA approved microparticle depot systems. Nutropin Depot®, however, is no longer commercially available due to high cost and manufacturing challenges [16, 17]. These issues are also encountered by other growth hormone products and, to some extent, several protein-based microparticle formulations [18]. Despite improved patient compliance afforded by Nutropin Depot®; this example highlights some of the after-market challenges that face microparticle formulations that must be overcome in order to remain competitive.
Table 2. Examples of FDA approved drugs.
List of approved drugs that fall into: (i) microparticle-based depot systems (biodegradable non-microparticle based formulations have been excluded), (ii) NP-based chemotherapies, (iii) transdermal devices [4], (iv) osmotically controlled oral formulations [82], (v) inhalers [114], (vi) implants (emphasis on intravitreal, cancer, and birth control formulations) and (vii) antibody drug conjugates.
| Part I | |||
|---|---|---|---|
| Microparticle-Based Depot | NP-based Chemotherapy | Transdermal Devices | Osmotically Controlled Oral Formulations |
|
|
|
|
| Part II | ||
| Inhalers | Implants | Antibody Drug Conjugates |
|
|
|
Alkermes has also developed Risperdal® Consta®, Vivitrol® and Bydureon® which are FDA approved and clinically available microsphere depot DDS. Risperdal® Consta®, approved in 2003 for the treatment of schizophrenia, delivers Risperdal® for a two-week period. It reduces inconsistencies associated with delivery using oral formulations. Since its approval for schizophrenia, Risperdal® Consta® has received approval for treatment of bipolar I disorder. Another Alkermes product, Vivitrol®, which delivers naltrexone, was approved by the FDA in 2006 for the treatment of alcohol dependence. Vivitrol® is taken once a month as an alternative to the naltrexone oral formulation, which suffered from severe compliance issues. As an anti-addiction medication, Vivitrol® addresses compliance issues by providing a 30-day treatment with a single injection. In 2010, Vivitrol® was approved to treat opioid-dependent patients. Bydureon®, the most recently approved of these Alkermes products, was FDA approved in 2012 and delivers Exenatide for the treatment of type 2 diabetes. Bydureon® is a weekly dose that provides an alternative to the twice daily injectable form, Byetta®. Bydureon® has the advantage of long-lasting effects. Other biodegradable, though non-microparticle, depot systems have been approved by the FDA. One example is Eligard®, an in situ forming biodegradable implant that delivers leuprolide for treatment of prostate cancer.
2. Nanoparticle-based cancer therapies
Nanoparticles (NPs) represent the most widely studied DDS. Over the last 10 years, more than 25,000 publications (Web of Science keyword: targeted nanoparticles, January 2014) have focused on targeted drug delivery using NPs. Even within the field of NP-based drug delivery systems, cancer remains the most studied target. NPs provide clear advantages for delivering chemotherapeutic drugs. NPs protect drugs from degradation in vivo, provide targeting to diseased tissue, and control drug release at the target site. They also benefit from synthesis methods that allow the scaled-up production of large batches of near identical properties. The large number of publications in this field is a combined result of the clinical need and the scientific difficulty that outlines the problem. The success rate, defined as the fraction of new nanotherapies that lead to clinical products, however, is very low. The path to FDA approval for nanomedicines is long and risky; however, a handful of technologies have made strong strides and are discussed here.
Doxil®: The First Cancer Nanomedicine
Doxil®, currently marketed by Johnson & Johnson, is the first FDA approved liposomal NP formulation for the treatment of certain cancers [19] and has experienced commercial success, often exceeding hundreds of millions of dollars in sales per year [20]. Doxil® is a liposomal formulation of doxorubicin and was approved by the FDA for the treatment of AIDS-related Kaposi’s sarcoma in 1995 and for recurrent ovarian cancer in 1998 [21]. The development of Doxil® was enabled by research that shed light on various aspects of liposomal formulations including long circulation [22], sufficient enhanced permeation and retention (EPR) effect [23], stable drug loading into liposomes [24] and releasing the encapsulated doxorubicin to target cells [25]. The early development efforts culminated in a study in Beagle dogs which confirmed that 2000 Da PEG-DSPE increased circulation time and reduced murine macrophage uptake in vitro better than any of the other long-circulating alternatives [26]. These findings eventually led to initial “first in man” (FIM) studies for Doxil®. In these FIM studies, published in 1994, Doxil® exhibited a half-life of about 45 hours compared to about 10 hours for free Doxorubicin. Further, Doxil® showed marked enhancement (4- to 16-fold increase) of Doxorubicin delivery to tumors compared to free doxorubicin [27]. Doxil® was approved by the FDA in 1995 and was available clinically in 1996.
Advantages and Limitations of Doxil®
Doxil® offers many advantages over its free drug counterpart, doxorubicin. It exhibits enhanced circulation, increased tumor persistence and improved half-life compared to free doxorubicin [19]. Also, as a nanoparticle, Doxil® can target certain tumors via the enhanced permeability and retention (EPR) effect which allows small particles (~100 nm) to accumulate in the leaky outer vasculature surrounding tumors [28]. This increases preferential accumulation of NP around tumors and facilitates NP delivery of drugs to tumors. This type of passive targeting significantly increases the amount of Doxil®-encapsulated doxorubicin that reaches tumors compared to free doxorubicin. As a result, Doxil® benefits from less off-target side effects and enhanced tumor killing. At the same time, there remain limitations. Despite being capable of EPR-mediated passive targeting, Doxil® lacks active targeting. The EPR effect is not exhibited to the same extent in all patients and can lead to differences in the tumor targeting, and subsequent therapeutic efficacy. Further, while Doxil® is PEGylated it is still cleared rapidly and could benefit from longer circulation, likely resulting in more passes around the tumor and interactions with the target tissue. More recently, production issues of Doxil® have led to widespread shortage of Doxil® in the US [29].
Lessons Learned from Doxil®--Current Academic Research
As the first NP-related commercial success, Doxil® inspires current nanomedicine research, and in fact, many NP-based DDS attempt to improve upon Doxil®. Specifically, many NPs utilizing antibodies, peptides and various other ligands are investigated to improve pharmacokinetics and target the delivery of therapeutics to specific sites in the body [30, 31]. Concomitantly, stealth particles that avoid rapid immune system clearance and circulate for longer times are being developed. Many strategies, ranging from hydrophilic coatings, such as PEG, to macrophage-avoiding ligands such as CD47 are being used to functionalize NPs for stealth applications [32, 33]. The mass production of therapeutic NPs has benefited greatly from fabrication advances in the semiconductor industry, as new methods utilizing mass producible nanofabrication techniques have been developed. A novel technology, PRINT, makes it possible to mass produce specifically sized, shaped, flexible, ligand-decorated and multi-layered NPs for therapeutic application [34, 35].
Current Clinical Landscape and Future Outlook
Many nanoparticle-based therapies are being developed for the treatment of cancer, yet few make it to a commercial product (Table 2). The most recent example is Marqibo®, liposomal vincristine, which was approved by the FDA in 2012 for treatment of a rare leukemia. Other than Doxil®, perhaps the most well-known NP used in the clinic is Abraxane®, originally FDA approved in 2005 for the treatment of breast cancer [36]. Abraxane®, currently marked by Celgene, is albumin-bound paclitaxel, an already approved chemotherapy drug. Abraxane® performed well in initial clinical trials [37-39] and, in the years since, has been approved for treatment of various other cancers. Abraxane® has shown excellent commercial success with preliminary annual sales in 2013 of $649 million [40].
Other promising nanoparticle formulations have made the successful translation from academic labs to clinical trials. BIND Therapeutics has developed a platform NP formulation (Accurins™) which combines active targeting ligands, protective and stealth functionality granted by PEG, controlled release via tuned polymer matrix and a therapeutic payload into a single NP. Early studies with Accurins™ showed low toxicity and stunted tumor growth in mice, comparable pharmacokinetics in mice, rats and non-human primates and tumor shrinkage in some human patients in a clinical trial [41]. Accurins™ are currently in Phase II clinical trials for treatment of non-small cell lung cancer and prostate cancer. Another promising NP is CRLX101 (originally IT-101). CRLX101 is a nanoparticulate chemotherapeutic formulation that consists of a camptothecin and cyclodextrin-based polymer conjugate. CRLX101 benefits from the efficacy of camptothecin, the EPR targeting granted from the size of CRLX101 and sustained release of camptothecin. These advantages limit many of the undesired side-effects of camptothecin while concomitantly providing a more stable formulation. The initial preclinical studies showed prolonged circulation time of CRLX101 compared to camptothecin alone, enhanced accumulation compared to camptothecin alone in tumors via the EPR effect and a prolonged release of camptothecin from CRLX101 following residence in tumor tissue [42]. Early clinical studies point to promising safety, pharmacokinetic and efficacy results. CRLX101 is currently being developed by Cerulean Pharma Inc. and is in Phase II clinical trials [43]. Another cancer therapeutic that builds on the same platform technology of a drug conjugated to a cyclodextrin-based polymer conjugate, CRLX301, is under development by Cerulean Pharma Inc. as well. In another example, Calando Pharmaceuticals is developing a cyclodextrin-based NP formulation that is able to encapsulate and protect small-interfering RNA (siRNA) in sub 100nm particles, CALAA-01. CALAA-01 is one of the first examples of an siRNA targeted nanoparticle used in clinical trials, and is comprised of: (i) linear cyclodextrin polymers that form the core, (ii) siRNA against RRM2 that inhibits growth of cancer cells, and surface modification in the form of (iii) PEG coating, for stability, and (iv) a transferrin ligand, for targeting to cancer cells [44]. CALAA-01 is currently undergoing clinical trials and has shown promising results, specifically showing that: (i) CALAA-01 is able to localize in tumors, (ii) reduce the expression of RRM2 when compared to pre-CALAA-01 dosed tissues and (iii) CALAA-01 mediates mRNA cleavage at predicted sites [44]. These results collectively illustrate that siRNA systemic administration, in nanoparticle form, can inhibit specific genes in humans. Many other NP formulations that have made significant progress and are undergoing clinical trials have been extensively reviewed elsewhere [45-47]. Several in vitro transfection reagents such as Lipofectamine 2000 have also been widely used in in vitro cell cultures [48] and are not reviewed here.
3. Transdermal Patches
Transdermal patches offer a painless, patient-compliant interface to facilitate the systemic administration of drugs. Many approved transdermal patches have a similar basic composition; a protective backing layer that prevents drug leakage, a reservoir which stores the drug which is ideally small and lipophilic, and an adhesive layer that facilitates skin contact [49]. Some systems may employ an extra layer that improves the controlled release of the drug. Many commercially successful transdermal patches have been developed for a variety of applications ranging from birth control to smoking cessation. The first FDA-approved patch was a 3-day patch that delivered scopolamine for motion sickness treatment in 1979. Well over a dozen patches are currently in use and approved by the FDA (Table 2). Of the various patches, fentanyl stands out as an example of a commercially successful transdermal patch used for chronic pain management. The fentanyl patch has exceeded $1 billion annual sales multiple times, highlighting its commercial success [50, 51].
Development of Fentanyl Patch
Fentanyl is a potent opioid analgesic first synthesized in 1960 [52] and was quickly approved in Western Europe due to its rapid onset and short duration, which made it an attractive choice as an analgesic for surgery. Alza Corporation and Janssen Pharmaceuticals began exploration of a fentanyl transdermal patch to both increase the duration of fentanyl and provide the patients with a potent opioid that could potentially be self-administered. A transdermal fentanyl patch, eventually known as Duragesic®, was developed by Alza Corporation and Janssen Pharmaceuticals and was approved by the FDA in 1990 for chronic pain [4]. This transdermal patch is a perfect example of a DDS that took an existing molecule (fentanyl) and improved its delivery, subsequently making it a commercial success. Several generic versions of fentanyl patches are also available. Another notable commercially successful transdermal patch is Lidoderm®, which was launched in 1999 and has reached annual sales of over $1 billion in 2012 [53].
Advantages and Limitations of Fentanyl Patch
Duragesic® offered two main advantages over its counterpart, both of which improved patient compliance: (i) controlled release up to 72 hours and (ii) formulated for self-administration. Fentanyl’s early success arose from the rapid onset time and short duration. By reformulating fentanyl as a patch, the applications were broadened from surgery room analgesic to potent, long lasting, pain relieving medication that the patient could apply themselves as a predetermined dose. The longer acting, dose-optimized fentanyl patch reduced the need for multiple doses and greatly improved patient compliance. Further, it offered at-home and on-demand application without the need of an expert to administer the formulation. In the past, defects in the production of fentanyl patches have led to their recall and changes in the patch design.
Lessons Learned from the Fentanyl Patch--Current Academic Research
The limitations of first generation transdermal patches have inspired the next generation of patches. The basic barrier function of the skin inherently limits drug transport from transdermal patches. The stratum corneum, the outermost layer of the skin, prevents the transport of molecules via its brick and mortar structure. Second generation patches have been designed to perform the same functions as first generation patches but also enhance transport of the drug through the skin. A number of techniques, which can be incorporated in or used alongside transdermal patches, are being developed to enhance drug transport via transdermal patch. These include: (i) ultrasound [54], (ii) chemical permeation enhancers [55], (iii) microneedles [56] and (iv) iontophoresis [57]. Chemical permeation enhancers disrupt the structure of the stratum corneum and significantly improve small molecule transport through this barrier. Hundreds of permeation enhancers are known and have been studied for this application. Sophisticated methods for high throughput screening of combinations of permeation enhancers have been developed and are currently used in industrial settings [58]. Iontophoresis uses electric fields to improve drug transport through the skin and the main advantage is the control over the quantity of drug transported, as it is directly proportional to the applied current. This technique has been used to deliver opioids [59], dopamine agonists [60], antiemetic agents [61], steroids [62] and peptides [63] in humans. Ultrasound induces cavitation on the skin surface and enhances transdermal transport. This technique has been used to deliver several drugs including lidocaine [64], cyclosporine A [65], hydrocortisone, dexamethasone and Ibuprofen in humans [66].
Current Clinical Landscape and Future Outlook
Newer, more sophisticated, transdermal patches and other transdermal DDS are being developed that are currently being tested in clinical trials. Notably, microneedles are investigated as a physical means of minimally piercing the skin, so as to avoid pain, prior to or jointly delivering a drug through the porated skin for systemic delivery. Clinical studies have confirmed the ability of microneedles to deliver variety of drugs including insulin [67-69], lidocaine [70] and vaccines [71]. Commercial development of microneedles is also noteworthy. Zosano has developed a titanium microneedle technology, applied via a reusable applicator, to deliver parathyroid hormone (PTH) across the skin [72] for the treatment of osteoporosis [73]. PTH delivery is desirable due to its potent bone building effect, but it is currently only available as daily subcutaneous injections. Zosano Pharma-PTH (ZP-PTH) offers a much improved non-invasive delivery method and a longer shelf life compared to subcutaneous PTH injections. Phase II studies for ZP-PTH have shown that ZP-PTH was effective in increasing spine bone mineral density, in a dose dependent manner. Further, hip bone mineral density was shown to increase at 6 months with ZP-PTH which has not been shown with the current approved subcutaneous treatment [73]. ZP-PTH has finished both Phase I and Phase II trials [74]. Corium International Inc. is developing biodegradable microneedles for PTH delivery.
Other device-based technologies have also advanced from the lab to the clinic (Table 2). For example, iontophoresis has been demonstrated to deliver fentanyl and lidocaine in humans [75, 76]. Commercial devices exist for topical delivery of lidocaine such as the active patch known as LidoSite® developed by Vyteris. Clinical trials have shown that a ten minute application delivered lidocaine via iontophoresis to induce effective anesthesia for venipuncture [76]. An ultrasound device, SonoPrep®, developed by Sontra Medical was also approved by the FDA for delivery of lidocaine [77]. Systemic delivery of fentanyl using iontophoresis has also made strong progress. IONSYS® was approved by the FDA and the European Medicines Agency (EMA) in 2006. However, IONSYS® has not been launched in the US and was recalled in Europe. This technology is currently under development at Incline therapeutics.
Methods are also being developed to use devices to perform continuous glucose monitoring across permeabilized skin. Symphony® (Echo Therapeutics) uses a non-invasive device to permeabilize the skin and then using a biosensor patch to monitor glucose levels for diabetic patients. It provides a means to avoid needle sticks and offer minute-by-minute glucose monitoring with limited skin irritation in a compact and lightweight package [78]. Symphony has demonstrated continuous transdermal glucose monitoring [79] and has applied for a CE mark. Microneedle-based technologies are also being developed for painless collection of blood. A device (Touch Activated Phlebotomy, TAP™) has recently received a CE mark approval.
4. Oral Drug Delivery
Oral delivery is by far the most commonly used mode of drug administration. This is evidenced by the number of oral formulations that breached the top 100 sold drugs in 2013 (Table 1). Predetermined doses, the simplicity offered by patient self-administration and systemic delivery, all incorporated in one tablet or capsule, are the main advantages of oral delivery. There are a variety of capsules in the market, and in fact, novel capsule designs offer an opportunity to develop novel oral DDS. No peptides or proteins, however, are currently delivered by the oral route due to rapid degradation in the stomach and size-limited transport across the epithelium. Yet, much effort has been spent on developing oral delivery systems for proteins, especially insulin.
Development of OROS®-Osmotically Driven Devices
Osmotically-driven DDS have been a topic of long-standing interest [80]. The interest in osmotically-driven pumps exhibited significant jump in the mid-70s [81]. The early designs of osmotic pumps eventually led to the establishment of the osmotic-controlled release oral delivery system (OROS®) technology. The controlled delivery offered by OROS®, which was developed by Alza, was tuned via a semi-permeable membrane which surrounds the drug and allows for water penetration into the drug reservoir. Water permeates into the drug reservoir and via osmotic pumping, facilitates drug release from an open pore. The rate of drug release is dependent on the osmotic properties of the drug and the design parameters can be tuned to control the release profile. The simplicity of the system paired with the advantages over simple oral tablets led to a lasting impact of OROS® on oral drug delivery. The first product, Osmosin, was launched in Europe in 1982 and withdrawn in 1983 due to severe GI tract irritation and, in some cases, intestinal wall perforation [82]. Since then, these issues have been addressed and, to date, numerous drugs in the clinic have been FDA approved and utilize osmotically driven capsule systems (Table 2) with many more in clinical trials. Collectively, these products speak for the high commercial impact of OROS®. Concerta®, one of approximately 10 products that utilize OROS® technology, has generated over $1 billion in sales multiple times [83].
Advantages and Limitations of OROS®
The main advantage of OROS® is that it allows controlled release of therapeutics. Several simpler drug pills suffer from non-controlled drug release which leads to sharp peaks and valleys in pharmacokinetic profiles. OROS® allowed improvements in terms of maintaining drug concentration in plasma within a safe and therapeutic range [84]. This allowed less frequent dosing and fewer side effects. Some OROS® capsules are designed to release the drug in certain regions of the GI Tract which provides another layer of sophistication. As with most oral DDS, OROS® offers a benefit of known predetermined dose. Further, OROS® provides an avenue for the delivery of poorly soluble drugs, as evidenced by the success of Procardia XL® [82]. At the same time, OROS® suffers from certain limitations that generally limit all oral delivery systems, namely poor delivery of peptides and proteins. In addition, the localized drug release has been shown to cause GI irritation and ulcers [85], though such issues have been relatively rare [86].
Lessons Learned from OROS®--Current Academic Research
Several oral drug delivery platforms have been developed, some inspired by OROS®. These include protective coatings for the drug reservoir [87] and functionalized surfaces for mucoadhesion to intestinal mucosa [88]. The primary excitement in the field of oral delivery, however, has been improving the ability to deliver proteins and peptides [89]. In general, most approaches have focused on improving protein absorption in the GI tract or limiting proteolytic degradation of proteins [2, 90]. One method to improve these aspects is the use of mucoadhesive systems that increase the residence time of oral DDS in the GI tract [91]. Many mucoadhesive systems have been proposed and studied in small animals, ranging from particle systems [92] to patches [93]. Other techniques include using chemical permeation enhances to improve protein adsorption in the GI tract [94], targeting endocytotic pathways for enhanced cellular uptake [95] or enhancing the lipophilicity of proteins to improve partitioning into epithelial cell membranes [2]. Some have used specific enteric capsule coatings designed to degrade in specific sections of the GI tractor even co-administer enzyme inhibitors, both with and without adsorption enhancers, so as to avoid significant proteolytic activity.
Current Clinical Landscape and Future Prospects
Oramed has developed a technology to deliver peptides and proteins by oral administration. The technology makes use of protease inhibitors to prevent degradation of the protein in the stomach, an absorption enhancer to facilitate passage through intestinal epithelium and an enteric coated capsule to ensure release of capsule contents in the small intestine. Recent studies in humans showed that an Oramed insulin pill taken prior to each meal in type I diabetes patients, in conjunction with normal subcutaneous insulin injections, were able to reduce glycemia throughout the day [96]. Oramed has recently finished Phase II trials in the US. Emisphere is developing Eligen® technology that can be applied for the oral delivery of molecules, including proteins. The Eligen® technology is based on compounds that interact with drugs and facilitate drug transport across the intestinal epithelium. Emisphere‘s technology has been used in clinical trials to transport insulin, salmon calcitonin, GLP-1 analogs and heparin via the oral route [97]. Clinical trials have also shown that Eligen®-delivered oral vitamin B12 performs just as well as the standard B12 regimen of ~9 injections over a 90 day period [98]. Several other innovative technologies are being tested for oral delivery of proteins and are at various stages of development. These include the use of mucoadhesive patches [99], FcRn-targeted nanoparticles [100] and hydrogels [101, 102].
5. Pulmonary Drug Delivery
Pulmonary drug delivery is mediated via the inhalation of drugs through a variety of means, ranging from nebulizers to inhalers. Pulmonary drug delivery has been explored for systemic as well as localized delivery of drugs to the lungs. The advantages for systemic delivery are that inhalation offers a rapid systemic onset, in some cases on the order of minutes, which results from the large surface area of lungs [103]. In case of localized delivery to lungs, which has been the primary application for inhaled medications, onset is even more rapid. This is essential to treat severe asthma attacks as, in many cases, people suffering from asthma attacks require simple to use/access (patient compliant) and fast acting DDS. Of the numerous inhalation DDS on the market, the metered dose inhalers have had the most impact, effectively making asthma medications simple for patients to administer to themselves. The metered dose inhaler has also inspired other inhalation DDS to push beyond what was originally thought possible, such as the inhaled delivery of peptides or proteins. Here, we highlight the development of the first metered dose inhaler.
Development of Asthma Inhalers
The metered dose inhaler (MDI) was first conceived in the mid 50’s [104]. While other pulmonary DDS such as nebulizers had been developed and used up to this point and found success due to localized lung delivery, they were limited as they were fragile and, most importantly, not able to precisely control the amount of therapeutic given to a patient. The MDI revolutionized pulmonary drug delivery by offering a controlled method to deliver a known amount of therapeutic with each dose. The first MDI was approved and marketed in 1956 [105]. The MDI design consists of a canister which contains the drug formulation, the metering valve which dispenses the pre-determined dose and a mouthpiece which facilitates the transport of the drug formulation to the patient. Nearly 10 years ago, it was estimated that asthma inhalers generate sales of about $25 billion a year [106]. More recently, the top two selling combination inhalers, Advair® and Symbicort®, generated $8 and $3 billion, respectively, in 2012 [83].
Advantages and Limitations of Inhalers
MDIs offer numerous advantages for delivery of asthma medication over nebulizers. The inability of older nebulizers to control the amount of therapeutic dose resulted in increased, potentially fatal, side effects of overdose or insufficient delivery. These inconsistencies allowed MDI inhalers to provide much needed ease of administration in terms of both consistent dosing and simple application procedures. The combination of the large dispersion area of aerosolized DDS that typically follow administration and the large surface area of the lungs synergistically contribute to the delivery of inhaled therapeutics by facilitating the rapid uptake of molecules into systemic circulation. Further, if local delivery to lungs is desired, inhalers provide a direct access. However, inhalers are limited in their ability to deliver peptides and proteins. Degradation of stored peptides and proteins is a particular issue for MDIs. Dry powder inhalers (DPIs) are potentially more advantageous than MDIs for protein storage as they allow for the storage of proteins in a dry form, effectively extending protein shelf life. Further, DPIs are typically activated by the patient’s air flow [107] as compared to MDIs which requires coordination between actuation and inhalation. The patient is responsible for this coordination and must know the proper ways to administer their own medication; however, this issue can be addressed with proper training.
Lessons Learned from Inhalers--Current Academic Research
Recent research has improved the use of MDIs and other inhalers by: (i) optimizing aerosol particle size for ideal aerodynamic diameters for enhanced local delivery to lungs [108], (ii) engineering of spacers to limit the loss of drug at target site during application [104] and (iii) improving on the actual design and technology of the original MDI, to name a few [109]. In a broader sense, inhalation research is focused on the delivery of larger molecules like peptides via the inhalation route for systemic administration. Inhalation is an attractive route for protein delivery due to the rapid absorption of drugs through the lungs, the lack of proteolytic enzymes in lungs compared to GI tract and the general non-invasive nature of inhalers. Techniques have been developed to improve macromolecule delivery via inhalation including absorption enhancers [110] and improved particle design for either deep lung penetration [111] or extended delivery of encapsulated therapeutics [112].
Current Clinical Landscape and Future Prospects
Inhalers have been predominately used, and FDA approved, for the treatment of asthma and chronic obstructive pulmonary disease (COPD) (Table 2). As of December 2013, the FDA has completed the phase-out of chlorofluorocarbon inhalers [113], with many products still remaining [114]. Many other devices have been approved or are in development for the treatment of other diseases including cystic fibrosis (Cayston® and TOBI™ Podhaler™), diabetes (Exubera®, approved in 2006 and withdrawn in 2007) and pulmonary arterial hypertension (Ventavis® and Tyvaso®) (Table 2) [115]. Inhalation offers a broad platform that can systemically deliver peptides and proteins in a patient-compliant, non-invasive manner. In particular, they offer rapid absorption, lesser interference from proteases compared to oral route and high permeability of the epithelium. These advantages over oral route for peptide and protein delivery influence the landscape of clinical inhalation research, as the search for non-invasive delivery of peptides and proteins continues to persist. Many inhalable DDS, as highlighted below, are making strong strides to achieve routine clinical delivery of insulin. In fact, one such product had been previously approved by the FDA.
The first FDA approved inhalable insulin formulation was Nektar’s Exubera®, which was manufactured and marketed by Pfizer. The Exubera® formulation stored and delivered insulin via a dry powder inhaler [115] so as to maintain peptide stability. Further, Exubera® provided appropriate aerosolized characteristics to ensure pulmonary delivery, a low-dose powder filling so as to deliver small doses and a physical device to control dispersion and reliable dosing to patients [116]. Despite successful clinical trials [117, 118], the additional cost of Exubera [119] caused the discontinuation of the product [120]. MannKind Inc. continued to develop an inhaled insulin product, AFREZZA® which potentially offers a compact inhalation device, low dosing and rapid absorption [121]. AFREZZA® has submitted a New Drug Application (NDA) and has recently announced positive Phase III clinical study results, namely weight advantages, reduced hypoglycemia and decrease in fasting blood glucose levels compared to insulin aspart [122].
Other future prospects for inhalable DDS focus on taking advantage of either: (i) the rapid drug onset for near immediate treatment or (ii) the localized delivery to lungs for the treatment of lung infections. Civitas is developing a pulmonary delivery platform called ARCUS® which is focused on treating OFF episodes associated with Parkinson’s disease with a formulation (CVT-301). CVT-301 is designed to deliver L-dopa and is to be used as needed and in tandem with standard L-dopa oral formulated Parkinson’s disease treatments. The ARCUS® inhaler is designed to provide immediate relief from OFF episodes. Briefly, ARCUS® provides reliable delivery of a large, precise dose via a breath activated device. ARCUS® utilizes a dry powder formulation that ensures both stability and delivery deep into the lungs for systemic delivery. Phase I studies have shown that CVT-301 was successful in achieving target L-dopa plasma levels and Phase II studies have shown improved motor functions in Parkinson’s disease patients that were in the OFF state [123]. Currently, CVT-301 is undergoing Phase II studies to determine safety and efficacy in treatment of OFF states in Parkinson’s disease patients. Cardeas is developing inhalable antibiotics for pneumonia treatment using a single-use nebulizer to deliver two synergistically acting antibiotics, amikacin and fosfomycin. Cardeas was recently granted fast track review for their antibiotic combination product and has begun enrollment for Phase II studies for treatment of multi-drug resistant bacterial infections [124]. Early clinical trials indicate that the Cardeas formulation is safe and tolerable for all tested doses.
6. Implantable Systems
Implantable DDS can be classified into two categories that define their release properties; either passive delivery or active delivery. Passive DDS control drug release via the material properties that constitute the implant. Passive DDS can tune drug release from the reservoir by controlling rates of diffusion, osmosis, or concentration gradients. These passive release methods are dependent on drug choice, membrane composition, size and tortuosity of membrane pores, and the combination of these design parameters. On the other hand, active implant DDS control drug release using a pump that can be activated by a number of methods ranging from simple manual actuation from physical pressure to electrochemically driven mechanisms that can vary drug delivery rates. Since some implantable DDS take advantage of micro and nano-fabrication technologies, this may lead to longer approval times as they may fall under the FDA Office of Combination Product review, which looks to review medical therapies that combines elements from independently established fields [125]. Implantable DDS have made a large impact in ocular drug delivery as traditional methods require numerous intravitreal injections which can potentially result in a number of issues as discussed below. Here, we will highlight the very first of these intravitreal implants, the Vitrasert® implant.
Development of Vitrasert®
The Vitrasert® implant was approved by the FDA in 1996 for the treatment of AIDS-related cytomegalovirus (CMV) retinitis. The conventional means of delivering ganciclovir has been intravitreal injections; however, the nature of CMV retinitis treatment requires many injections that result in complications that can arise from repeated intravitreal injections. Theoretically, reservoir implants provide an attractive means to deliver ganciclovir while limiting the number of intravitreal procedures, but no such device existed at the time. The Vitrasert® implant consists of a ganciclovir reservoir that is coated in both polyvinyl alcohol (PVA) and ethylene vinyl acetate (EVA) that is sutured on the inside wall of the eye. The PVA mediates the release of drug and the EVA controls the surface area that the drug can pass through. The implant can maintain controlled drug release for a period of 5-8 months, after which the implant must be removed and replaced in order to continue treatment. In early studies, Vitrasert® showed zero order release of ganciclovir [126] and in a Phase I trial showed efficacy for controlling CMV retinitis in patients that showed little to no response to conventional ganciclovir therapies [127]. Vitrasert® was eventually advanced by Control Delivery Systems and has been approved by the FDA.
Advantages and Limitations of Vitrasert®
Vitrasert® was the first implant approved for CMV retinitis treatment. The alternative, intravitreal ganciclovir injections, require frequent (weekly) administration over a period of months or years [128]. The issues associated with these repeated intravitreal injections ranged from hemorrhaging to retinal detachment [129]. Further, rapid drug clearance from the vitreous region led to more frequent injections, on the order of 1-2 per week [128]. The Vitrasert® implant circumvents the majority of these issues. Controlled release allows for better drug absorption which lowers the number of required procedures compared to intravitreal injections. This results in improved sustained release and better intraocular concentrations over a longer period of time compared to both the combination of initial intravitreal injection alongside maintenance intravenous injections [128]. Other advantages include localized delivery to the eye and specified dosing. Vitrasert® lasts between 5-8 months before replacement is necessary. The initial clinical trials showed that ganciclovir implants limited progression of CMV retinitis nearly 3-fold longer than intravenous ganciclovir alone could offer [130]. The disadvantages of Vitrasert® include the lack of systemic treatment as CMV infection can also appear as a systemic infection and oral supplementation of ganciclovir is often required to prevent CMV extraocular infection. Two separate invasive surgeries for the implantation and removal of Vitrasert® are required and, independent of the Vitrasert® implant, can cause complications due to the nature of eye surgery.
Lessons Learned from Vitrasert®--Current Academic Research
Retisert®, approved by the FDA in 2005 for the treatment of chronic non-infectious uveitis, followed Vitrasert® as the second generation of reservoir based implants. Retisert® provides reliable drug delivery for up to 3 years, well past that of the 5-8 month limit of Vitrasert®. Retisert® is also smaller compared to Vitrasert® which makes implantation and removal less invasive. Both of these products address areas of the pharmaceutical market where there is truly no competitive alternative that offers long-term drug release. More recently, active release implants which offer on demand drug release have been developed in academic labs [131-133]. These devices can control drug release via a variety of mechanisms including, but not limited to: physical forces, electrochemical methods, magnetic fields, laser induced or electrothermal means. Further, currently researched devices can be fabricated via semiconductor technology for more precise and reliable synthesis.
Current Clinical Landscape and Future Prospects
Other implantable DDS have been developed and approved by the FDA (Table 2), and a few examples are highlighted here. Iluvien®, a microfabrication-based device has been developed to deliver fluocinolone acetonide. Iluvien® does not require suturing as in the case with both Vitrasert® and Retisert®, which provides a much less invasive procedure. The implant is designed to last up to 3 years without requiring replacement. Iluvien® delivers fluocinolone acetonide to patients suffering from diabetic macular edema, which is typically treated via laser photocoagulation that often leaves patients with irreversible blind spots. Similar to its predecessors, Iluvien® targets a disease that offers virtually no alternative in the form of FDA approved drug therapies. Iluvien® has been approved and is being marketed in many countries including the UK, Germany and Spain and is currently being considered for FDA approval. Ongoing Phase II clinical trials aimed at treating wet-age related macular edema and dry-age related macular edema are also under way. Another FDA approved implant, Ozurdex®, is used to deliver corticosteroid dexamethasone for treatment of retinal vein occlusion. Ozurdex® is the first, and only, biodegradable implant that delivers dexamethasone [134]. Following drug release from the implant, no surgery is required to remove the implant from the body.
The Gliadel® wafer was FDA approved in 1996 for use as an adjunct to surgery in patients with recurrent glioblastoma multiforme, an aggressive type of brain tumor. In 2003, the Gliadel® wafer was approved for use as a first time treatment for brain tumors. It was the first localized chemotherapeutic for initial treatment of brain cancer. The dime sized wafers, which are comprised of the chemotherapeutic agent carmustine and the polymer matrix made of poly (carboxyphenoxy-propane/sebacic acid) [135], are surgically inserted in the cavity that remains following removal of brain tumors. Gliadel® has shown to increase patient survival for up to 6 months in some cases, but also increased incidences of postoperative wound infections and cerebral edema [136]. Current clinical Gliadel® research looks to combine Gliadel® wafers with systemic chemotherapies and radiation treatment for enhanced tumor cell death following surgery.
MicroCHIPS is developing a platform of implantable DDS, which utilizes a MEMS-based technology for drug delivery [137]. These implants allow active control over drug release, either at predetermined times or on-demand. Release of PTH has been demonstrated using MicroCHIPs technology and offers precise dosing at scheduled intervals to overcome the compliance issues associated with the daily injections conventional PTH treatments currently require. Human studies have shown that PTH was delivered as programmed and showed similar pharmacokinetics while offering less variation in dosing as compared to multiple PTH injections. Further, bone formation was increased in the short, 20 day, study [138].
7. Antibody-Drug Conjugates
Antibody-drug conjugates (ADCs) are targeted bioconjugate pharmaceuticals that combine the benefits of monoclonal antibodies and cytotoxic drugs to treat cancer. ADCs involve a chemical linker that conjugates the antibody to the drug and can either be cleavable or non-cleavable. Indeed, the vast number of monoclonal antibodies and drugs allow for near endless combinations of ADCs. ADCs potentially offer a development path for highly toxic drugs that are otherwise difficult to clinically implement due to off-target toxicity. As an example, the ADC Brentuximab vedotin (Adcetris®) facilitated the use of the highly toxic Monomethyl auristatin E (MMAE) in the clinic, which is otherwise challenging due to the high toxicity of MMAE. To date, three ADCs have been approved by the FDA; the first one, Gemtuzumab ozogamicin (Mylotarg®), has been withdrawn from the market in 2010. Here, we will highlight the development of the most recently approved ADC, Trastuzumab emtansine (commercialized by Roche), which has seen early commercial success.
Development of Trastuzumab Emtansine (Kadcyla®)
Trastuzumab emtansine (Kadcyla®) is comprised of the monoclonal antibody trastuzumab, the potent cytotoxic drug mertansine (DM1) and a linker that covalently attaches trastuzumab and DM1 together via reactive succinimide ester and maleimide groups. trastuzumab is a monoclonal antibody (FDA approved in 1998) that is used for the treatment of certain breast cancers by targeting HER2 receptors. The main limitation of trastuzumab is eventual tumor resistance. Combination of trastuzumab with DM1 can address this issue. DM1, is a derivative of maytansine which are known to be a toxic class of therapeutics and have essentially no therapeutic window [139]. The derivative itself, DM1, is 100 to 10000 fold more potent than typical chemotherapeutics [139], which limits clinical use of DM1 to targeted applications. The linker component utilizes activated maleimide reactive groups on trastuzumab’s surface to bind to thiol groups on DM1 [140]. The Phase III studies of Kadcyla® showed an increase in progression-free survival of 3.2 months, overall survival of 5.8 months and lower toxicity when compared to treatment with lapatinib plus capecitabine in patients that were previously treated with trastuzumab and a taxane [141]. Kadcyla® was approved by the FDA in 2013 and generated a revenue of over $150 million within a few months of launch [142].
Advantages and Limitations of Trastuzumab Emtansine
Kadcyla® offers the benefits of a highly potent cytotoxic drug alongside the targeted abilities of the monoclonal antibody trastuzumab. The main advantages are being able to combine two separately established technologies to synergistically increase the therapeutic efficacy beyond what is offered by individual components. The ADC directly addresses the clinical limitations of each individual composition, namely the lack of long term efficacy of antibodies upon repeated dosing and the lack of clinical utility afforded the highly potent DM1 drug due to its high toxicity which typically results from lack of specific tumor targeting. What remains is a highly potent targeted therapy, which chemotherapy cannot provide, for the treatment of HER2 positive breast cancer. ADCs offer a larger therapeutic window and lower side effects compared to traditional chemotherapies, stemming from their selective tumor targeting. Unfortunately, the main issues that challenge successful monoclonal antibodies also limit ADCs, specifically: (i) poor access to hypoxic tumor areas and generally poor tumor penetration, (ii) issues concerning the non-target site uptake, and (iii) undesirable immune system responses via Fc interactions [143].
Lessons Learned from Kadcyla®--Current Academic Research
The early success of Kadcyla® highlights the importance of selecting a highly specific antibody that had previously shown clinical success and the selection of a highly toxic drug that likely could not be delivered in its free, non-targeted, form. Research continues to focus on the linker technology that conjugates the drug to the antibody. This is because both highly specific and highly toxic drugs already exist, so cutting edge research is focused on linker technology that can directly improve ADCs by improving homogenous attachment between antibody and drug, thereby facilitating more predictable circulation, cell internalization and drug release of ADCs. Some specific efforts involve chemically limiting the number of potential binding sites on antibodies to have better control of drug-antibody stoichiometry [144] and genetically encoding unnatural amino acids into antibodies for site-specified drug binding [145]. Optimization of ADCs has been investigated for over 20 years [146] and so the majority of research efforts are focused on ADCs currently in clinical trials [147, 148].
Current Clinical Landscape and Future Prospects
There are over 25 clinical trials currently underway for ADCs [147, 148]. Many of them utilize the same, or similar, cytotoxic drugs as the two currently approved ADCs, Kadcyla® and Adcetris®. The other currently FDA approved ADC, Adcetris®, was approved in 2011 for the treatment of Hodgkin’s lymphoma and anaplastic large cell lymphomas. Adcetris® is comprised of a CD30-specific antibody, a highly potent antitubulin agent monomethyl auristatin E (MMAE) and an enzyme cleavable linker that releases the drug when inside the target cells [149]. The combination of these provides a potent therapy, as the drug component of Adcetris®, MMAE, is too potent to have any clinical utility on its own and the antibody portion, cAC10, has limited therapeutic effect on its own. Adcetris® generated sales of $136 million in its first year on the market (October 2011 to September 2012) [150].
Gemtuzimab ozogamicin (Mylotarg®) was the first FDA approved ADC; however, it was withdrawn from the market in 2010, 10 years after its initial approval. The market withdrawal was spurred by a clinical trial that indicated that Mylotarg® not only increased mortality, but also added no benefit over alternative therapies [151]. Mylotarg® was used for the treatment of acute myelogenous leukemia and consisted of an antibody directed toward CD33, a highly potent cytotoxic calicheamicin derivative and an acid-labile hydrazone linker [152]. Mylotarg® is still being tested in clinical trials and showing promise in combination with chemotherapy [153]. As stated earlier, over 25 ADCs are currently in clinical trials [148], and some of the ones in later development stages include: (i) Inotuzumab ozogamicin, developed by Pfizer, which targets CD22 with a calicheamicin payload attached via a hydrazone linker, (ii) Glembatumumab vedotin which targets transmembrane protein NMB with an MMAE agent attached via an enzyme cleavable dipeptide and has received FDA Fast Track designation for breast cancer treatment and (iii) Lorvotuzumab mertansine which targets CD56 with DM1 attached via a cleavable disulfide linker and has been granted orphan drug status for the treatment of small cell lung cancer.
Summary and Outlook
There is no doubt that DDS have had a strong impact on pharmaceutical market. Novel DDS have provided several advantages including local delivery, use of drugs that are otherwise difficult to use and increased patient compliance. In addition, drug delivery systems also provide means of patent extension, which further adds the value of new drug molecules. A review of the field reveals several clinical and commercial success stories. Many sub-fields of drug delivery, i.e., transdermal drug delivery, depot injections, and oral drug delivery, have commercially successful products with annual sales exceeding a billion dollars. At the same time, it should also be noted that the journey from an idea to a commercially successful DDS is long and difficult, as evidenced by the few FDA approved products in spite of a strong early research pipeline. At each step there are many roadblocks that can spell immediate failure (Figure 3), thus preventing the clinical or commercial translation of many promising DDS.
Figure 3.
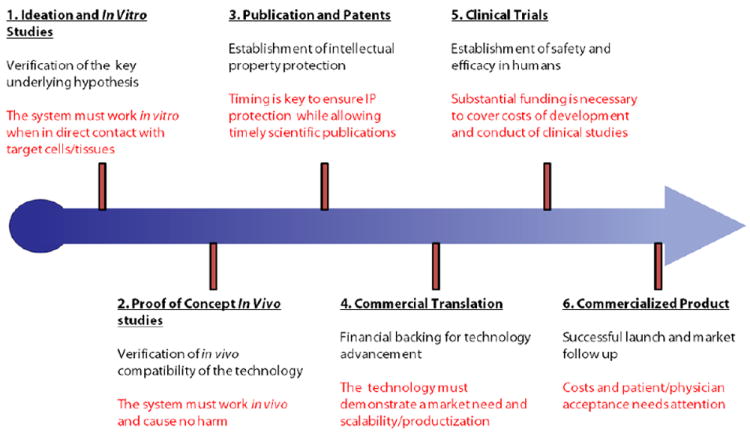
The typical path that academic discoveries follow from initial ideation to a final commercialized product. The challenges at each step are highlighted.
Strong academic research has provided numerous early-stage technologies for DDS. Naturally, many commercially successful DDS began as novel ideas originating in an academic research lab. The hypotheses that form the foundation of these ideas need to be tested and verified as soon as possible. Proof of concept studies are essential in confirming that the DDS is viable and can provide therapeutic benefits in vivo, even if just in small animals. Given the high rate of fall-out during in vitro to in vivo translation, it is critical that the idea be put to the ‘in vivo test’ as soon as possible. In vitro optimization of the DDS must typically occur in order to provide the best chance for success at expensive, and often lengthy, in vivo experiments. Further, success in small animal models does not guarantee success in humans. Following up with publications and patents is also key. Strong publications often provide validation of significant scientific breakthroughs through peer-review [154] while patents are essential to secure commercial development.
Investors and industrial partners play a strong role in commercial as well as clinical translation. While the two do not necessarily have to correlate, the high costs of clinical studies and often requires strong financial commitments. Unlike technologies originated in large pharmaceutical companies, academic inventions lack substantial capital and require outside sources of funding and support in order to progress the technology. Further, companies likely have access to therapeutic molecules that are in need of novel drug delivery systems. Clinical trials are the ultimate test of efficacy and utility of a novel DDS. Prior to FDA approval, DDS candidates must show efficacy and tolerability in pre-clinical studies. Even after successful studies and commercial launch, side-effects still need to be monitored and recorded, as it is difficult to cover all details in clinical trials. This is evidenced by the recall of many drugs and DDS formulations after commercialization. Indeed, many DDS fail when undergoing clinical trials for a variety of reasons, ranging from: (i) lack of funds to support expensive clinical trials, (ii) lack of tolerability or high toxicity in patients even in spite of therapeutically successful outcomes, (iii) lack of therapeutic effect or (iv) study design failures stemming from patient selection, dosing issues or even selecting an appropriate endpoint. Timeline of development also needs to be closely monitored in view of the finite patent life and extended regulatory approval times.
Many of the DDS showcased here (Table 3) were developed or inspired by research at the academic level. While the process from lab-level research to a commercial product is long, there are many examples that illustrate the potential of ideas and proof-of-concept studies in building the foundation for these commercialized products or even facilitating the development of new sub-fields in drug delivery. Partnering between pharmaceutical companies and academic labs has the potential to further both fundamental drug delivery research and translate these findings in the form of improved clinical therapeutics.
Table 3. Case study summary.
Advantages, disadvantages and specific contributions to drug delivery of the 7 highlighted DDS.
| DDS | Case Study (Target Disease) | Advantages Compared to Alternatives | General Disadvantages | Case Study Contribution |
|---|---|---|---|---|
| Microparticle Depots | Lupron Depot® (Cancer) |
|
|
|
| Nanoparticles for Cancer Treatment | Doxil® (Cancer) |
|
|
|
| Transdermal Devices | Duragesic® (Pain Relief) |
|
|
|
| Oral Delivery Systems | OROS® (Various, see Table 5) |
|
|
|
| Pulmonary Delivery Systems | MDI Inhalers (Asthma) |
|
|
|
| Implants | Vitrasert (CMV Retinitis) |
|
|
|
| Antibody Drug Conjugates | Kadcyla® (Cancer) |
|
|
|
Acknowledgments
This work was supported by the National Institute of Health under Grant No. R01DK097379 and National Science Foundation Graduate Research Fellowship under Grant No. DGE-1144085 to ACA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health promotion international. 2004;19(1):95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2(4):289–95. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- 3.Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6(1):67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 4.Prausnitz MR, Langer R. Transdermal drug delivery. Nature biotechnology. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudana R, et al. Ocular drug delivery. AAPS J. 2010;12(3):348–60. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today. 2002;7(23):1184–9. doi: 10.1016/s1359-6446(02)02529-1. [DOI] [PubMed] [Google Scholar]
- 7.Talmadge JE. The pharmaceutics and delivery of therapeutic polypeptides and proteins. Advanced drug delivery reviews. 1993;10(2):247–299. [Google Scholar]
- 8.Langer R. Controlled release of a therapeutic protein. Nat Med. 1996;2(7):742–3. doi: 10.1038/nm0796-742. [DOI] [PubMed] [Google Scholar]
- 9.Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263(5580):797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 10.Wright JC, Hoffman AS. Long Acting Injections and Implants. Springer; 2012. Historical overview of long acting injections and implants; pp. 11–24. [Google Scholar]
- 11.Chaubal M. Polylactides/glycolides-excipients for injectable drug delivery and beyond. Drug Deliv Technol. 2002;2:34–36. [Google Scholar]
- 12.Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide- co-glycolide) Nat Biotechnol. 2000;18(1):52–7. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 13.Sinha V, Trehan A. Biodegradable microspheres for protein delivery. Journal of Controlled Release. 2003;90(3):261–280. doi: 10.1016/s0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 14.van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly (lactic-co-glycolic acid) microparticles. Pharmaceutical research. 2000;17(10):1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 15.Johnson OL, et al. A month-long effect from a single injection of microencapsulated human growth hormone. Nat Med. 1996;2(7):795–9. doi: 10.1038/nm0796-795. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, et al. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57(3):391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Jordan F, et al. Sustained release hGH microsphere formulation produced by a novel supercritical fluid technology: in vivo studies. J Control Release. 2010;141(2):153–60. doi: 10.1016/j.jconrel.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Tracy MA. Development and scale-up of a microsphere protein delivery system. Biotechnol Prog. 1998;14(1):108–15. doi: 10.1021/bp9701271. [DOI] [PubMed] [Google Scholar]
- 19.Barenholz Y. Doxil(R)--the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Johnson & Johnson Annual Report 2012. 2014 Feb 9; Available from: https://www.jnj.com/sites/default/files/pdf/JNJ2012annualreport.pdf.
- 21.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–22. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 22.Allen T, Chonn A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS letters. 1987;223(1):42–46. doi: 10.1016/0014-5793(87)80506-9. [DOI] [PubMed] [Google Scholar]
- 23.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer research. 1986;46(12 Part 1):6387–6392. [PubMed] [Google Scholar]
- 24.Haran G, et al. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1993;1151(2):201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz AT, Barenholz Y, Gabizon AA. In vitro cytotoxicity of liposome-encapsulated doxorubicin: dependence on liposome composition and drug release. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1992;1109(2):203–209. doi: 10.1016/0005-2736(92)90084-y. [DOI] [PubMed] [Google Scholar]
- 26.Gabizon AA, Barenholz Y, Bialer M. Prolongation of the circulation time of doxorubicin encapsulated in liposomes containing a polyethylene glycol-derivatized phospholipid: pharmacokinetic studies in rodents and dogs. Pharmaceutical research. 1993;10(5):703–708. doi: 10.1023/a:1018907715905. [DOI] [PubMed] [Google Scholar]
- 27.Gabizon A, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Research. 1994;54(4):987–992. [PubMed] [Google Scholar]
- 28.Maeda H, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of Controlled Release. 2000;65(1):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 29.Important Update Regarding Supply Outage of DOXIL®. 2014 Feb 9; Available from: http://www.doxil.com/doxil-supply-shortage.
- 30.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 31.Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv Mater. 2012;24(28):3747–56. doi: 10.1002/adma.201200454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez PL, et al. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339(6122):971–5. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 34.Rolland JP, et al. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127(28):10096–100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 35.Morton SW, et al. Scalable manufacture of built-to-order nanomedicine: spray-assisted layer-by-layer functionalization of PRINT nanoparticles. Adv Mater. 2013;25(34):4707–13. doi: 10.1002/adma.201302025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 37.Gradishar WJ, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. Journal of clinical oncology. 2005;23(31):7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 38.Green M, et al. Abraxane®, a novel Cremophor®-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Annals of Oncology. 2006;17(8):1263–1268. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 39.Nyman DW, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. Journal of clinical oncology. 2005;23(31):7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 40.Celgene Corporation Announces 2014 Financial Outlook and Preliminary 2013 Results. 2014 Feb 9; Available from: http://ir.celgene.com/releasedetail.cfm?ReleaseID=821044.
- 41.Hrkach J, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128):128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 42.Svenson S, et al. Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101. J Control Release. 2011;153(1):49–55. doi: 10.1016/j.jconrel.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Eliasof S, et al. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc Natl Acad Sci U S A. 2013;110(37):15127–32. doi: 10.1073/pnas.1309566110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis ME, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–98. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 46.Matsumura Y, Kataoka K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009;100(4):572–9. doi: 10.1111/j.1349-7006.2009.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heidel JD, Davis ME. Clinical developments in nanotechnology for cancer therapy. Pharm Res. 2011;28(2):187–99. doi: 10.1007/s11095-010-0178-7. [DOI] [PubMed] [Google Scholar]
- 48.Dalby B, et al. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004;33(2):95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 49.Venkatraman S, Gale R. Skin adhesives and skin adhesion. 1. Transdermal drug delivery systems. Biomaterials. 1998;19(13):1119–36. doi: 10.1016/s0142-9612(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 50.Watkinson AC. A commentary on transdermal drug delivery systems in clinical trials. J Pharm Sci. 2013;102(9):3082–8. doi: 10.1002/jps.23490. [DOI] [PubMed] [Google Scholar]
- 51.Stanley TH. Fentanyl. J Pain Symptom Manage. 2005;29(5 Suppl):S67–71. doi: 10.1016/j.jpainsymman.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Stanley TH. The history and development of the fentanyl series. J Pain Symptom Manage. 1992;7(3 Suppl):S3–7. doi: 10.1016/0885-3924(92)90047-l. [DOI] [PubMed] [Google Scholar]
- 53.Lidoderm Sales Data. 2014 Feb 9; Available from: http://www.drugs.com/stats/lidoderm.
- 54.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nature Reviews Drug Discovery. 2005;4(3):255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 55.Karande P, Jain A, Mitragotri S. Insights into synergistic interactions in binary mixtures of chemical permeation enhancers for transdermal drug delivery. Journal of controlled release. 2006;115(1):85–93. doi: 10.1016/j.jconrel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Prausnitz MR. Microneedles for transdermal drug delivery. Advanced drug delivery reviews. 2004;56(5):581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 57.Kalia YN, et al. Iontophoretic drug delivery. Advanced drug delivery reviews. 2004;56(5):619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 58.Karande P, Jain A, Mitragotri S. Discovery of transdermal penetration enhancers by high-throughput screening. Nature biotechnology. 2004;22(2):192–197. doi: 10.1038/nbt928. [DOI] [PubMed] [Google Scholar]
- 59.Ashburn MA, et al. Iontophoretic delivery of morphine for postoperative analgesia. J Pain Symptom Manage. 1992;7(1):27–33. doi: 10.1016/0885-3924(92)90104-p. [DOI] [PubMed] [Google Scholar]
- 60.van der Geest R, et al. Iontophoretic delivery of apomorphine. II: An in vivo study in patients with Parkinson’s disease. Pharm Res. 1997;14(12):1804–10. doi: 10.1023/a:1012152401715. [DOI] [PubMed] [Google Scholar]
- 61.Cormier M, et al. Effect of transdermal iontophoresis codelivery of hydrocortisone on metoclopramide pharmacokinetics and skin-induced reactions in human subjects. J Pharm Sci. 1999;88(10):1030–5. doi: 10.1021/js980491+. [DOI] [PubMed] [Google Scholar]
- 62.James MP, Graham RM, English J. Percutaneous iontophoresis of prednisolone--a pharmacokinetic study. Clin Exp Dermatol. 1986;11(1):54–61. doi: 10.1111/j.1365-2230.1986.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 63.Green PG. Iontophoretic delivery of peptide drugs. Journal of controlled release. 1996;41(1):33–48. [Google Scholar]
- 64.Spierings EL, Brevard JA, Katz NP. Two-Minute Skin Anesthesia Through Ultrasound Pretreatment and Iontophoretic Delivery of a Topical Anesthetic: A Feasibility Study. Pain Medicine. 2008;9(1):55–59. doi: 10.1111/j.1526-4637.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 65.SantoiannI P, Nino M, Calabro G. Intradermal drug delivery by low frequency sonophoresis (25KHz) 2004 [PubMed] [Google Scholar]
- 66.Polat BE, et al. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release. 2011;152(3):330–48. doi: 10.1016/j.jconrel.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Norman JJ, et al. Faster pharmacokinetics and increased patient acceptance of intradermal insulin delivery using a single hollow microneedle in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2013;14(6):459–65. doi: 10.1111/pedi.12031. [DOI] [PubMed] [Google Scholar]
- 68.Gupta J, Felner EI, Prausnitz MR. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol Ther. 2011;13(4):451–6. doi: 10.1089/dia.2010.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta J, Felner EI, Prausnitz MR. Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles. Diabetes Technol Ther. 2009;11(6):329–37. doi: 10.1089/dia.2008.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gupta J, et al. Rapid local anesthesia in humans using minimally invasive microneedles. Clin J Pain. 2012;28(2):129–35. doi: 10.1097/AJP.0b013e318225dbe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–68. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ameri M, Fan SC, Maa YF. Parathyroid hormone PTH(1-34) formulation that enables uniform coating on a novel transdermal microprojection delivery system. Pharm Res. 2010;27(2):303–13. doi: 10.1007/s11095-009-0019-8. [DOI] [PubMed] [Google Scholar]
- 73.Daddona PE, et al. Parathyroid hormone (1-34)-coated microneedle patch system: clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm Res. 2011;28(1):159–65. doi: 10.1007/s11095-010-0192-9. [DOI] [PubMed] [Google Scholar]
- 74.Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. Int J Pharm. 2008;364(2):227–36. doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mayes S, Ferrone M. Fentanyl HCl patient-controlled iontophoretic transdermal system for the management of acute postoperative pain. Ann Pharmacother. 2006;40(12):2178–86. doi: 10.1345/aph.1H135. [DOI] [PubMed] [Google Scholar]
- 76.Zempsky WT, et al. Evaluation of a low-dose lidocaine iontophoresis system for topical anesthesia in adults and children: a randomized, controlled trial. Clin Ther. 2004;26(7):1110–9. doi: 10.1016/s0149-2918(04)90183-x. [DOI] [PubMed] [Google Scholar]
- 77.Becker BM, et al. Ultrasound with topical anesthetic rapidly decreases pain of intravenous cannulation. Academic emergency medicine. 2005;12(4):289–295. doi: 10.1197/j.aem.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 78.Vashist SK. Non-invasive glucose monitoring technology in diabetes management: a review. Anal Chim Acta. 2012;750:16–27. doi: 10.1016/j.aca.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 79.Chuang H, et al. Pilot studies of transdermal continuous glucose measurement in outpatient diabetic patients and in patients during and after cardiac surgery. J Diabetes Sci Technol. 2008;2(4):595–602. doi: 10.1177/193229680800200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rose S, Nelson JF. A continuous long-term injector. Aust J Exp Biol Med Sci. 1955;33(4):415–9. doi: 10.1038/icb.1955.44. [DOI] [PubMed] [Google Scholar]
- 81.Theeuwes F. Elementary osmotic pump. J Pharm Sci. 1975;64(12):1987–91. doi: 10.1002/jps.2600641218. [DOI] [PubMed] [Google Scholar]
- 82.Malaterre V, et al. Oral osmotically driven systems: 30 years of development and clinical use. Eur J Pharm Biopharm. 2009;73(3):311–23. doi: 10.1016/j.ejpb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 83.Rosenthal E. The Soaring Cost of a Simple Breath. 2014 Feb 9; Published: October 12, 2013]; Available from: http://www.nytimes.com/2013/10/13/us/the-soaring-cost-of-a-simple-breath.html?_r=0.
- 84.Theeuwes F. Orososmotic system development. Drug Development and Industrial Pharmacy. 1983;9(7):1331–1357. [Google Scholar]
- 85.Park CG, et al. A nanofibrous sheet-based system for linear delivery of nifedipine. J Control Release. 2011;149(3):250–7. doi: 10.1016/j.jconrel.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 86.Bass DM, Prevo M, Waxman DS. Gastrointestinal safety of an extended-release, nondeformable, oral dosage form (OROS: a retrospective study) Drug Saf. 2002;25(14):1021–33. doi: 10.2165/00002018-200225140-00004. [DOI] [PubMed] [Google Scholar]
- 87.Agyilirah GA, Banker GS. Polymers for Controlled Drug Delivery. CRC Press; Boca Raton: 1991. Polymers for enteric coating applications; pp. 39–66. [Google Scholar]
- 88.Ponchel G, Irache J-M. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Advanced drug delivery reviews. 1998;34(2):191–219. doi: 10.1016/s0169-409x(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 89.Morishita M, Peppas NA. Is the oral route possible for peptide and protein drug delivery? Drug discovery today. 2006;11(19):905–910. doi: 10.1016/j.drudis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 90.Bernkop-Schnürch A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. Journal of controlled release. 1998;52(1):1–16. doi: 10.1016/s0168-3659(97)00204-6. [DOI] [PubMed] [Google Scholar]
- 91.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557–70. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mathiowitz E, et al. Biologically erodable microspheres as potential oral drug delivery systems. Nature. 1997;386(6623):410–4. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]
- 93.Gupta V, et al. Mucoadhesive intestinal devices for oral delivery of salmon calcitonin. J Control Release. 2013;172(3):753–62. doi: 10.1016/j.jconrel.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 94.Ward PD, Tippin TK, Thakker DR. Enhancing paracellular permeability by modulating epithelial tight junctions. Pharmaceutical science & technology today. 2000;3(10):346–358. doi: 10.1016/s1461-5347(00)00302-3. [DOI] [PubMed] [Google Scholar]
- 95.Swaan PW. Recent advances in intestinal macromolecular drug delivery via receptor-mediated transport pathways. Pharmaceutical research. 1998;15(6):826–834. doi: 10.1023/a:1011908128045. [DOI] [PubMed] [Google Scholar]
- 96.Eldor R, et al. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLoS One. 2013;8(4):e59524. doi: 10.1371/journal.pone.0059524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.The Eligen® Library. 2014 Feb 9; Available from: http://www.emisphere.com/eligen_library.html.
- 98.Castelli MC, et al. Comparing the Efficacy and Tolerability of a New Daily Oral Vitamin B12 Formulation and Intermittent Intramuscular Vitamin B12 in Normalizing Low Cobalamin Levels: A Randomized, Open-Label, Parallel-Group Study. Clinical therapeutics. 2011;33(3):358–371. e2. doi: 10.1016/j.clinthera.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Whitehead K, Shen Z, Mitragotri S. Oral delivery of macromolecules using intestinal patches: applications for insulin delivery. Journal of controlled release. 2004;98(1):37–45. doi: 10.1016/j.jconrel.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 100.Pridgen EM, et al. Transepithelial transport of fc-targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci Transl Med. 2013;5(213):213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Torres-Lugo M, et al. pH-Sensitive Hydrogels as Gastrointestinal Tract Absorption Enhancers: Transport Mechanisms of Salmon Calcitonin and Other Model Molecules Using the Caco-2 Cell Model. Biotechnology progress. 2002;18(3):612–616. doi: 10.1021/bp0101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Torres-Lugo M, et al. Physicochemical behavior and cytotoxic effects of p (methacrylic acid–g-ethylene glycol) nanospheres for oral delivery of proteins. Journal of controlled release. 2002;80(1):197–205. doi: 10.1016/s0168-3659(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 103.Agu RU, et al. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respiratory research. 2001;2(4):198. doi: 10.1186/rr58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dalby R, Suman J. Inhalation therapy: technological milestones in asthma treatment. Advanced drug delivery reviews. 2003;55(7):779–791. doi: 10.1016/s0169-409x(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 105.Anderson PJ. History of aerosol therapy: liquid nebulization to MDIs to DPIs. Respiratory care. 2005;50(9):1139–1150. [PubMed] [Google Scholar]
- 106.Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respiratory care. 2005;50(10):1360–1375. [PubMed] [Google Scholar]
- 107.Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respiratory care. 2005;50(9):1209–1227. [PubMed] [Google Scholar]
- 108.Edwards DA, Ben-Jebria A, Langer R. Recent advances in pulmonary drug delivery using large, porous inhaled particles. Journal of Applied Physiology. 1998;85(2):379–385. doi: 10.1152/jappl.1998.85.2.379. [DOI] [PubMed] [Google Scholar]
- 109.Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. The Lancet. 2011;377(9770):1032–1045. doi: 10.1016/S0140-6736(10)60926-9. [DOI] [PubMed] [Google Scholar]
- 110.Hussain A, et al. Absorption enhancers in pulmonary protein delivery. J Control Release. 2004;94(1):15–24. doi: 10.1016/j.jconrel.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 111.Cleland JL, Daugherty A, Mrsny R. Emerging protein delivery methods. Curr Opin Biotechnol. 2001;12(2):212–9. doi: 10.1016/s0958-1669(00)00202-0. [DOI] [PubMed] [Google Scholar]
- 112.Patton JS, Bukar J, Nagarajan S. Inhaled insulin. Adv Drug Deliv Rev. 1999;35(2-3):235–247. doi: 10.1016/s0169-409x(98)00074-x. [DOI] [PubMed] [Google Scholar]
- 113.FDA to complete phase-out of chlorofluorocarbon inhalers. 2014 Feb 9; Published: October 23, 2013]; Available from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm371901.htm.
- 114.Drug Treatments for Asthma and Chronic Obstructive Pulmonary Disease that Do Not Use Chlorofluorocarbons. 2014 Feb 9; Available from: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm082370.htm.
- 115.Hickey AJ. Back to the future: inhaled drug products. J Pharm Sci. 2013;102(4):1165–72. doi: 10.1002/jps.23465. [DOI] [PubMed] [Google Scholar]
- 116.White S, et al. EXUBERA: pharmaceutical development of a novel product for pulmonary delivery of insulin. Diabetes Technol Ther. 2005;7(6):896–906. doi: 10.1089/dia.2005.7.896. [DOI] [PubMed] [Google Scholar]
- 117.Quattrin T, et al. Efficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 1 diabetes: results of a 6-month, randomized, comparative trial. Diabetes Care. 2004;27(11):2622–7. doi: 10.2337/diacare.27.11.2622. [DOI] [PubMed] [Google Scholar]
- 118.Hollander PA, et al. Efficacy and safety of inhaled insulin (exubera) compared with subcutaneous insulin therapy in patients with type 2 diabetes: results of a 6-month, randomized, comparative trial. Diabetes Care. 2004;27(10):2356–62. doi: 10.2337/diacare.27.10.2356. [DOI] [PubMed] [Google Scholar]
- 119.Black C, et al. The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation. Health Technol Assess. 2007;11(33):1–126. doi: 10.3310/hta11330. [DOI] [PubMed] [Google Scholar]
- 120.Opar A. Another blow for inhaled protein therapeutics. Nature Reviews Drug Discovery. 2008;7(3):189–190. [Google Scholar]
- 121.Kling J. Dreamboat sinks prospects for fast approval of inhaled insulin. Nature biotechnology. 2011;29(3):175–176. doi: 10.1038/nbt0311-175. [DOI] [PubMed] [Google Scholar]
- 122.Press Releases: MannKind Reports Positive Data from a Phase 3 Clinical Study of AFREZZA in Patients with Type 1 Diabetes. 2014 Feb 9; Published: August 14, 2013]; Available from: http://www.news.mannkindcorp.com/phoenix.zhtml?c=147953&p=irol-newsArticle&ID=1847441&highlight=
- 123.Available from: http://www.civitastherapeutics.com/CVT-301.html.
- 124.Cardeas Pharma announces that FDA Grants QIDP Designation and Fast Track Review for the Company’s Antibiotic Combination Product. 2014 Feb 9; Published: January 8, 2014]; Available from: http://cardeaspharma.com/press-releases/cardeas-pharma-announces-that-fda-grants-qidp-designation-and-fast-track-review-for-the-companys-antibiotic-combination-product/
- 125.Staples M, et al. Application of micro- and nano-electromechanical devices to drug delivery. Pharm Res. 2006;23(5):847–63. doi: 10.1007/s11095-006-9906-4. [DOI] [PubMed] [Google Scholar]
- 126.Smith TJ, et al. Intravitreal sustained-release ganciclovir. Arch Ophthalmol. 1992;110(2):255–8. doi: 10.1001/archopht.1992.01080140111037. [DOI] [PubMed] [Google Scholar]
- 127.Sanborn GE, et al. Sustained-release ganciclovir therapy for treatment of cytomegalovirus retinitis. Use of an intravitreal device. Arch Ophthalmol. 1992;110(2):188–95. doi: 10.1001/archopht.1992.01080140044023. [DOI] [PubMed] [Google Scholar]
- 128.Velez G, Whitcup SM. New developments in sustained release drug delivery for the treatment of intraocular disease. Br J Ophthalmol. 1999;83(11):1225–9. doi: 10.1136/bjo.83.11.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Geroski DH, Edelhauser HF. Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci. 2000;41(5):961–4. [PubMed] [Google Scholar]
- 130.Musch DC, et al. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. The Ganciclovir Implant Study Group. N Engl J Med. 1997;337(2):83–90. doi: 10.1056/NEJM199707103370203. [DOI] [PubMed] [Google Scholar]
- 131.Stevenson CL, Santini JT, Jr, Langer R. Reservoir-based drug delivery systems utilizing microtechnology. Advanced drug delivery reviews. 2012;64(14):1590–1602. doi: 10.1016/j.addr.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Receveur RA, Lindemans FW, de Rooij NF. Microsystem technologies for implantable applications. Journal of Micromechanics and Microengineering. 2007;17(5):R50. [Google Scholar]
- 133.Richards Grayson AC, et al. Electronic MEMS for triggered delivery. Advanced drug delivery reviews. 2004;56(2):173–184. doi: 10.1016/j.addr.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 134.How Ozurdex(R) Works. 2014 Mar 11; Available from: http://www.ozurdex.com/HowItWorks.aspx.
- 135.Giese A, et al. Pattern of recurrence following local chemotherapy with biodegradable carmustine (BCNU) implants in patients with glioblastoma. J Neurooncol. 2004;66(3):351–60. doi: 10.1023/b:neon.0000014539.90077.db. [DOI] [PubMed] [Google Scholar]
- 136.Weber EL, Goebel EA. Cerebral edema associated with Gliadel wafers: two case studies. Neuro Oncol. 2005;7(1):84–9. doi: 10.1215/S1152851704000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Santini JT, Jr, Cima MJ, Langer R. A controlled-release microchip. Nature. 1999;397(6717):335–8. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 138.Farra R, et al. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci Transl Med. 2012;4(122):122ra21. doi: 10.1126/scitranslmed.3003276. [DOI] [PubMed] [Google Scholar]
- 139.Hughes B. Antibody-drug conjugates for cancer: poised to deliver? Nat Rev Drug Discov. 2010;9(9):665–7. doi: 10.1038/nrd3270. [DOI] [PubMed] [Google Scholar]
- 140.Junutula JR, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925–32. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 141.Verma S, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.ImmunoGen, Inc. Reports First Quarter Fiscal Year 2014 Financial Results and Provides Quarterly Update. 2014 Feb 9; Published: October 25, 2013] Available from: http://investor.immunogen.com/releasedetail.cfm?ReleaseID=800370.
- 143.Chames P, et al. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157(2):220–33. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McDonagh CF, et al. Engineered antibody-drug conjugates with defined sites and stoichiometries of drug attachment. Protein Eng Des Sel. 2006;19(7):299–307. doi: 10.1093/protein/gzl013. [DOI] [PubMed] [Google Scholar]
- 145.Axup JY, et al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci U S A. 2012;109(40):16101–6. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14(4):529–37. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 147.Lambert JM. Drug-conjugated antibodies for the treatment of cancer. Br J Clin Pharmacol. 2013;76(2):248–62. doi: 10.1111/bcp.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mullard A. Maturing antibody-drug conjugate pipeline hits 30. Nature Reviews Drug Discovery. 2013;12(5):329–332. doi: 10.1038/nrd4009. [DOI] [PubMed] [Google Scholar]
- 149.Younes A, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–21. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 150.Zolot RS, Basu S, Million RP. Antibody-drug conjugates. Nat Rev Drug Discov. 2013;12(4):259–60. doi: 10.1038/nrd3980. [DOI] [PubMed] [Google Scholar]
- 151.FDA: Pfizer Voluntarily Withdraws Cancer Treatment Mylotarg from U.S. Market. 2014 Feb 9; Published: June 21, 2010]; Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm216448.htm.
- 152.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23(9):1137–46. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 153.Burnett AK, et al. Blood. AMER SOC HEMATOLOGY; 1900 M STREET. NW SUITE 200, WASHINGTON, DC 20036 USA: 2011. The addition of gemtuzumab ozogamicin to intensive chemotherapy in older patients with AML produces a significant improvement in overall survival: results of the UK NCRI AML16 randomized trial. [Google Scholar]
- 154.Langer R. A personal account of translating discoveries in an academic lab. Nat Biotechnol. 2013;31(6):487–9. doi: 10.1038/nbt.2609. [DOI] [PubMed] [Google Scholar]
- 155.U.S. Pharmaceutical Sales - 2013. 2014 Jan 17; Available from: http://www.drugs.com/stats/top100/2013/sales.


