Abstract
We have previously shown that human herpesvirus 8 (HHV-8) uses DC-SIGN as an entry receptor for dendritic cells, macrophages and B cells. The viral attachment protein for DC-SIGN is unknown. HHV-8 virions contain 5 conserved herpesvirus glycoproteins, a single unique glycoprotein, and 2 predicted glycoproteins. Previous studies have shown that DC-SIGN binds highly mannosylated glycoproteins. The HHV-8 glycoprotein B (gB) has been reported to be highly mannosylated, and therefore we hypothesized that gB will bind to DC-SIGN. In this report we confirm that gB has a high mannose carbohydrate structure and demonstrate for the first time that it binds DC-SIGN in a dose-dependent manner. We also identify key amino acids in the DC-SIGN carbohydrate recognition domain that are required for HHV-8 infection and compare these results with published binding regions for ICAM-2/3 and HIV-1 gp120. These results clarify some of the initial events in HHV-8 entry and can be used for the design of targeted preventive therapies.
Introduction
We have previously demonstrated that DC-SIGN is a cellular receptor for human herpesvirus-8 (HHV-8, also known as Kaposi’s sarcoma-associated herpesvirus, KSHV), the causative agent of Kaposi’s sarcoma, primary effusion lymphoma and a subset of multicentric Castleman’s disease (Knowlton et al., 2012; Moore and Chang, 2001; Rappocciolo et al., 2008; Rappocciolo et al., 2006). In addition to its expression on monocyte-derived dendritic cells (MDDCs), DC-SIGN is also expressed on activated macrophages and B cells and its isomer, DC-SIGNR, is expressed on endothelial cells (Rappocciolo et al., 2008; Rappocciolo et al., 2006; Soilleux et al., 2002; van den Berg et al., 2012). These cell types represent natural targets for HHV-8 in vivo. Studies on the interactions between DC-SIGN and other viruses known to use DC-SIGN as an entry receptor, such as human immunodeficiency virus (HIV), Ebola virus, hepatitis C virus and cytomegalovirus (CMV), have demonstrated that viral glycoproteins are the viral attachment proteins responsible for binding to DC-SIGN, or its endothelial cell-expressed homologue, DC-SIGNR (Cassol et al., 2012; Samreen et al., 2012) (Curtis et al., 1992; Gardner et al., 2003; Geijtenbeek et al., 2000; Halary et al., 2002; Lin et al., 2003; Pohlmann et al., 2003; Simmons et al., 2003). The majority of these studies have demonstrated that viral glycoproteins with a high mannose glycan structure bind to DC-SIGN/DC-SIGNR (Anderluh et al., 2012; Feinberg et al., 2001). Like other herpesviruses, HHV-8 encodes a variety of glycoproteins that are expressed on the virion. There are 6-to-8 HHV-8 virion-associated glycoproteins known to date: the herpesvirus conserved glycoproteins gB, gH, gL, gM, and gN, the HHV-8 unique glycoprotein K8.1A, and the gene products of open reading frames (ORFs) 28 and 68, which are predicted to be glycoproteins (Zhu et al., 2005). Studies in our laboratory as well as others, have determined that the HHV-8 gB glycoprotein produced in B cells has a high mannose glycan structure while other glycoproteins such as K8.1A and gN have a predominately complex structure (Baghian et al., 2000; Koyano et al., 2003; Wu et al., 2000). The glycan structure of the remaining 5 HHV-8 glycoproteins is not known. As gB has a high mannose glycan structure, it is a prime candidate for a viral attachment protein that binds DC-SIGN.
Studies investigating the binding site of DC-SIGN’s natural ligands, ICAM-2/3 and the HIV gp120 protein, have reported that the binding of gp120 is separate but overlapping with ICAM-2/3, and that the conserved Ca+2 binding residues of DC-SIGN were important to binding of both molecules (Geijtenbeek et al., 2002; Su et al., 2004). DC-SIGN contains two predicted Ca+2 binding sites, one formed by amino acids E347, N349, E354, N365, and D366 and another by residues D320, Q323, N350, and D355 (Geijtenbeek et al., 2002). For the most part, these studies agree on important residues for binding of both molecules, with the exception of V351, an amino acid located in the “binding pocket” of DC-SIGN and which was suggested to be important in ICAM binding by Geitenbeek, et al.(Geijtenbeek et al., 2002), but not by Su, et al (Su et al., 2004). Additionally, the crystal structure of DC-SIGN suggests that the “binding pocket” is formed with N311 on one end (Feinberg et al., 2001). This mutation was suggested to confer preferential ligand binding, but was surprisingly shown not to be involved in either gp120 or ICAM binding (Feinberg et al., 2001; Su et al., 2004). Interestingly, a mutation at the other end of the pocket in D367, was shown to be important to ICAM binding, but not gp120 binding (Su et al., 2004). As we have shown that HHV-8 binds DC-SIGN (Rappocciolo et al., 2008; Rappocciolo et al., 2006), we wished to determine whether HHV-8 binds in a similar or distinct manner to the other two ligands. To this end, we have made cell lines expressing a panel of 6 point mutation-containing DC-SIGN proteins, in which the amino acid has been mutated to an alanine, along with the corresponding wild-type DC-SIGN protein to determine which of these amino acids are important in DC-SIGN-mediated HHV-8 infection.
In this report we demonstrate for the first time, using a soluble form of gB, that it binds DC-SIGN in a dose-dependent manner. We identified several amino acids in the carbohydrate recognition domain (CRD) of DC-SIGN that are important in HHV-8 infection and that overlap the ICAM and gp120 binding sites.
Materials and Methods
Soluble proteins
Baculovirus-expressed soluble DC-SIGN was obtained in purified form via a Material Transfer Agreement with the Centers for Disease Control and Prevention, Atlanta, GA (Promadej-Lanier et al., 2008). Baculovirus expressing the soluble HHV-8 glycoprotein gB (Wang et al., 2003) to which 6X Histidine tags have been introduced, were obtained from B. Chandran (Rosalind Franklin University, North Chicago, IL). Baculoviruses were used to infect Sf9 cells and incubated for 6 days at 28°C. Cell pellets were lysed using I-PER lysis solution (ThermoFisher/Pierce, Rockford, IL) in the presence of protease inhibitors and proteins were purified using a His-binding Cobalt Column (ThermoFisher/Pierce, Rockford, IL). Proteins were dialyzed overnight against PBS to remove imidizole using a 10,000 MWCO Slide-a-lyzer (ThermoFisher/Pierce, Rockford, IL). Proteins were stored at −80°C until use. Protein concentration was determined using the Bio-Rad Protein Assay kit per the manufacturer’s protocol (Bio-Rad, Hercules, CA) and relative amounts confirmed by western blot and coomassie staining.
Endoglycosidase digests
Soluble gB was digested with Endoglycosidase H or PNGaseF (NEB, Ipswich, MA) at 37°C for 1 hr according to the manufacturer’s instructions. Lysates were subjected to SDS-PAGE and western blots were performed as described.
Western blots
Proteins or cell lysates were subjected to SDS-PAGE and transferred to Immobilon PVDF membrane (Millipore, Billerica, MA). Membranes were blocked in 3% milk/PBS/0.05% Tween-20 and incubated with anti-gB-N1 rabbit antisera (1:2500), or anti-DC-SIGN polyclonal antibody (1:5000, Calbiochem, Gibbstown, NJ) in PBS/0.05% Tween-20. The anti-gB-N1 rabbit polyclonal antisera was produced by Sigma-Genosys against the HHV-8 gB peptide TFQTSSSPTPPGSSS (aa 30-46) as described (Wang et al., 2003). Membranes were then incubated with secondary antibodies goat anti-rabbit IgG-HRP (gB and DC-SIGN, 1:70,000) (Thermo-Fisher/Pierce, Rockford, IL) in PBS/0.05% Tween-20 and subsequently developed using the West Pico Chemiluminescence kit (Thermo-Fisher/Pierce, Rockford, IL).
Far Western Blot
Soluble proteins were subjected to 12% SDS-PAGE and transferred to Immobilon PVDF membrane (Millipore, Billerica, MA). Soluble DC-SIGN protein was labeled in vitro with EZ-Link NHS-LC-LC-Biotin according to the manufacturer’s instructions at a molar coupling ratio of 5:1 for 30 minutes at room temperature (ThermoFisher/Pierce, Rockford, IL). Membranes were blocked for 7.5 minutes in Superblock T20 (ThermoFisher/Pierce, Rockford, IL) at room temperature and washed twice in PBS/0.05% Tween-20. Membranes were then incubated with DC-SIGN-biotin in probe dilution buffer (0.3% BSA/1% normal goat serum/PBS) at 10mg/ml at 37°C for 1 hr. Five washes were performed using PBS/0.05% Tween-20. The membranes were then incubated with avidin-HRP substrate diluted into 7.5ml PBS/0.05% Tween-20 according to the manufacturer’s instructions (ABC Elite kit, Vector labs, Burlingame, CA) for 1 hour at 37°C. Five washes were performed using PBS/0.05% Tween-20. Blots were developed using Vector Red diluted into 14ml of deionized water according to the manufacturer’s instructions (Vector labs, Burlingame, CA) and rinsed in tap water to stop the reaction when sufficient color change had developed.
ELISA
Polysorp 96 well plates (Nunc, Roskilde, Denmark) were coated with the soluble gB glycoproteins in 1X bicarbonate buffer (ThermoFisher/Pierce, Rockford, IL) overnight at 4°C. Plates were washed with PBS/0.05% Tween-20, air-dried and stored at −20°C. Plates were blocked using 1% bovine serum albumin in PBS/0.05% Tween-20 for 30 minutes at room temperature and subsequently incubated with DC-SIGN that had been conjugated with biotin (ThermoFisher/Pierce, Rockford, IL) for 1 hour at room temperature. Plates were washed thoroughly and incubated with strepavidin-HRP (R&D Systems, Minneapolis, MN) for 1 hour at room temperature. Plates were developed using color-change substrate (R&D Systems, Minneapolis, MN) and the reaction was stopped using 2N H2SO4. Plates were read at 450nm/540nm. ELISAs to demonstrate protein presence were performed by blocking using 1% bovine serum albumin in PBS/0.05% Tween-20 for 30 minutes at room temperature. Wells were then incubated with an anti-gB antisera (Sigma-Aldrich, St. Louis, MO) at 1:500 in PBS/0.05% Tween-20 for 1hr at room temperature and followed with goat anti-rabbit peroxidase conjugated IgG antibodies (ThermoFisher/Pierce, Rockford, IL) at 1:350 in PBS/0.05% Tween-20 for 1hr at room temperature. Plates were developed using color-change substrate (R&D Systems, Minneapolis, MN) and the reaction was stopped using 2N H2SO4. Plates were read at 450nm/540nm.
Cell lines
Sf9 cells were purchased from Invitrogen (Carlsbad, CA). K562 cells were obtained from ATCC (Manassas, VA). Stable K562 transfectants of both wild type and point mutants of DC-SIGN were made by transfecting plasmids containing individual point mutations in DC-SIGN (obtained from Dr. Benhur Lee, UCLA, Los Angeles, CA)(Su et al., 2004) into K562 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and selecting single cell derived G418 resistant clones. Clones were screened for surface expression of DC-SIGN using an anti-DC-SIGN polyclonal antibody (Calbiochem, Gibbstown, NJ) recognizing an epitope outside of the mutated regions (data not shown). DNA was purified from the cell lines and sequenced to confirm the identity of each of the clones (data not shown). TRex BCBL-1-RTA cells were obtained from Dr. Jae Jung (Harvard University, Boston, MA) (Nakamura et al., 2003).
HHV-8 Infection
TRex BCBL-1 RTA cells (Nakamura et al., 2003) were induced using 1 μg/ml Doxycycline and incubated for 3 days. Supernatant (containing infectious HHV-8) was collected and concentrated to approximately 1/100 of the original volume using 100,000 MWCO or 1,000,000 MWCO Centricons (Millipore, Billerica, MA). Virus was stored at −80°C until use. Infectivity was tested via infection of K562-DC-SIGN cells and visualization of viral protein production by immunofluorescence (IFA). Infections were performed by infecting 105 cells in the smallest possible volume at 37°C for 1-4 hrs, followed by removal of the virus. Infected cells were incubated at 37°C for 48 hrs.
Immunofluorescence
Infection of target cells was visualized by IFA using antibodies against the HHV-8 proteins ORF59, K8.1 and LANA-1 (Advanced Biologics Inc, Columbia, MD). Initially, infected cells were stained with antibodies against all three proteins. These results demonstrated that ORF59 expression was always present in infected cells and therefore, in later experiments, we only used antibodies against ORF59 to demonstrate viral infection. For immunofluorescence, cells were fixed in 1% paraformaldehyde, permeabilized in 0.1% Triton-X100 in PBS and dried to poly-L-lysine coated slides. Wells were blocked in 10% goat serum in PBS for 30 min at 37°C and subsequently incubated with the primary antibody at 37°C for 1 hr. Wells were washed in PBS and incubated with goat anti-mouse-IgG-FITC (ORF59 and K8.1, Santa Cruz Biotechnology, Santa Cruz, CA) or goat anti-rat-IgG-FITC (LANA-1, Dako, Glostrup, Denmark) at 37°C for 1 hr. K562 cells expressing DC-SIGN mutants were confirmed for DC-SIGN expression by IFA using a polyclonal anti-DC-SIGN antibody (Calbiochem, Gibbstown, NJ) and goat-anti-rabbit IgG-FITC (Santa Cruz Biotechnology, Santa Cruz, CA) and/or monoclonal anti-DC-SIGN (R&D Systems, Minneapolis, MN) and goat-anti-mouse IgG-FITC (Santa Cruz Biotechnology, Santa Cruz, CA) in a similar fashion.
Flow Cytometry
Antibodies used for flow cytometry were purchased from Advanced Biotechnologies (anti-HHV-8 ORF 59), Calbiochem (anti-DC-SIGN), Pharmingen (anti-mouse), and Invitrogen (anti-rabbit, Alexa Fluor 488). 2×106 were washed and resuspended in phosphate buffered saline containing 2% fetal bovine serum and 0.02% sodium azide. Cells were fixed with 2% paraformaldehyde, and permeabilized with 0.2% Triton-X-100. Cells were then incubated with the appropriate primary antibody, washed 3 times in phosphate buffered saline, then incubated with the appropriate secondary antibody. Cells were washed and resuspended in phosphate buffered saline containing 2% fetal bovine serum and 0.02% sodium azide until flow cytometric analysis via a Coulter EPICS XL-MCL Flow Cytometer. The population of cells viable for analysis was determined by gating according to a typical forward and side scatter for the cells observed. Each experiment was performed three times. Results were analyzed using Cyflogic (CyFlo Ltd. Finland).
In order to determine the number of DC-SIGN positive cells, the parental K562 cells (which do not express DC-SIGN) were compared to K562 cells that expressed wild type DC-SIGN. Gates were set so that the non-DC-SIGN expressing parental K562 cells demonstrated no background staining, and were set at 0%. The same methodology was used to determine the positive number of ORF 59 expressing cells. In order to determine the percentage of cells positive for HHV-8 post infection, the number of ORF 59 positive cells (48 hrs p.i.) was divided by the percentage of ‘available for infection’ DC-SIGN positive cells.
Results
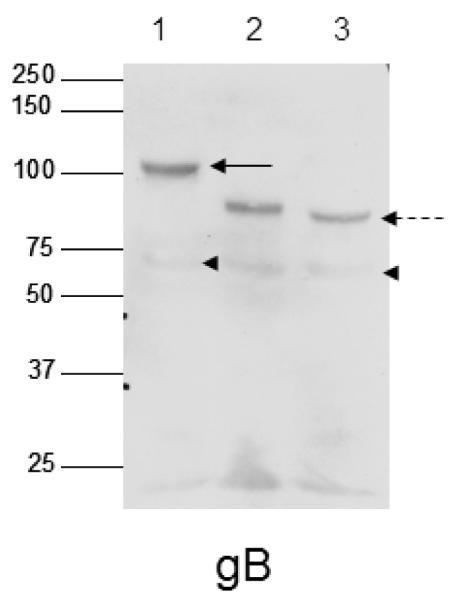
As it had been previously shown that HHV-8 gB was highly mannosylated (Baghian et al., 2000), we sought to confirm that the baculovirus constructs which we have obtained were consistent with this observation under our expression conditions. To this end, purified soluble gB protein made in Sf9 cells was digested with Endoglycosidase H (EndoH), which cleaves N-linked glycoproteins at the chitobiose core of high mannose and some hybrid oligosaccharides, or PNGaseF, which cleaves N-linked glycoproteins between the innermost GlcNAc and asparagine residues of high mannose, hybrid and complex oligosaccharides. As shown in Fig. 1, soluble gB was cleaved to a similar extent by both PNGaseF and Endoglycosidase H, as demonstrated by the migration of a lower molecular weight band (solid arrows vs. dotted arrow), indicating that gB glycosylation is predominantly high mannose.
Fig. 1.
gB is highly mannosylated. Soluble gB was left untreated (lane 1) or digested with PNGaseF (lane 2) or EndoH (lane 3) and western blotted with the anti-gB antibody. Arrows show full length bands, dotted arrows show digested full length proteins, and arrowheads show undigested and digested sizes of gB ~68kDa cleaved portion.
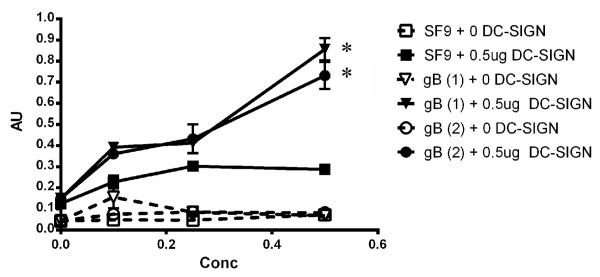
Next, we sought to determine if soluble gB was capable of binding DC-SIGN. To this end, we constructed an ELISA in which soluble gB was bound to a plastic plate and allowed to interact with biotin labeled soluble DC-SIGN. Binding of the soluble DC-SIGN was detected using Streptavidin-HRP as described in Materials and Methods. Two negative controls were used; uninfected Sf9 lysate was His-purified in the same manner as the soluble proteins and bound to the plate in place of the purified soluble gB (black lines), and wells containing bound gB or the uninfected Sf9 lysate were exposed to blocking buffer rather than DC-SIGN (dotted lines). As shown in Fig. 2, soluble gB from two separate preparations (denoted 1 and 2) bound at significantly higher levels (Oneway ANOVA, p< 0.05) to DC-SIGN in a dose-dependent manner, while little binding was seen with the uninfected Sf9 lysate.
Fig. 2.
Soluble gB binds DC-SIGN in a dose-dependent manner. ELISAs were performed using plates bound with uninfected Sf9 lysates 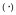 , 2 independent preparations of soluble gB (1 & 2, ▼ &
, 2 independent preparations of soluble gB (1 & 2, ▼ & 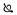 ) at varying amounts. Plates were incubated with 0
) at varying amounts. Plates were incubated with 0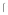 g (open symbols, dotted lines) or 0.5
g (open symbols, dotted lines) or 0.5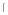 g (solid symbols, solid lines) of soluble DC-SIGN. Error bars represent results of duplicate wells. Representative of multiple experiments. *=p<0.05 as determined by one way ANOVA compared to Sf9 lysate plus 0
g (solid symbols, solid lines) of soluble DC-SIGN. Error bars represent results of duplicate wells. Representative of multiple experiments. *=p<0.05 as determined by one way ANOVA compared to Sf9 lysate plus 0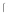 g DC-SIGN.
g DC-SIGN.
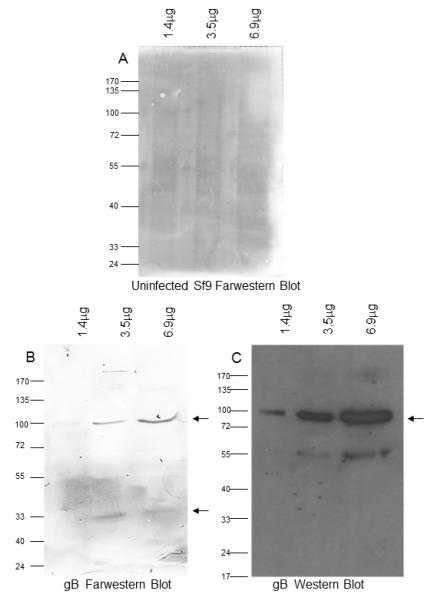
As a second method to demonstrate gB binding to DC-SIGN, we performed far western blot analyses. Briefly, varying amounts of soluble gB or uninfected Sf9 lysate were subjected to SDS-PAGE, transferred to membrane and subsequently incubated with biotinylated soluble DC-SIGN. As seen in Fig. 3b, DC-SIGN bound only to the immobilized soluble gB and this binding was dose-responsive as shown by bands of increasing density. There was no binding detected of soluble DC-SIGN to the blot containing the uninfected Sf9 lysate at similar protein levels (Fig. 3a). The full length gB protein is approximately 112 kDa. When expressed on virions, it is often cleaved into two smaller proteins with molecular weights of approximately 75 and 59 kDa (Baghian et al., 2000). The soluble version we have used has a full length of about 100 kDa and cleaved forms of 68 and 36 kDa (Wang et al., 2003). An advantage of the far western blot analyses is that it allows determination of which gB bands bind DC-SIGN. The soluble DC-SIGN bound two of the three gB bands; the full length undigested band of ~100 kDa and the smaller cleaved band of ~36 kDa (Fig. 3b). A Western blot of the soluble gB proteins analyzed in Fig 3(b) is shown in Fig. 3(c). The soluble gB proteins were detected using the gB-N1 antisera which is directed against a peptide located in the ~68 kDa cleaved portion. As a result, the only gB bands detected by this sera are the full length and 68 kDa bands as shown Fig. 3(c). These results suggest that soluble gB can bind DC-SIGN through a region between amino acids 440-702.
Fig. 3.
gB binds DC-SIGN in a far western blot. (a-b). Lysates (Lane 1, 1.4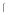 g, lane 2, 3.5
g, lane 2, 3.5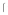 g and lane 3, 6.9
g and lane 3, 6.9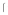 g) of uninfected Sf9 (a), or soluble gB (b) were treated with soluble DC-SIGN in a Far Western blot. (c). Corresponding western blot of soluble gB at the same amounts. Arrow in b & c show the full length soluble gB band (~100kDa) and low molecular weight cleaved band (~36kDa). Representative of multiple experiments.
g) of uninfected Sf9 (a), or soluble gB (b) were treated with soluble DC-SIGN in a Far Western blot. (c). Corresponding western blot of soluble gB at the same amounts. Arrow in b & c show the full length soluble gB band (~100kDa) and low molecular weight cleaved band (~36kDa). Representative of multiple experiments.
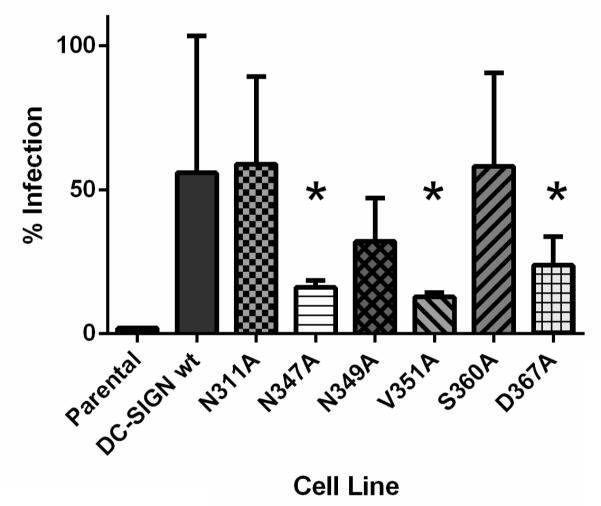
Previous studies have shown that DC-SIGN binds host (ICAM-2/3) and pathogenic (HIV gp120) ligands in separate but overlapping sites (Geijtenbeek et al., 2002; Su et al., 2004). Therefore, we sought to determine whether any of these sites were important for HHV-8 infection. To this end, K562 cell lines expressing 6 different mutations in the DC-SIGN carbohydrate recognition domain were infected with HHV-8. As described in Materials and Methods, the expression and genotype of each mutant cell line was confirmed by IFA and DNA sequencing (data not shown). It follows, that if the particular cell line is permissive for HHV-8 infection, the DC-SIGN mutation expressed in that cell line is not in a position which affects HHV-8 entry. Conversely, if the cell line is not permissive, it suggests that the mutation does affect HHV-8 entry, likely due to its inability to bind DC-SIGN. Parental K562 cells and K562-DC-SIGN expressing cells served as negative and positive controls respectively. Table 1 shows both of these cell types stained for expression of the viral protein ORF59 at 48 hours post-infection and then assayed by flow cytometry. These cells served as the basis for the determination of infection of the mutant cell lines. The cell lines expressing the different DC-SIGN mutants were assayed for infectivity by flow cytometry by staining for both DC-SIGN and ORF59 expression. We analyzed the cells lines for expression of DC-SIGN prior to infection as we have previously demonstrated that HHV-8 infection results in downregulation of DC-SIGN expression (Rappocciolo et al., 2006). Table 1 shows the results of the flow cytometry experiments, in which DC-SIGN was stained prior to infection, and ORF 59 expression was assayed 48 hours post infection. Fig. 4 shows the averages of three separate experiments. As shown in Fig. 4, several of the DC-SIGN mutations affect HHV-8 infectivity. Mutations in amino acids E347, V351, and D367 are important for HHV-8 infectivity, while mutations in amino acids N311, N349, and S360 did not reduce HHV-8 infectivity. These results were confirmed in multiple immunofluorescence assays (data not shown).
Table 1.
Flow cytometry analysis of HHV-8 infection of K562 cells expressing DC-SIGN mutations. Cells were stained at time 0 for DC-SIGN and at 48 hpi for ORF59. (a) Percentages of DC-SIGN expressing K562 cells prior to infection and ORF 59 expressing cells post-infection are shown in triplicate.
| Experiment Number |
K562 | Clone % of DC-SIGN + Cells Post- Transfection |
% of ORF 59 + Cells Post Infection |
Ratio of Virus + Cells to Population of Infectable Cells |
|---|---|---|---|---|
| 1 | Parental | 0 | 0 | NA |
| DC-SIGN wt | 36.77 | 10.99 | 29.89% | |
| N311A | 14.81 | 3.78 | 25.52% | |
| N347A | 23.38 | 4.37 | 18.69% | |
| N349A | 9.42 | 2.7 | 28.66% | |
| V351A | 74.45 | 8.52 | 11.44% | |
| S360A | 38.09 | 13.87 | 36.41% | |
| D367A | 74.75 | 15.55 | 20.80% | |
| 2 | Parental | 0 | 0 | NA |
| DC-SIGN wt | 36.77 | 9.86 | 26.82% | |
| N311A | 14.81 | 12.55 | 84.74% | |
| N347A | 23.38 | 3.69 | 15.78% | |
| N349A | 9.42 | 4.57 | 48.51% | |
| V351A | 74.45 | 10.7 | 14.37% | |
| S360A | 38.09 | 36.36 | 95.46% | |
| D367A | 74.75 | 11.69 | 15.64% | |
| 3 | Parental | 0 | 0 | NA |
| DC-SIGN wt | 20.3 | 22.49 | 110.79% | |
| N311A | 11.36 | 7.56 | 66.55% | |
| N347A | 28.02 | 3.89 | 13.88% | |
| N349A | 5.97 | 1.13 | 18.93% | |
| V351A | 21.23 | 2.68 | 12.62% | |
| S360A | 18.69 | 7.97 | 42.64% | |
| D367A | 13.32 | 4.64 | 34.83% |
Fig. 4.
Flow cytometry analysis of HHV-8 infection of K562 cells expressing DC-SIGN mutations. Cells were stained at time 0 for DC-SIGN and at 48 hpi for ORF59. Results calculated from Table 1 are shown. Results were confirmed by immunofluorescence assay (data not shown). Levels are presented based on: % infection = (% Virus+/% DC-sign+)*100. Statistical analysis were done in GraphPad. Asterick denoted bars (E347A, V351A and D367A) were shown to be significantly different from DC-SIGN wt. (One Sample T-test, alpha=0.05).
Discussion
In this study, we have demonstrated that soluble HHV-8 gB serves as a viral attachment protein responsible for binding to DC-SIGN. This interaction was shown to be dose-responsive both by ELISA and far western blot. These results agree with previously published reports that DC-SIGN binds high mannose glycoproteins (such as gB) preferentially over complex sugars (Feinberg et al., 2001). Additionally, two other studies have shown that DC-SIGN binds to the CMV and herpes simplex virus types 1 and 2 gB homologues (de Jong et al., 2008; Halary et al., 2002). Glycosylation patterns of the remaining 7 HHV-8 glycoproteins have not yet been determined, save for K8.1A and gN, which have been shown to have complex glycosylation (Koyano et al., 2003; Wu et al., 2000). We cannot discount that other glycoproteins can bind DC-SIGN, as we have only investigated gB. Future studies will address whether other HHV-8 glycoproteins can be used in place of gB for entry of HHV-8.
Interestingly, it appears that the lower molecular weight (~36kDa) cleaved portion of soluble gB (corresponding to aa. 440-702 of the soluble protein) binds to DC-SIGN based on our far western results. This is somewhat unexpected as this region is nearest to the transmembrane region of the protein in the sequence (Wang et al., 2003), and is predicted to have less glycosylation sites than the larger molecular weight portion. However, as the structure of HHV-8 gB has not been resolved, we cannot determine the precise location of this region once the protein has folded. Alignment with the sequences of HSV-1 and EBV gB, whose structures are known, suggests that this region would correspond to the Domains III-V (core, crown, and arm) which are located on the main stalk and top surface of the protein, rather than the cleaved flexible base regions (Domains I & II) which contain the fusion loops (Backovic et al., 2007; Backovic et al., 2009; Hannah et al., 2007; Heldwein et al., 2006). However, the published structures correspond to what is thought to be “post-fusion” conformations, based on similarities to VSV G protein, but are predicted to still be at least partially exposed in the hypothetical “pre-fusion” conformation (Backovic et al., 2009). Therefore, based on these results we propose that gB binds DC-SIGN at an exposed portion furthest from the viral membrane, which would avoid hindering the flexibility of the arm regions and subsequent insertion of the fusion loops into the cell membrane. Future studies are required to prove this hypothesis, as well as, whether gB binds DC-SIGN in a glycosylation dependent manner.
We have shown that HHV-8 infects cells by binding to the DC-SIGN CRD in a region that overlaps both ICAM and gp120 binding sites. As the N311 mutant is not inside the CRD, it does not affect binding of ICAM, gp120 or HHV-8 infection, and similarly, S360 was in a region unimportant for all three ligands (Geijtenbeek et al., 2002; Su et al., 2004). Amino acid N349 is a calcium binding site and was found to be important to binding ICAM and gp120. It demonstrated reduced infectivity with HHV-8. The other amino acids tested had various discrepancies, as E347 and D367 were important for all three ligands, while V351 was important for ICAM binding and HHV-8 infectivity. Previously published studies assessed comparative binding levels of ICAMs and gp120 and found that both of these ligands have low to mid-level binding to most of the mutants (Geijtenbeek et al., 2002; Su et al., 2004). As shown in Fig. 5, all mutants were susceptible to HHV-8 infection to some extent, which likely reflects lower binding ability of gB as similar to the other studies.
Fig. 5.

HHV-8, ICAM and gp120 binding sites on DC-SIGN CRD. The amino acid sequence of the DC-SIGN CRD is shown. Amino acids shown to be involved in ICAM or gp120 binding by previous studies and HHV-8 soluble gB from the present study are shown as solid bars below the single amino acid codes. White asterisk refers to disputed mutation between two published studies. Letters in bold refer to c-type lectin conserved CA+2 binding sites. Hatched bars show mutations tested in each study that did not affect binding or infection. Mutation N311A (which is outside the CRD) is not shown.
This study has identified key components of the initial protein-protein interactions required for initiation of HHV-8 infection. Identification of the kinetics of early infection can lead to the development of specific anti-viral therapies designed to prevent infection of susceptible cells which may, in turn, also prevent the development of HHV-8-related cancers.
Highlights.
Glycoprotein B (gB) of human herpesvirus 8 (HHV-8) has a high mannose carbohydrate structure.
The HHV-8 gB protein binds to the viral entry receptor, DC-SIGN.
HHV-8 infects cells by binding to the DC-SIGN carbohydrate domain in a region that overlaps both ICAM and HIV gp120 binding sites.
Acknowledgements
This work was supported by NIH grants R01 CA 82053 and U01 AI 35041.
The authors thank Drs. Sal Butera and Hongwei Jia (Centers for Disease Control and Prevention, Atlanta, GA) for providing the soluble DC-SIGN protein, Dr. Bala Chandran (Rosalind Franklin University, North Chicago, IL) for a baculovirus expressing gB, and Dr. Benhur Lee (UCLA, Los Angeles, CA) for the DC-SIGN point mutation constructs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderluh M, Jug G, Svajger U, Obermajer N. DC-SIGN antagonists, a potential new class of anti-infectives. Current medicinal chemistry. 2012;19(7):992–1007. doi: 10.2174/092986712799320664. [DOI] [PubMed] [Google Scholar]
- Backovic M, Jardetzky TS, Longnecker R. Hydrophobic residues that form putative fusion loops of Epstein-Barr virus glycoprotein B are critical for fusion activity. Journal of virology. 2007;81(17):9596–9600. doi: 10.1128/JVI.00758-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backovic M, Longnecker R, Jardetzky TS. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(8):2880–2885. doi: 10.1073/pnas.0810530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghian A, Luftig M, Black JB, Meng YX, Pau CP, Voss T, Pellett PE, Kousoulas KG. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology. 2000;269(1):18–25. doi: 10.1006/viro.2000.0198. [DOI] [PubMed] [Google Scholar]
- Cassol E, Cassetta L, Rizzi C, Gabuzda D, Alfano M, Poli G. Dendritic Cell-Specific ICAM-3 Grabbing Nonintegrin mediates HIV-1 Infection of and Transmission by M2a-Polarized Macrophages In Vitro. AIDS. 2012 doi: 10.1097/QAD.0b013e32835cfc82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis BM, Scharnowske S, Watson AJ. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc.Natl.Acad.Sci.U.S.A. 1992;89(17):8356–8360. doi: 10.1073/pnas.89.17.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MA, de Witte L, Bolmstedt A, van Kooyk Y, Geijtenbeek TB. Dendritic cells mediate herpes simplex virus infection and transmission through the C-type lectin DC-SIGN. The Journal of general virology. 2008;89(Pt 10):2398–2409. doi: 10.1099/vir.0.2008/003129-0. [DOI] [PubMed] [Google Scholar]
- Feinberg H, Mitchell DA, Drickamer K, Weis WI. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294(5549):2163–2166. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- Gardner JP, Durso RJ, Arrigale RR, Donovan GP, Maddon PJ, Dragic T, Olson WC. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(8):4498–4503. doi: 10.1073/pnas.0831128100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TBH, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GCF, Middel J, Cornelissen ILMH, Nottet HSLM, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100(5):587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TBH, van Duijnhoven GCF, van Vliet SJ, Krieger E, Vriend G, Figdor CG, van Kooyk Y. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. Journal of Biological Chemistry. 2002;277(13):11314–11320. doi: 10.1074/jbc.M111532200. [DOI] [PubMed] [Google Scholar]
- Halary F, Amara A, Lortat-Jacob H, Messerle M, Delaunay T, Houles C, Fieschi F, Arenzana-Seisdedos F, Moreau JF, Dechanet-Merville J. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity. 2002;17(5):653–664. doi: 10.1016/s1074-7613(02)00447-8. [DOI] [PubMed] [Google Scholar]
- Hannah BP, Heldwein EE, Bender FC, Cohen GH, Eisenberg RJ. Mutational evidence of internal fusion loops in herpes simplex virus glycoprotein B. Journal of virology. 2007;81(9):4858–4865. doi: 10.1128/JVI.02755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313(5784):217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- Knowlton ER, Lepone LM, Li J, Rappocciolo G, Jenkins FJ, Rinaldo CR. Professional antigen presenting cells in human herpesvirus 8 infection. Frontiers in immunology. 2012;3:427. doi: 10.3389/fimmu.2012.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano S, Mar EC, Stamey FR, Inoue N. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J.Gen.Virol. 2003;84(Pt 6):1485–1491. doi: 10.1099/vir.0.18941-0. [DOI] [PubMed] [Google Scholar]
- Lin G, Simmons G, Pohlmann S, Baribaud F, Ni HP, Leslie GJ, Haggarty B, Bates P, Weissman D, Hoxie JA, Doms RW. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. Journal of virology. 2003;77(2):1337–1346. doi: 10.1128/JVI.77.2.1337-1346.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 4th. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 2803–2833. [Google Scholar]
- Nakamura H, Lu M, Gwack Y, Souvlis J, Zeichner SL, Jung JU. Global changes in Kaposi’s sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J.Virol. 2003;77(7):4205–4220. doi: 10.1128/JVI.77.7.4205-4220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann S, Zhang J, Baribaud F, Chen ZW, Leslie G, Lin G, Granelli-Piperno A, Dom RW, Rice CM, McSKeating JA. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. Journal of virology. 2003;77(7):4070–4080. doi: 10.1128/JVI.77.7.4070-4080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promadej-Lanier N, Srinivasan P, Curtis K, Adams DR, Kim C, Luo W, Jia H, Subbarao S, Otten RA, Butera S. Systemic and mucosal immunological responses during repeated mucosal SHIV(162P3) challenges prior to and following infection in pigtailed macaques. Virology. 2008;375(2):492–503. doi: 10.1016/j.virol.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. Journal of virology. 2008;82(10):4793–4806. doi: 10.1128/JVI.01587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, Borowski L, Watkins SC, Rinaldo CR., Jr. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol. 2006;176(3):1741–1749. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- Samreen B, Khaliq S, Ashfaq UA, Khan M, Afzal N, Shahzad MA, Riaz S, Jahan S. Hepatitis C virus entry: role of host and viral factors. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2012;12(8):1699–1709. doi: 10.1016/j.meegid.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Simmons G, Reeves JD, Grogan CC, Vandenberghe LH, Baribaud F, Whitbeck JC, Burke E, Buchmeier MJ, Soilleux EJ, Riley JL, Doms RW, Bates P, Pohlmann S. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology. 2003;305(1):115–123. doi: 10.1006/viro.2002.1730. [DOI] [PubMed] [Google Scholar]
- Soilleux EJ, Morris LS, Trowsdale J, Coleman N, Boyle JJ. Human atherosclerotic plaques express DC-SIGN, a novel protein found on dendritic cells and macrophages. Journal of Pathology. 2002;198(4):511–516. doi: 10.1002/path.1205. [DOI] [PubMed] [Google Scholar]
- Su SV, Hong P, Baik S, Negrete OA, Gurney KB, Lee B. DC-SIGN binds to HIV-1 glycoprotein 120 in a distinct but overlapping fashion compared with ICAM-2 and ICAM-3. J.Biol.Chem. 2004;279(18):19122–19132. doi: 10.1074/jbc.M400184200. [DOI] [PubMed] [Google Scholar]
- van den Berg LM, Gringhuis SI, Geijtenbeek TB. An evolutionary perspective on C-type lectins in infection and immunity. Annals of the New York Academy of Sciences. 2012;1253:149–158. doi: 10.1111/j.1749-6632.2011.06392.x. [DOI] [PubMed] [Google Scholar]
- Wang FZ, Akula SM, Sharma-Walia N, Zeng L, Chandran B. Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. J.Virol. 2003;77(5):3131–3147. doi: 10.1128/JVI.77.5.3131-3147.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Renne R, Ganem D, Forghani B. Human herpesvirus 8 glycoprotein K8.1: expression, post-translational modification and localization analyzed by monoclonal antibody. J.Clin.Virol. 2000;17(2):127–136. doi: 10.1016/s1386-6532(00)00085-8. [DOI] [PubMed] [Google Scholar]
- Zhu FX, Chong JM, Wu L, Yuan Y. Virion proteins of Kaposi’s sarcoma-associated herpesvirus. J.Virol. 2005;79(2):800–811. doi: 10.1128/JVI.79.2.800-811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]