Abstract
The controlled drug delivery technology has progressed over the last six decades. It began in 1952 with the introduction of the first sustained release formulation. The 1st generation (1950-1980) of drug delivery was focused on developing oral and transdermal sustained release systems and establishing the controlled drug release mechanisms. Attention of the 2nd generation (1980-2010) was dedicated to development of zero-order release systems, self-regulated drug delivery systems, long-term depot formulations, and nanotechnology-based delivery systems. The latter part of the 2nd generation was consumed mostly for studying nanoparticle formulations. The Journal of Controlled Release (JCR) has played a pivotal role during the 2nd generation of drug delivery technologies, and it will continue playing a leading role for the next generation. Taking the right path towards the productive 3rd generation of drug delivery technologies requires honest open dialogues without any preconceived ideas of the past. The drug delivery field needs to take a bold approach of designing the future drug delivery formulations first, based on today’s necessities, and produce necessary innovations. The JCR will provide the forum for sharing the new ideas that will shape the 3rd generation of drug delivery technologies.
Keywords: Evolution of drug delivery, smart polymers, modulated delivery, targeted delivery, depot formulations
1. Historical Perspective
The first issue of the Journal of Controlled Release (JCR) was published in 1984. As stated clearly in the first editorial by Jorge Heller and Jan Feijen, the two founding editors, JCR was designed to serve as the leading forum for the drug delivery scientists to exchange their ideas through high quality manuscripts [1]. Since then, the JCR has grown to be one of the most influential journals in pharmaceutics and drug delivery fields. The key to the success of the JCR has been its emphasis on the high quality research, and this tradition was continued with Colin G. Pitt, who succeeded the Founding Editors in 1996 and served as the Editor-in-Chief until 2005. The passions and dedications by the three editors during the first two decades have been essential in establishing the foundation for the journal to grow as the forum to publish new information in drug delivery.
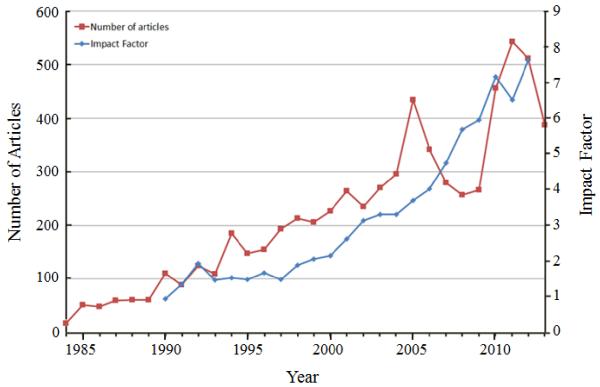
The number of publications has gradually increased over the years, as shown in Fig 1. The JCR receives substantially more manuscripts than it publishes. The main criteria for publication in the JCR are the quality and the novelty of the research presented in the submitted manuscripts. One parameter in measuring the influence of the journal to the field is the impact factor, and the JCR impact factor has grown over the years. In 2013, the impact factor reached above 7, and it places the JCR at the top of the research journals in the pharmaceutics and drug delivery fields. The success of the JCR would not have been possible without the loyalty of the authors, the dedication of reviewers, and the diligence of all editors for the last 30 years.
Fig. 1.
The number of articles published annually in the JCR since 1984.
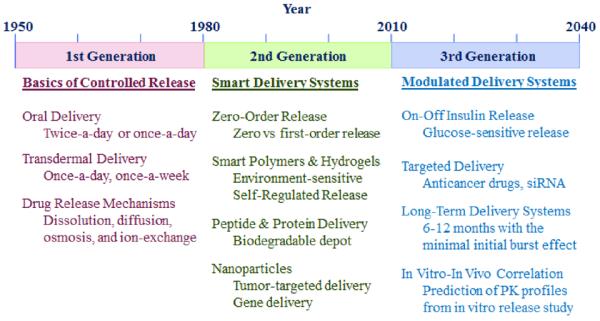
The contribution of the JCR to the drug delivery field can be best understood by examining the history of controlled drug delivery technologies. Table 1 describes the three generations of drug delivery. The first controlled release formulation was introduced by Smith Kline & French in 1952 for 12-hour delivery of dextroamphetamine (Dexedrine) [2, 3]. Since then, until the end of the 1970s, the basic understanding of controlled drug delivery was established, such as different drug release mechanisms including dissolution-, diffusion-, osmosis-, and ion exchange-based mechanisms. The technologies developed during the 1st generation were used to develop numerous twice-a-day and once-a-day oral delivery systems. The same drug release mechanisms were also used to develop once-a-day and once-a-week transdermal patches. The JCR was launched at the beginning of the 2nd generation of controlled drug delivery technologies. At the time, the research efforts were focused on developing zero-order delivery systems. It was thought that the delivery systems with zero-order release kinetics would be superior because they would maintain the steady drug concentration in the blood (as illustrated in Fig. 2), and the common thinking was “the flatter the better”. After a decade of extensive efforts it was realized that zero-order delivery was not really necessary to develop sustained drug delivery systems. First, zero-order release does not result in maintenance of the constant drug concentration in the blood. This is particularly obvious for oral delivery systems. Because of the ever decreasing drug absorption properties as the formulation moves down from the upper small intestine to the large intestine, the drug concentration slowly decreases after reaching a peak. Second, maintaining the constant drug concentration in the blood is not really required for most drugs because the drug efficacy remains the same as long as the drug concentration is above the minimum effective level and below the maximum safe concentration (Fig. 2). For some drugs, such as nitroglycerin, pituitary gland hormones, and insulin, the constant blood level may not be even desired. It took a decade to understand this simple and intuitive fact, but it allowed a lot of flexibility in the design of future drug delivery systems.
Table 1.
Evolution of controlled drug delivery systems since 1950.
|
Fig. 2.
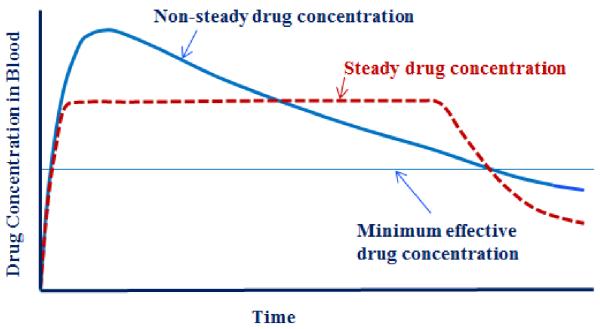
The drug is effective as long as its concentration in blood is above the minimum effective concentration regardless of the pharmacokinetic profiles. (This is assuming that the maximum drug concentration is lower than the toxic level of the drug).
During the 2nd generation, many other drug delivery technologies were developed. The so-called “smart” polymers and hydrogels were developed to make delivery systems that are triggered by changes in environmental factors, such as pH, temperature, or glucose. Biodegradable microparticles, solid implants, and in situ gel-forming implants were used to deliver peptides and proteins for months. Zoladex® Depot was the first implant that was introduced in 1989 to deliver goserelin acetate for 1 month and 3 months. Since then, only fewer than 10 clinical products were introduced to deliver other peptides and proteins, indicating the difficulties associated with product development. The last decade of the 2nd generation was dedicated to the development of nanotechnology-based drug delivery systems. The JCR has been at the center of shaping the 2nd generation of drug delivery technologies. The 3rd generation of drug delivery is yet to be established, and thus, the technologies listed in Table 1 are only predictions. For the 3rd generation of drug delivery to be beneficial, however, it should deal with overcoming the hurdles associated with the current drug delivery systems listed in Table 1.
2. The 2nd generation of drug delivery research
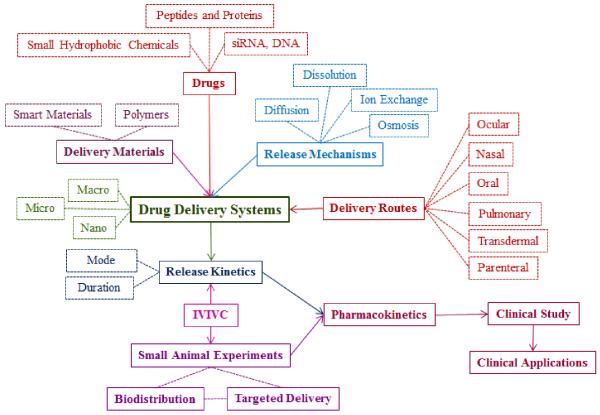
During the last 30 years, numerous articles on various topics have been published. The topics cover all aspects of drug delivery science. Because of the complexity and interdependence of the topics, it is difficult to classify the published articles in certain categories, e.g., based on the drug release mechanisms, drug types, polymers, or drug delivery routes. Such classification may be useful in understanding the trend in drug delivery research over time, but the large number of articles with numerous variations in formulations and applications makes it very difficult. Since the ultimate goal of drug delivery research is to develop formulations that can be used in clinical applications to treat various diseases, the drug delivery research can be cataloged as shown in Fig. 3.
Fig. 3.
Overview of the drug delivery system development from basic research to clinical applications. The main components of drug delivery systems and processes are shown in bold face and a solid box, and subsections of each component are shown in a dotted box. (IVIVC: In vitro - in vivo correlation).
The outline described in Fig. 3 provides an overview of drug delivery research. The most important ingredient in clinical applications is the drug. The drug, however, will not be useful without suitable delivery systems. Delivering a drug at desired release kinetics requires understanding of the physicochemical properties of the drug, which, in turn, determine the type of a delivery material and a drug release mechanism. The in vitro drug release experiment is followed by an in vivo pharmacokinetic study in animals and humans to determine the suitability of a formulation for clinical application. As shown in Fig. 3, numerous parameters need to be considered, and furthermore, their interdependence has to be taken into account to develop successful drug delivery systems for intended applications. Because of the interconnectivity without a clear starting point, development of a new drug delivery system requires simultaneous considerations of multiple factors. For example, after a drug is selected, a suitable delivery route, drug release mechanism, drug release kinetics, and drug delivery materials have to be taken into account simultaneously. It is important to understand the complexity associated with the development of a suitable drug delivery system that can ultimately be used in human patients.
From the first issue of the JCR in 1984 till the last issue in 2013, 6,569 articles have been published. The top cited papers in each year during 1984 ~ 2013 are listed in Table 2. The topics in Table 2 represent research trends in drug delivery for the last 30 years. In the 1980s, the popular topics included theoretical analysis of drug release kinetics, pH- and temperature-sensitive (i.e., smart) polymers, nasal delivery, and bioadhesive (or mucoadhesive) oral drug delivery systems. In the 1990s, the research interest in smart polymers and hydrogels and mucoahesion continued. But a new trend emerged in dealing with nanoparticles made of biodegradable polymers, polymeric micelles, lipids, chitosan, and dendrimers. From the turn of the new century, the research topics have centered on nanotechnology, in particular, targeted drug delivery to tumors and gene delivery using various nanoparticles. As the topics in Table 2 indicate, technologies in drug delivery have advanced from understanding the drug release mechanisms to manipulation of nanosized delivery vehicles for targeted drug delivery, such as pH- or temperature-sensitive nanoparticles.
Table 2.
Progression of the research topics as examined by top cited papers.
| Year | Title of Top Cited Paper | |
|---|---|---|
| 1984 | Powder dosage form of insulin for nasal administration | [4] |
| 1985 | Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues | [5] |
| 1986 | Thermally reversible hydrogels: II. Delivery and selective removal of substances from aqueous solutions |
[6] |
| 1987 | A simple equation for description of solute release II. Fickian and anomalous release from swellable devices |
[7] |
| 1988 | pH-controlled release from hydrophobic/polyelectrolyte copolymer hydrogels | [8] |
| 1989 | Solute and penetrant diffusion in swellable polymers. IX. The mechanisms of drug release from pH-sensitive swelling-controlled systems |
[9] |
| 1990 | Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patches |
[10] |
| 1991 | A novel approach for preparation of pH-sensitive hydrogels for enteric drug delivery |
[11] |
| 1992 | A new class of drug carriers: Micelles of poly(oxyethylene)-poly(oxypropylene) block copolymers as microcontainers for drug targeting from blood in brain |
[12] |
| 1993 | Block copolymer micelles as vehicles for drug delivery | [13] |
| 1994 | Enhanced tumor accumulation and prolonged circulation times of micelle-forming poly(ethylene oxide-aspartate) block copolymer-adriamycin conjugates |
[14] |
| 1995 | In vitro cytotoxicity of macromolecules in different cell culture systems | [15] |
| 1996 | The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: Effects of chitosanglutamate and carbomer on epithelial tight junctions in vitro |
[16] |
| 1997 | Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential |
[17] |
| 1998 | Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery |
[18] |
| 1999 | PLGA nanoparticles prepared by nanoprecipitation: Drug loading and release studies of a water soluble drug |
[19] |
| 2000 | Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo |
[20] |
| 2001 | Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency |
[21] |
| 2002 | Release of tetracycline hydrochloride from electrospun poly(ethylene-co- vinylacetate), poly(lactic acid), and a blend |
[22] |
| 2003 | Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: Comparison of physicochemical properties, transfection efficiency and in vivo distribution with high molecular-weight polyethylenimine |
[23] |
| 2004 | Micellar carriers based on block copolymers of poly(ε-caprolactone) and poly(ethylene glycol) for doxorubicin delivery |
[24] |
| 2005 | Block copolymer micelles: Preparation, characterization and application in drug delivery |
[25] |
| 2006 | PEG-modified gold nanorods with a stealth character for in vivo applications | [26] |
| 2007 | Coated microneedles for transdermal delivery | [27] |
| 2008 | Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles | [28] |
| 2009 | Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles |
[29] |
| 2010 | Size and shape effects in the biodistribution of intravascularly injected particles | [30] |
| 2011 | Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery |
[31] |
| 2012 | Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model |
[32] |
| 2013 | Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa - A first in vivo study in human patients |
[33] |
3. The current status of drug delivery technology
For more than the last 10 years the most popular topic in the drug delivery field has been nanoparticles. This is a result of intensive support from the governmental funding agencies on nanotechnologies since the year 2000 [34]. Significant advances have been made in manipulating properties of nanoparticles that can be administered directly to the blood in a hope to deliver most of the drug to the target site. After all, the success of tumor treatment and gene therapy, among others, depends entirely on the ability of drug delivery systems to reach their intended targets. As listed in Table 1, the majority of the nanotechnology-based research has been focused on targeted drug delivery, such as anticancer drug delivery to tumors and siRNA delivery to target cells, using nanoparticles. Although all nanoparticle drug delivery systems showed improved efficacy over the control in shrinking the tumor size in small animal models, none of the nanoparticle formulations have been successfully translated into clinical applications. As shown in Fig. 3, the ultimate goal of the drug delivery research is to produce clinically useful formulations that can help patients in treating various diseases. Thus, the lack of successful translation to clinical applications by nanoparticle formulations requires careful review of the limitations associated with the current nanoparticle systems.
In the drug delivery field, Jörg Kreuter may have been the first to use the term “nanoparticle” in 1976 [35]. In the JCR, Robert Gurny published the first research article using nanoparticulate systems for ocular drug delivery in 1986 [36]. Apparently, the term “nanoparticle” was not new in 2000 when the current nanotechnology revolution began, including the nanoparticle-based drug delivery systems. The properties of nanoparticles in drug delivery have not changed significantly over the years, and yet for more than a decade, nanoparticles have been hailed as a new tool revolutionizing drug delivery. In the hindsight analysis of the progresses made to date, one wonders what caused such frenzy on nanotechnology or nanoparticles in the first place. There was no evidence or proof that nanoparticles would be better drug delivery systems than other formulations. Nanoparticles were simply assumed to have “different” properties from those of micro/macro particles simply because of their huge surface area. Nanoparticles with enormous surface area may be useful for certain applications, such as increasing the dissolution rate of poorly soluble drugs, but other than that, no substantial advantages have been observed. Thus, a question is raised as to whether nanoparticle-based drug delivery systems will achieve truly targeted drug delivery. Numerous nanoparticle systems have been shown to accumulate at the tumor site more than the control non-particulate formulations due to the so-called enhanced permeation and retention (EPR) effect [37, 38]. It needs to be understood, however, that the increase in nanoparticle accumulation at the tumor by nanoparticles is only marginal and the total drug found at the tumor site is only a very small fraction of the total administered dose at best [34, 39-41]. While more efficient targeted drug delivery systems need to be developed, the drug delivery research has to move forward to deal with many other equally important topics.
Most topics listed in the 2nd generation of Table 1 still require answers to advance the field. Significant advances in smart polymers and hydrogels were achieved, but their clinical applications are yet to be realized. Despite introduction of numerous biodegradable polymers and better understanding on the microparticle formulations, long-term delivery of drugs, whether small or large and whether hydrophobic or hydrophilic, has been limited. For the drug delivery field to make tangible impacts in helping patients to live better, several drug delivery technologies have to be perfected.
4. Future Back
The advances that will take place in drug delivery technologies during the next 30 years are difficult to predict. Regardless of the new technologies developed, however, the diseases to treat and the hurdles to overcome for improved drug delivery will not change significantly from our current needs. Improved drug delivery technologies will have to solve the problems listed in Table 1. The demand for developing modulated insulin delivery systems will continue to increase, as the number of patients with diabetes continues to rise. Targeted drug delivery to tumors, which has been the main research focus for more than a decade will not suddenly diminish. The ability to deliver a drug for long-term, i.e., 6 months or longer, for treating chronic diseases will be essential in improving the compliance of patients. Furthermore, innovative in vitro testing methods will have to be developed to accurately predict the in vivo pharmacokinetic of drugs and drug formulations in humans.
The drug delivery scientists can wait and see what new technologies are developed in the future to solve the problems at hand. This passive approach, however, will not allow us to achieve the goals in a timely manner. Instead, the drug delivery scientists can take a bold new approach known as “future back”. The future back approach is not about imagining the future, but is more about knowing what is possible or clearly impossible, and thus finding a way to achieve the goal [42]. If the scientists rely on future innovations yet to be made, the progress will be limited to the priorities and conventions at the time, leading to limited progress. This is especially true when the innovations are incremental and quickly outdated [42]. Thus, the scientists can first describe an ultimate drug delivery system with all desirable properties, and work backward to find out how to achieve the goal, i.e., to define what innovations are necessary and how to assemble those innovations to achieve the ultimate drug delivery system.
There are at least 4 modulated delivery systems to be developed during the 3rd generation. They are glucose-sensitive transient insulin delivery with on-off switching capability, targeted delivery of anticancer agents or siRNA to tumors, long-term drug delivery ranging from 6 months to a year, and in vitro testing methods that can predict in vivo pharmacokinetic profiles. Of these, developing a modulated insulin delivery system is, technically speaking, the most challenging. Insulin delivery is different from delivery of all other drugs, in that insulin has to be delivered at the right time, i.e., when the blood glucose level increases, in an accurate amount just enough to reduce the glucose level in blood. As shown in Fig. 4, the insulin level in blood should not be constant, but pulsatile. The insulin concentration in blood should be reduced after the glucose concentration in blood is decreased. Otherwise, hypoglycemia will occur. Despite significant advances, the pulsatile drug release systems useful in clinical applications have not been achieved to date [43]. A compounding difficulty in developing successful modulated insulin delivery systems is the fact that the system has to be implanted in the body for extended periods of time, and the system should not cause any biocompatibility problems. This requires the use of biocompatible materials, which can be either biodegradable or not, that can respond to fluctuations in blood glucose level with the ability to turn on or off in a matter of minutes, if not seconds. This is a tall order by any standard, and requires multiple innovations.
Fig. 4.
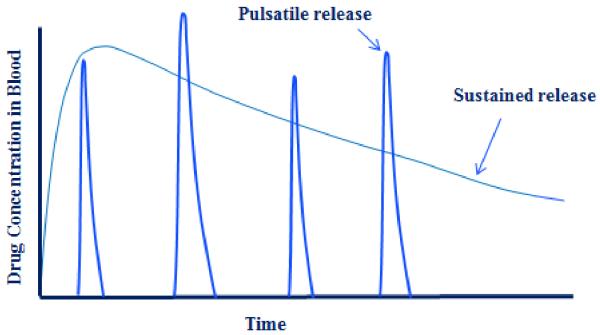
The drug concentration profiles in blood by pulsatile drug release systems as compared with the sustained release systems. The pulsatile release system requires a sensor, an on-off switch and an ability to deliver an accurate amount at the right time.
Targeted drug delivery research will continue, but the methods to achieve it need to be changed. Thus far, the scientists rely solely on nanoparticle formulations for targeted delivery to tumors. As mentioned above, this has been based on a too naïve assumption that the nanoparticles would have different properties than larger sized particles. Achieving true targeted delivery requires understanding of how foreign materials are distributed throughout the body and how to minimize the distribution of the administered nanoparticles to the unwanted tissues. The drug delivery scientists have not paid enough attention to understanding the biodistribution of nanoparticle formulations. Altering the biodistribution of administered formulations will be able to lower the side effects, even if the drug efficacy may not increase significantly. Reducing the side effects of a drug is as important as delivering more of the drug to the target site. Development of PEGylated liposome formulation of doxorubicin is a good example [44].
The number of long-term depot formulations for peptide and protein delivery is limited to only a dozen of products. This is trivial as compared with thousands of products developed for oral sustained release products. There are clearly differences in technical challenges. For oral drug delivery systems, the formulations do not have to be degradable or deliver a drug more than 24 hours. The long-term depot formulations need to deliver a drug for months. The most pressing challenge to overcome in this area is to reduce the initial burst release. Almost all formulations for depot formulations are made by double emulsion methods which usually result in substantial initial burst release. The drug concentration in the blood in the first few days is orders of magnitude higher than the steady state drug concentration. Eliminating the initial burst release for depot formulations is the key to development of more products. Recent advances in microfabrication technologies are expected to achieve this goal. Small animal models have not been a good predictor of the drug efficacy in humans. This is especially significant in targeted drug delivery. Development of in vitro models that can allow prediction of in vivo pharmacokinetic profiles will revolutionize the development of new drugs and new drug delivery systems.
5. The role of the JCR in the future of drug delivery research
The JCR has played a pivotal role in advancing the drug delivery field for the last 30 years, and will continue to play an essential role for the next 30 years. The role of the JCR in the 3rd generation of drug delivery can be best understood by asking a simple question: what the drug delivery field will miss if the JCR no longer exists. If the JCR did not exist, then the scientists would not have a primary means of publishing their best work in drug delivery. There are other journals for publication, but the significance of publishing in the JCR cannot be replaced for its focus on drug delivery technologies and its long history. It is the first journal that publishes articles only on drug delivery. The absence of the JCR will slow, if not jeopardize, the steady advances of the drug delivery field. The JCR is the only journal that has a cover story which is selected from the papers in each issue. Since 2008 when the first cover story was published, more than 100 cover stories have been published. The JCR is also the only journal that publishes concept papers which present innovative new ideas but with limited data to accelerate the publication process [45]. The JCR is the only journal that features perspective reviews which are different from regular review articles in that the authors’ opinions on a topic are reflected. It has been the only journal that has raised valuable questions against the common belief, e.g., questions about the validity of nanoparticle approaches [40, 46, 47]. Furthermore, the JCR has been publishing special issues regularly based on international symposiums, including the International Symposium on Recent Advances in Drug Delivery Systems held in the U.S.A. [48], the European Symposium on Controlled Drug Delivery held in the Netherlands [49], International Nanomedicine and Drug Delivery Symposium held in the U.S.A. [50], Asian 3 Foresight Program held in Asia [51], and Innovative Polymers for Controlled Delivery held in China [52]. Special issues or thematic issues have also been published based on symposiums on specific topics, such as nanotechnology [53], liposomes [54], and tumors [55], or research by scientists in Japan [56] and Europe [57]. The JCR will continue its tradition of excellence and leadership through publishing innovative new ideas and controversial topics.
Drug delivery scientists can make real differences in treating various diseases by doing their job in developing new drug delivery systems. It requires relentless curiosity and experiments, thinking bigger, and embracing a paradox [42]. It also requires not being trapped by dogma, which is living with the results of other people’s thinking [58]. The answers to solving the problems listed in Table 1 may already exist and all we need is simply connecting the dots. Connecting the dots is not possible looking forward, but is possible only looking backward [58]. This is all the more reason why the “future back” approach is necessary for the drug delivery scientists to make “insanely good” drug delivery systems. The drug delivery scientists should have open minds that accept different views and allow uninterrupted communication. The JCR promises to provide the forum for the free flow of new ideas and different points of views in the years to come.
Acknowledgment
This work was supported by the Showalter Research Trust Fund and the National Institute of Health through CA129287 and GM095879.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Heller J, Feijen J. Editorial. J. Control. Release. 1984;1:1. [Google Scholar]
- [2].Helfand WH, Cowen DL. Evolution of pharmaceutical oral dosage forms. Pharm. Hist. 1983;25:3–18. [PubMed] [Google Scholar]
- [3].Lee PI, Li J-X. Evolution of oral controlled release dosage forms. In: Wen H, Park K, editors. Oral Controlled Release Formulation Design and Drug Delivery. John Wiley & Sons, Inc.; Hoboken, NJ: 2010. pp. 21–31. [Google Scholar]
- [4].Tsuneji N, Yuji N, Naoki N, Yoshiki S, Kunio S. Powder dosage form of insulin for nasal administration. Journal of Controlled Release. 1984;1:15–22. [Google Scholar]
- [5].Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. Journal of Controlled Release. 1985;2:257–275. [Google Scholar]
- [6].Hoffman AS, Afrassiabi A, Dong LC. Thermally reversible hydrogels: II. Delivery and selective removal of substances from aqueous solutions. Journal of Controlled Release. 1986;4:213–222. [PubMed] [Google Scholar]
- [7].Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. Journal of Controlled Release. 1987;5:37–42. [PubMed] [Google Scholar]
- [8].Siegel RA, Falamarzian M, Firestone BA, Moxley BC. pH-Controlled release from hydrophobic/polyelectrolyte copolymer hydrogels. Journal of Controlled Release. 1988;8:179–182. [Google Scholar]
- [9].Peppas L. Brannon, Peppas NA. Solute and penetrant diffusion in swellable polymers. IX. The mechanisms of drug release from pH-sensitive swelling-controlled systems. Journal of Controlled Release. 1989;8:267–274. [Google Scholar]
- [10].Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, Tice TR. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the peyer’s patches. Journal of Controlled Release. 1990;11:205–214. [Google Scholar]
- [11].Dong L.-c., Hoffman AS. A novel approach for preparation of pH-sensitive hydrogels for enteric drug delivery. Journal of Controlled Release. 1991;15:141–152. [Google Scholar]
- [12].Kabanov AV, Batrakova EV, Nubarov N.S. Melik, Fedoseev NA, Dorodnich TY, Alakhov VY, Chekhonin VP, Nazarova IR, Kabanov VA. A new class of drug carriers: micelles of poly(oxyethylene)-poly(oxypropylene) block copolymers as microcontainers for drug targeting from blood in brain. Journal of Controlled Release. 1992;22:141–157. [Google Scholar]
- [13].Kataoka K, Kwon GS, Yokoyama M, Okano T, Sakurai Y. Block copolymer micelles as vehicles for drug delivery. Journal of Controlled Release. 1993;24:119–132. [Google Scholar]
- [14].Kwon G, Suwa S, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Enhanced tumor accumulation and prolonged circulation times of micelle-forming poly (ethylene oxide-aspartate) block copolymer-adriamycin conjugates. Journal of Controlled Release. 1994;29:17–23. [Google Scholar]
- [15].Choksakulnimitr S, Masuda S, Tokuda H, Takakura Y, Hashida M. In vitro cytotoxicity of macromolecules in different cell culture systems. Journal of Controlled Release. 1995;34:233–241. [Google Scholar]
- [16].Borchard G, Lueβen HL, Boer A.G. de, Verhoef JC, Lehr C-M, Junginger HE. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: Effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. Journal of Controlled Release. 1996;39:131–138. [Google Scholar]
- [17].Westesen K, Bunjes H, Koch MHJ. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. Journal of Controlled Release. 1997;48:223–236. [Google Scholar]
- [18].MacLaughlin FC, Mumper RJ, Wang J, Tagliaferri JM, Gill I, Hinchcliffe M, Rolland AP. Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. Journal of Controlled Release. 1998;56:259–272. doi: 10.1016/s0168-3659(98)00097-2. [DOI] [PubMed] [Google Scholar]
- [19].Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. Journal of Controlled Release. 1999;57:171–185. doi: 10.1016/s0168-3659(98)00116-3. [DOI] [PubMed] [Google Scholar]
- [20].Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers:: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. Journal of Controlled Release. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- [21].Mao H-Q, Roy K, Le V.L. Troung, Janes KA, Lin KY, Wang Y, August JT, Leong KW. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. Journal of Controlled Release. 2001;70:399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- [22].Kenawy E-R, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, Wnek GE. Release of tetracycline hydrochloride from electrospun poly(ethylene-covinylacetate), poly(lactic acid), and a blend. Journal of Controlled Release. 2002;81:57–64. doi: 10.1016/s0168-3659(02)00041-x. [DOI] [PubMed] [Google Scholar]
- [23].Kunath K, von Harpe A, Fischer D, Petersen H, Bickel U, Voigt K, Kissel T. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. Journal of Controlled Release. 2003;89:113–125. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- [24].Shuai X, Ai H, Nasongkla N, Kim S, Gao J. Micellar carriers based on block copolymers of poly(ε-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. Journal of Controlled Release. 2004;98:415–426. doi: 10.1016/j.jconrel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- [25].Gaucher G, Dufresne M-H, Sant VP, Kang N, Maysinger D, Leroux J-C. Block copolymer micelles: preparation, characterization and application in drug delivery. Journal of Controlled Release. 2005;109:169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- [26].Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. PEG-modified gold nanorods with a stealth character for in vivo applications. Journal of Controlled Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- [27].Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. Journal of Controlled Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kratz F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. Journal of Controlled Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- [29].Nam HY, Kwon SM, Chung H, Lee S-Y, Kwon S-H, Jeon H, Kim Y, Park JH, Kim J, Her S, Oh Y-K, Kwon IC, Kim K, Jeong SY. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. Journal of Controlled Release. 2009;135:259–267. doi: 10.1016/j.jconrel.2009.01.018. [DOI] [PubMed] [Google Scholar]
- [30].Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, Ferrari M. Size and shape effects in the biodistribution of intravascularly injected particles. Journal of Controlled Release. 2010;141:320–327. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- [31].Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. Journal of Controlled Release. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- [32].Ranjan A, Jacobs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS, Gacchina CE, Sharma KV, Frenkel V, Wood BJ, Dreher MR. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. Journal of Controlled Release. 2012;158:487–494. doi: 10.1016/j.jconrel.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schmidt C, Lautenschlaeger C, Collnot E-M, Schumann M, Bojarski C, Schulzke J-D, Lehr C-M, Stallmach A. Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa—A first in vivo study in human patients. Journal of Controlled Release. 2013;165:139–145. doi: 10.1016/j.jconrel.2012.10.019. [DOI] [PubMed] [Google Scholar]
- [34].Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7:7442–7447. doi: 10.1021/nn404501g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kreuter J, Speiser P. In vitro studies of poly(methylmethacrylate) adjuvants. J. Pharm. Sci. 1976;65:1624–1627. doi: 10.1002/jps.2600651115. [DOI] [PubMed] [Google Scholar]
- [36].Gurny R, Boye T, Ibrahim H. Ocular therapy with nanoparticulate systems for controlled drug delivery. Journal of Controlled Release. 1985;2:353–361. [Google Scholar]
- [37].Maeda H, Ueda M, Morinaga T, Matsumotog T. Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein-neocarzinostatin: Pronounced improvements in pharmacological properties. J. Med. Chem. 1985;28:455–461. doi: 10.1021/jm00382a012. [DOI] [PubMed] [Google Scholar]
- [38].Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- [39].Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J. Control. Release. 2012;164:108–114. doi: 10.1016/j.jconrel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hollis CP, Weiss HL, Leggas M, Evers BM, Gemeinhart RA, Li T. Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: lessons learned of the EPR effect and image-guided drug delivery. J. Control. Release. 2013;172:12–21. doi: 10.1016/j.jconrel.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stirland DL, Nichols JW, Miura S, Bae YH. Mind the gap: A survey of how cancer drug carriers are susceptible to the gap between research and practice. J. Control. Release. 2013;172:1045–1064. doi: 10.1016/j.jconrel.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fisk P. Creative Genius: An Innovation Guide for Business Leaders, Border Crossers and Game Changers. Capstone Publishing Ltd. (a Wiley Company); Chichester, West Sussex, United Kingdom: 2011. [Google Scholar]
- [43].Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution. Advanced Drug Delivery Reviews. doi: 10.1016/j.addr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- [44].Barenholz Y. Doxil® - The first FDA-approved nano-drug: Lessons learned. J. Control. Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- [45].Sommer AP, Zhu D, Scharnweber T. Laser modulated transmembrane convection: Implementation in cancer chemotherapy. J. Control. Release. 2010;148:131–134. doi: 10.1016/j.jconrel.2010.10.010. [DOI] [PubMed] [Google Scholar]
- [46].Bae YH, Park K. Targeted Drug Delivery to Tumors: Myths, Reality, and Possibility. J. Control. Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Park K. Lessons learned from thermosensitive liposomes for improved chemotherapy. J. Control. Release. 2014;174:219. doi: 10.1016/j.jconrel.2014.01.001. [DOI] [PubMed] [Google Scholar]
- [48].Bae YH, Grainger DW. Journal of Controlled Release; The 16th International Symposium on Recent Advances in Drug Delivery; Salt Lake City, UT, USA. February 3–6, 2013; 2013. pp. 393–394. [DOI] [PubMed] [Google Scholar]
- [49].Engbersen JFJ. Preface. Journal of Controlled Release. 2012;164:247. doi: 10.1016/j.jconrel.2012.08.024. [DOI] [PubMed] [Google Scholar]
- [50].Minko T, Hatefi A. Preface-tenth international nanomedicine and drug delivery symposium (NanoDDS’12) Journal of Controlled Release. 2013;171:259–260. doi: 10.1016/j.jconrel.2013.08.008. [DOI] [PubMed] [Google Scholar]
- [51].Chen X, Park TG, Maruyama A. Preface. Journal of Controlled Release. 2011;155:1. doi: 10.1016/j.jconrel.2011.08.034. [DOI] [PubMed] [Google Scholar]
- [52].Zhong Z, Feijen J. Journal of Controlled Release; The Second Symposium on Innovative Polymers for Controlled Delivery; Suzhou, China. September 11–14, 2012; 2013. pp. 163–164. [DOI] [PubMed] [Google Scholar]
- [53].Crommelin DJA, Park K, Florence A. Pharmaceutical nanotechnology: Unmet needs in drug delivery. Journal of Controlled Release. 2010;141:263–264. doi: 10.1016/j.jconrel.2009.11.019. [DOI] [PubMed] [Google Scholar]
- [54].Barenholz Y, Peer D. Liposomes and other assemblies as drugs and nano-drugs: From basic and translational research to the clinics. Journal of Controlled Release. 2012;160:115–116. doi: 10.1016/j.jconrel.2012.03.025. [DOI] [PubMed] [Google Scholar]
- [55].Bergstrom DE, Wei A. Preface. Journal of Controlled Release. 2012;164:107. doi: 10.1016/j.jconrel.2012.10.004. [DOI] [PubMed] [Google Scholar]
- [56].Harashima H, Kikuchi A, Kataoka K. Editorial. Journal of Controlled Release. 2011;149:1. doi: 10.1016/j.jconrel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- [57].Lammers T. Drug delivery research in Europe. Journal of Controlled Release. 2012;161:151. doi: 10.1016/j.jconrel.2012.05.019. [DOI] [PubMed] [Google Scholar]
- [58].Jobs S. The Commencement address at Stanford University. 2005 http://news.stanford.edu/news/2005/june15/jobs-061505.html.