Abstract
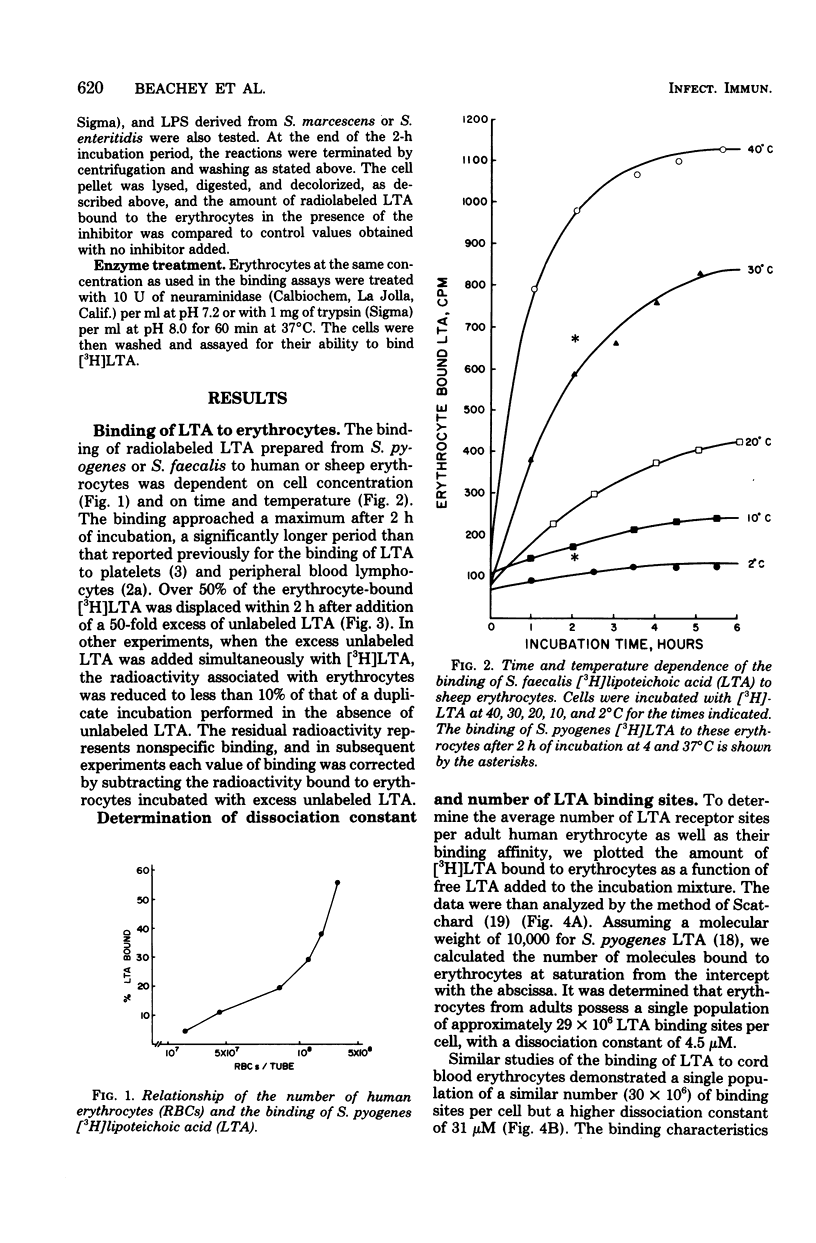
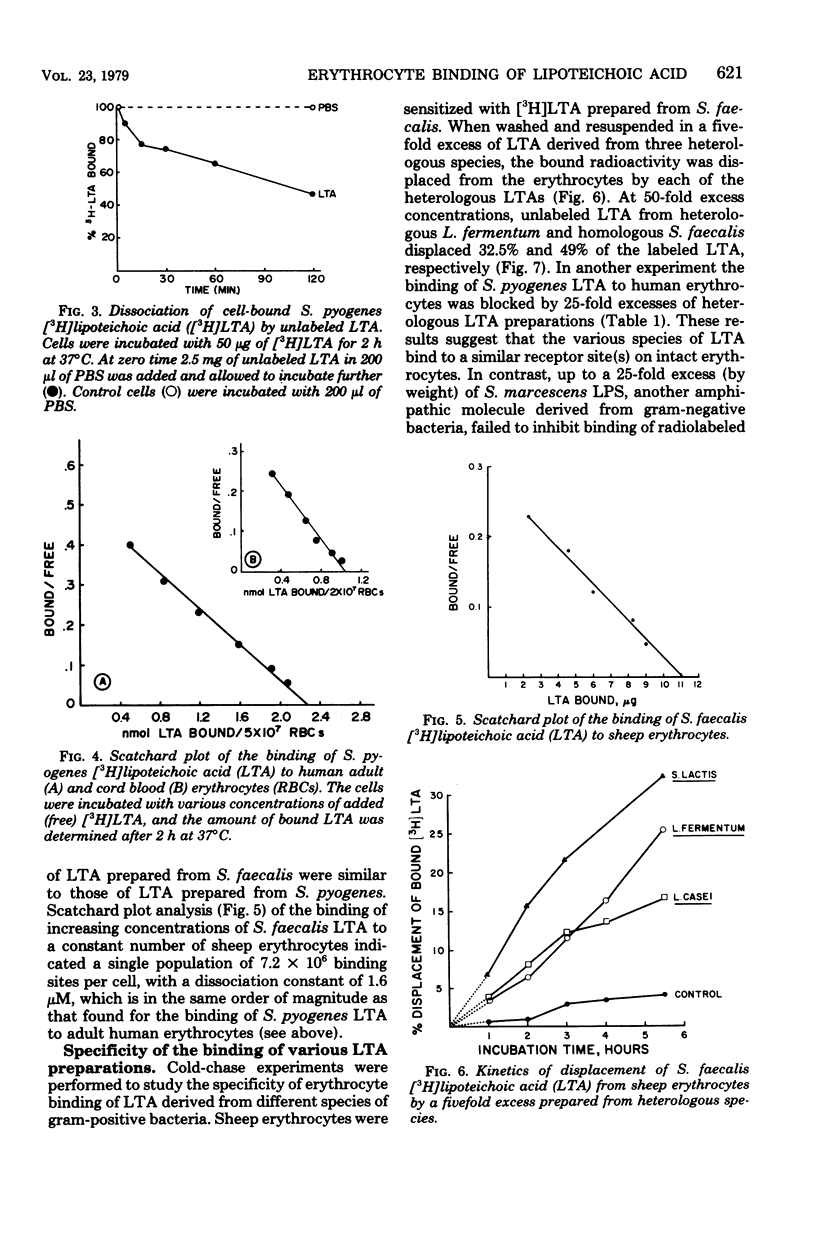
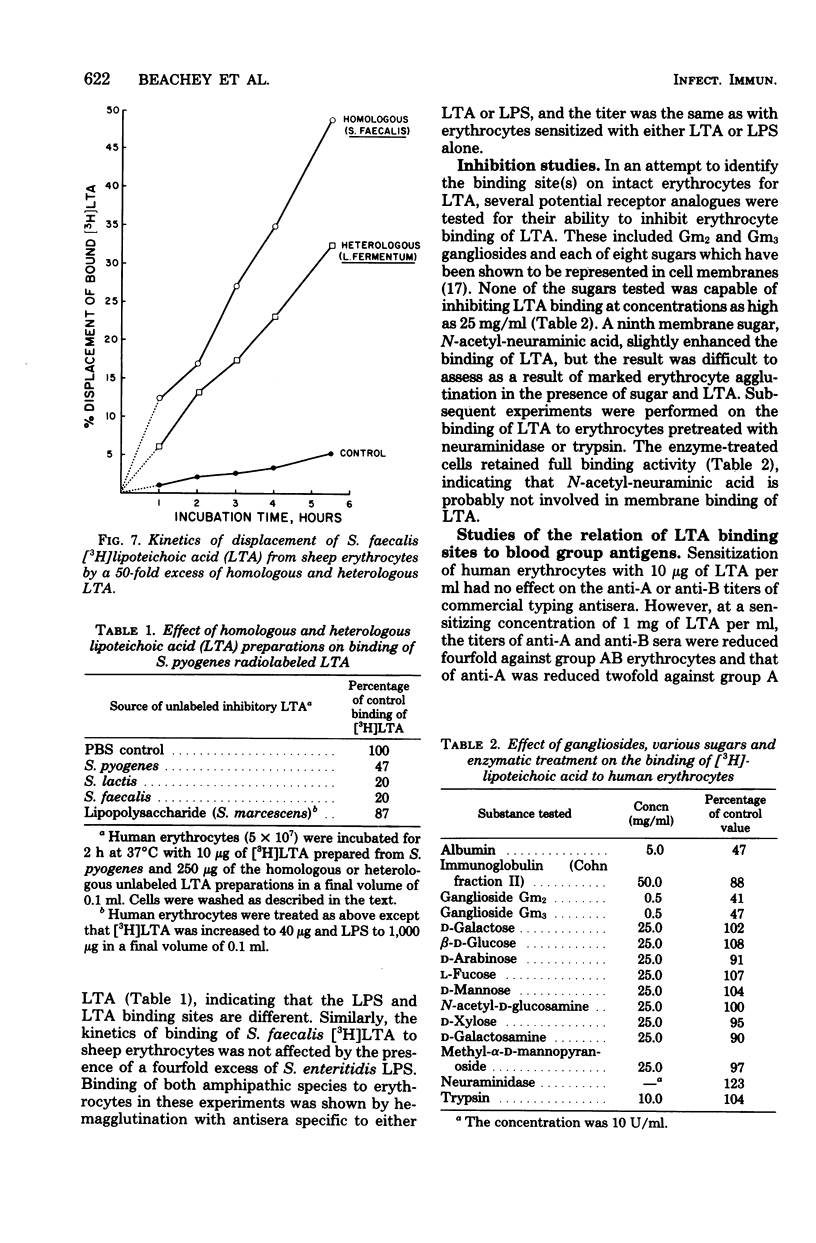
The lipoteichoic acids (LTA) of gram-positive bacteria are known to bind spontaneously to a variety of animal cell membranes. We investigated the biological and biochemical characteristics of the binding of LTA of Streptococcus pyogenes and S. faecalis to human and sheep erythrocytes. The kinetics of the binding of the radiolabeled LTA ([3H]LTA) from each of these organisms to erythrocytes was similar. The dissociation constants for sheep and adult human erythrocytes were 1.6 μM and 4.5 μM, respectively, whereas that of human cord blood erythrocytes was approximately 10-fold higher, 31 μM. The number of binding sites for sheep erythrocytes was calculated to be 7.2 × × 106 per cell, and that of human erythrocytes, 29 × 106 per cell. Binding was reversible. More than 50% of bound [3H]LTA was displaced from erythrocytes by a 50-fold excess of unlabeled LTA. LTA prepared from heterologous species of gram-positive bacteria were all inhibitory to the binding of [3H]LTA whether derived from S. pyogenes or from S. faecalis. Among a number of potential receptor analogues and other inhibitors tested, including serum albumin, gangliosides Gm2 and Gm3, lipopolysaccharide of gram-negative bacteria, and various sugars, only albumin and the gangliosides significantly inhibited LTA binding. Trypsin or neuraminidase treatment of erythrocytes had no effect on LTA binding. Deacylation of [3H]LTA abolished binding ability and binding was restored by esterification of the deacylated material with stearoyl chloride, indicating that ester-linked lipids are necessary for membrane binding.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M. L., Beachey E. H. Excretion of lipoteichoic acid by group A streptococci. Influence of penicillin on excretion and loss of ability to adhere to human oral mucosal cells. J Clin Invest. 1978 Mar;61(3):671–677. doi: 10.1172/JCI108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkan M., Ofek I., Beachey E. H. Adherence pharyngeal and skin strains of group A streptococci to human skin and oral epithelial cells. Infect Immun. 1977 Nov;18(2):555–557. doi: 10.1128/iai.18.2.555-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Chiang T. M., Ofek I., Kang A. H. Interaction of lipoteichoic acid of group A streptococci with human platelets. Infect Immun. 1977 May;16(2):649–654. doi: 10.1128/iai.16.2.649-654.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Dale J. B., Grebe S., Ahmed A., Simpson W. A., Ofek I. Lymphocytes binding and T cell mitogenic properties of group A streptococcal lipoteichoic acid. J Immunol. 1979 Jan;122(1):189–195. [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling U., Westphal O. Synthesis and use of O-stearoyl polysaccharides in passive hemagglutination and hemolysis. Eur J Biochem. 1967 Mar;1(1):46–50. doi: 10.1007/978-3-662-25813-2_9. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Serological properties of the wall and membrane teichoic acids from Lactobacillus helveticus NCIB 8025. J Gen Microbiol. 1970 Oct;63(2):237–248. doi: 10.1099/00221287-63-2-237. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Serological studies on the teichoic acids of Lactobacillus plantarum. Infect Immun. 1972 Jul;6(1):43–49. doi: 10.1128/iai.6.1.43-49.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kościelak J., Plasek A., Górniak H., Gardas A., Gregor A. Structures of fucose-containing glycolipids with H and B blood-group activity and of sialic acid and glucosamine-containing glycolipid of human-erythrocyte membrane. Eur J Biochem. 1973 Aug 17;37(2):214–225. doi: 10.1111/j.1432-1033.1973.tb02978.x. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Lehmann V., Rietschel E. T. Recent findings on the chemical structure and biological activity of bacterial lipopolysaccharides. J Hyg Epidemiol Microbiol Immunol. 1974;18(4):381–390. [PubMed] [Google Scholar]
- Miller G. A., Jackson R. W. The effect of a streptococcus pyogenes teichoic acid on the immune response of mice. J Immunol. 1973 Jan;110(1):148–156. [PubMed] [Google Scholar]
- Ne'eman N., Ginsburg I. Red cell-sensitizing antigen of group A streptococci. II. Immunological and immunopathological properties. Isr J Med Sci. 1972 Nov;8(11):1807–1816. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Eyal F., Morrison J. C. Postnatal development of binding of streptococci and lipoteichoic acid by oral mucosal cells of humans. J Infect Dis. 1977 Feb;135(2):267–274. doi: 10.1093/infdis/135.2.267. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. THE GANGLIOSIDES. J Lipid Res. 1964 Apr;5:145–155. [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Teichoic acid of a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1973 Jun;114(3):934–942. doi: 10.1128/jb.114.3.934-942.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A. Fatty acid binding to plasma albumin. J Lipid Res. 1975 May;16(3):165–179. [PubMed] [Google Scholar]
- Springer G. F., Adye J. C., Bezkorovainy A., Jirgensons B. Properties and activity of the lipopolysaccharide-receptor from human erythrocytes. Biochemistry. 1974 Mar 26;13(7):1379–1389. doi: 10.1021/bi00704a011. [DOI] [PubMed] [Google Scholar]
- Waltersdorff R. L., Fiedel B. A., Jackson R. W. Induction of nephrocalcinosis in rabbit kidneys after long-term exposure to a streptococcal teichoic acid. Infect Immun. 1977 Sep;17(3):665–667. doi: 10.1128/iai.17.3.665-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Gibbens J. W., Knox K. W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973 Jan;113(1):365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]
- Wiegandt H. Structure and specificity of gangliosides. Adv Exp Med Biol. 1976;71(00):3–14. doi: 10.1007/978-1-4614-4614-9_1. [DOI] [PubMed] [Google Scholar]



