SUMMARY
Serotonin and dopamine are major neuromodulators. Here we used a modified rabies virus to identify monosynaptic inputs to serotonin neurons in the dorsal and median raphe (DR and MR). We found that inputs to DR and MR serotonin neurons are spatially shiftedin the forebrain, with MRserotonin neurons receiving inputs from more medial structures. We then compared these data with inputs to dopamine neurons in the ventral tegmental area (VTA) and substantianigra pars compacta (SNc). We found that DR serotonin neurons receive inputs from a remarkably similar set of areas as VTA dopamine neurons, apart from the striatum, which preferentially targets dopamine neurons. Ourresults suggest three majorinput streams: amedial stream regulates MR serotonin neurons, anintermediate stream regulatesDR serotonin and VTA dopamine neurons, and alateral stream regulatesSNc dopamine neurons. These results providefundamental organizational principlesofafferent control forserotonin and dopamine.
INTRODUCTION
Serotonin and dopamine are major neuromodulators essential for flexible behavior. Both are released from small populations of neurons in the midbrain and brainstem. A unique feature of these neurons is that they receiveandintegrate inputs from many brain areas, and broadcast their outputsthrough long axons to many brain areas(Jacobs and Azmitia, 1992). Despite the importance of these neurotransmitters in normal behaviors and psychiatric disorders, their regulationremainspoorly understood.
Forebrain-projecting serotonin neurons are found in the dorsal raphe (DR) and median raphe (MR). They are thought to be involved in diverse functions including the regulation of sleep-wake cycles(Lydic et al., 1983; McGinty and Harper, 1976), motor facilitation (Jacobs and Fornal, 1997), defensive behavior(Deakin and Graeff, 1991), behavioral inhibition (Soubrie, 1986), learning from negative reinforcement(Daw et al., 2002; Dayan and Huys, 2008; Deakin and Graeff, 1991; den Ouden et al., 2013), processing reward value(Nakamura et al., 2008; Seymour et al., 2012), andtemporal discounting(Doya, 2002; Miyazaki et al., 2011). Although it is known thatDR and MR project to overlapping, yet distinct forebrain structures(Azmitia and Segal, 1978; Vertes and Linley, 2008; Vertes et al., 1999), experimental manipulations in DR and MR have yielded equivocal results, and howserotonin neurons in these areasfunction remainselusive.
Forebrain-projecting dopamine neurons are mainlyfound inthe ventral tegmental area (VTA) and the substantianigra pars compacta (SNc). Neurophysiological recordings in behaving animals have demonstrated that many putative dopamine neurons signalthe discrepancy between actual and expected reward, that is, reward prediction error(Bayer and Glimcher, 2005; Matsumoto and Hikosaka, 2009; Schultz et al., 1997). Although VTA and SNccontain diverse cell types forming complex circuits, recent studies have clarified the regulation and functional roles of dopamine neurons (Cohen et al. 2012;Lammel et al., 2012; Steinberg et al., 2013; Tan et al., 2012; Tsai et al., 2009; van Zessen et al., 2012). Furthermore, monosynaptic inputs to dopamine neurons in VTA and SNc were identified from the whole brain using a rabies-virus-based transsynaptic tracing method(Watabe-Uchida et al., 2012). These studies have provided a foundation of our understanding of the anatomy and physiology of dopamine neurons as well as their diversity(Lammel et al., 2013; Roeper, 2013).
Compared to dopamine, our understanding of serotonin has been limited. One reason is that DR and MR contain a diverse collection of cell types(Hioki et al., 2010). It has thus been difficult to identify serotonin neurons while recording in behaving animals(Allers and Sharp, 2003; Kocsis et al., 2006; Nakamura et al., 2008; Ranade and Mainen, 2009). Furthermore, although previous studies identified afferents to the DR and MR (Aghajanian and Wang, 1977; Gervasoni et al., 2000; Marcinkiewicz et al., 1989; Peyron et al., 1998; Soiza-Reilly and Commons, 2011; Vertes and Linley, 2008),technical limitations of conventional tracershave made it difficult to distinguish between synaptic inputs to serotonin versus non-serotonin neurons.
To understandthe organizing principles of afferents to serotonin neurons, we applied a rabies-virus-based tracing method (Watabe-Uchida et al., 2012; Wickersham et al., 2007)to identify monosynaptic inputs to DR and MR serotonin neuronsthroughout the brain. We thencompared the distributions of inputs to DR and MR serotonin neurons.
In addition, dopamine and serotonin are thought to be involved in related functions such as processing reward and punishment, and are thought to interact (Boureau and Dayan, 2011; Kapur and Remington, 1996). However, little is known about the anatomical basis of these interactions. We therefore compared the dataobtained for serotonin with those obtained fordopamine neuronsin a previous study(Watabe-Uchida et al., 2012). These results provide foundational information as to the global organization of monosynaptic inputs to subdivisions of the serotonin and dopamine systems.
RESULTS
Whole-brain mapping of monosynaptic inputs to serotonin neurons
To visualize monosynaptic inputs to DR and MR serotonin neurons, we used a retrograde transsynaptic tracing system based on amodified rabies virus(SADΔG-EGFP(EnvA); Wickersham et al., 2007). This virus is pseudotypedwith an avian virus envelope protein (EnvA), so that, in mammalian brains, initial infection is restricted to cells that are engineered to express a cognate receptor (TVA protein). In addition, this rabies virus lacks the gene encoding the rabies virus envelope glycoprotein (RG), which is required for transsynaptic spread. This allows for the restriction of transsynaptic spread only from cells that exogenously express RG (thus, only mono-, but not polysynaptic, inputs are labeled). To express TVA and RG in serotonin neurons, we injected two helper viruses that express TVA and RG under the control ofCrerecombinase(AAV5-FLEX-TVA-mCherry and AAV8-FLEX-RG; Watabe-Uchida et al., 2012) intomice expressing Crespecifically in serotonin neurons (Sert-Cre mice; Zhuang et al., 2005). Injections were targeted to either DR or MR. After 14 days, we injected SADΔG-EGFP(EnvA) into the same area, and analyzed the brains 7 dayslater.
Here, neurons that express TVA are labeled by a red fluorescent protein, mCherry. Neurons that are infected by the rabies virus express an enhanced green fluorescent protein, EGFP. We identified starter neurons based on co-expression of mCherry and EGFP (Figures 1A-I). Almost all double-positive neurons (95.8%) were found to be serotonin neurons(Figure 1C), by co-staining of an antibody against serotonin. Near the centerofinjection sites, 30.5% of serotonin neurons were double-positive for mCherry and EGFP. We found a small number of mCherry- and EGFP-double-positive neurons in neighboring serotonin-containing nuclei:thepontine reticular nucleus (Pn), mesencephalic reticular formation (mRt),caudal linear nucleus of the raphe (CLi) and parts of the interpeduncular nucleus (IPN). However,these neuronsmade upa small fraction of total starter neurons in most animals (Figure 1I). Starter neurons tiledalmost the entire DR or MR (Supplemental note).Across animals, the number of EGFP-positive neurons was roughly proportional to the number of starter neurons (Input=a ·Starter+b;a=8.2, p<0.05; b=1075, p=0.42)(Figures 1F-H).
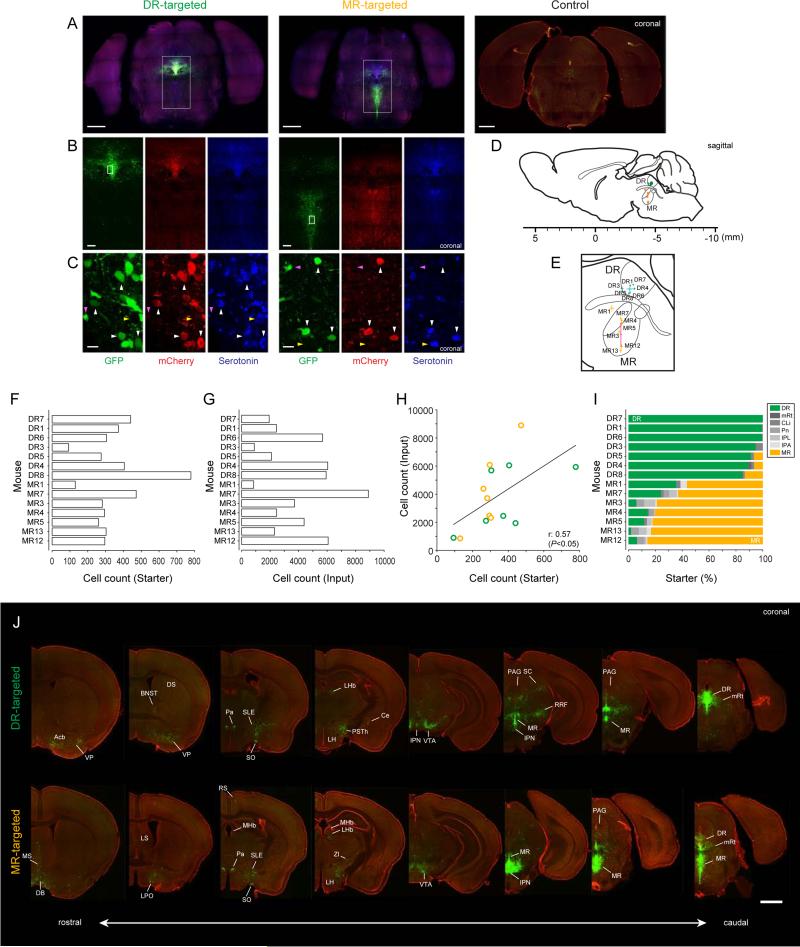
Figure 1. Identification of monosynaptic inputs to serotonin neurons using rabies virus and Sert-Cre mice.
Injection site inthe raphe nuclei of DR-targeted and MR-targetedSert-Cre micebrainsin low- (A), middle- (B) andhigh-magnification images (C). Bregma -4.65 mm. The rv-EGFP expression and immunoreactivity to mCherry and serotonin are shown in green, red and blue, respectively. The white rectangles indicate the magnified regions. White arrowheads point at neurons that are triple-positive for rv-EGFP, mCherry and serotonin (starter neurons that are serotonergic), and magenta arrowheads indicate rv-EGFP-positive but mCherry- and serotonin-negative neurons (input neurons). The yellow arrowheads point at rv-EGFP-negative serotonergic neurons..Scale bars, 1 mm in (A) 0.2 mm in (B) and 10 m in (C). (A, control) Low-magnification image of the injection site (DR) in wild type mice.Red, Nissl stain. Green, rv-EGFP.Bregma -4.5 mm. Scale bar, 1 mm.
(D and E) Centers of injection sites from individual animals.Geometric means are shown by circles (D) or crosses (E, mean ± SEM). Green, DR-targeted animals; orange, MR-targeted animals.Scale bar in (D) represents distance from bregma. Cyan and magenta crosses in (E) are mean ± SEM of centers of injection sites from 7 animals in DR-targeted group (DR1, DR3, DR4, DR5, DR6, DR7 and DR8) and 5 animals in MR-targeted group (MR3, MR4, MR5, MR12 and MR13).
(F) Numbers of starter neurons.
(G) Numbers of transsynaptically labeled neurons (“input neurons”).
(H) Relationship between numbers of starter and input neurons.
(I) Proportions of labeled neurons in each of the serotonin-neuron containing nuclei. DR, dorsal raphe;mRt, mesencephalic reticular formation;CLi, caudal linear nucleus of the raphe; Pn, pontine reticular nucleus; IPL,interpeduncular nucleus lateral subnucleus; IPA, interpeduncular nucleus apical subnucleus; MR, median raphe.
(J) Coronal sections for DR- and MR-targeted cases (DR4 and MR5, respectively).Scale bar, 1 mm. Acb, nucleus accumbens; MS, medial septal nucleus; DB diagonal band of Broca; VP, ventalpallidum; BNST, bed nucleus of the striaterminalis; DS,dorsal striatum; LS, lateral septal nucleus; LPO, lateral preoptic area; Pa, paraventricular hypothalamic nucleus; SLE, sublenticular extended amygdala; SO, supraoptic nucleus; RS, retrosplenial cortex; MHb, medial habenula; LHb, lateral habenula; PSTh, parasubthalamic nucleus; LH, lateral hypothalamus; Ce, central amygdala nucleus;ZI, zonaincerta; VTA, ventral tegmental area; IPN, interpeduncular nucleus; SC, superior colliculus; PAG, periaqueductal gray; RRF, retrorubral field.Left to right corresponds torostral to caudal (coordinates:Bregma, 0.7, 0.14, □0.88, □2.00, □3.28, □4.00, □4.30, □4.60 mm).
These results, together withprevious studies (Miyamichi et al., 2013; Wall et al., 2013; Watabe-Uchida et al., 2012; Wickersham et al., 2007), indicate that EGFP-positive neurons outside injection sitesrepresenttranssynaptically-labeled monosynaptic inputs toserotonin neurons. Because there wasslight non-specific labeling in serotonergic nucleiadjacent to injection sites, we excluded datafromthese areas for the following analysis. IPN is located just anterior to MR, and the caudal apical and caudal ventrolateralsubnuclei of IPN(IPA and IPVL; Hale and Lowry, 2011)contain some serotonin neurons(Groenewegen and Steinbusch, 1984). IPA and IPL contained a small number of starter neurons but other IPN subnuclei did not. Therefore, we counted input neurons in IPN after excluding IPA and IPL.The following analysis uses twelve animals, seven with preferential injections into DR and five with preferential injections into MR (Figure 1I). All results reported below were further verified using the two or three animals with highest specificity for either DR or MR.
For both DR and MR serotonin neurons, EGFP-positiveneurons (which we refer to as “input neurons”) were distributed throughoutthe brain (Figure 1J). However, they were mostly found at relatively ventral portions of the forebrainand in midbrain and brainstem structures close to DR and MR. Interestingly, although DR and MR are both midline structures, inputs toDR serotonin neurons weregenerally more lateral than inputs toMR serotonin neurons. This roughly matches their axonal projection patterns; MR neurons project mainly to midline structures and the hippocampus whereas DR neurons project to a broader array of regions, including more lateral areas (Azmitia and Segal, 1978; Vertes and Linley, 2008; Vertes et al., 1999).
Comparison between inputs to DR and MR serotonin neurons
To quantify the distributions of monosynaptic inputs, we identified areas based on a standard mouse atlas (Franklin and Paxinos, 2008). We then registered the locations of labeled neurons tostandard anatomical coordinates. To correct for variability in the total number of neurons (Figure 1G), the datawere normalized by the total number of input neurons in each animal (Figures 2,S1,S2).
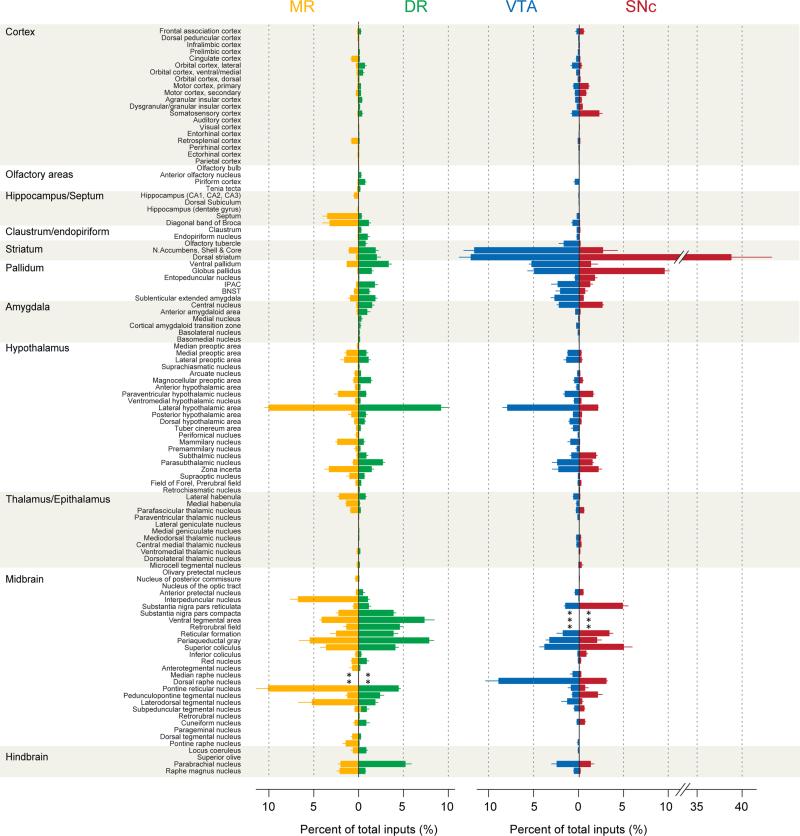
Figure 2. Summary of monosynaptic inputs to DR andMR serotonin neurons, and VTA andSNc dopamine neurons.
(Left) Monosynaptic inputs to MR and DR serotonin neurons (orange and green, respectively). Mean±SEM (n=7 and 5 mice for DRand MR groups, respectively). Asterisks (*) indicate areas that are excluded fromthe analysis.
(Right) Monosynaptic inputs to VTA and SNcdopamine neuron (blue and red, respectively). Data from Watabe-Uchida et al. (2012).
The values are the percentage of total inputsin each area. Brain areas analyzed for MR and DR inputs are matched to the brain areas analyzed for the VTA- and SNc-targeted data set(Watabe-Uchida et al., 2012). An analysis containing a more comprehensive set of areas in shown in Figure S2.
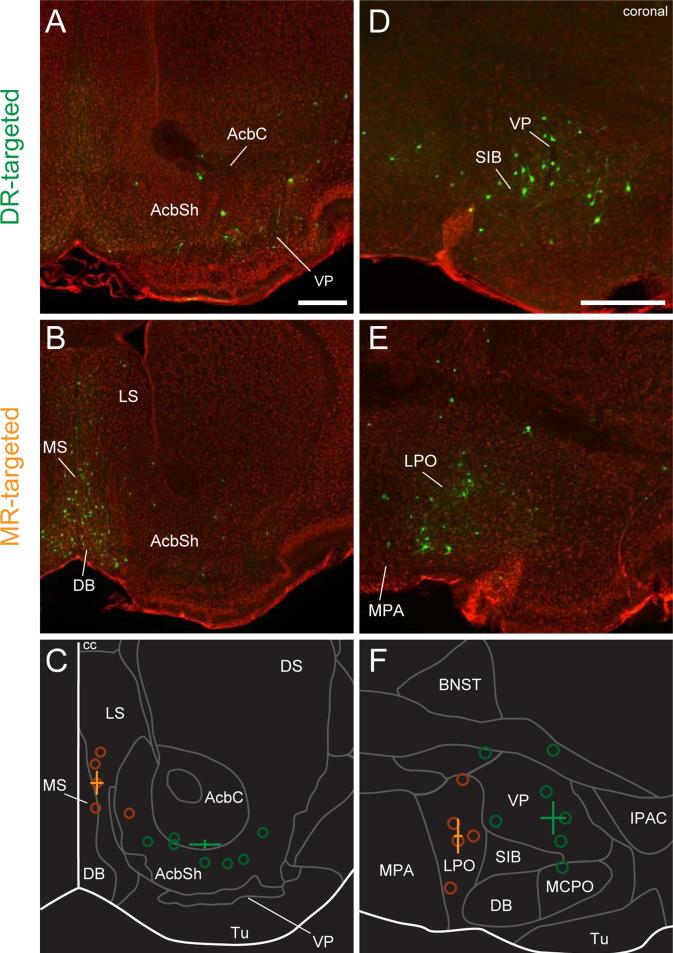
We found striking differences in rostral forebrain areas, particularlyin the basal ganglia and septum (Figure 2):inputs to DR serotonin neurons weredistributed widely acrossthe basal ganglia, whereasmanyfewer inputs toMR serotonin neurons were found there. For instance, DR serotonin neurons receivedmany inputs from the ventral pallidum (VP), globuspallidus (GP), dorsal striatum(DS) and nucleus accumbens(Acb),although labeled neurons in the lattertwo structuresmay be “spill-over” from the VP and GP (Figures 2,3). That is, labeled neurons were very sparse in the center of DS and Acb, but some were found at the periphery of DS and Acb, borderingVP and GP. DR serotonin neurons also receivedmany inputs from areasin the extended amygdala, such as the interstitial nucleus of the posterior limb of the anterior commissure (IPAC), the bed nucleus of the striaterminalis (BNST), the sublenticularextended amygdala(SLE) (Figures 2,3), and the central nucleus of the amygdala(Ce) (Figures 2,S3). In contrast, MR serotonin neurons received very few inputs from these areas.Incontrast, MR serotonin neurons received inputs from more medial structures(Figure 3), such as themedial and lateral portions of the septum (mainly the medial septum, MS) and the diagonal band of Broca (DB).
Figure 3. Spatial shift of input areas for DR and MR serotonin neurons in the forebrain.
(A-C) Septal and striatal areas. (A) DR-targeted. (B) MR-targeted. Green, rv-EGFP; Red, fluorescent Nissl staining. (C) The median of the coordinatesof all input neurons. Open circles indicate the medians from individual animals. Crosses indicate the mean±SEMof medians in DR- or MR-targeted groups (n=7 and 5 mice for DR and MR groups, respectively). Green, DR targeted; orange, MR-targeted. Scale bar, 0.5 mm.Bregma:1.1 mm.
(D-F) Pallidal and hypothalamic areas. (D) DR-targeted. (E) MR-targeted. Same conventions as (A) through (C).Bregma: 0.14 mm.
Abbreviations as in Figure 1; AcbC, nucleus accumbens core; AcbSh, nucleus accumbens shell; cc, corpus callosum; Tu, olfactory tubercle; MPA, medial preoptic nucleus; SIB, substantiainnominata basal part; MCPO, magnocellularpreoptic nucleus; IPAC, interstitial nucleus of the posterior limb of the anterior commissure.
We also observed many inputs in the hypothalamus. Indeed, the largest numbers of inputs from the forebrain to both DR and MR serotonin neurons came from the lateral hypothalamus (LH) (Figures 2,S3). MR serotonin neurons received more inputs from the medial and lateral preoptic areas (MPA and LPO), which are medial to the areas that contain inputs to DR, such as VP and the extended amygdala (Figure 3). Other midline structures, such as the paraventricular hypothalamic nucleus(Pa) and supramammillary nucleus (SUM), provided moderate levels of input to MR serotonin neurons, whereas DR serotonin neurons received preferential inputs from the subthalamic nucleus (STh) and the parasubthalamic nucleus (PSTh)(Figures 2,S3).
There werevery few inputs from the thalamus to either MR or DR serotonin neurons. However, in the epithalamus, both the lateral and medial habenula(LHb and MHb) provideddense inputs to MR serotonin neurons, with sparser projections to DR serotonin neurons (Figures 2,4).
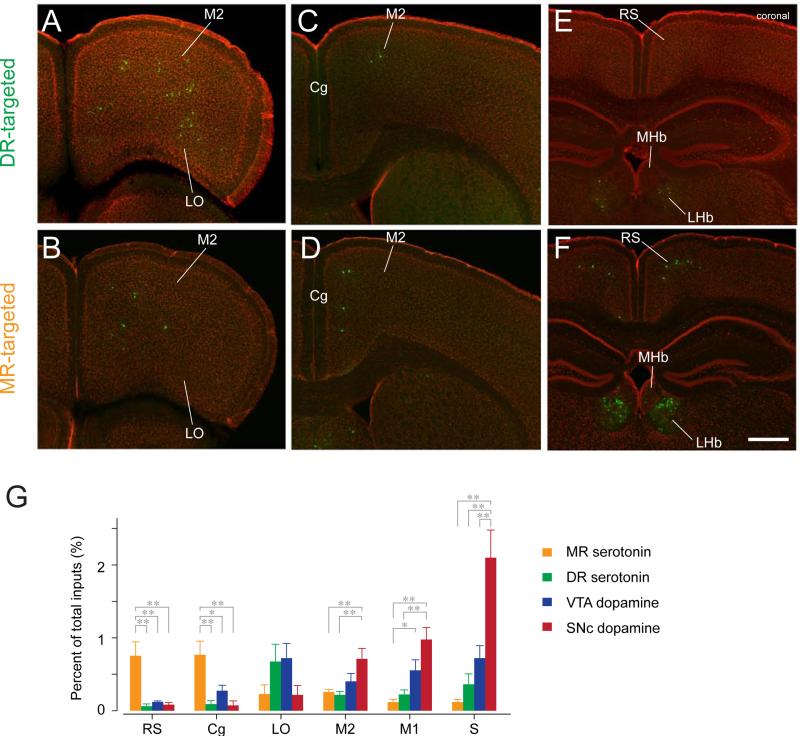
Figure 4. Monosynaptic inputs from the cortex and habenula.
(A-F) Distributions of input neurons toDR (A, C and E) and MR (B, D and F) serotonin neurons.Scale bar, 0.5 mm.Abbreviations as in Figure 1. LO, lateral orbital cortex; M2, secondary motor cortex; Cg, cingulate cortex; RS, retrosplenial cortex.Bregma: 2.58 mm (A, B), 0.86 mm (C, D) and □1.70 mm (E, F). (G) Percent of total inputsin six cortical areas that contained relatively large numbers of input neurons (>0.7% in at least one of the four experimental groups). MR (orange), DR (green), VTA (blue) and SNc(red). Mean ± SEM (n=7 and 5 mice for DR and MR groups,n=4 mice each for VTA andSNc groups, respectively).** p<0.01 and * p<0.05,one-way ANOVA, Tukey-Kramer multiple comparison test.
In the midbrain and brainstem, IPN, the laterodorsaltegmentum (LDTg), and Pnprovidedmany inputs to MR, but fewer to DR. VTA, the retrorubral field(RRF), SNc,and substantianigra pars reticulata(SNr) preferentially projected to DRversusMR serotonin neurons (Figure2). The periaqueductal gray (PAG) projected to both DR and MR, althoughitsventrolateral part preferentially projectedto DR serotonin neurons (Figure S3).MRt, the superior colliculus (SC) and the pedunculopontine tegmental area (PPTg) projected strongly to both DR and MR serotonin neurons. The parabrachial nucleus (PB)had a slight preference to DR whereas the raphe magnus nucleus (RMg) preferentially projected to MR (Figure 2).
Fewer EGFP-positive neurons were found in theneocortex (Figure 2). However, there were significant differences in the distributions of inputs to DR versus MR serotonin neurons. MR serotonin neurons receivedmore inputs from more medial cortical areas, such as the cingulate (Cg) and retrosplenialcortices (RS), whereasDR serotonin neuronsreceivedmore from the orbitofrontal cortex (in particular, its lateral part, LO) and somatosensory cortex (S)(Figure 4).
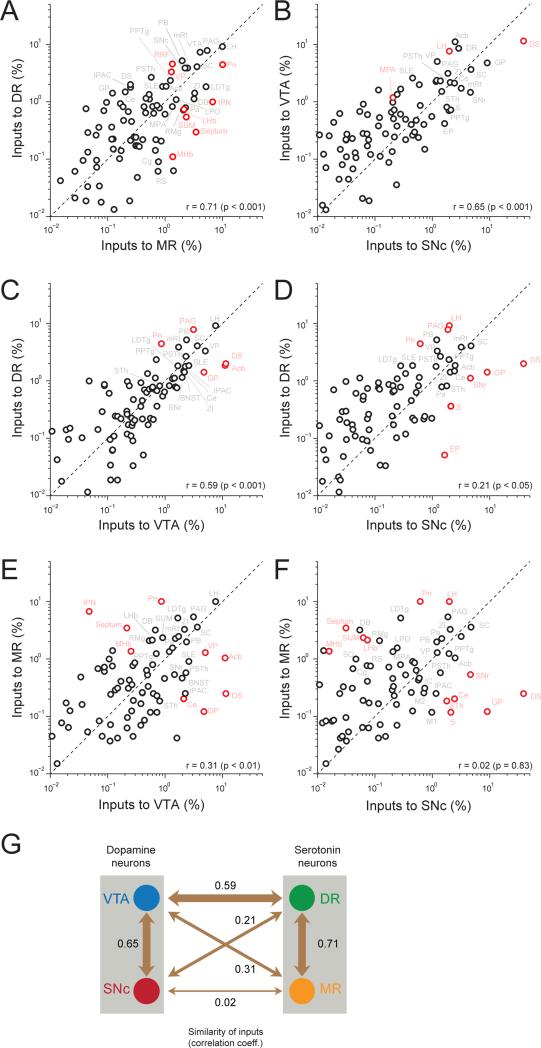
To quantify the similarity in the distributions of inputs to DR and MR serotonin neurons, we calculated the correlation coefficient between the numbers of input neurons across areas (Figure 5A;r = 0.71, p < 0.001). In the scatter plot, each pointrepresentsone area, and the diagonal represents the line of unity.Areas represented by points close to the diagonal provided similar numbers of inputs to DR and MR serotonin neurons, while areas far from the diagonal provided distinct numbers of inputs(significant differences in red, p < 0.05, corrected for multiple comparisons using a Bonferroni-correction, n = 7 and 5 for DR and MR groups, respectively). DR serotonin neurons received significantly more inputs fromRRF and VP, whereas MR serotonin neurons receivedmore from Pn, IPN, septum, LHb, SUMandMHb. Large common inputs camefrom LH, PAG, mRtand SC.
Figure 5. Comparisons of monosynaptic inputs across four groups.
(A) Comparison between inputs to DR and MR serotonin neurons.(DR, n=7 mice; MR, n=5 mice).
(B)Comparison between inputs to VTAandSNc dopamine neurons (VTA, n=4; SNc n=4).
(C)Comparison between inputs to DR serotonin and VTA dopamine neurons.
(D)Comparison between inputs to DR serotonin andSNc dopamine neurons.
(E)Comparison between inputs to MR serotonin and VTA dopamine neurons.
(F)Comparison between inputs to MR serotonin and SNc dopamine neurons.
Values are the means of percent of total inputs from each region. Red circles indicate significant differences(p < 0.05, Bonferroni-corrected). r: Pearson's correlation coefficients.
(G)Summary of similarities between input patterns. Numbers indicatecorrelation coefficients. The thickness of each arrow indicates the similarity.
In summary, DR and MR serotonin neurons receive inputs from largely segregated areas: MR serotonin neurons from more medial structures, often close to the midline, DR serotonin neurons from more lateral structures. LH provides many inputs to both DR and MR serotonin neurons.
Comparison between inputs to serotonin and dopamine neurons
We noticed a striking similarity in the inputs to DR serotonin neurons and inputs to VTA dopamine neuronsthat we obtained in a previous study (Watabe-Uchida et al., 2012). For instance, areas in the basal ganglia that were identified as major inputs to VTA dopamine neurons also provided many inputs to DR serotonin neurons.
To quantify similarities in the inputs in the four data sets (inputs to DR and MR serotonin neurons and VTA and SNcdopamine neurons), we calculated correlation coefficients for all pairs of areas (Figures 5B-F). Correlations were higher for within-serotonin or within-dopamine comparisons (that is, larger values between inputs to DR versus MR serotonin neurons or between inputs to VTA versus SNc dopamineneurons;r =0.71 and 0.65, respectively, p < 0.001; Figures 5A,B). It should be noted, however, that these pairs contained common starter neurons because viral injectionsresulted in some labeling of the other, non-targeted structure (Figure 1I).
In addition to these pairs, we found a remarkable similarity between inputs to DR serotonin and VTA dopamine neurons (correlation coefficient, r = 0.59, p < 0.001; Figure 5C). This correlation comes from common inputsfrom hypothalamus (LH, PSTh, ZI), extended amygdala (Ce, BNST, IPAC, SLE), basal ganglia (VP, STh, SNr) and other midbrain and brainstem structures (SC, PB, mRt, LDTg). A major difference, however, existed in inputs from the striatum (Acb and DS). For VTA dopamine neurons, Acb and DSprovided the largest number of inputs (Figure 2). In contrast, DR serotonin neurons received many fewer inputs from Acb and DS. Upon removing these two areas (Acb and DS), the correlation value increased to 0.81 (p < 0.001). On the other hand, DR serotonin neurons received more inputs from PAG and Pn.
This high similarity between DR serotonin and VTA dopamine neurons was in contrast to low similarities between other pairs of neuron populations (Figures 5D-F). While correlations were found between inputs to DR serotonin and SNc dopamine neurons (r = 0.21, p< 0.05)and between inputsto VTA dopamine and MR serotonin neurons (r = 0.31, p < 0.01)(Figures 5D,E),inputs to SNc dopamine and MR serotonin neurons had essentially no correlation (r = 0.02, p = 0.83; Figure 5F), suggesting a gradual difference of the input patterns of MR, DR, VTA and SNc (Figure 5G).
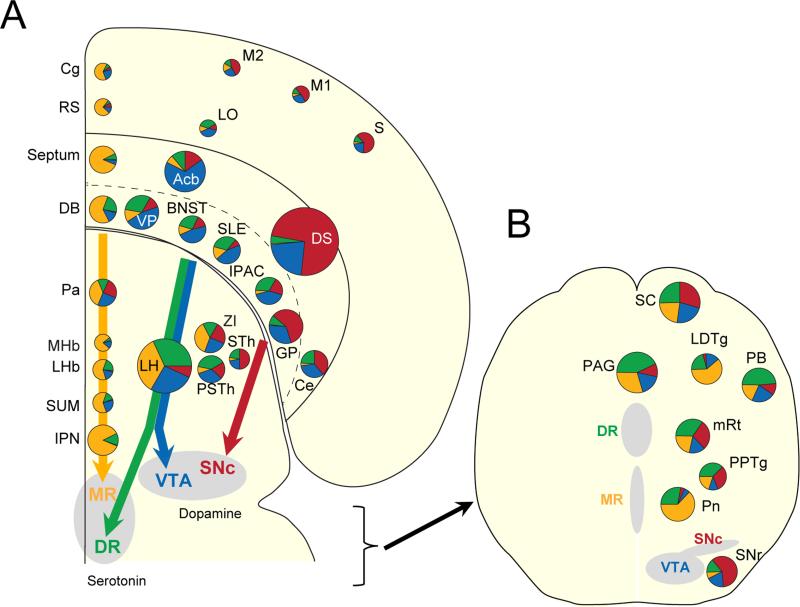
To compare the spatial distributions of input neurons across brains, we transformedeach coronal section to matchasection from a standard atlas(Franklin and Paxinos, 2008). The distributions of EGFP-labeled neurons were then compared by making horizontal slices using the morphed brains for each population of neurons (Figure S4).We also generated a flat map representation (after Swanson, 2000)to indicatethe proportion of inputs to each of the four postsynaptic neuron types,conserving the rough locations in the anterior-posterior and medial-lateral axes in the forebrain (Figure 6A) and the dorsal-ventral and medial-lateral axis on a coronal section at the level of midbrain and brainstem (Figure 6B). These representations showedthat (1) more medial structures (Cg, RS, Septum, DB, Pa, MHb, LHb, SUM and IPN) projected to MR serotonin neurons; (2) intermediate structures (LO, Acb, VP,BNST, SLE, IPAC, LH and PSTh) projected to VTA dopamine and DR serotonin neurons, although Acbdid not project strongly to DR serotonin neurons; and (3) more lateral and dorsal structures (M2, M1, S, DS, GP, Ce, STh and SNr) projected to SNc dopamine neurons. These observations show that the correlation valuesmentioned above correspond to spatial distributions along the medial-lateral axis.
Figure 6. Three input“axes” for serotonin and dopamine.
Each pie chart representsthe percentage of total inputs for each postsynaptic neuron type, and are placed at their rough locations in the anterior-posterior and medial-lateral axes on a horizontal section ofthe forebrain(A) and the dorsal-ventral and medial-lateral axes on a coronal section at the level of midbrain and brainstem (B). Color represents postsynaptic neuron types: inputs toMR serotonin neurons (orange), DR serotonin neurons (green), VTA dopamine neurons (blue), and SNc dopamine neurons (red). The size of the pie chartsreflects thesum of thepercentages. Colored arrows indicate the main axes of input streams.
In summary, asimilar set of areas projects directly to DR serotonin and VTA dopamine neurons (Figure 5G), with the exception of the striatum. In contrast, MR serotonin neurons and SNc dopamine neurons receive different sets of inputs.
Serotonin-dopamine interactions
The similar inputsto VTA dopamine and DR serotonin neuronsraise the possibility thatthese common sources similarly regulate the activity of VTA dopamine and DR serotonin neurons. In addition to these common inputs, previous studies indicated that interactions between serotonin and dopamine neuronsmay play an important role in behavior(Di Giovanni et al., 2010).Therefore, weexamined monosynaptic connections between serotonin and dopamine neurons.
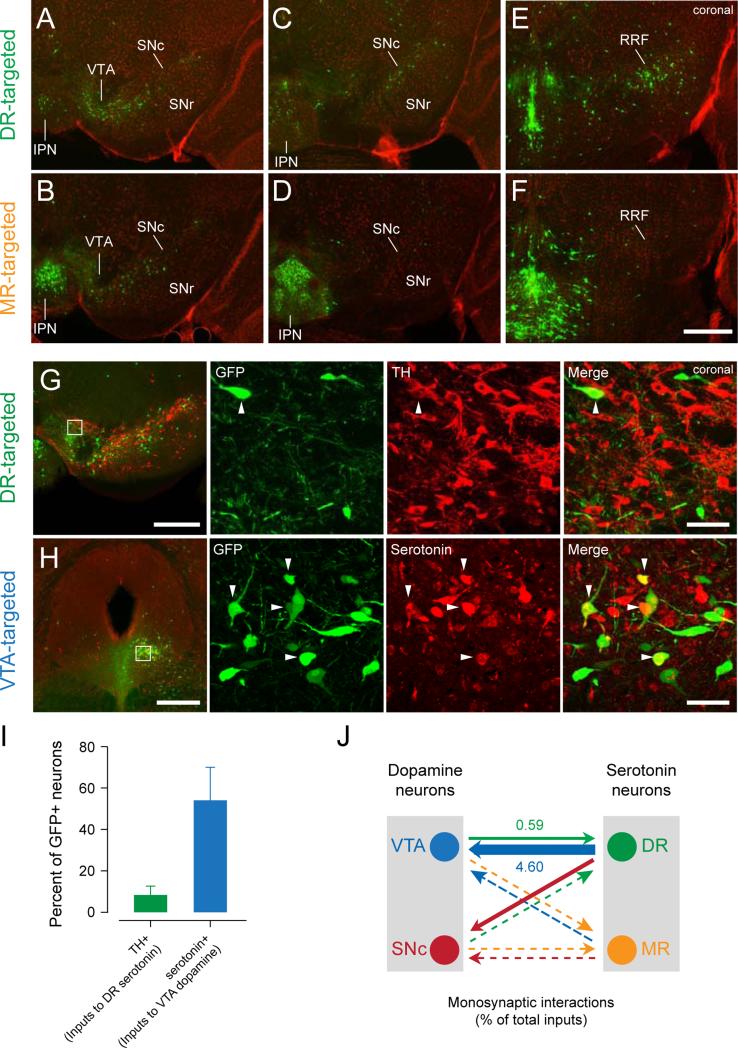
A previous study showedthatVTA (and, to a lesser extent, SNc) dopamine neurons receive heavymonosynaptic inputs from DR(Watabe-Uchida et al., 2012). In contrast, both VTA and SNc dopamine neurons receive many fewer inputs from MR (Watabe-Uchida et al., 2012). Here, we examined their interactions in the opposite direction:which of the midbrain areas that contain dopamine neurons (VTA, SNc, and RRF) projects to serotonin neurons?We found that DR serotonin neurons receivedheavy inputs from all three areas (VTA, SNc, and RRF; Figures 7A-F). MR serotonin neurons also received heavy inputs from VTA but fewer from SNc and RRF. Thus, DR serotonin neurons have strong reciprocal connections with bothVTA and SNcwhereas MR serotonin neurons havea unidirectional connection from VTA.
Figure 7. Interactions between serotonin and dopamine neurons.
(A-F) Distributions of input neurons toDR (A, C and E) and MR (B, D and F) serotonin neurons.Abbreviations as in Figure 1; SNr, substantianigraparsreticulata. Scale bar, 0.5 mm.Bregma□3.40 mm (A, B), □3.64 mm (C, D) and □4.04 mm (E, F).
(G) Monosynaptic inputs to DR serotonin neurons stained againsttyrosine hydroxylase (TH). White square indicates the location of the high-magnification view on the right. White arrowhead indicates a TH-positive input neuron. Scale bars, 0.5 mm and 50 m (low and high magnification images).Bregma□3.52 mm.
(H) Monosynaptic inputs to VTA dopamine neurons stained againstserotonin.White arrowheads indicate serotonin-positive input neurons.Bregma□4.72 mm.
(I)Percentage of dopamine (among all VTA and SNc inputs) or serotonin (among all DR inputs) neurons in rv-EGFP positive neurons.Mean±SEM.(n=39±15, 54±4 neurons,n=2 animals each).
(J) Summary of monosynaptic connections between serotonin and dopamine neurons. Numbers indicate percent of total inputs that are serotonergic or dopaminergic, estimated by multiplying the percent of inputs from each area by thepercentof dopamine- or serotonin positive-neurons amongrv-EGFP-labeled neurons. Arrowthickness reflects the numberof connections.Dotted lines indicate weak (<0.5%) connections.
The reciprocal connection between DR and VTA hasbeen reported (Geisler and Zahm, 2005; Vertes et al., 1999) but which cell types contribute to these interactions is unknown. In particular, while serotonergicregulation of VTA dopamine neurons was confirmed by multiple methods (Boureau and Dayan, 2011), projectionsfrom dopamine to serotonin neurons have remained unclear(Ferreira et al., 2008; Kalén et al., 1988). Our results thus farhave specified postsynaptic cell types (dopamine or serotonin neurons).In the following, we examined presynaptic cell types by immunostaining in combination with transsynaptic tracing with the modified rabies virus using Sert-Cre mice and DAT-Cre mice.
We stained against tyrosine hydroxylase (TH) in sections in which monosynaptic inputs to DR serotonin neurons were labeled andagainst serotoninsections in which monosynaptic inputs to VTA dopamine neurons were labeled(Figures 7G,H). The results showed that many monosynaptic inputs todopamine neurons were serotonergic (54.0%)(Figure 7I). In contrast, few (only 8.1%)monosynaptic inputs to serotonin neurons weredopaminergic(Figure 7I), consistent with previous observations(Kalén et al., 1988). Interestingly, VTA inputs to serotonin neurons clustered in the posterior-ventral-medial part of the SNc and in the ventral-lateral part of VTA, surrounding the medial leminiscus (Figures 7A,B).
Thus, inputs from DR to VTA dopamine neurons include a large number of serotonergic projections, while inputs from VTA (and SNc) to DR serotonin neurons do not contain many dopaminergic projections, suggesting a largely unidirectional projection from serotonin to dopamine neurons (Figure 7J).
DISCUSSION
Using a modified rabies virus, we mapped the whole-brain monosynaptic inputs to DR and MR serotonin neurons. We found that MR serotonin neurons receive inputs from more medial forebrain areas than DR serotonin neurons. We next compared the distributions of inputs to serotonin neurons with those to dopamine neurons obtained in a previous study (Watabe-Uchida et al., 2012). We found a remarkable overall similarity between inputs to VTA dopamine and DR serotonin neurons. This comparison also revealed an important difference: compared to serotonin neurons, dopamine neurons receive many more direct inputs from the striatum. These results demonstrate a global organizing principle of inputs to two major ascending neuromodulator systems. There are roughly three descending input streams: medial areas project to MR serotonin neurons, intermediate areas project to DR serotonin and VTA dopamine neurons, and more lateral areas project to SNc dopamine neurons.
Similarity between inputs to DR serotonin and VTA dopamine neurons
Comparing the relative numbers of inputs across areas, we found that DR serotonin and VTA dopamine neurons receive quantitatively similar patterns of inputs compared to MR serotonin and SNc dopamine neurons (Figure 5G).These findingsnotably advance the literature in two ways: first, our analysis is based on direct inputs to serotonin and dopamine neurons, a specificity that has been difficult to achieve with conventionaltracers(Wickersham et al., 2007). Second, although differences in inputs tosubareas of eitherdopamine or serotonin systemhave been noted qualitatively in previous studies (Graybiel and Ragsdale, 1979; Ikemoto, 2007; Vertes and Linley, 2008; Watabe-Uchida et al., 2012), to our knowledge, our analysis is the first quantitative comparison between different neurotransmitter systems (here, dopamine and serotonin).
Thesimilarities and differencesobserved between inputs to DR serotonin and VTA dopamine neurons mayprovide insight into their functions. Serotonin has been proposed to be involved in diversefunctionsbut it has been difficult to pin-pointanyspecific one. Proposed functions of serotonin partially overlap with those of dopamine,but their roles often appear opposed. For instance, dopamine is associated with positive reinforcement and promoting approach/exploration behavior,whereas serotonin is associated with negative reinforcement and behavioral inhibition (Daw et al., 2002; Dayan and Huys, 2008; Deakin and Graeff, 1991; den Ouden et al., 2013; Soubrie, 1986). This idea of opponencybetween the two systemshas been supported by the observation that serotonin neurons are activated by noxious stimuli(Montagne-Clavel et al., 1995; Schweimer and Ungless, 2010) while dopamine neurons are activated by reward. It remains to be determined how these observations can be reconciled withrecent studies showing that many DR neurons (which likely included serotonin neurons) were excited by reward(Miyazaki et al., 2011; Nakamura et al., 2008; Ranade and Mainen, 2009). Importantly, unlike dopamine neurons, their response to reward was not modulated by whether reward was expected (Nakamura et al., 2008). In addition, the observation that LH provided very dense input to DR serotonin and VTA dopamine neurons suggests that their role in reward prediction (Ono et al., 1986) may similarly influence serotonin and dopamine neurons. These results suggest that the way DR serotonin neurons respond during behavior bearssomesimilarity to that of dopamine neurons, although critical differences exist.
A striking difference between inputs to DR serotonin and VTA dopamine neurons is the much smaller number of direct inputs from the striatum (Acb and DS) to DR serotonin neurons. Forboth VTA and SNc dopamine neurons, the largest number of inputs comes from the striatum (Watabe-Uchida et al., 2012). In contrast, DR serotonin neurons receive more inputs from pallidal/extended amygdala structures and from LH. Dopamine neurons receive inputs from the striatum both directly and indirectly through pallidal and hypothalamic structures(Watabe-Uchida et al., 2012). Our results suggest that DR serotonin neurons receive primarily indirect inputsfrom the striatum. It has been proposed that striatal neurons provide reward expectation signals to directly inhibit dopamine neurons to calculate reward prediction errors (Doya, 1999; Houk et al., 1995), although the functional role of this input remains to be clarified. Thesmall numberof direct inputs from the striatum to DR serotonin neurons might be related to the observation that DR serotonin neurons respond to reward even when reward is expected(Nakamura et al., 2008). In addition to the striatum, serotonin neurons do not receive inputs from other areas to which they project, such as hippocampus, thalamus and amygdala. In contrast, dopamine neurons receive inputs from most of their projection sites (that is, they have reciprocal connections). This may explain the longer timescale responses in serotonin neurons because of the lack of immediate negative feedback as found in dopamine neurons(Haber et al., 2000).
Forebrain-habenula-raphe serotonin pathways
The habenula (Hb) has long been considered a node of major descending pathways to serotonin neurons emanating from various forebrain regions. Whereas theMHb-IPN-raphe route conveys information from the hippocampal system to serotonin neurons, the LHb-raphe route conveys different kinds of information from the basal ganglia and hypothalamus to serotonin neurons(Herkenham and Nauta, 1979). We found two major differences between Hb inputs to DR versus MR serotonin neurons as described below.
We found that LHb sends strong monosynaptic inputs to MR serotonin neurons, consistent with previous findings(Behzadi et al., 1990). On the other hand, although LHb projects strongly to DR (Vertes et al., 1999), we found that DR serotonin neurons (andVTA and SNcdopamine neurons) receive few monosynaptic inputs from LHb. Recent studies found that the rostromedial tegmental nucleus (RMTg)relays inputs from LHb to DR as well as to dopamine neurons (Jhou et al., 2009). Because most LHbneurons are excitatory and RMTg neurons are inhibitory, our data suggest that activation of LHbneurons canexert opponent control over MR and DR serotonin neurons. That is, LHb neuronsdirectly excite MR serotonin neurons and, at the same time, inhibit DR serotonin neurons (and dopamine neurons) via RMTg.
Although previous studies indicated that IPN projects to both MR and DR (Groenewegen et al., 1986), our results showed that IPN projects preferentially to MR, over DR, serotonin neurons. Most inputs originate from rostral IPN (Figures 7A-D), which receives strong projections from MHb. We also found that MHb projects directly to MR (Figures 2,4E,F). These results show that MR serotonin neurons receive strong inputs directly and indirectly (via IPN) from MHb (Figure S5).
Previous studiesfound that MR receives input from extended amygdala (e.g., BNST) and basal ganglia (e.g., VP)(Marcinkiewicz et al., 1989; Vertes and Linley, 2008). However, our data showed few monosynaptic inputs from these areas to MR serotonin neurons. Recentstudies showed that these areas project to RMTg, which is adjacent to MR (Jhou et al., 2009). Other studies using anterograde tracers indicated thatAcb and VP project to the lateral part of MR but not to the midline where most serotonin neurons reside (Behzadi et al., 1990). These results suggest that differences between studies can be explained by the higher specificity of labeling in the present study.
Hierarchical organization between dopamine and serotonin
Serotonin and dopamine systems are thought to interact (Boureau and Dayan, 2011; Kapur and Remington, 1996). IOur data showed that bothDR serotonin and VTA dopamine neurons receive a large number of monosynaptic inputs from VTA and DR, respectively. However, althoughinputs from DR to VTA dopamine neurons includedmany serotonin neurons, inputs from VTA (and SNc) to DR serotonin neurons did not contain many dopamine neurons. These results indicate alargely onedirectionalinformation flow from DR serotonin neurons to VTA and SNcdopamine neurons (Figure 7J).
It is interesting that, although both DR serotonin and VTA and SNcdopamine neurons project to the striatum, the striatum sends back massive projectionsprimarily to dopamine (Watabe-Uchida et al., 2012),and not serotonin neurons (present study). Therefore, serotonin could also control dopamine neurons by regulating striatal activity (Kapur and Remington, 1996)but not vice versa. This suggests a hierarchical relationship between DR serotonin and VTA and SNcdopamine neurons; overall, serotonin is in a stronger position to controldopamine than vice versa both through direct and indirect connections. Our data match observations that lesions of DR or MR or pharmacological manipulations of serotonin affect dopamine release(Boureau and Dayan, 2011; Di Giovanni et al., 1999; Hervé et al., 1979).This serotonin-dopamine interaction is important for understanding neural circuits for reinforcement learning. It has been proposed that serotoninadds affective tone by inhibiting dopamine signals(Boureau and Dayan, 2011; Daw et al., 2002).Moreover, drugs for psychiatric disorders such as schizophrenia, depression, and addiction act directly or indirectly on both serotonin and dopamine(Kapur and Remington, 1996). The anatomical basis of serotonin-dopamine interactionsanalyzed in the present study can provide insight into normal reward processing as well as brain disorders.
Three axes ofdescending control of dopamine and serotonin
Our data suggestthat three axes of descending projections control serotonin and dopamine (Figure 6). The most medial axis originates from the septo-hippocampal system and controls MR serotonin neurons. The intermediate axis originates from the ventral basal ganglia/extended amygdala and LH, and controls VTA dopamine and DR serotonin neurons. The most lateral axis originates from the dorsal part of the basal ganglia and the subthalamic nucleus, and controlsSNc dopamine neurons. In addition to these subcortical descending projections, we also observed direct projections from the neocortexthat follow similar segregation: somatosensory and motor cortices to SNc dopamine neurons, orbitofrontal cortex to VTA dopamine and DR serotonin neurons and medial cortical areas such as the Cgand RS to MR serotonin neurons (Figure 4G).
In this study, we observed global afferent control for serotonin and dopamine systems. Our results demonstrate that three parallel pathways form largely segregated control systems for these two pathways.Across and within these pathways, DR serotonin neurons appear to exert hierarchically greater control over VTA and SNc dopamine neurons than vice versa. We compared serotonin and dopamine systems because they are thought to interact. Similar comparisons with other neuromodulators may provide insight into the global organization of brain connectivity.
EXPERIMENTAL PROCEDURES
Viral injections
We used 10 adult (2 to 6 months old) female Sert-Cre mice (Slc6a4tm1(cre)Xz; Zhuang et al., 2005) that express Crerecombinase under the transcriptional control of the serotonin transporter gene. These micewere backcrossed with C57BL/6J mice. For some control experiments, C57BL6 mice were used.All procedures were in accordance with Harvard University Institutional Animal Care and Use Committee.
To visualize monosynaptic inputs to DR and MR serotonin neurons, we used a transsynaptic tracing system based on the modified rabies virus (Watabe-Uchida et al., 2012; Wickersham et al., 2007). First, 0.3-0.5 μl of AAV8-FLEX-RG (2×1012 particles/ml) and 0.3-0.5 μl AAV5-FLEX-TVA-mCherry (4×1012 particles/ml) were stereotaxically injected into the DR or MR (4.5 mm and 4.2 mm posterior to thebregma, 0mm and 0mm to the midline, and 2.1 mm and 3.6 mm ventral to the dura, respectively) using a micromanipulator with a pulled glass needle. Fourteen days later, 0.8-1 μl of pseudotyped rabies virus, SADΔG-EGFP(EnvA) (5×107 plaque-forming units [pfu] per milliliter)(Wickersham et al., 2007), was injected into the same area. All surgeries were performed under aseptic conditions with animals under ketamine/medetomidine (60 and 0.5 mg/kg, I.P., respectively)or isoflurane (1-3% at 600 ml/min) anesthesia. Analgesia (ketofen 5mg/kg, I.P., buprenorphine, 0.1 mg/kg, I.P.) was administered postoperatively. The data for monosynaptic inputsto VTA and SNcdopamine neurons were obtained using a similar method usingDAT-Cremice (Watabe-Uchida et al., 2012).
Histology
One week after injection of rabies virus, mice were perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. After 1 day of postfixation in 4% PFA,100-μm-thick coronal slices were prepared using a vibratome. Every third section was counterstained with NeuroTrace Fluorescent Nissl Stains (Molecular Probes). To identify starter neurons infected by the rabies virus, immunohistochemistry was performed. TVA-mCherry signal was detected using either anti-mCherrymouse monoclonal antibody (1:100; Clontechoranti-DsRed rabbit polyclonal antibody (1:200; Rockland Immunochemicals Inc.), with Alexa Fluor 594 goat anti-mouse secondary antibody, or Alexa Fluor 555 goat anti-rabbit secondary antibody (1:200; Molecular Probes). Serotonin neurons were identified using ananti-serotonin rabbit polyclonal antibody (1:200; Sigma-Aldrich), or anti-serotonin rat monoclonal antibody (1:100; Millipore, Temecula, CA, USA), with biotinylated goat anti-rabbit secondary antibody (1:200; Jackson ImmunoResearch), streptavidin-conjugated Alexa Fluor 405, or Alexa Fluor 633 goat anti-rat secondary antibody (1:200; Molecular Probes). Slices were permeabilized with 0.5% Triton X-100, and incubation with antibodies and washing was done with 0.05% Triton X-100. Whole-section mosaics of low-magnification images were taken semiautomatically with AxioImager Z2, Axio Scan Z1 or LSM 700 Inverted Confocal microscope (Zeiss), and assembled using software (Axiovision or Zen, Zeiss). High magnification images were taken by an LSM 510 or LSM 700 Inverted Confocal microscope (Zeiss). Starter neurons were identified based on coexpression of EGFP and mCherry (Figures 1C,F,I).
For identification of cell types of rabies-infected neurons, we used anti-serotonin and anti-TH rabbit polyclonal (1:200; Millipore) antibodies. We performed the following control experiments to quantify the specificity of these antibodies. We first crossed Sert-Cre andDAT-Cre mice (B6.SJLSlc6a3tm1.1(cre)Bkmn/J, Jackson Lab)(Bäckman et al., 2006) with tdTomato-reporter mice (Gt(ROSA)26 Sortm9(CAG-tdTomato)Hze, Jackson Lab) to express tdTomato in serotonin and dopamine neurons, respectively. After fixation in 4% PFA/PBS and slicing, immunohistochemistry was performed. In Sert-Cre/tdTomato brain slices, 90.75 ± 4.2 % (mean±SEM, n=3 animals) of tdTomato-positive neurons were labeled by the anti-serotonin antibody. InDAT-Cre/tdTomato brain slices, 95.7±3.2% (mean±SEM, n=2 animals) of tdTomato-positive neurons were labeled by the anti-TH antibody.
To quantify the specificity of initial infection of starter neurons by the rabies virus,AAV5-FLEX-TVA-mCherry and pseudotyped rabies virus, SADΔG-EGFP(EnvA), were injected withoutAAV8-FLEX-RG into DR of Sert-Cre mice.Immunohistochemistry using the anti-serotonin antibody showed that 90.77±1.9% (mean±SEM, n=3 animals) of rv-EGFP-positive neurons were labeled by the anti-serotonin antibody, which is close to the efficiency at which the antibody can label serotonin neurons.
Image Analysis
The locations of labeled neurons and the outlines of brain areas were manually registered using custom software written in MATLAB (Mathworks)and R (http://www.r-project.org/). Nomenclature, abbreviations and outlines of brain areas are according to a standard atlas (Franklin and Paxinos, 2008). Starter and input neurons in Figures 1F-H were countedfrom both hemispheres, but other data for quantitative analysis were from onehemisphere. Centers of injection sites were calculatedas the arithmetic mean of the coordinates ofrv-EGFP- and mCherry-double positive neurons in each animal.Positions of neuronswere measured usingthe center of the aqueduct as the landmark in each brain slice.
For quantitative comparisonsof input neurons between DR and MR, we used 7 brains from the DR group and 5 brains from theMR group thatcontained relatively large numbers of starter neurons and transsynaptically-labeled neurons, and that had highest specificities of starter neurons (DR1, DR3, DR4, DR5, DR6, DR7 and DR8; and MR3, MR4, MR5, MR12 and MR13; Figure 1I). The numbers of input neuronswere normalized by the total number of inputs (excluding the injection sites) in each animal to obtain the percentage of total inputs. Because the data for inputs to dopamine neurons were based on brain areas anterior tobregma -5.34 mm (Watabe-Uchida et al., 2012), for the main analyses, we used data fromthe corresponding areas for inputs to serotonin as well. Analyses including morecaudal areasare shown in Figure S2.The data for monosynaptic inputs to VTA and SNcdopamine neurons were reported previously (Watabe-Uchida et al., 2012). The four brains from VTA and SNc group that were used in the previous report were also used for the analysis here (VTA: v001, v004, v009, v010; and SNc: s001, s003, s004, s006).
To compare distributions of input neurons in the forebrain (Figure 3), the median of the coordinates of input neurons except those in the cortex was obtained. To superimpose results from different animals onto a standard atlas, positions of neurons were normalized by three landmarks: the corpus callosum at the midline, the ventral-most part of the midline and the lateral-most part of the dorsal striatum.
For statistical analysis of cortical inputs (Figure 4G), multiple group comparisons were assessed using a one-way analysis of variance (ANOVA),followed by post-hocTukey-Kramer tests.
For statistical comparisons of the number of inputs between the different starter groups (Figure 5), areas that contained<1% of the total inputs in any of the four starter groups were excluded.Corrections for multiple comparisons were performed using Bonferroni correctionsbased on the number of all of these areas used for statistical comparisons.To quantify the similarity in input patterns, we calculated Pearson's correlation coefficients without excluding any area.
Supplementary Material
HIGHLIGHTS.
Monosynaptic inputs to serotonin neurons in the dorsal and median raphe (DR and MR)
Inputs to DR and MR serotonin neurons are spatially shifted in the forebrain
DR serotonin and VTA dopamine neurons receive similar inputs, except from striatum
Three input streams control serotonin and dopamine
ACKNOWLEGEMENTS
We are grateful to Dr. E. Callaway for providing us with the rabies virus andother reagents. We thank Dr. J.A.T. Young for pCMMP-TVA950, Dr. X. Zhuang for theSert-Cre mouse, and Dr. C. Dulacfor support andreagents. We thank Drs. K. Commons, S. Ikemoto and D. Wang for critical comments on the manuscript andN.Eshel, W.Menegas,and other members of the Uchida lab for discussion. This work was supported by a Howard Hughes Medical Institute Fellowship from the Helen Hay Whitney Foundation (J.Y.C.); a Howard Hughes Medical Institute Collaborative Innovation Award; and NIH (R01MH095953, R01MH101207) (N.U.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
S.K.O., J.Y.C., N.U., and M.W.-U. designed experiments, analyzed data, and wrote the paper. S.K.O., D.H., and M.W.-U. collected data.
SUPPLEMENTARY INFOMRATION
Supplemental data for this article includes fivefigures, one note,one experimental procedure and references.
REFERENCES
- Aghajanian GK, Wang RY. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1977;122:229–242. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast-and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bäckman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3’ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi G, Kalén P, Parvopassu F, Wiklund L. Afferents to the median raphe nucleus of the rat: retrograde cholera toxin and wheat germ conjugated horseradish peroxidase tracing, and selective D-[3H]aspartate labelling of possible excitatory amino acid inputs. Neuroscience. 1990;37:77–100. doi: 10.1016/0306-4522(90)90194-9. [DOI] [PubMed] [Google Scholar]
- Boureau Y-L, Dayan P. Opponency revisited: competition and cooperation between dopamine and serotonin. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011;36:74–97. doi: 10.1038/npp.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Dayan P, Huys QJM. Serotonin, inhibition, and negative mood. PLoS Comput. Biol. 2008;4:e4. doi: 10.1371/journal.pcbi.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JF, Graeff FG. 5-HT and mechanisms of defence. J. Psychopharmacol. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- den Ouden HEM, Daw ND, Fernandez G, Elshout JA, Rijpkema M, Hoogman M, Franke B, Cools R. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80:1090–1100. doi: 10.1016/j.neuron.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, De Deurwaerdére P, Di Mascio M, Di Matteo V, Esposito E, Spampinato U. Selective blockade of serotonin-2C/2B receptors enhances mesolimbic and mesostriatal dopaminergic function: a combined in vivo electrophysiological and microdialysis study. Neuroscience. 1999;91:587–597. doi: 10.1016/s0306-4522(98)00655-1. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Esposito E, Di Matteo V. Role of serotonin in central dopamine dysfunction. CNS Neurosci. Ther. 2010;16:179–194. doi: 10.1111/j.1755-5949.2010.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K. What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Netw. 1999;12:961–974. doi: 10.1016/s0893-6080(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Doya K. Metalearning and neuromodulation. Neural Netw. 2002;15:495–506. doi: 10.1016/s0893-6080(02)00044-8. [DOI] [PubMed] [Google Scholar]
- Ferreira JGP, Del-Fava F, Hasue RH, Shammah-Lagnado SJ. Organization of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neuroscience. 2008;153:196–213. doi: 10.1016/j.neuroscience.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates. Elsevier Academic Press; San Diego: 2008. [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J. Comp. Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J. Neurosci. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW., Jr Fiber connections of the basal ganglia. Prog. Brain Res. 1979;51:237–283. [PubMed] [Google Scholar]
- Groenewegen HJ, Steinbusch HW. Serotonergic and non-serotonergic projections from the interpeduncular nucleus to the ventral hippocampus in the rat. Neurosci. Lett. 1984;51:19–24. doi: 10.1016/0304-3940(84)90256-8. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJH. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J. Comp. Neurol. 1986;249:65–102. doi: 10.1002/cne.902490107. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal Pathways in Primates Form an Ascending Spiral from the Shell to the Dorsolateral Striatum. J. Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl.) 2011;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJH. Efferent connections of the habenular nuclei in the rat. J. Comp. Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Hervé D, Simon H, Blanc G, Lisoprawski A, Le Moal M, Glowinski J, Tassin JP. Increased utilization of dopamine in the nucleus accumbens but not in the cerebral cortex after dorsal raphe lesion in the rat. Neurosci. Lett. 1979;15:127–133. doi: 10.1016/0304-3940(79)96101-9. [DOI] [PubMed] [Google Scholar]
- Hioki H, Nakamura H, Ma Y-F, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J. Comp. Neurol. 2010;518:668–686. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- Houk JC, Adams JL, Barto AG. A model of how the basal ganglia generate and use neural signals that predict reinforcement. Models Inf. Process. Basal Ganglia. 1995:249–270. [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Curr. Opin. Neurobiol. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalén P, Skagerberg G, Lindvall O. Projections from the ventral tegmental area and mesencephalic raphe to the dorsal raphe nucleus in the rat. Evidence for a minor dopaminergic component. Exp. Brain Res. 1988;73:69–77. doi: 10.1007/BF00279662. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am. J. Psychiatry. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Varga V, Dahan L, Sik A. Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1059–1064. doi: 10.1073/pnas.0508360103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydic R, McCarley RW, Hobson JA. The time-course of dorsal raphe discharge, PGO waves, and muscle tone averaged across multiple sleep cycles. Brain Res. 1983;274:365–370. doi: 10.1016/0006-8993(83)90720-5. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz M, Morcos R, Chrétien M. CNS connections with the median raphe nucleus: retrograde tracing with WGA-apoHRP-Gold complex in the rat. J. Comp. Neurol. 1989;289:11–35. doi: 10.1002/cne.902890103. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- Miyamichi K, Shlomai-Fuchs Y, Shu M, Weissbourd BC, Luo L. Dissecting local circuits: Parvalbumin interneurons underlie broad feedback control of olfactory bulb output. Neuron. 2013;80:1232–1245. doi: 10.1016/j.neuron.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Miyazaki KW, Doya K. Activation of dorsal raphe serotonin neurons underlies waiting for delayed rewards. J. Neurosci. 2011;31:469–479. doi: 10.1523/JNEUROSCI.3714-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne-Clavel J, Oliveras JL, Martin G. Single-unit recordings at dorsal raphe nucleus in the awake-anesthetized rat: spontaneous activity and responses to cutaneous innocuous and noxious stimulations. Pain. 1995;60:303–310. doi: 10.1016/0304-3959(94)00129-3. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J. Neurosci. 2008;28:5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Nakamura K, Nishijo H, Fukuda M. Hypothalamic neuron involvement in integration of reward, aversion, and cue signals. J. Neurophysiol. 1986;56:63–79. doi: 10.1152/jn.1986.56.1.63. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Ranade SP, Mainen ZF. Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor, and reward events. J. Neurophysiol. 2009;102:3026–3037. doi: 10.1152/jn.00507.2009. [DOI] [PubMed] [Google Scholar]
- Roeper J. Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 2013;36:336–342. doi: 10.1016/j.tins.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schweimer JV, Ungless MA. Phasic responses in dorsal raphe serotonin neurons to noxious stimuli. Neuroscience. 2010;171:1209–1215. doi: 10.1016/j.neuroscience.2010.09.058. [DOI] [PubMed] [Google Scholar]
- Seymour B, Daw ND, Roiser JP, Dayan P, Dolan R. Serotonin selectively modulates reward value in human decision-making. J. Neurosci. 2012;32:5833–5842. doi: 10.1523/JNEUROSCI.0053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiza-Reilly M, Commons KG. Glutamatergic drive of the dorsal raphe nucleus. J. Chem. Neuroanat. 2011;41:247–255. doi: 10.1016/j.jchemneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubrie P. Reconciling the role of central serotonin neurons in human and animal behavior. Behav. Brain Sci. 1986;9:319–364. [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci. 2013;16:966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. GABA Neurons of the VTA Drive Conditioned Place Aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA Neurons Disrupts Reward Consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- Vertes RP, Linley SB. Efferent and afferent connections of the dorsal and median raphe nuclei in the rat. In: Monti JM, Pandi-Perumal SR, Jacobs BL, Nutt DJ, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Birkhäuser; Basel: 2008. pp. 69–102. [Google Scholar]
- Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 2013;79:347–360. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann K-K, Young JAT, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J. Neurosci. Methods. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.