Abstract
Biopharmaceuticals are making increasing impact on medicine, including treatment of indications in the eye. Macromolecular drugs are typically given by physician-administered invasive delivery methods, because non--invasive ocular delivery methods, such as eye drops, and systemic delivery, have low bioavailability and/or poor ocular targeting. There is a need to improve delivery of biopharmaceuticals to enable less-invasive delivery routes, less-frequent dosing through controlled-release drug delivery and improved drug targeting within the eye to increase efficacy and reduce side effects. This review discusses the barriers to drug delivery via various ophthalmic routes of administration in the context of macromolecule delivery and discusses efforts to develop controlled-release systems for delivery of biopharmaceuticals to the eye. The growing number of macromolecular therapies in the eye needs improved drug delivery methods that increase drug efficacy, safety and patient compliance.
1. Introduction
Since the FDA approval of human insulin for the management of diabetes mellitus in 1982, over 100 clinically-approved biopharmaceuticals [1] have been introduced to the U.S. market. As a class of active pharmaceutical ingredients (APIs), biopharmaceuticals often allow for the treatment of previously incurable diseases with fewer side effects. Now, the demand for biopharmaceuticals is greater than ever before. Total U.S. biologics sales have reached ~$63 billion in 2012 [2], an 18.2% increase over 2011 sales and a 92.7% increase over 2005 sales [2]. Monoclonal antibodies account for the highest percentage, with annual sales reaching $24.6 billion [2]. Likewise, the market for ophthalmic biopharmaceutical drugs has grown tremendously since the introduction of the anti-vascular endothelial growth factor (anti-VEGF) aptamer, pegatanib in 2004, and monoclonal antibody, ranibizumab in 2006 [3]. Currently, sales of macromolecular drugs for ophthalmic indications have reached $4 billion a year in 2011 and are expected to exceed $8 billion in 2016, with an annual growth rate of almost 16% between 2011–2016 [3].
Significant growth in the number of biopharmaceuticals in recent years will allow better treatment of many chronic ocular diseases that currently do not have treatments. While there may be many new biopharmaceutical entities in the pipeline, current ophthalmic drug delivery technologies are tailored for the delivery of small molecules and/or deliver drugs in a non-targeted manner throughout the eye. For this reason, there is a need to develop drug delivery technologies suitable for macromolecular therapies, ideally targeting them to biologically relevant tissues within the eye. However, ophthalmic delivery of macromolecules is difficult because (i) the large size of the macromolecule limits diffusion and renders topical therapies highly inefficient if not impossible; (ii) tissue barriers, such as the blood retinal barrier, limit the penetration of applied pharmacotherapies to the target site; and (iii) the small size of the eye and presence of many distinct tissues makes targeting necessary. For this reason, ophthalmic drug delivery technology must evolve alongside the significant arket growth of biopharmaceutical therapies [2, 3]. This article seeks to describe available ophthalmic drug delivery routes and sustained release systems in development and current use, especially for macromolecules, so that the reader can better design and evaluate systems for particular macromolecule delivery needs. This review builds off other recent reviews of ocular drug delivery [4–6]
1.1 Ocular diseases: present and future treatments
Ocular diseases affect many people worldwide, and many of these ocular diseases directly impact the patient’s vision and quality of life. It is estimated that 285 million people worldwide are visually impaired or blind, and the number of blind individuals increases by approximately 7 million people per year [7]. In the United States alone, about 3.4 million people over the age of 40 are blind or have significant visual impairment (defined as best corrected visual acuity of 20/200 in the better-seeing eye) [7, 8]. The major diseases found in the industrialized world that significantly impact vision include age-related macular degeneration (AMD), diabetic retinopathy, cataract, uveitis, keratitis, and glaucoma.
Currently, the approved macromolecular therapies for the eye involve the use of anti-VEGF agents, of which there are: pegatanib (Macugen®), ranibizumab (Lucentis®) and aflibercept (Eylea®), while bevacizumab (Avastin®) is used off label [3]. Anti-VEGF therapies bind to the VEGF signaling peptide with high affinity to neutralize VEGF’s downstream effect of promoting the growth of leaky immature vessels [9]. VEGF has been demonstrated to play a central role in the pathogenesis of choroidal neovascularization (CNV), which is the primary mode of vision loss in wet AMD [10]. VEGF is sufficient to induce CNV formation, and blockade of VEGF signaling can inhibit the formation of CNV in animal models. Anti-VEGF treatments are currently FDA-approved for neovascular AMD, but can be used off-label for other diseases, such as corneal neovascularization [11–13] and neovascular glaucoma [14–16].
There are number of emerging macromolecular drugs that are in clinical trials. For example, Fovista® (Ophthotech, Princeton, NJ) is a platelet-derived growth factor (PDGF) aptamer (50 kDa) that strongly binds to PDGF-B, which regulates neovascular pericytes [17], is currently in phase 3 clinical trials to treat AMD[18]. MP0112 (Molecular Partners, Zurich, Switzerland; and Allergan, Fort Worth, TX) uses designed ankyrin repeat proteins (DARPins), which are in a novel class of proteins that combine the high specificity and affinity binding to a target protein associated with antibody therapeutics with good molecular stability, tissue penetration and ease of manufacturing) [19]. MP0112 selectively binds all VEGF-A isoforms [20] and has completed Phase I/IIa clinical trials in wet AMD and diabetic macular edema.
ARC1905 (Ophthotech), an anti-C5 aptamer, completed a phase I study for dry AMD[21] and combination therapy with Lucentis® for wet AMD[22]. FCFD4514S (Genentech, South San Francisco, CA) which selectively inhibits complement factor D, completed a phase II clinical trial for geographic atrophy[23]. Adalimumab (Humira®, AbbVie, North Chicago, IL) is a TNF-alpha inhibitor, which was approved for rheumatoid arthritis, has been studied in a phase II clinical trial for CMV secondary to AMD [24]. There are other macromolecular drugs under development as well that are not listed here [3, 4, 25].
2. Ocular anatomy and drug delivery barriers
2.1. Ocular anatomy
The human eye is an approximately globular structure with a diameter of 24 mm, and a mass of about 7.5 grams [26]. Each ocular tissue has a distinct structure that plays a necessary function in enabling visual perception. As the eyes occupy less than 0.05% of the total body weight [27], each ocular tissue is compact and only several cell layers thick. Furthermore, since the eye is a part of the central nervous system and thus “immune privileged”, there are barriers in the eye meant to keep the systemic circulation separate from ocular tissues [28–30].
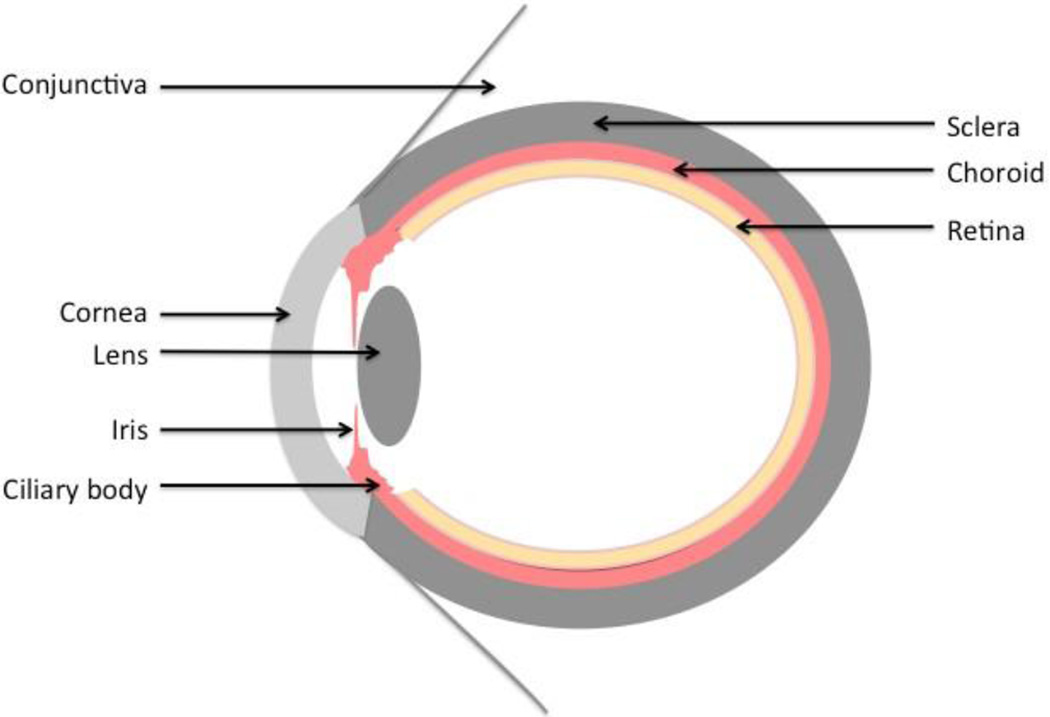
The smaller anterior portion is primarily responsible for collecting and focusing light, and the larger posterior portion is responsible for detecting light. The eye is comprised of three concentric adjoining tissue layers. The outermost layer is a collagenous layer that encircles the eye and provides it with mechanical strength. In the anterior portion, the collagen layer, called the cornea, is transparent to visible light and focuses the light on the retina. In the posterior portion, the collagenous layer is opaque and called the sclera. The middle layer, termed the uvea, is a pigmented layer that comprises the iris and ciliary body in the anterior portion and the vascular choroid in the posterior portion. The iris serves as a biological aperture to control the amount of light entering the eye. The ciliary body secretes aqueous humor, which provides the nutrients to the avascular tissues in the anterior segment and maintains the intraocular pressure. The choroid is a vast network of capillaries that supplies the retina with nutrients. The innermost layer is the neurosensory retina, which detects and transduces light signal to the brain. In between the anterior and posterior segments is the lens, which is responsible for further refracting the light entering the eye. The anterior segment is filled with aqueous humor, and the posterior segment is filled with the gelatinous vitreous humor.
2.2. Cornea and anterior barriers
The cornea is a ~500-µm-thick transparent collagenous structure that provides the majority of the refractive power of the eye and is the primary barrier to topic drug absorption [31]. The main layers of the cornea, from anterior to posterior, are the corneal epithelium, corneal stroma, and the corneal endothelium. Only moderately-charged small molecules are able to penetrate through the cornea [32, 33]. The corneal epithelium forms tight junctions that limit the passage of hydrophilic molecules [34, 35]. The collagen fibers of the stroma are charged (limiting the passage of hydrophobic molecules) and highly organized (acting to sieve larger molecules). Furthermore, there is a constant flow of a tear film across the outer surface of the cornea [36]. The limited diffusion across the cornea and limited capacity of the lacrimal lake result in a low bioavailability of 1–7% for most approved drugs and much lower bioavailiabity for other compounds, including macromolecules [37].
The conjunctiva is a thin translucent layer that is superficial to the sclera but not the cornea. Conjuntival epithelium also poses tight junctions that prevent easy penetration of the molecules [38]. The intercellular spaces in the conjunctival epithelium are wider than cornea and therefore more permeable to larger molecules [38]. In addition, there are many blood and lymphatic vessels throughout the subconjunctiva that remove molecules or particulates that land on the scleral surface of the eye [39, 40]. For this reason, drug molecules that are absorbed across the conjunctiva are often taken up by this vasculature and carried away from the eye into systemic circulation.
The intraocular environment, such as aqueous humor, is protected by the blood-aqueous barrier, which is comprised of the endothelial cells in the uvea and non-pigmented layer of the epithelium of the ciliary body. The blood--aqueous barrier allows both active and paracellular transport, which is controlled by the tight junctions. Fluorescently labeled dextrans as large as 150 kDa were able to cross the blood-aqueous barrier [41].
2.3 Sclera and choriocapillaries
Scleral also poses a barrier to diffusion of macromolecules. In vitro studies have shown a variety of molecules able to penetrate the sclera, although permeability drops off steeply at high molecular weight [33, 42–47] (See Table 1). In vivo, proteins as large as 145 kDa were shown to penetrate though the sclera and were detected in the choroid in rabbits [48, 49]. In ex vivo human sclera, up to 150 kDa dextran and 149 kDa bevacizumab were able to penetrate across the sclera [47].
Table 1.
Permeability of macromolecules across ocular barriers
| Compound | Tissue | Animala | MWb (kDa) | Permeability (cm/s) | ref |
|---|---|---|---|---|---|
| Serum Albumin | Cornea | H | 66 | 5.74E-07 | [52] |
| Immunoglobulin G | Stroma | R* | 140 | 8.00E-07 | [53] |
| Serum Albumin | Stroma | R* | 65 | 1.40E-07 | [53] |
| Serum Albumin | Sclera | C | 65 | 1.30E-07 | [45] |
| Hemoglobin | Sclera | C | 64.5 | 3.60E-07 | [45] |
| Dextran 10 | Sclera | H | 10 | 6.20E-06 | [42] |
| Dextran 70 | Sclera | H | 70 | 1.90E-06 | [42] |
| Bevacizumab | Sclera | H | 145 | 5.30E-07 | [54] |
| FITC-Dextran | RPE-Choroid | C | 9.3 | 2.14E-07 | [55] |
| FITC-Dextran | RPE-Choroid | C | 21.2 | 1.34E-07 | [55] |
| FITC-Dextran | RPE-Choroid | C | 38.2 | 4.60 E 08 | [55] |
| FITC-Dextran | RPE-Choroid | C | 77 | 2.70E 08 | [55] |
animal model used (R) Rabbit, (C) Cow (H) Human (*) in vivo,
molecular weight.
Choriocapillaries, composed of fenestrated endothelial cells, can hinder passage of macromolecules. In vivo experiment with various proteins in rats showed 40 kDa horseradish perdoxidase was able to penetrate rapidly across the capillaries but penetration of hemoglobin (68 kDa) and lactoperoxidase (84 kDa) was significantly restricted [50]. In another study, permeation of ferritin (480 kDa) in mouse, rabbit and guinea pig was examined and most of the tracer remained in the choriocapillaries [51].
2.4. Retina and blood-retinal barriers
The retina is the photosensitive layer that detects photons, processes light information, and transduces electrical impulses to the brain that are interpreted as vision. The retina is organized into layers with the photoreceptors in the outermost layer, interneurons in the middle, and the retinal ganglion cells in the innermost layer. The inner limiting membrane separates the retina from the vitreous humor. Retina itself is also a significant diffusion barrier to macromolecules. Diffusion of a compound with a molecular weight of larger than 76 kDa is severely limited in human retina [56]. The inner and outer plexform layers are the sites with highest resistance to the diffusion of macormolecules [56, 57]. Diffusion studies in human retina showed macromolecules larger than 150 kDa were largely arrested at the inner limiting membrane of the retina [56–58].
The blood-retinal barrier separates the neurosensory retina from the systemic circulation [29, 59]. The blood retinal barrier is subdivided into the inner and outer blood-retinal barriers. The inner blood-retinal barrier lines the retinal vasculature, which supplies the inner retina, and is comprised of the tight junctions between the endothelium of the retinal vasculature [60, 61]. Fluorescently labeled dextrans (3 kDa to 150 kDa) were tested for permeation across the inner blood-retinal barrier, however, labeled dextran of any sizes were not detectable [41]. The outer blood-retinal barrier is comprised of the retinal pigment epithelium (RPE), which lies between the photoreceptors and the choriocapillaries [62–64]. The RPE is a hexagonal monolayer whose function is to (i) physically and metabolically support the photoreceptors, (ii) selectively transport nutrients to photoreceptors and waste out of the subretinal space and (iii) absorb scattered light [63, 64]. The tight junctions in RPE pose significant barriers to macromolecules. Permeation of 376 Da to 77 kDa fluorescently tagged molecules showed exponential decrease in permeability in excised bovine eyes. The 77 kDa fluorescently-labeled dextran showed 35-fold smaller permeability compared to the 376 Da molecule [55].
3. Routes of Administration
3.1. Systemic delivery
Oral administration and parenteral injections are the two most common methods of delivery that can achieve systemic dosing. Systemic delivery of drugs can be used to treat ocular conditions, however the small size of the eye and ocular barriers prevent the favorable partitioning of drugs into the eye even for small molecules [55, 65, 66]. Furthermore, systemic treatments are subjected to modification by the liver and clearance by the kidney. Though a larger dose may be used to overcome these challenges, this can result in systemic side effects and possible toxicity if the therapeutic window is exceeded.
Oral delivery is used as a noninvasive method to deliver drugs systemically; however, it has been shown that limited penetration into the targeting tissue and systemic side effects are often associated ith oral delivery [67]. Drugs administered orally are subjected to the harsh environment of the gastrointestinal tract and to the first-pass metabolism of the liver. Due to extremely poor absorption across the gastrointestinal tract, macromolecular therapies are rarely given as an oral medication. Furthermore, few compounds, usually small molecules therapeutics of the analgesic, antibiotic, and antiviral classes, have been investigated as oral medications for ocular diseases [68–70].
Following parenteral administration, the blood-aqueous barrier and blood-retinal barrier are the major obstacles preventing drugs from entering the eye [6]. Both layers contain tight junctions that prevent the drugs from penetrating into the eye [66]. Due to the large size of macromolecules and poor absorption across many ocular barriers, macromolecules are typically not given systemically for ocular delivery [6]. In addition, increased doses of macromolecules can increase the severity of systemic side events due to off-target effects. Bioavailability of macromolecules in the back of the eye is very low when given systemically [71]. In one study, systemic administration of a radiolabeled protein showed very high drug levels in the blood and liver, but no drug was detected in ocular tissues [72].
The efficacy of the systemic delivery will be impacted by the integrity of the ocular barriers in disease. For example, a clinical study was done to test the efficacy of systemic bevacizumab in an uncontrolled open-label study in 18 patients with classic CNV with compromised RPE layer. In this study, patients were treated at baseline with 3 doses of bevacizumab by intravenous infusion over a period of 6 weeks. The assessment showed an increase in visual acuity by 14 letters and decreased thickness of the retina by 112 microns, indicating successful delivery to the back of the eye by systemic administration, probably enabled by the compromised RPE [73]. Although no serious systemic adverse events were identified through 24 weeks in this study, careful consideration has to be given before using systemic administration due to the high systemic concentration of the injected drug.
3.2. Extraocular delivery
3.2.1. Topical delivery
Topical instillation of ophthalmic drops can be the most convenient method to administer pharmaceutical agents for the treatment of ocular disease that manifest on the ocular surface or in the anterior segment. The limited capacity of the lacrimal lake in comparison with the typical volume of an eye drop limits the contact time of the eye drop with the eye. The vast majority of the eye drop is washed away within minutes, though viscosity enhancers can increase this residence time. Diffusion, and sometimes other applied forces, are used to drive drug molecules through the corneal barriers into the eye. Because of limited tissue penetration with these methods, topical delivery is usually used for external, corneal, and anterior segment diseases. This route has been used clinically to treat diseases found in the cornea, conjunctiva, sclera, iris, ciliary body, and aqueous humor, though efficacy has been demonstrated in some studies for posterior segment diseases experimentally [74, 75].
Topical administration is simple enough that patients are generally able to self-administer eye drops, although compliance with daily regimens can be low [76, 77]. The structure of the cornea (see previous section) only allows significant passage of small molecules that are moderately lipophilic. As a result, macromolecule solutions penetrate through the corneal barriers of the eye at very low rates, which in most cases are insufficient for therapy. An ex vivo human cornea diffusion study of serum albumin (66 kDa) and myoglobin (16 kDa) showed low diffusivities of 3.10E-8 and 5.5E-8 cm2/s [52]. In another study, bevacizumab was barely detected beyond the superficial layer of corneal epithelium in mice with intact corneas even after 7 days of topical administration [78]. In vivo, a pharmacokinetic study also showed aggressive delivery (1.25 mg/0.05 mL six times daily) of topical bevacizumab failed to reach therapeutic concentration in iris, choroid, retina, and vitreous [49].
3.2.2. Subconjunctival delivery
An injection into the subconjunctival space is a widely used periocular route of delivery. The subconjunctival space can be accessed with an injection deep to the bulbar conjunctiva and superficial to the sclera. The human subconjunctival space is highly expandable and is able to accommodate up to 500 µL [79]. Subconjunctival routes can be used for sustained-release delivery because a drug depot can be formed in these spaces. Subconjunctival administration is a potential way of delivering drugs to targets in the anterior segment and/or posterior segment [59] [80]. In vivo, proteins as large as 145 kDa were shown to penetrate though the sclera and were detected in the choroid, but mostly cleared by the systemic circulation and, thus, very small and well below the therapeutic concentration was detected in the retina [48, 49]. However, drugs injected into this space are often rapidly cleared into the systemic circulation. Micro-/nano-technology and/or physical methods, such as ultrasound and iontophoresis, can be combined with periocular administration to improve bioavailability after periocular delivery of macromolecules [81, 82].
3.3. Intraocular delivery
Intraocular drug delivery techniques seek to deposit the therapeutic agent in the eye, in some cases targeted directly at the site of action. This can shorten the distance drugs need to diffuse to increase local drug concentration, reduce drug delivery to other off-target sites to lessen side effects, and bypass ocular epithelial and other barriers to increase bioavailability.
3.3.1. Intrastromal delivery
The corneal epithelium along with tear fluid drainage poses a significant barrier to drug delivery into the cornea. To overcome the low penetration of topically applied drug, intrastromal injections can be utilized. Furthermore, the cornea can serve as a reservoir for large molecular weight drugs. Densely acked corneal stromal structure and proteoglycans in the corneal stroma hinder the diffusion of macromolecules inside the stroma [53, 83]. Intrastromal injection of immunoglobulin G and serum albumin showed extremely high half--lives of 26 and 9 days, respectively, inside the stroma due to hindered diffusion inside the densely packed stromal structure [53]. Recently, anti-VEGF treatments have been used to treat corneal neovascularization [84]. Intrastromal delivery of anti-VEGF therapy (bevacizumab) showed a dramatic regression of corneal neovascularization with an increase in visual acuity [85]. Intrastromal delivery of macromolecules is an attractive modality to deliver drugs because of their extended half-life inside the avascular corneal stroma [86]. Thus, intrastromal injection is a viable modality to deliver macromolecules directly into the cornea.
3.3.2. Intracameral delivery
Intracameral administration is the injection of a drug into the anterior chamber of the eye. Intracameral injection has been explored to improve the delivery of low bioavailability drugs to both the anterior and posterior segments of the eye, although intracameral injections are not able to deliver significant concentrations of drugs to the posterior segment of the eye [87]. They can, however, be used to deliver drug into the anterior segment, such as prophylactic delivery of antibiotics after cataract surgery to prevent endophthalmitis [86, 88]. Intracameral injection of antibiotic [89] and antifungal [90] agents has been used to treat deep corneal infections.
A few studies have investigated intracameral administration of macromolecules (bevacizumab). These studies showed that intracameral administration was more effective in reducing neovascularization of the cornea [91] and iris [14–16] than subconjunctival injections [91]. Intracameral administration of anti-VEGF therapy did not cause morphologic changes of corneal endothelial cells in the rabbit model [92, 93]. However, to combat the rapid turnover of fluid in the anterior chamber, repeated intracameral injections would be needed to maintain therapeutic concentrations of drug over time, which brings an increased risk of infection. Care should be taken when injecting polymeric sustained-release formulations into the anterior chamber, because injection of microparticles can cause physical clogging of the aqueous outflow facility and consequently increase intraocular pressure [94].
3.3.3. Intrascleral delivery
Intrascleral drug delivery has been explored as a possible delivery route to the back of the eye [39, 95–97]. The sclera is permeable to many drugs, including macromolecules (Table 1), and can potentially act as a drug reservoir for extended-release delivery [98, 99]. Diffusion of molecules deposited in a sclera of the eye is mediated by the drug molecule’s size and/or its binding characteristics to the sclera. Studies have shown that periocular and peribulbar (i.e., extraocular) injections that rely on transscleral movement of drugs have been used as a method to deliver drugs to the chorioretina [39, 87, 100, 101]. However, extraocular delivery did not result in significant chorioretinal targeting.
Other studies have hypothesized that placement of drug within the sclera would result in higher concentrations of drug delivered to the chorioretina [95–97]. One study demonstrated that a hollow microneedle (200–300 µm in length) that partially penetrated the sclera could be used to inject drugs intrasclerally [97]. The microneedle allowed for a more simplified approach (i.e., potentially can be done in a clinic setting) to inject up to 35 µL of drug solution or suspension (~1 mg solids). No in vivo or clinical application of this delivery route has been further explored. See section 3.2.2 for more information on scleral permeability.
3.3.4. Intravitreal delivery
Intravitreal administration is commonly done by injecting a drug solution or suspension into the vitreous cavity in the center of the eye. In 1998, the FDA approved an intravitreal injection of an antisense oligonucleotide compound to treat retinitis [102]. The injection procedure is generally done in the clinic under local anesthetic. A 27-- or 30-gauge needle is pierced through the pars plana, which is a relatively avascular zone in the eye approximately 3 mm posterior from the limbus. A volume of 20–100 µL can generally be injected into the vitreous humor without adversely affecting vision [103, 104]. Intravitreal injection is the main modality to deliver macormolecules to the posterior segment and into the eye.
An intravitreal injection is an invasive procedure that requires the penetration of all layers of the ocular globe, which, therefore, can be associated with complications, including endophthalmitis, retinal detachment, iritis, uveitis, intraocular hemorrhage, cataract, and hypotony, which can be caused by either the injection procedure or injected drug. The prevalence of endophthalmitis is estimated to be 0.3% per injection and 0.9% per eye [103]. Should these complications arise, permanent vision loss in that eye is possible. Although repeated injections potentially increase the rate of complications, it is not uncommon to have intravitreal injection on a monthly basis. Therefore, novel delivery method that can deliver long-term controlled--release formulations could significantly reduce complications caused by repeated injections. Care must be taken to ensure the injected formulation is transparent and does not impair vision.
Currently, many biopharmaceuticals to treat choroidal and retinal diseases are given as intravitreal injections [105], including the macromolecules pegaptanib sodium [106], bevacizumab [107], ranibizumab [108], and aflibercept [109] for the treatment of neovascular AMD. Anti-VEGF drugs bevacizumab (149 kDa) and ranibizumab (48 kDa) have reported half-lives in the rabbit eye in the vitreous of 4.32 and 2.88 days [110]. Pharmacokinetic analysis after a single intravitreal injection of 1.25 mg bevacizumab was able to reach peak concentration 93 µg/mL in choroid/retina and maintained the minimum concentration to fully inhibit neovascularization (500 ng/mL) [111] for 7 weeks in the rabbit [49]. To have their therapeutic effect, the drug molecules must diffuse through the vitreous and to the chorioretina. Furthermore, the drug must permeate across the multiple sub-layers of the retina and the RPE to reach the choroid; these two layers represent significant barriers to diffusion, especially for macromolecules [28, 112–115]. The barrier ability of the RPE layer has been demonstrated by several studies, which show that intravitreal delivery of anti-VEGF therapies is more effective with a compromised RPE layer [116–118].
Drugs injected into the intravitreal space are cleared either by the anterior or posterior route [119]. The anterior elimination is through aqueous humor via the trabecular meshwork and uveoscleral outflow. The posterior elimination involves passive diffusion of molecules across the blood-retinal barriers. Large molecular weight drugs tend to have half-lives inside the vitreous humor of days to weeks, although this depends on the molecule and formulation [120]. Small drugs are likely to escape from the vitreous more easily than macromolecular pharmacotherapies. In order to increase the half-life, pegaptanib was PEGylated to increase its molecular weight [121].
3.3.5. Suprachoroidal delivery
Suprachoroidal injections are designed to place drugs in the suprachoroidal space (SCS), which is a potential space found between the sclera and choroid. As demonstrated previously, the SCS can be accessed by surgically cutting through the conjunctiva and sclera, and a catheter can be snaked to the SCS behind the macula [122, 123]. More recent studies have shown that a microneedle can also be used to penetrate the sclera and deliver a drug suspension or solution into the SCS [124, 125]. Normally, the SCS is collapsed down due to the deformability of the chorioretina and the hydrostatic pressure in the eye [126, 127]. However, positive pressure from the injection can cause the space to expand and incorporate fluid [124, 125, 128]. SCS delivery is expected to be advantageous compared with other methods because of higher bioavailability, higher local concentration of drug in the choroid, fewer side effects due to focal targeting, and no obstruction of the visual axis [122–125]. SCS injections may enable improved efficacy in treating choroidal diseases, compared with intravitreal injections, since the drugs are delivered directly to the target tissue of the choroid [122, 124, 125]. However, high blood flow in the choriocapillaries (Table 2) can act as a sink that washes away small molecules and macromolecules deposited in the SCS on a timescale as fast as hours [59, 129, 130]. Thus, SCS injections may be best used with sustained-release delivery systems. The pharmacokinetics of SCS delivery would also be affected by the integrity of the choroid and RPE layer in disease state.
Table 2.
Blood flow in ocular tissue.
Because SCS delivery targets the vascular choroid, this route of administration results in faster drug clearance with a limited drug reservoir, compared with intravitreal injections. For example, a bevacizumab delivered into the SCS did not have the sustained release profile seen for bevacizumab injected intravitreally [130]. Bevacizumab delivered into the SCS was not detectable one week post injection [130]. Similar results were seen with an injection of ketorolac in a perfused ex vivo porcine eye [129]. These drugs were likely cleared from the SCS by high blood flow of the choriocapillaries (Table 2), which forms the deep border of the SCS.
In contrast studies with polymeric particles injected into the SCS using microneedles showed that nanoparticles as small as 20 nm and microparticles as large as 10 µm could be injected into the SCS and were not cleared for up to at least 2 months [124, 125]. The unfenestrated choriocapillaries have pores that are capable of clearing nanoparticles up to 12 nm in diameter [62]. Thus, bolus delivery of macromolecules to the SCS may be achieved without special formulation, but sustained-release delivery may require drug-encapsulation and release from particles with diameters significantly greater than 12 nm or other controlled-release formulations.
3.3.6. Subretinal delivery
The subretinal space is the extracellular space that exists between the photoreceptors of the retina and the RPE layer [133, 134]. The subretinal space is a loosely organized space several microns in thickness [135]. In order to overcome the barrier properties of the retinal inner limiting membrane and RPE, subretinal administration can be utilized. Subretinal administration is often used for targeting macromolecules specifically to retinal cells [136], often in the context of gene therapy [137, 138]. Subretinal injections are generally made via transcorneal or transscleral routes. Injection through the transcorneal route passes through the iris, lens and vitreous [139], whereas the transscleral route enters through the pars plana and vitreous [140]. Subretinal space injection has been used clinically [141], but long--term safety of this injection procedure has not been fully studied. Subretinal injection can lead to detachment of the photoreceptors from the RPE, which can result in the irreversible death of photoreceptors if not reversed quickly.
4. Controlled-release Delivery
4.1. Polymeric formulations
4.1.1. Biodegradable polymeric particles
Biodegradable particles for ocular drug delivery can be composed of biocompatible polymers, which degrade into their monomers and other byproducts that are safely cleared by the body [142]. In the case of small molecules, numerous compounds have been encapsulated in polymeric particles such as polylactic-co-glycolic acid (PLGA) for sustained release purposes with high encapsulation efficiency [143, 144]. When formulated as particles, these particles can be mixed with a fluid carrier and delivered via many routes, such as topical, periocular, suprachoroidal, and intravitreal injections [124, 143, 145–147]. The rate of drug release is thus determined by the degradation rate of the polymeric particle, which is often designed to provide sustained drug delivery for weeks or months [145]. To date, microsphere formulations for ophthalmic diseases have reached the pre-clinical stage, but have not yet reached a market [148]. One barrier to ocular applications has been the need to establish safety of biodegradable particles and their biodegradation products in the eye, formulation stability, and control of release rate [148–152].
Intravitreal injection of polymeric particles could cause clouding of the vitreous because the particles can scatter light [153]. Microparticles tend to sink to the lower part of the vitreal cavity, while nanoparticles are more susceptible to cause clouding in the vitreous [153]. Periocular injections, such as a subconjunctival injection, are also an attractive modality to deliver sustained release formulation. A study showed 20 nm particles were rapidly cleared from the rat periocular space following posterior subconjunctival injection, whereas 200 nm particles persisted for at least two months [154]. Periocular circulation (episcleral and lymphatic) was suggested to play an important role in the clearance of the smaller nanoparticles. Due to the systemic clearance of molecules from this space, subconjunctival injections cannot typically be considered targeted only to the eye.
A short fragment (40 amino acids) of anti-angiogenic pigment epithelium-derived factor (PEDF) has been loaded into nanospheres and showed release over 40 days in vitro [155]. Biodegradable microspheres have been engineered to release pegaptanib sodium (anti-VEGF aptamer) continuously at the scleral surface for up to 20 days [156]. Intravitreal PLGA microspheres released pegaptanib over several weeks after injection [157]. Bevacizumab was released for 2 months in vivo using nanoparticles using a porous microparticle system [147]. Microspheres have also been used to deliver anti-TGF-beta2 oligonucleotide using subconjunctival injection to increase bleb survival rate after glaucoma filtering surgery, which achieved in vitro release of the oligonucleotide for 30 days using PLGA microspheres [158].
There are significant hurdles for integrating macromolecules in a carrier matrix without compromising structural integrity and activity and controlling release rate and duration of release [159]. Microsphere technology is one of the most studied areas in sustained release technology. However, it is difficult to get long-term release of macromolecules for more than a few weeks to months at a therapeutic level.
4.1.2. Biodegradable polymeric Implants
Biodegradable polymers can also be formulated as implants that encapsulate drugs for controlled release. The main advantage of using biodegradable implants is that the larger drug loading, due to the larger dosage form size, and the smaller surface-to-volume ratio of the biodegradable implants compared to polymeric particles, allows prolonged drug release. Biodegradable implants are capable of releasing drug for many months. Implants can be introduced into the body by minor surgical techniques and many implants are placed directly in the vitreous or on the sclera for the drug formulation to release into the eye. Currently ophthalmic biodegradable implants for macromolecular drugs are not available clinically.
Biodegradable implants suffer from similar challenges as biodegradable polymeric particle systems, which include maintaining stability, control of release rate, duration of release and safety of biodegradation products [160]. Similar to biodegradable polymeric particle systems, the release profile from implants can be affected by different parameters such as drug loading, surface area and volume of implant, polymer composition and molecular weight, and solubility of the drug [161]. Biodegradable intravitreal implants for macromolecules have been previously demonstrated in a preclinical study. Human recombinant tissue plasminogen activator (t-PA, a thrombolytic agent) loaded into an implant was inserted into the vitreous and shown to release t-PA at a rate up to 0.5µg/day for 2 weeks [162].
A few ophthalmic implants for delivering small molecular weight drugs to the eye are available on the market. Ozurdex (Allergan, Irvine, CA) is approved for the treatment of macular edema but has been used off-label for uveitis too [163, 164]. Ozurdex is a biodegradable implant consisting of 0.7 mg of dexamethasone within a PLGA copolymer matrix that is implanted in the vitreal cavity and releases drug for 6 months [165]. The formulation approach used in Ozurdex was also evaluated for delivery of brimonidine tartrate in clinical trials [166]. Biodegradable implants are more susceptible to non-linear release kinetics and burst release compared to nonbiodegradable implants [160, 161]. If the implants can be made small enough, they can be inserted into the eye using a minimal surgical procedure.
4.1.3. Non-biodegradable implants
Non-biodegradable implants (i.e., reservoir type) typically contain a drug core surrounded by a semipermeable membrane allowing steady release of drug with zero-order kinetics for up to months to years [167]. However, these implants must be removed or refilled, which can involve a second surgical intervention, after they are expended. There are a few clinically approved non-biodegradable implants that release small-molecule drugs into the vitreous for a prolonged period of time. Minor surgery is required to place the implant at the pars plana and typically anchor it to the sclera via a suture. Due to this surgical procedure, non-biodegradable implants are more prone to complications, such as retinal detachment. For the extraocular non-biodegradable implants, possible chronic irritation and scar formation are drawbacks [168, 169].
To date, non-biodegradable implants for macromolecular weight drugs have not yet reached the market. An osmotic pump implant has been used to deliver IgG across sclera for 28 days. However, due to large size of the implant, the main compartment was implanted in the subcutaneous space and connected using a brain infusion kit to the sclera [101]. For small molecules, there are numerous implants available on the market. The first reservoir-type implant approved was the Vitrasert® (Bausch & Lomb, Rochester, NY) [170]. This ganciclovir-releasing implant was extensively used in the treatment of cytomegalovirus retinitis [171] and is capable of releasing drug for up to 5 to 8 months [172]. Another reservoir-type non-biodegradable implant is Retisert® (Bausch & Lomb). Retisert is used to release fluocinolone acetonide for chronic non-infectious uveitis [173, 174], but it has also been used for diabetic macular edema off-label [175]. Non--biodegradable implant systems for controlled release delivery of macromolecules could be an attractive dosage form if suitable implant design can achieve smaller form factor and maintain stability of the biopharmaceuticals during the implant’s lifetime in the body.
4.1.4. In situ gelling formulations
In situ-forming gels involve low-viscosity solutions that undergo phase transition to form a gel after a stimulus. The phase transition can be mediated by various stimuli, such as changes in temperature, pH, and ionic composition. Numerous in situ polymeric gelling (or thickening) systems, such as chitosan [176], poloxamer [177], hydroxypropylmethylcellulose [178] and polycaprolactone [179], have been developed for use in the eye. In situ gelling systems are generally used as a method to increase the precorneal residence time of topically applied drugs, which can increase bioavailability of small-molecule drugs [180, 181], but may not be applicable to macromolecules because of their extremely poor permeability across the corneal epithelium. In situ gelling formulations have been studied for delivering macromolecules as an injectable, which avoid surgical implantation and enables extended release. Recently, suprachoroidal delivery of anti-VEGF therapy was demonstrated for 60 days in vivo [182] using a light-activated in situ forming gel. A thermosensitive in situ gelling injectable was developed for ocular delivery of bevacizumab and demonstrated in vitro release of bevacizumab for 18 days [183]. In situ gelling system allows easy administration of sustained release materials to the desired site. However, it is difficult to get long-term release of macromolecules for more than a few weeks or months at a therapeutic level.
4.2. Delivery using cells
For lifelong diseases, encapsulated cell technology offers a technique that can result in the delivery of therapeutic agents in perpetuity. The principle of encapsulated cell technology (ECT) is to entrap genetically engineered cells within a semipermeable matrix such that they are immunologically isolated from the host’s body [184]. The cells are genetically designed to produce and release therapeutic substances (e.g., proteins). The encapsulated cells are protected from the host immune system by the matrix and cannot transmigrate away from the implant. The semipermeable membrane that surrounds the implant allows the passage of nutrients into the cells in the matrix and the release of the therapeutic substance from the implant. Encapsulated cell technology allows sustained delivery of pharmacotherapies by continuous expression of the protein without genetic alteration of the host cells [184]. Other studies have examined delivery of gene-based therapies to the patient’s endogenous cells, which is beyond the scope of this review, but has been discussed elsewhere [185, 186]
Previously, ciliary neurotrophic factor (CNTF) has been shown to protect the retina from degeneration in animal model of retinitis pigmentosa [184, 187]. This technology has now proceeded into clinical trials for the treatment of AMD [188] and retinitis pigmentosa [189]. In this study, each of the 10 patients was implanted with a device for 6 months. During this time, the patients reached and maintained visual acuity increased by 10–15 letters [187]. ECT is a potentially powerful and attractive modality for long-term delivery of macromolecules.
5. Conclusion
Over the past decade, macromolecular pharmacotherapeis have gained significant interest and increasing impact on clinical medicine. A few macromolecular drugs are already used in the eye, especially for anti-VEGF therapy of AMD, and many new macromolecular therapies are currently under development. However, ocular delivery of these biopharmaceuticals is challenging due to their large molecular weight. Conventional extraocular delivery methods, such as a topical eye drops and periocular injection, have low bioavailability and limited targeting of macromolecules. Intraocular delivery methods, including intravitreal injection, with the possible use of polymeric controlled release systems to minimize the frequency of injection, are of significant interest for delivery of macromolecules to the eye. Future challenges must address reducing the frequency of treatment when administered by healthcare personnel (e.g., through controlled release), increasing drug targeting to the intended sites of action to increase efficacy and safety (e.g., through physical and chemical targeting approaches) and increasing the bioavailability of extraocular delivery methods (e.g., through novel formulations).
Figure 1.
Anatomy of the eye.
Acknowledgements
This work was supported in part by the National Eye Institute (R24-EY017045 and R01-EY022097) and the National Natural Science Foundation of China (project numbers 81000369 and 81271036). We thank Henry Edelhauser for helpful discussions and Donna Bondy for administrative support. Mark Prausnitz is an inventor on patents and has a significant financial interest and serves on the Board of Directors of a company that is developing microneedle--based products for ocular delivery (Clearside Biomedical). This potential conflict of interest has been disclosed and is overseen by Georgia Tech and Emory University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryu JK, Kim HS, Nam DH. Current status and perspectives of biopharmaceutical drugs. Biotechnol Bioprocess Eng. 2012;17:900–911. [Google Scholar]
- 2.Aggarwal RS. What’s fueling the biotech engine-2012 to 2013. Nat Biotechnol. 2014;32:32–39. doi: 10.1038/nbt.2794. [DOI] [PubMed] [Google Scholar]
- 3.Syed BA, Evans JB, Bielory L. Wet AMD market. Nat Rev Drug Discov. 2012;11:827. doi: 10.1038/nrd3790. [DOI] [PubMed] [Google Scholar]
- 4.El Sanharawi M, Kowalczuk L, Touchard E, Omri S, de Kozak Y, Behar-Cohen F. Protein delivery for retinal diseases: from basic considerations to clinical applications. Prog Retin Eye Res. 2010;29:443–465. doi: 10.1016/j.preteyeres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Kaur IP, Kakkar S. Nanotherapy for posterior eye diseases. J Control Release. 2014 doi: 10.1016/j.jconrel.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12:348–360. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 8.Congdon N, O’Colmain B, Klaver CCW, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P, Hyman L. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 10.Castro MR, Lutz D, Edelman JL. Effect of COX inhibitors on VEGF-induced retinal vascular leakage and experimental corneal and choroidal neovascularization. Exp Eye Res. 2004;79:275–285. doi: 10.1016/j.exer.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Oh JY, Kim MK, Wee WR. Subconjunctival and intracorneal bevacizumab injection for corneal neovascularization in lipid keratopathy. Cornea. 2009;28:1070–1073. doi: 10.1097/ICO.0b013e31819839f9. [DOI] [PubMed] [Google Scholar]
- 12.Kim WJ, Jeong HO, Chung SK. The effect of bevacizumab on corneal neovascularization in rabbits. Korean J Ophthalmol. 2010;24:230–236. doi: 10.3341/kjo.2010.24.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acar BT, Halili E, Acar S. The effect of different doses of subconjunctival bevacizumab injection on corneal neovascularization. Int Ophthalmol. 2013;33:507–513. doi: 10.1007/s10792-013-9732-8. [DOI] [PubMed] [Google Scholar]
- 14.Duch S, Buchacra O, Milla E, Andreu D, Tellez J. Intracameral bevacizumab (Avastin) for neovascular glaucoma: a pilot study in 6 patients. J Glaucoma. 2009;18:140–143. doi: 10.1097/IJG.0b013e318170a747. [DOI] [PubMed] [Google Scholar]
- 15.Yuzbasioglu E, Artunay O, Rasier R, Sengul A, Bahcecioglu H. Simultaneous intravitreal and intracameral injection of bevacizumab (Avastin) in neovascular glaucoma. J Ocul Pharmacol Ther. 2009;25:259–264. doi: 10.1089/jop.2008.0088. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez Jimenez-Ortiz H, Perucho Martinez S, Toledano Fernandez N, Martin Giral E. Intracameral bevacizumab (Avastin(R)) in the management of neovascular glaucoma surgery. Arch Soc Esp Oftalmol. 2012;87:396–400. doi: 10.1016/j.oftal.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson GS, Adamis AP, Shima DT. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006;168:2036–2053. doi: 10.2353/ajpath.2006.050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A phase 3 safety and efficacy study of fovista™ (E10030) intravitreous administration in combination with either avastin® or eylea® compared to avastin® or eylea® monotherapy. U.S. National Institutes of Health; [Google Scholar]
- 19.Binz HK, Stumpp MT, Forrer P, Amstutz P, Pluckthun A. Designing repeat proteins: well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J Mol Biol. 2003;332:489–503. doi: 10.1016/s0022-2836(03)00896-9. [DOI] [PubMed] [Google Scholar]
- 20.Campochiaro PA, Channa R, Berger BB, Heier JS, Brown DM, Fiedler U, Hepp J, Stumpp MT. Treatment of diabetic macular edema with a designed ankyrin repeat protein that binds vascular endothelial growth factor: a phase I/II study. Am J Ophthalmol. 2013;155:697–704. doi: 10.1016/j.ajo.2012.09.032. 704 e691–692. [DOI] [PubMed] [Google Scholar]
- 21.A study of arc1905 (anti-c5 aptamer) in subjects with dry age-related macular degeneration. U.S. National Institutes of Health; [Google Scholar]
- 22.Arc1905 (anti-c5 aptamer) given either in combination therapy with lucentis® 0.5 mg/eye in subjects with neovascular age-related macular degeneration. U.S. National Institutes of Health; [Google Scholar]
- 23.A study of safety, tolerability, and evidence of activity of fcfd4514s administered monthly or every other month to patients with geographic atrophy. U.S. National Institutes of Health; [Google Scholar]
- 24.Intravitreal adalimumab in patients with choroidal neovascularization secondary to age-related macular degeneration. U.S. National Institutes of Health; [Google Scholar]
- 25.Rodrigues EB, Farah ME, Maia M, Penha FM, Regatieri C, Melo GB, Pinheiro MM, Zanetti CR. Therapeutic monoclonal antibodies in ophthalmology. Prog Retin Eye Res. 2009;28:117–144. doi: 10.1016/j.preteyeres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Todd TW, Beecher H, Williams GH, Todd AW. The weight and growth of the human eyeball. Hum Biol. 1940;12:1–20. [Google Scholar]
- 27.Todd TW, Beecher H, Williams GH, Todd AW. The weight and growth of the human eyeball. 1940 [Google Scholar]
- 28.Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979;23:279–296. doi: 10.1016/0039-6257(79)90158-9. [DOI] [PubMed] [Google Scholar]
- 29.Runkle EA, Antonetti DA. The blood-retinal barrier: structure and functional significance. Methods Mol Biol. 2011;686:133–148. doi: 10.1007/978-1-60761-938-3_5. [DOI] [PubMed] [Google Scholar]
- 30.Benhar I, London A, Schwartz M. The privileged immunity of immune privileged organs: the case of the eye. Front Immunol. 2012;3:296. doi: 10.3389/fimmu.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 32.Edwards A, Prausnitz MR. Predicted permeability of the cornea to topical drugs. Pharm Res. 2001;18:1497–1508. doi: 10.1023/a:1013061926851. [DOI] [PubMed] [Google Scholar]
- 33.Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87:1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- 34.Yi X, Wang Y, Yu FS. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest Ophthalmol Vis Sci. 2000;41:4093–4100. [PubMed] [Google Scholar]
- 35.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 36.van Haeringen NJ, Glasius E. Lysosomal hydrolases in tears and the lacrimal gland: effect of acetylsalicylic acid on the release from the lacrimal gland. Invest Ophthalmol Vis Sci. 1980;19:826–829. [PubMed] [Google Scholar]
- 37.Ghate D, Edelhauser HF. Barriers to glaucoma drug delivery. J Glaucoma. 2008;17:147–156. doi: 10.1097/IJG.0b013e31814b990d. [DOI] [PubMed] [Google Scholar]
- 38.Huang AJ, Tseng SC, Kenyon KR. Paracellular permeability of corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1989;30:684–689. [PubMed] [Google Scholar]
- 39.Kim SH, Galban CJ, Lutz RJ, Dedrick RL, Csaky KG, Lizak MJ, Wang NS, Tansey G, Robinson MR. Assessment of subconjunctival and intrascleral drug delivery to the posterior segment using dynamic contrast-enhanced magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2007;48:808–814. doi: 10.1167/iovs.06-0670. [DOI] [PubMed] [Google Scholar]
- 40.Nakao S, Hafezi-Moghadam A, Ishibashi T. Lymphatics and lymphangiogenesis in the eye. J Ophthalmol. 2012;2012:783163. doi: 10.1155/2012/783163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellhorn RW. Permeability of blood-ocular barriers of neonatal and adult cats to fluorescein-labeled dextrans of selected molecular sizes. Invest Ophthalmol Vis Sci. 1981;21:282–290. [PubMed] [Google Scholar]
- 42.Olsen TW, Edelhauser HF, Lim JI, Geroski DH. Human scleral permeability. Effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Invest Ophthalmol Vis Sci. 1995;36:1893–1903. [PubMed] [Google Scholar]
- 43.Aurélie Edwards MRP. Fiber matrix model of sclera and corneal stroma for drug delivery to the eye. AIChE J. 1998;44:12. [Google Scholar]
- 44.Rudnick DE, Noonan JS, Geroski DH, Prausnitz MR, Edelhauser HF. The effect of intraocular pressure on human and rabbit scleral permeability. Invest Ophthalmol Vis Sci. 1999;40:3054–3058. [PubMed] [Google Scholar]
- 45.Maurice DM, Polgar J. Diffusion across the sclera. Exp Eye Res. 1977;25:577–582. doi: 10.1016/0014-4835(77)90136-1. [DOI] [PubMed] [Google Scholar]
- 46.Pescina S, Ferrari G, Govoni P, Macaluso C, Padula C, Santi P, Nicoli S. In-vitro permeation of bevacizumab through human sclera: effect of iontophoresis application. Journal of Pharmacy and Pharmacology. 2010;62:1189–1194. doi: 10.1111/j.2042-7158.2010.01153.x. [DOI] [PubMed] [Google Scholar]
- 47.Wen H, Hao J, Li SK. Characterization of human sclera barrier properties for transscleral delivery of bevacizumab and ranibizumab. J Pharm Sci. 2013;102:892–903. doi: 10.1002/jps.23387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demetriades AM, Deering T, Liu H, Lu L, Gehlbach P, Packer JD, Mac Gabhann F, Popel AS, Wei LL, Campochiaro PA. Trans-scleral delivery of antiangiogenic proteins. J Ocul Pharmacol Ther. 2008;24:70–79. doi: 10.1089/jop.2007.0061. [DOI] [PubMed] [Google Scholar]
- 49.Nomoto H, Shiraga F, Kuno N, Kimura E, Fujii S, Shinomiya K, Nugent AK, Hirooka K, Baba T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2009;50:4807–4813. doi: 10.1167/iovs.08-3148. [DOI] [PubMed] [Google Scholar]
- 50.Pino RM, Essner E. Permeability of rat choriocapillaris to hemeproteins. Restriction of tracers by a fenestrated endothelium. J Histochem Cytochem. 1981;29:281–290. doi: 10.1177/29.2.7252121. [DOI] [PubMed] [Google Scholar]
- 51.Essner E, Gordon SR. Observations on the permeability of the choriocapillaris of the eye. Cell Tissue Res. 1983;231:571–577. doi: 10.1007/BF00218115. [DOI] [PubMed] [Google Scholar]
- 52.Charalel RA, Engberg K, Noolandi J, Cochran JR, Frank C, Ta CN. Diffusion of protein through the human cornea. Ophthalmic Res. 2012;48:50–55. doi: 10.1159/000329794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allansmith M, de Ramus A, Maurice D. The dynamics of IgG in the cornea. Invest Ophthalmol Vis Sci. 1979;18:947–955. [PubMed] [Google Scholar]
- 54.Pescina S, Ferrari G, Govoni P, Macaluso C, Padula C, Santi P, Nicoli S. In-vitro permeation of bevacizumab through human sclera: effect of iontophoresis application. J Pharm Pharmacol. 2010;62:1189–1194. doi: 10.1111/j.2042-7158.2010.01153.x. [DOI] [PubMed] [Google Scholar]
- 55.Pitkanen L, Ranta VP, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest Ophthalmol Vis Sci. 2005;46:641–646. doi: 10.1167/iovs.04-1051. [DOI] [PubMed] [Google Scholar]
- 56.Jackson TL, Antcliff RJ, Hillenkamp J, Marshall J. Human retinal molecular weight exclusion limit and estimate of species variation. Invest Ophthalmol Vis Sci. 2003;44:2141–2146. doi: 10.1167/iovs.02-1027. [DOI] [PubMed] [Google Scholar]
- 57.Tao Y, Li XX, Jiang YR, Bai XB, Wu BD, Dong JQ. Diffusion of macromolecule through retina after experimental branch retinal vein occlusion and estimate of intraretinal barrier. Curr Drug Metab. 2007;8:151–156. doi: 10.2174/138920007779815968. [DOI] [PubMed] [Google Scholar]
- 58.Mordenti J, Cuthbertson RA, Ferrara N, Thomsen K, Berleau L, Licko V, Allen PC, Valverde CR, Meng YG, Fei DT, Fourre KM, Ryan AM. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999;27:536–544. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- 59.Ranta VP, Mannermaa E, Lummepuro K, Subrizi A, Laukkanen A, Antopolsky M, Murtomaki L, Hornof M, Urtti A. Barrier analysis of periocular drug delivery to the posterior segment. J Control Release. 2010;148:42–48. doi: 10.1016/j.jconrel.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 60.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 61.Motiejunaite R, Kazlauskas A. Pericytes and ocular diseases. Exp Eye Res. 2008;86:171–177. doi: 10.1016/j.exer.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 62.Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res. 2010;2:14. doi: 10.1186/2040-2384-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 64.Binder S, Stanzel BV, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Prog Retin Eye Res. 2007;26:516–554. doi: 10.1016/j.preteyeres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Raviola G. The structural basis of the blood-ocular barriers. Exp Eye Res. 1977;(25 Suppl):27–63. doi: 10.1016/s0014-4835(77)80009-2. [DOI] [PubMed] [Google Scholar]
- 66.Barar J, Javadzadeh AR, Omidi Y. Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin Drug Deliv. 2008;5:567–581. doi: 10.1517/17425247.5.5.567. [DOI] [PubMed] [Google Scholar]
- 67.Kaur IP, Smitha R, Aggarwal D, Kapil M. Acetazolamide: future perspective in topical glaucoma therapeutics. Int J Pharm. 2002;248:1–14. doi: 10.1016/s0378-5173(02)00438-6. [DOI] [PubMed] [Google Scholar]
- 68.Coppens M, Versichelen L, Mortier E. Treatment of postoperative pain after ophthalmic surgery. Bull Soc Belge Ophtalmol. 2002:27–32. [PubMed] [Google Scholar]
- 69.Kampougeris G, Antoniadou A, Kavouklis E, Chryssouli Z, Giamarellou H. Penetration of moxifloxacin into the human aqueous humour after oral administration. Br J Ophthalmol. 2005;89:628–631. doi: 10.1136/bjo.2004.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chong DY, Johnson MW, Huynh TH, Hall EF, Comer GM, Fish DN. Vitreous penetration of orally administered famciclovir. Am J Ophthalmol. 2009;148:38–42. doi: 10.1016/j.ajo.2009.02.010. e31. [DOI] [PubMed] [Google Scholar]
- 71.Furrer E, Berdugo M, Stella C, Behar-Cohen F, Gurny R, Feige U, Lichtlen P, Urech DM. Pharmacokinetics and posterior segment biodistribution of ESBA105, an anti-TNF-alpha single-chain antibody, upon topical administration to the rabbit eye. Invest Ophthalmol Vis Sci. 2009;50:771–778. doi: 10.1167/iovs.08-2370. [DOI] [PubMed] [Google Scholar]
- 72.BenEzra D, Maftzir G, Hochberg E, Anteby I, Lorberboum-Galski H. Ocular distribution of the chimeric protein IL2-PE40. Curr Eye Res. 1995;14:153–158. doi: 10.3109/02713689508999927. [DOI] [PubMed] [Google Scholar]
- 73.Moshfeghi AA, Rosenfeld PJ, Puliafito CA, Michels S, Marcus EN, Lenchus JD, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twenty-four-week results of an uncontrolled open-label clinical study. Ophthalmology. 2006;113:2002. doi: 10.1016/j.ophtha.2006.05.070. e2001–2012. [DOI] [PubMed] [Google Scholar]
- 74.Lau D, Leung L, Ferdinands M, Allen PJ, Fullinfaw RO, Davies GE, Kong DC, Penetration of 1% voriconazole eye drops into human vitreous, humour: a prospective. open-label study. Clin Experiment Ophthalmol. 2009;37:197–200. doi: 10.1111/j.1442-9071.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- 75.Ottiger M, Thiel MA, Feige U, Lichtlen P, Urech DM. Efficient intraocular penetration of topical anti-TNF-alpha single-chain antibody (ESBA105) to anterior and posterior segment without penetration enhancer. Invest Ophthalmol Vis Sci. 2009;50:779–786. doi: 10.1167/iovs.08-2372. [DOI] [PubMed] [Google Scholar]
- 76.Reardon G, Kotak S, Schwartz GF. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer Adher. 2011;5:441–463. doi: 10.2147/PPA.S23780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 78.Dastjerdi MH, Sadrai Z, Saban DR, Zhang Q, Dana R. Corneal penetration of topical and subconjunctival bevacizumab. Invest Ophthalmol Vis Sci. 2011;52:8718–8723. doi: 10.1167/iovs.11-7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srirangam R, Majumdar S. Transscleral drug delivery to the posterior segment of the eye: Particulate and colloidal formulations and biopharmaceutical considerations. In: Mitra AK, editor. Advances in Ocular Drug Delivery, Research Signpost, Kerala, India. 2012. pp. 33–36. [Google Scholar]
- 80.Mac Gabhann F, Demetriades AM, Deering T, Packer JD, Shah SM, Duh E, Campochiaro PA, Popel AS. Protein transport to choroid and retina following periocular injection: theoretical and experimental study. Ann Biomed Eng. 2007;35:615–630. doi: 10.1007/s10439-006-9238-x. [DOI] [PubMed] [Google Scholar]
- 81.Eljarrat-Binstock E, Orucov F, Aldouby Y, Frucht-Pery J, Domb AJ. Charged nanoparticles delivery to the eye using hydrogel iontophoresis. J Control Release. 2008;126:156–161. doi: 10.1016/j.jconrel.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 82.Peeters L, Lentacker I, Vandenbroucke RE, Lucas B, Demeester J, Sanders NN, De Smedt SC. Can ultrasound solve the transport barrier of the neural retina? Pharm Res. 2008;25:2657–2665. doi: 10.1007/s11095-008-9684-2. [DOI] [PubMed] [Google Scholar]
- 83.Edwards A, Prausnitz MR. Fiber matrix model of sclera and corneal stroma for drug delivery to the eye. AIChE J. 1998;44:214–225. [Google Scholar]
- 84.Gupta D, Illingworth C. Treatments for corneal neovascularization: a review. Cornea. 2011 doi: 10.1097/ICO.0b013e318201405a. [DOI] [PubMed] [Google Scholar]
- 85.Hashemian MN, Zare MA, Rahimi F, Mohammadpour M. Deep intrastromal bevacizumab injection for management of corneal stromal vascularization after deep anterior lamellar keratoplasty, a novel technique. Cornea. 2011;30:215–218. doi: 10.1097/ICO.0b013e3181e291a6. [DOI] [PubMed] [Google Scholar]
- 86.Avisar I, Weinberger D, Kremer I. Effect of subconjunctival and intraocular bevacizumab injections on corneal neovascularization in a mouse model. Curr Eye Res. 2010;35:108–115. doi: 10.3109/02713680903429007. [DOI] [PubMed] [Google Scholar]
- 87.Lee TW, Robinson JR. Drug delivery to the posterior segment of the eye: some insights on the penetration pathways after subconjunctival injection. J Ocul Pharmacol Ther. 2001;17:565–572. doi: 10.1089/10807680152729257. [DOI] [PubMed] [Google Scholar]
- 88.Liesegang TJ. Prophylactic antibiotics in cataract operations. Mayo Clin Proc. 1997;72:149–159. doi: 10.4065/72.2.149. [DOI] [PubMed] [Google Scholar]
- 89.Daily MJ, Kachmaryk MM, Foody RJ. Successful prevention of visual loss with emergency management following inadvertent intracameral injection of gentamicin. Arch Ophthalmol. 1995;113:855–856. doi: 10.1001/archopht.1995.01100070025016. [DOI] [PubMed] [Google Scholar]
- 90.Yoon KC, Jeong IY, Im SK, Chae HJ, Yang SY. Therapeutic effect of intracameral amphotericin B injection in the treatment of fungal keratitis. Cornea. 2007;26:814–818. doi: 10.1097/ICO.0b013e31806c791e. [DOI] [PubMed] [Google Scholar]
- 91.Dratviman-Storobinsky O, Lubin BC, Hasanreisoglu M, Goldenberg-Cohen N. Effect of subconjuctival and intraocular bevacizumab injection on angiogenic gene expression levels in a mouse model of corneal neovascularization. Mol Vis. 2009;15:2326–2338. [PMC free article] [PubMed] [Google Scholar]
- 92.Shin JP, Lee JW, Sohn BJ, Kim HK, Kim SY. In vivo corneal endothelial safety of intracameral bevacizumab and effect in neovascular glaucoma combined with Ahmed valve implantation. J Glaucoma. 2009;18:589–594. doi: 10.1097/IJG.0b013e3181996ed2. [DOI] [PubMed] [Google Scholar]
- 93.Park HY, Kim SJ, Lee HB, Kim ES, Tchah H. Effect of intracameral bevacizumab injection on corneal endothelium in rabbits. Cornea. 2008;27:1151–1155. doi: 10.1097/ICO.0b013e318180e572. [DOI] [PubMed] [Google Scholar]
- 94.Sappington RM, Carlson BJ, Crish SD, Calkins DJ. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci. 2010;51:207–216. doi: 10.1167/iovs.09-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okabe J, Kimura H, Kunou N, Okabe K, Kato A, Ogura Y. Biodegradable intrascleral implant for sustained intraocular delivery of betamethasone phosphate. Invest Ophthalmol Vis Sci. 2003;44:740–744. doi: 10.1167/iovs.02-0375. [DOI] [PubMed] [Google Scholar]
- 96.Jiang J, Gill HS, Ghate D, McCarey BE, Patel SR, Edelhauser HF, Prausnitz MR. Coated microneedles for drug delivery to the eye. Invest Ophthalmol Vis Sci. 2007;48:4038–4043. doi: 10.1167/iovs.07-0066. [DOI] [PubMed] [Google Scholar]
- 97.Jiang J, Moore JS, Edelhauser HF, Prausnitz MR. Intrascleral drug delivery to the eye using hollow microneedles. Pharm Res. 2009;26:395–403. doi: 10.1007/s11095-008-9756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ambati J, Canakis CS, Miller JW, Gragoudas ES, Edwards A, Weissgold DJ, Kim I, Delori FC, Adamis AP. Diffusion of high molecular weight compounds through sclera. Invest Ophthalmol Vis Sci. 2000;41:1181–1185. [PubMed] [Google Scholar]
- 99.Edelhauser HF, Rowe-Rendleman CL, Robinson MR, Dawson DG, Chader GJ, Grossniklaus HE, Rittenhouse KD, Wilson CG, Weber DA, Kuppermann BD, Csaky KG, Olsen TW, Kompella UB, Holers VM, Hageman GS, Gilger BC, Campochiaro PA, Whitcup SM, Wong WT. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci. 2010;51:5403–5420. doi: 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robinson MR, Lee SS, Kim H, Kim S, Lutz RJ, Galban C, Bungay PM, Yuan P, Wang NS, Kim J, Csaky KG. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp Eye Res. 2006;82:479–487. doi: 10.1016/j.exer.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 101.Ambati J, Gragoudas ES, Miller JW, You TT, Miyamoto K, Delori FC, Adamis AP. Transscleral delivery of bioactive protein to the choroid and retina. Invest Ophthalmol Vis Sci. 2000;41:1186–1191. [PubMed] [Google Scholar]
- 102.insert P. Vitravene (Fomivirsen Sodium Intravitreal Injectable) Injection. Isis Pharmaceuticals, Inc.; 1998. [Google Scholar]
- 103.Jager RD, Aiello LP, Patel SC, Cunningham ET., Jr. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676–698. doi: 10.1097/00006982-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 104.Benz MS, Albini TA, Holz ER, Lakhanpal RR, Westfall AC, Iyer MN, Carvounis PE. Short-term course of intraocular pressure after intravitreal injection of triamcinolone acetonide. Ophthalmology. 2006;113:1174–1178. doi: 10.1016/j.ophtha.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 105.Anderson OA, Bainbridge JW, Shima DT. Delivery of anti-angiogenic molecular therapies for retinal disease. Drug Discov Today. 2010;15:272–282. doi: 10.1016/j.drudis.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 106.Eyetech Study G. Preclinical and phase 1A clinical evaluation of an anti-VEGF pegylated aptamer (EYE001) for the treatment of exudative age-related macular degeneration. Retina. 2002;22:143–152. doi: 10.1097/00006982-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 107.Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL., 3rd Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 108.Chang TS, Tonnu IQ, Globe DR, Fine J. longitudinal changes in self-reported visual functioningin AMD patients in a randomized controlled Phase I/II trial of lucentisTM (ranizumab; rHuFAB v2) Invest Ophthalmol Vis Sci. 2004;45:3098. [Google Scholar]
- 109.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U. View, V.S. Groups, Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 110.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis) Ophthalmology. 2007;114:2179–2182. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 111.Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7:335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 112.Heiduschka P, Feitz H, Hofmeister S, Schultheiss S, Mack AF, Peters S, Zeimssen F, Niggeman B, Julien S, Bartz-Schmidt KU. Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Invest Ophthalmol Vis Sci. 2007;48:2814–2823. doi: 10.1167/iovs.06-1171. [DOI] [PubMed] [Google Scholar]
- 113.Ruponen M, Honkakoski P, Ronkko S, Pelkonen J, Tammi M, Urtti A. Extracellular and intracellular barriers in non-viral gene delivery. J Control Release. 2003;93:213–217. doi: 10.1016/j.jconrel.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 114.Olsen TW, Gilger BC. Suprachoroidal and intrascleral drug delivery. In: Kompella UB, Edelhauser HF, editors. Drug product development for the back of the eye. London: Springer; 2011. pp. 173–184. [Google Scholar]
- 115.Dalkara D, Kolstad KD, Caporale N, Visel M, Klimczak RR, Schaffer DV, Flannery JG. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther. 2009;17:2096–2102. doi: 10.1038/mt.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Otsuji T, Nagai Y, Sho K, Tsumura A, Koike N, Tsuda M, Nishimura T, Takahashi K. Initial non-responders to ranibizumab in the treatment of age-related macular degeneration (AMD) Clin Ophthalmol. 2013;7:1487–1490. doi: 10.2147/OPTH.S46317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nigam N, Hedaya J, Freeman WR. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol. 2008;92:864–865. [PMC free article] [PubMed] [Google Scholar]
- 118.Lux A, Llacer H, Heussen FM, Joussen AM. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol. 2007;91:1318–1322. doi: 10.1136/bjo.2006.113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chader G, Sears ML. Pharmacology of the eye. Berlin ; New York: Springer-Verlag; 1984. [Google Scholar]
- 120.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 121.Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22:315–329. doi: 10.2165/00063030-200822050-00004. [DOI] [PubMed] [Google Scholar]
- 122.Olsen TW, Feng X, Wabner K, Conston SR, Sierra DH, Folden DV, Smith ME, Cameron JD. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol. 2006;142:777–787. doi: 10.1016/j.ajo.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 123.Einmahl S, Savoldelli M, D’Hermies F, Tabatabay C, Gurny R, Behar-Cohen F. Evaluation of a novel biomaterial in the suprachoroidal space of the rabbit eye. Invest Ophthalmol Vis Sci. 2002;43:1533–1539. [PubMed] [Google Scholar]
- 124.Patel SR, Lin AS, Edelhauser HF, Prausnitz MR. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm Res. 2011;28:166–176. doi: 10.1007/s11095-010-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012;53:4433–4441. doi: 10.1167/iovs.12-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fatt I, Shantinath K. Flow conductivity of retina and its role in retinal adhesion. Exp Eye Res. 1971;12:218–226. doi: 10.1016/0014-4835(71)90094-7. [DOI] [PubMed] [Google Scholar]
- 127.Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci. 1989;30:233–238. [PubMed] [Google Scholar]
- 128.Kim YC, Edelhauser HF, Prausnitz MR. Particle-stabilized emulsion droplets for gravity-mediated targeting in the posterior segment of the eye. Adv Healthc Mater. 2014 doi: 10.1002/adhm.201300696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abarca EM, Salmon JH, Gilger BC. Effect of choroidal perfusion on ocular tissue distribution after intravitreal or suprachoroidal injection in an arterially perfused ex vivo pig eye model. J Ocul Pharmacol Ther. 2013;29:715–722. doi: 10.1089/jop.2013.0063. [DOI] [PubMed] [Google Scholar]
- 130.Olsen TW, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron JD. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci. 2011;52:4749–4756. doi: 10.1167/iovs.10-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stjernschantz J, Nilsson SF, Astin M. Vasodynamic and angiogenic effects of eicosanoids in the eye. Prog Clin Biol Res. 1989;312:155–170. [PubMed] [Google Scholar]
- 132.Thorig L, Bill A. Effects of B-HT 920 in the eye and on regional blood flows in anaesthetized and conscious rabbits. Curr Eye Res. 1986;5:565–573. doi: 10.3109/02713688609015120. [DOI] [PubMed] [Google Scholar]
- 133.Fischbarg J. Advances in organ biology. Greenwich, Conn: JAI Press; 1996. [Google Scholar]
- 134.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Progress in retinal and eye research. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 135.Adler AJ, Martin KJ. Retinol-binding proteins in bovine interphotoreceptor matrix. Biochemical and biophysical research communications. 1982;108:1601–1608. doi: 10.1016/s0006-291x(82)80091-0. [DOI] [PubMed] [Google Scholar]
- 136.Kompella UB, Amrite AC, Pacha Ravi R, Durazo SA. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Progress in retinal and eye research. 2013;36:172–198. doi: 10.1016/j.preteyeres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johnson CJ, Berglin L, Chrenek MA, Redmond TM, Boatright JH, Nickerson JM. Technical brief: subretinal injection and electroporation into adult mouse eyes. Molecular vision. 2008;14:2211–2226. [PMC free article] [PubMed] [Google Scholar]
- 138.Shen WY, Rakoczy PE. Uptake dynamics and retinal tolerance of phosphorothioate oligonucleotide and its direct delivery into the site of choroidal neovascularization through subretinal administration in the rat. Antisense Nucleic Acid Drug Dev. 2001;11:257–264. doi: 10.1089/108729001317022250. [DOI] [PubMed] [Google Scholar]
- 139.Timmers AM, Zhang H, Squitieri A, Gonzalez-Pola C. Subretinal injections in rodent eyes: effects on electrophysiology and histology of rat retina. Mol Vis. 2001;7:131–137. [PubMed] [Google Scholar]
- 140.Schlichtenbrede FC, da Cruz L, Stephens C, Smith AJ, Georgiadis A, Thrasher AJ, Bainbridge JWB, Seeliger MW, Ali RR. Long-term evaluation of retinal function in Prph2Rd2/Rd2 mice following AAV-mediated gene replacement therapy. J Gene Med. 2003;5:757–764. doi: 10.1002/jgm.401. [DOI] [PubMed] [Google Scholar]
- 141.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kimura H, Ogura Y. Biodegradable polymers for ocular drug delivery. Ophthalmologica. 2001;215:143–155. doi: 10.1159/000050849. [DOI] [PubMed] [Google Scholar]
- 143.Kompella UB, Bandi N, Ayalasomayajula SP. Subconjunctival nano-and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest Ophthalmol Vis Sci. 2003;44:1192–1201. doi: 10.1167/iovs.02-0791. [DOI] [PubMed] [Google Scholar]
- 144.Saishin Y, Silva RL, Saishin Y, Callahan K, Schoch C, Ahlheim M, Lai H, Kane F, Brazzell RK, Bodmer D, Campochiaro PA. Periocular injection of microspheres containing PKC412 inhibits choroidal neovascularization in a porcine model. Invest Ophthalmol Vis Sci. 2003;44:4989–4993. doi: 10.1167/iovs.03-0600. [DOI] [PubMed] [Google Scholar]
- 145.Moshfeghi AA, Peyman GA. Micro-and nanoparticulates. Adv Drug Deliv Rev. 2005;57:2047–2052. doi: 10.1016/j.addr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 146.Bin Choy Y, Park JH, Prausnitz MR. Mucoadhesive microparticles engineered for ophthalmic drug delivery. J Phys Chem Solids. 2008;69:1533–1536. doi: 10.1016/j.jpcs.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yandrapu SK, Upadhyay AK, Petrash JM, Kompella UB. Nanoparticles in porous microparticles prepared by supercritical infusion and pressure quench technology for sustained delivery of bevacizumab. Mol Pharm. 2013;10:4676–4686. doi: 10.1021/mp400487f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eljarrat-Binstock E, Pe’er J, Domb AJ. New techniques for drug delivery to the posterior eye segment. Pharm Res. 2010;27:530–543. doi: 10.1007/s11095-009-0042-9. [DOI] [PubMed] [Google Scholar]
- 149.Short BG. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol Pathol. 2008;36:49–62. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- 150.Bu HZ, Gukasyan HJ, Goulet L, Lou XJ, Xiang C, Koudriakova T. Ocular disposition, pharmacokinetics, efficacy and safety of nanoparticle-formulated ophthalmic drugs. Curr Drug Metab. 2007;8:91–107. doi: 10.2174/138920007779815977. [DOI] [PubMed] [Google Scholar]
- 151.Mainardes RM, Urban MC, Cinto PO, Khalil NM, Chaud MV, Evangelista RC, Gremiao MP. Colloidal carriers for ophthalmic drug delivery. Curr Drug Targets. 2005;6:363–371. doi: 10.2174/1389450053765914. [DOI] [PubMed] [Google Scholar]
- 152.Rawas-Qalaji M, Williams CA. Advances in ocular drug delivery. Curr Eye Res. 2012;37:345–356. doi: 10.3109/02713683.2011.652286. [DOI] [PubMed] [Google Scholar]
- 153.Hsu J. Drug delivery methods for posterior segment disease. Curr Opin Ophthalmol. 2007;18:235–239. doi: 10.1097/ICU.0b013e3281108000. [DOI] [PubMed] [Google Scholar]
- 154.Amrite AC, Edelhauser HF, Singh SR, Kompella UB. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis. 2008;14:150–160. [PMC free article] [PubMed] [Google Scholar]
- 155.Li H, Tran VV, Hu Y, Mark Saltzman W, Barnstable CJ, Tombran-Tink J. A PEDF N-terminal peptide protects the retina from ischemic injury when delivered in PLGA nanospheres. Exp Eye Res. 2006;83:824–833. doi: 10.1016/j.exer.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 156.Carrasquillo KG, Ricker JA, Rigas IK, Miller JW, Gragoudas ES, Adamis AP. Controlled delivery of the anti-VEGF aptamer EYE001 with poly(lactic-co-glycolic)acid microspheres. Invest Ophthalmol Vis Sci. 2003;44:290–299. doi: 10.1167/iovs.01-1156. [DOI] [PubMed] [Google Scholar]
- 157.Cook GP, Burgess L, Wing J, Dowie T. Preparation and characterization of pegaptanib sustained release microsphere formulations for intraocular application. Association for research in vision and ophthalmology (ARVO; 2006. p. B521. [Google Scholar]
- 158.Gomes dos Santos AL, Bochot A, Doyle A, Tsapis N, Siepmann J, Siepmann F, Schmaler J, Besnard M, Behar-Cohen F, Fattal E. Sustained release of nanosized complexes of polyethylenimine and anti-TGF-beta 2 oligonucleotide improves the outcome of glaucoma surgery. J Control Release. 2006;112:369–381. doi: 10.1016/j.jconrel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 159.Taluja A, Youn YS, Bae YH. Novel approaches in microparticulate PLGA delivery systems encapsulating proteins. J Mater Chem. 2007;17:4002. [Google Scholar]
- 160.Yasukawa T, Ogura Y, Sakurai E, Tabata Y, Kimura H. Intraocular sustained drug delivery using implantable polymeric devices. Adv Drug Deliv Rev. 2005;57:2033–2046. doi: 10.1016/j.addr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 161.Manickavasagam D, Oyewumi MO. Critical assessment of implantable drug delivery devices in glaucoma management. J Drug Deliv. 2013;2013:895013. doi: 10.1155/2013/895013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou T, Lewis H, Foster RE, Schwendeman SP. Development of a multiple-drug delivery implant for intraocular management of proliferative vitreoretinopathy. J Control Release. 1998;55:281–295. doi: 10.1016/s0168-3659(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 163.Siddique SS, Shah R, Suelves AM, Foster CS. Road to remission: a comprehensive review of therapy in uveitis. Expert Opin Investig Drugs. 2011;20:1497–1515. doi: 10.1517/13543784.2011.617741. [DOI] [PubMed] [Google Scholar]
- 164.Williams GA, Haller JA, Kuppermann BD, Blumenkranz MS, Weinberg DV, Chou C, Whitcup SM, Dexamethasone DDSPIISG. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am J Ophthalmol. 2009;147:1048–1054. doi: 10.1016/j.ajo.2008.12.033. 1054 e1041–1042. [DOI] [PubMed] [Google Scholar]
- 165.Christoforidis JB, Chang S, Jiang A, Wang J, Cebulla CM. Intravitreal devices for the treatment of vitreous inflammation. Mediators Inflamm. 2012;2012:126463. doi: 10.1155/2012/126463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Safety and efficacy of brimonidine intravitreal implant in patients with geographic atrophy due to age-related macular degeneration (AMD) U.S. National Institutes of Health; [Google Scholar]
- 167.Driot JY, Novack GD, Rittenhouse KD, Milazzo C, Pearson PA. Ocular pharmacokinetics of fluocinolone acetonide after Retisert intravitreal implantation in rabbits over a 1-year period. J Ocul Pharmacol Ther. 2004;20:269–275. doi: 10.1089/1080768041223611. [DOI] [PubMed] [Google Scholar]
- 168.Baino F, Perero S, Ferraris S, Miola M, Balagna C, Verne E, Vitale-Brovarone C, Coggiola A, Dolcino D, Ferraris M. Biomaterials for orbital implants and ocular prostheses: overview and future prospects. Acta Biomater. 2014;10:1064–1087. doi: 10.1016/j.actbio.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 169.Lloyd AW, Faragher RG, Denyer SP. Ocular biomaterials and implants. Biomaterials. 2001;22:769–785. doi: 10.1016/s0142-9612(00)00237-4. [DOI] [PubMed] [Google Scholar]
- 170.Bourges JL, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, Gurny R, BenEzra D, Behar-Cohen F. Intraocular implants for extended drug delivery: therapeutic applications. Adv Drug Deliv Rev. 2006;58:1182–1202. doi: 10.1016/j.addr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 171.Kedhar SR, Jabs DA. Cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Herpes. 2007;14:66–71. [PubMed] [Google Scholar]
- 172.Lee SS, Hughes P, Ross AD, Robinson MR. Biodegradable implants for sustained drug release in the eye. Pharm Res. 2010;27:2043–2053. doi: 10.1007/s11095-010-0159-x. [DOI] [PubMed] [Google Scholar]
- 173.Jaffe GJ, Martin D, Callanan D, Pearson PA, Levy B, Comstock T. G. Fluocinolone Acetonide Uveitis Study, Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113:1020–1027. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 174.Taban M, Lowder CY, Kaiser PK. Outcome of fluocinolone acetonide implant (Retisert) reimplantation for chronic noninfectious posterior uveitis. Retina. 2008;28:1280–1288. doi: 10.1097/IAE.0b013e31817d8bf2. [DOI] [PubMed] [Google Scholar]
- 175.Pearson PA, Comstock TL, Ip M, Callanan D, Morse LS, Ashton P, Levy B, Mann ES, Eliott D. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011;118:1580–1587. doi: 10.1016/j.ophtha.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 176.Chen X, Li X, Zhou Y, Wang X, Zhang Y, Fan Y, Huang Y, Liu Y. Chitosan-based thermosensitive hydrogel as a promising ocular drug delivery system: preparation, characterization, and in vivo evaluation. J Biomater Appl. 2012;27:391–402. doi: 10.1177/0885328211406563. [DOI] [PubMed] [Google Scholar]
- 177.Qian Y, Wang F, Li R, Zhang Q, Xu Q. Preparation and evaluation of in situ gelling ophthalmic drug delivery system for methazolamide. Drug Dev Ind Pharm. 2010;36:1340–1347. doi: 10.3109/03639041003801893. [DOI] [PubMed] [Google Scholar]
- 178.Boddu SH, Jwala J, Vaishya R, Earla R, Karla PK, Pal D, Mitra AK. Novel nanoparticulate gel formulations of steroids for the treatment of macular edema. J Ocul Pharmacol Ther. 2010;26:37–48. doi: 10.1089/jop.2009.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yin H, Gong C, Shi S, Liu X, Wei Y, Qian Z. Toxicity evaluation of biodegradable and thermosensitive PEG-PCL-PEG hydrogel as a potential in situ sustained ophthalmic drug delivery system. J Biomed Mater Res B Appl Biomater. 2010;92:129–137. doi: 10.1002/jbm.b.31498. [DOI] [PubMed] [Google Scholar]
- 180.Pahuja P, Arora S, Pawar P. Ocular drug delivery system: a reference to natural polymers. Expert Opin Drug Deliv. 2012;9:837–861. doi: 10.1517/17425247.2012.690733. [DOI] [PubMed] [Google Scholar]
- 181.Achouri D, Alhanout K, Piccerelle P, Andrieu V. Recent advances in ocular drug delivery. Drug Dev Ind Pharm. 2013;39:1599–1617. doi: 10.3109/03639045.2012.736515. [DOI] [PubMed] [Google Scholar]
- 182.Tyagi P, Barros M, Stansbury JW, Kompella UB. Light-activated, in situ forming gel for sustained suprachoroidal delivery of bevacizumab. Molecular pharmaceutics. 2013;10:2858–2867. doi: 10.1021/mp300716t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang CH, Hwang YS, Chiang PR, Shen CR, Hong WH, Hsiue GH. Extended release of bevacizumab by thermosensitive biodegradable and biocompatible hydrogel. Biomacromolecules. 2012;13:40–48. doi: 10.1021/bm2009558. [DOI] [PubMed] [Google Scholar]
- 184.Tao W. Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin Biol Ther. 2006;6:717–726. doi: 10.1517/14712598.6.7.717. [DOI] [PubMed] [Google Scholar]
- 185.McClements ME, MacLaren RE. Gene therapy for retinal disease. Transl Res. 2013;161:241–254. doi: 10.1016/j.trsl.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roy K, Stein L, Kaushal S. Ocular gene therapy: an evaluation of recombinant adeno-associated virus-mediated gene therapy interventions for the treatment of ocular disease. Hum Gene Ther. 2010;21:915–927. doi: 10.1089/hum.2010.041. [DOI] [PubMed] [Google Scholar]
- 187.Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, Bush RA. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.A study of an encapsulated cell technology (ECT) implant for patients with atrophic macular degeneration. U.S. National Institutes of Health; [Google Scholar]
- 189.Retinal imaging of subjects implanted with ciliary neurotrophic factor (cntf)-releasing encapsulated cell implant for early-stage retinitis pigmentosa. U.S. National Institutes of Health; [Google Scholar]