Abstract
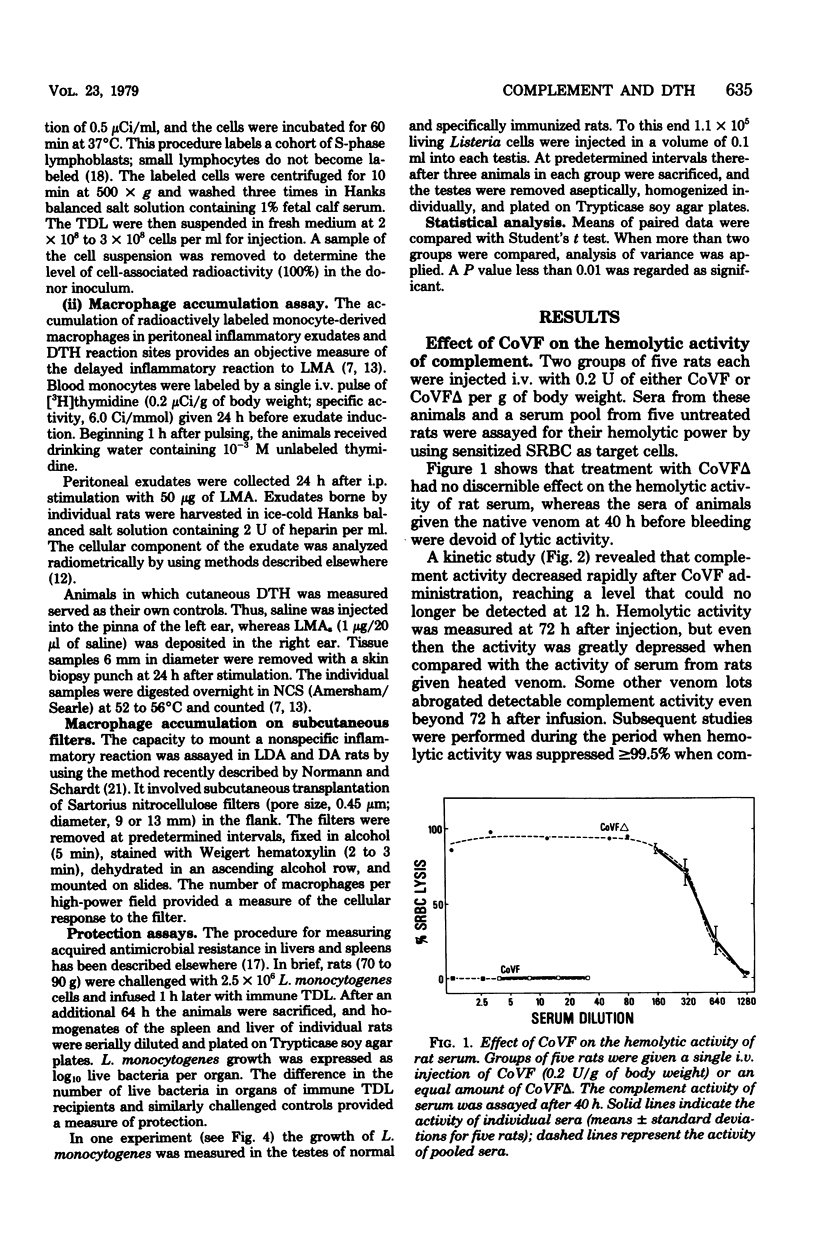
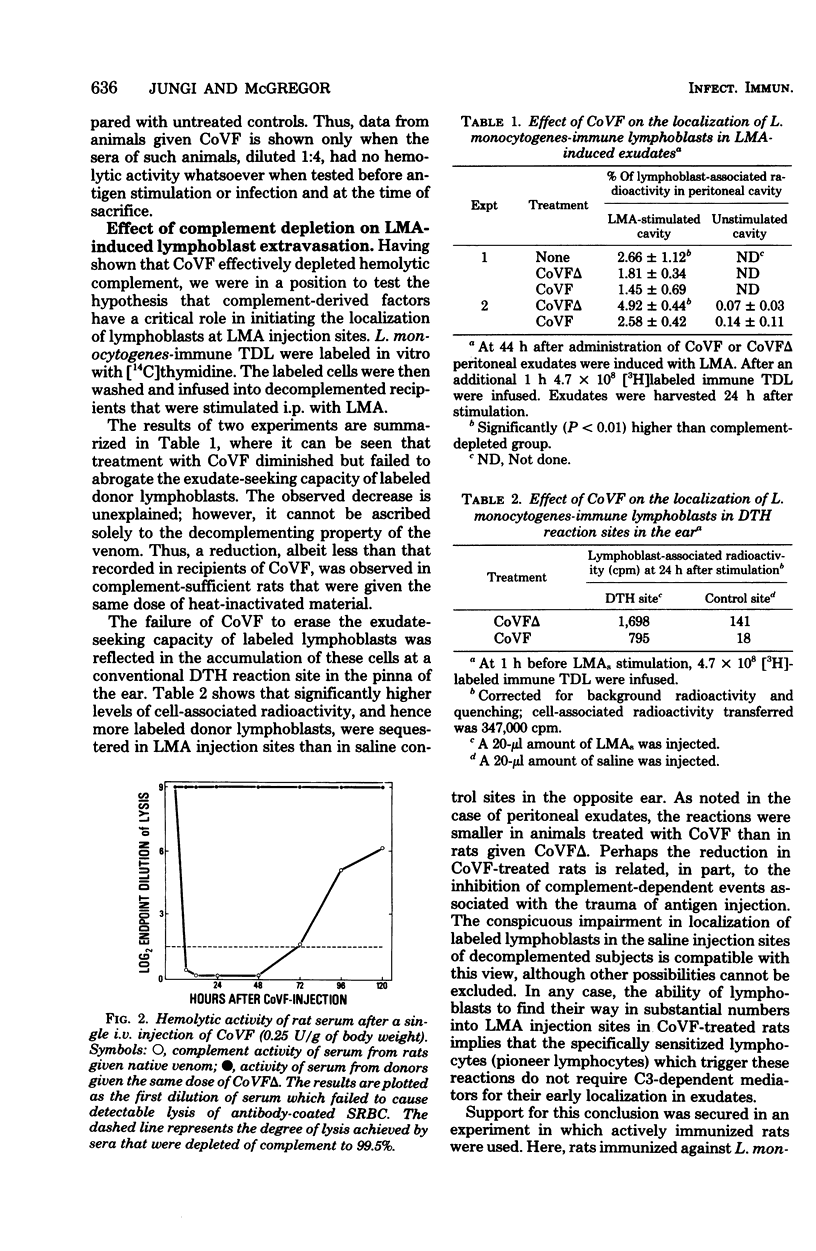
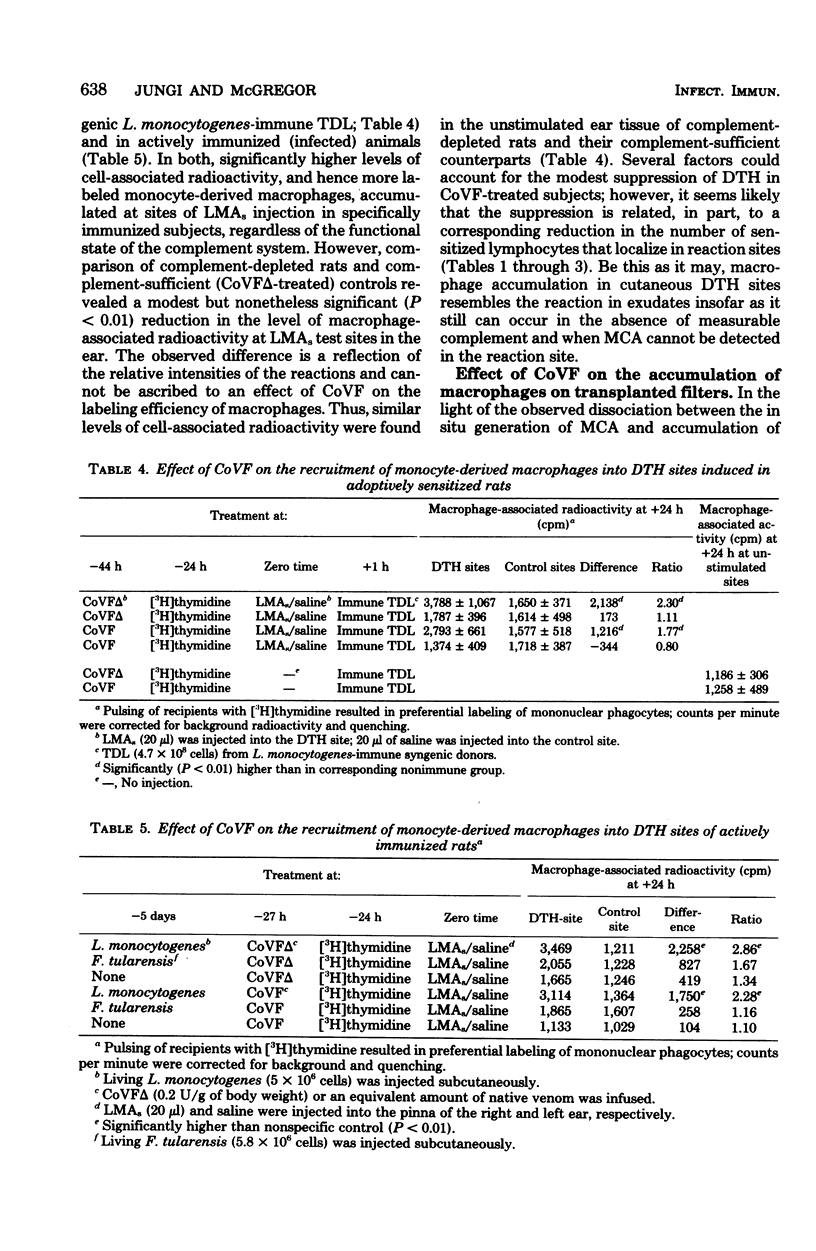
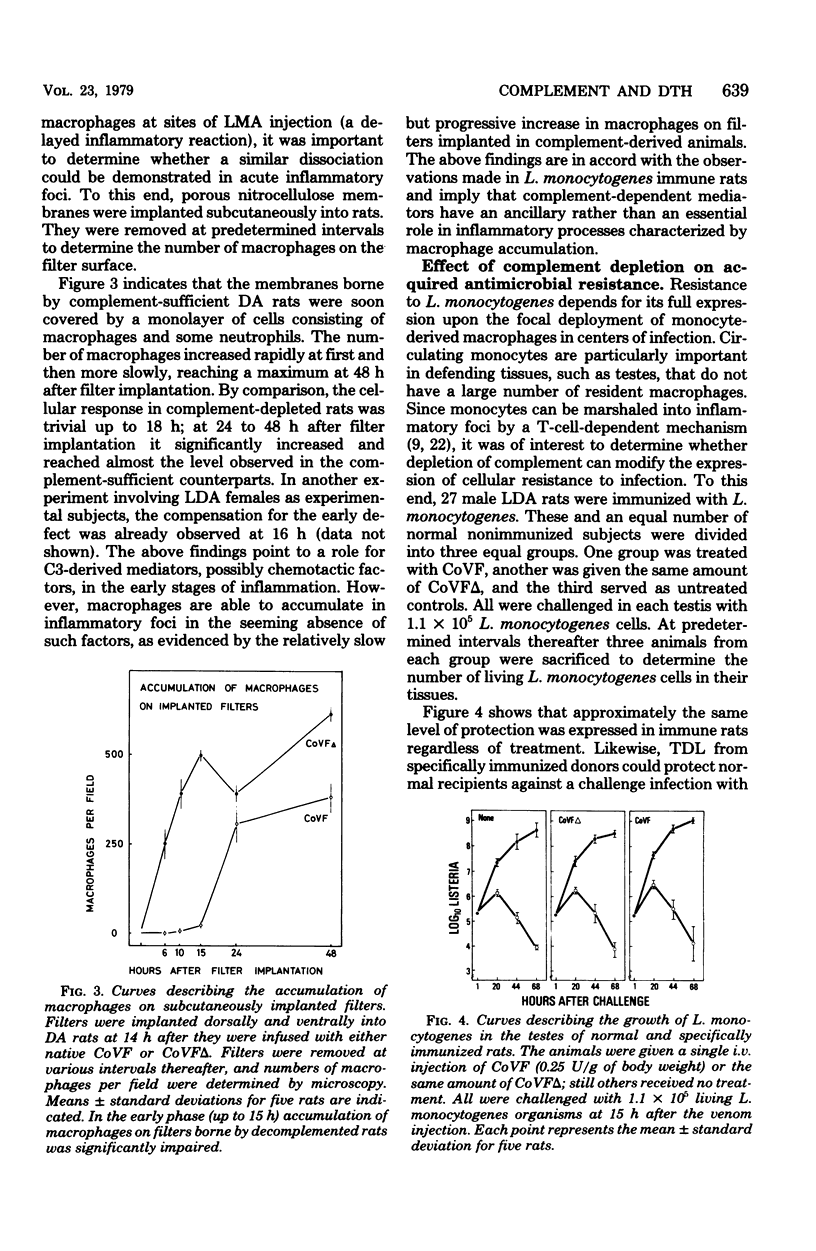
The hypothesis was tested that delayed-type hypersensitivity (DTH) to the complement-activating bacterium Listeria monocytogenes is initiated by complement-derived mediators that attract sensitized lymphocytes to reaction sites. To this end DTH and acquired resistance to L. monocytogenes were measured in rats injected with cobra venom factor, a potent inactivator of C3. Treatment with cobra venom factor reduced the hemolytic power of serum to less than 0.5% of the control value. Such decomplemented animals expressed both DTH and antimicrobial resistance, although expression of DTH was reduced (ca. 50%) when compared with complement-sufficient controls. The observed depression of DTH in cobra venom factor-treated rats was associated with a reduction in the number of recently activated lymphocytes (lymphoblasts) and macrophages that accumulated in DTH reaction sites. The above findings are explained, in part, by inhibition of inflammation during the early postinduction period. Supporting evidence came from measurements of labeled lymphoblast sequestration in saline injection sites and the slower accumulation of macrophages in nitrocellulose filters that were implanted subcutaneously in complement-depleted rats. The ability of cobra venom factor-treated rats to express DTH and protect themselves against a Listeria challenge seems to exclude C3-dependent factors as essential mediators in the attraction of antigen-reactive lymphocytes to reaction sites.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballow M., Day N. K., Good R. A. Effect of cobra venom factor on the local GVH reaction. I. Partial characterization of a cytotoxic factor from cobra venom for rat lymphocytes. J Immunol. 1973 Feb;110(2):354–361. [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Henson P. M., Cochrane C. G. Acute immune complex disease in rabbits. The role of complement and of a leukocyte-dependent release of vasoactive amines from platelets. J Exp Med. 1971 Mar 1;133(3):554–571. doi: 10.1084/jem.133.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. C. The influence of the cellular infiltrate on the evolution and intensity of delayed hypersensitivity reactions. J Exp Med. 1969 Feb 1;129(2):363–370. doi: 10.1084/jem.129.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Activated lymphocytes trigger lymphoblast extravasation. Cell Immunol. 1978 Jun;38(1):76–83. doi: 10.1016/0008-8749(78)90033-3. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Allogeneic restriction of the delayed inflammatory reaction in the rat. J Immunol. 1978 Aug;121(2):456–463. [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Generation of macrophage chemotactic activity in situ in Listeria-immune rats. Cell Immunol. 1977 Oct;33(2):322–339. doi: 10.1016/0008-8749(77)90162-9. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Austen K. F. Chemotaxis of human basophil leucocytes. Clin Exp Immunol. 1972 Aug;11(4):557–563. [PMC free article] [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D., Mackaness G. B. The mediator of cellular immunity. II. Migration of immunologically committed lymphocytes into inflammatory exudates. J Exp Med. 1971 Feb 1;133(2):400–409. doi: 10.1084/jem.133.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostiala A. A., McGregor D. D., Logie P. S. Tularaemia in the rat. I. The cellular basis on host resistance to infection. Immunology. 1975 May;28(5):855–869. [PMC free article] [PubMed] [Google Scholar]
- Kostiala A. A., McGregor D. D. The mediator of cellular immunity. IX. The relationship between cellular hypersensitivity and acquired cellular resistance in rats infected with Listeria monocytogenes. J Exp Med. 1975 Jun 1;141(6):1249–1260. doi: 10.1084/jem.141.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Hahn H. H., Mackaness G. B. The mediator of cellular immunity. V. Development of cellular resistance to infection in thymectomized irradiated rats. Cell Immunol. 1973 Feb;6(2):186–199. doi: 10.1016/0008-8749(73)90021-x. [DOI] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T., Mackaness G. B. The mediator of cellular immunity. I. The life-span and circulation dynamics of the immunologically committed lymphocyte. J Exp Med. 1971 Feb 1;133(2):389–399. doi: 10.1084/jem.133.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Logie P. S. The mediator of cellular immunity. VII. Localization of sensitized lymphocytes in inflammatory exudates. J Exp Med. 1974 Jun 1;139(6):1415–1430. doi: 10.1084/jem.139.6.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Logie P. S. The mediator of cellular immunity. X. Interaction of macrophages and specifically sensitized lymphocytes. Cell Immunol. 1975 Aug;18(2):454–465. doi: 10.1016/0008-8749(75)90072-6. [DOI] [PubMed] [Google Scholar]
- Nelson R. A., Jr A new concept of immunosuppression in hypersensitivity reactions and in transplantation immunity. Surv Ophthalmol. 1966 Aug;11(4):498–505. [PubMed] [Google Scholar]
- Normann S. J., Schardt M. A macrophage inflammation test using subcutaneous nitrocellulose filters. J Reticuloendothel Soc. 1978 Feb;23(2):153–160. [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTLETHWAITE A. E., Snyderman R. Characterization of chemotactic activity produced in vivo by a cell-mediated immune reaction in the guinea pig. J Immunol. 1975 Jan;114(1 Pt 2):274–278. [PubMed] [Google Scholar]
- Rumjanek V. M., Brent L., Pepys M. B. Cell-mediated immunological responsiveness in mice decomplemented with cobra venom factor. Immunology. 1978 Jun;34(6):1117–1123. [PMC free article] [PubMed] [Google Scholar]
- Russell R. J., Wilkinson P. C., Sless F., Parrott D. M. Chemotaxis of lymphoblasts. Nature. 1975 Aug 21;256(5519):646–648. doi: 10.1038/256646a0. [DOI] [PubMed] [Google Scholar]
- Schwartz H. J., Naff G. B. The effect of complement depletion by cobra venom factor on delayed hypersensitivity reactions. Proc Soc Exp Biol Med. 1971 Dec;138(3):1041–1043. doi: 10.3181/00379727-138-36046. [DOI] [PubMed] [Google Scholar]
- Scollay R., Hall J., Orlans E. Studies on the lymphocytes of sheep. II. Some properties of cells in various compartments of the recirculating lymphocyte pool. Eur J Immunol. 1976 Feb;6(2):121–125. doi: 10.1002/eji.1830060210. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Gewurz H., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide. Generation of a factor chemotactic for polymorphonuclear leukocytes. J Exp Med. 1968 Aug 1;128(2):259–275. doi: 10.1084/jem.128.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Phillips J. K., Mergenhagen S. E. Biological activity of complement in vivo. Role of C5 in the accumulation of polymorphonuclear leukocytes in inflammatory exudates. J Exp Med. 1971 Nov 1;134(5):1131–1143. doi: 10.1084/jem.134.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Shin H. S., Hausman M. H. A chemotactic factor for mononuclear leukocytes. Proc Soc Exp Biol Med. 1971 Nov;138(2):387–390. doi: 10.3181/00379727-138-35903. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Shin H., Dannenberg A. M., Jr Macrophage proteinase and inflammation: the production of chemotactic activity from the fifth complement by macrophage proteinase. J Immunol. 1972 Oct;109(4):896–898. [PubMed] [Google Scholar]
- Waldmann H., Lachmann P. J. The failure to show a necessary role for C3 in the in vitro antibody response. Eur J Immunol. 1975 Feb;5(2):185–193. doi: 10.1002/eji.1830050307. [DOI] [PubMed] [Google Scholar]
- Wissler J. H., Stecher V. J., Sorkin E. Biochemistry and biology of a leucotactic binary serum peptide system related to anaphylatoxin. Int Arch Allergy Appl Immunol. 1972;42(5):722–747. doi: 10.1159/000230652. [DOI] [PubMed] [Google Scholar]



