Abstract
Varicocele is defined as abnormally dilated scrotal veins. It is present in 15 % of normal males and in 40 % of males with infertility. This disorder is a challenge for the physicians involved in the diagnosis and treatment, as the pathophysiology of varicocele is not yet completely understood. For this reason, accurate diagnostic criteria and clear indications for treatment in asymptomatic adolescents or adults with clinical or subclinical varicocele are still not defined. Ultrasonography (US) is considered the best method for calculating the volume of the testicles, measuring vein diameter and monitoring the growth of the testis in adolescent patients. Color-Doppler US is the method of choice for detecting spermatic vein reflux and for classifying the grade of varicocele. Various classification systems have been published with recommendations on how to perform US imaging of the scrotum. Currently, color-Doppler US and spectral analysis are the most effective, non-invasive diagnostic procedures as they allow detection of subclinical varicocele associated with infertility. Various techniques are used in the treatment of varicocele including open surgery, laparoscopic procedures and interventional radiology. However, there is no consensus among physicians on which technique is the most effective in terms of outcome and complication rates. This review shows that color-Doppler US is currently the most widely employed diagnostic method for detection and classification of varicocele caused by venous reflux, as it is reliable and easily performed. The review also highlights the role of varicocelectomy in the management of adult male infertility.
Keywords: Varicocele, Ultrasonography, Color-Doppler, Testis
Riassunto
Il varicocele è una dilatazione abnorme delle vene scrotali. Esso si riscontra nel 15 % degli uomini normali e nel 40 % dei soggetti che presentano infertilità. Rappresenta ancora oggi un problema clinico per gli specialisti che sono coinvolti nella diagnosi e nel trattamento di questa affezione, in quanto i meccanismi fisiopatologici che stanno alla base di questa patologia non sono stati ancora chiariti in maniera completa. Per questi motivi non esistono ancora indicazioni condivise tra i medici sulle possibilità diagnostiche e sulle scelte terapeutiche da applicare negli adolescenti asintomatici o negli adulti con varicocele clinico o subclinico. L’esame ultrasonografico dello scroto viene considerato attualmente come la metodica migliore per calcolare il volume dei testicoli, per misurare il diametro dei vasi venosi e per seguire l’accrescimento testicolare negli adolescenti. Il Color-Doppler è il metodo più semplice e preciso per studiare e documentare l’inversione del flusso nelle vene spermatiche e serve per una precisa classificazione del varicocele. Sono state proposte numerose classificazioni cliniche ed ecografiche del varicocele e sono state pubblicare raccomandazioni su come condurre l’esame ecografico dello scroto per l’identificazione del varicocele. Attualmente il color-Doppler associato all’analisi spettrale è la procedura diagnostica più valida e non invasiva, in quanto consente di evidenziare anche i varicoceli subclinici associati ad infertilità. Per il trattamento del varicocele sono state proposte diverse procedure chirurgiche, a cielo aperto o laparoscopiche e procedure di radiologia interventistica. Non esiste però un consenso su quale è la tecnica migliore in termini di risultati e di complicanze. Questa revisione evidenzia come l’ecografia color-Doppler sia attualmente la procedura diagnostica più diffusa, semplice ed affidabile per la ricerca del varicocele refluente e come la varicocelectomia rappresenti la terapia iniziale negli adulti con infertilità.
Introduction
Male infertility is a social problem, which is in steady and progressive increase in the industrialized countries, and varicocele is the most common cause of this condition [1, 2]. It can be treated or at least improved through surgery or vein embolization leading to a significant increase in conception. However, there are no definite reports in the literature determining which patients will benefit from treatment of varicocele. In clinical practice, varicocele size, testicular volume and semen analysis are the three most important clinical parameters [3].
The etiology of varicocele is still unknown, but it is probably multifactorial [4]. The clinical picture is characterized by an abnormal enlargement of the spermatic veins of the venous plexus, which drains the blood from the testicles. This condition is associated with an anomalous intermittent or continuous backflow of blood into the plexus. As a result, venous stasis increases the temperature of the seminiferous tubules, thereby reducing sperm quality. Apoptosis of the germ-line cells is the main cause of changes in sperm count and morphology, while sperm chromatin abnormalities may be attributable to oxidative stress in the germinal elements [5, 6].
Definition
Varicocele is clinically defined as an abnormal dilation of the veins of the pampiniform venous plexus and the testicular veins with continuous or intermittent reflux of venous blood. This disorder may be present at birth or in young children, but the incidence substantially increases in adolescents coinciding with pubertal development.
The incidence of varicocele in adolescents and adult males ranges between 10 and 20 %, and this condition has also been observed in 4.8 % of patients who undergo vasectomy for birth control [7]. Varicocele is the most common andrological disorder among adolescents and adult males. The clinical relevance of varicocele is mainly linked to fertility, as it is one of the most common causes of impaired male fertility [8]. For this reason, parents often request pediatricians or general physicians to refer young adolescents to further diagnostic tests.
Etiology and pathogenesis
The cause of the onset of varicocele is not clear [9]. However, scrotal varices are formed as a result of increased venous pressure occurring in the veins which drain the blood from the testicles. The initial finding is increased thickness of the vein wall without dilation, as hypertrophy of the media will compensate and prevent the vein from bulging (Compensated stage).
If this situation persists and there is a steady increase in venous pressure, this will cause a progressive bulging of the veins, which will slow down the blood circulation. This will result in blood stagnation, but not yet in the formation of varicose veins (Concealed varicocele). Already in this pre-clinical stage, which may eventually lead to clinical varicocele, accumulation of excessive blood and venous stasis may cause spermatogenetic damage.
Over time, venous stasis and dilation will cause hyalinization and weakening of the vein walls, thereby giving rise to clinically manifest varicocele. This condition will be more or less evident depending on the degree of dilation and tortuosity of the vessels due to progressive atrophy and fibrosis affecting the vein walls (Manifest varicocele).
The veins running in the spermatic cord become involved in different phases. The condition initially involves the cremasteric venous vessels located outside the cremasteric fascia. They are devoid of valves and are, therefore, the most prone to injury due to hypertension in the pampiniform venous plexus with which they are connected through communicating veins (Stage I varicocele––Cremasteric varicocele). Only later, there is a progressive dilation of the pampiniform venous plexus (Stage II varicocele––Pampiniform varicocele). The veins running along the vas deferens are the last to be dilated as they are less sensitive to venous hypertension in the pampiniform venous plexus (Stage III varicocele––Vas deferens Varicocele).
Classification of varicocele
Varicocele is a condition that has been known for many years, but there is still no universally accepted classification, as the criteria and objectives applied by the various authors are different. The only gold standard in the diagnosis of varicocele is retrograde phlebography of the spermatic veins, but this examination is not adequate as a routine screening test.
The classification published by Dubin and Amelar [10] is the best known. It is applied by clinical urologists and pediatricians and is based on detection by palpation and visual examination of the dilation of the venous plexus of the testicular cord. However, the true incidence of varicocele tends to be underestimated, as diagnostic accuracy is closely linked to the physician’s experience. False-positive and false-negative outcomes are, therefore, frequent. Physical examination consists of palpation performed with the patient in the standing position and observation of the scrotum during the Valsalva maneuver. This classification system describes three degrees of varicocele:
Grade 1: varicocele is detectable by palpation only during the Valsalva maneuver.
Grade 2: varicocele is detectable by simple palpation.
Grade 3: varicocele is visible on inspection and palpation.
This clinical classification does not take into consideration subclinical varicocele, a condition that is easily identified at diagnostic imaging which can detect reflux of blood in the spermatic and scrotal veins.
The development of US imaging and particularly the introduction of high frequency probes associated with color-Doppler US has led to the widespread use of this diagnostic method in the search and characterization of varicocele. The examination evaluates the diameter of the venous vessels as well as colorimetric and blood flow changes [11].
The most commonly applied US classification systems are those proposed by Sarteschi et al. [12] and Chiou et al. [13]. Other classification systems have been proposed by scientific societies and by several authors, and the most recent is the hemodynamic classification system proposed by Iosa and Lazzari [14].
The classification system proposed by Sarteschi et al. divides varicocele into five grades on the basis of color-Doppler US findings obtained with the patient at rest and during the Valsalva maneuver (Table 1). The classification system proposed by Chiou et al. [13] is based on a score system, which takes into consideration the vessel diameter (in mm), the sum of the venous diameters and the changes in blood flow velocity during the Valsalva maneuver. Other US classification systems are those proposed by Hoekstra [15], Hirsh [16], Oyen [17] and the recent system proposed by Iosa et al. [14] (Table 2).
Table 1.
US classification of varicocele according to Sarteschi et al. [12]
| Grade 1: venous reflux at the emergence of the scrotal vein only during the Valsalva maneuver; hypertrophy of the venous wall without stasis |
| Grade 2: supratesticular reflux only during the Valsalva maneuver; venous stasis without varicosities |
| Grade 3: peritesticular reflux during the Valsalva maneuver; overt varicocele with early stage varices of the cremasteric vein |
| Grade 4: spontaneous basal reflux that increases during the Valsalva maneuver, possible testicular hypotrophy, overt varicocele, varicosities in the pampiniform plexus |
| Grade 5: spontaneous basal reflux that does not increase during the Valsalva maneuver, testicular hypotrophy, overt varicocele, varicosities in the pampiniform plexus |
Table 2.
Comparison of the US classification of varicocele proposed in the literature
| Classification by Hoekstra et al. [15] |
| Grade 0: absence of venous dilation |
| Grade 1: venous dilation <2.5 mm without reflux during the Valsalva maneuver |
| Grade 2: venous dilation and tortuosity ranging from 2.5 to 3.5 mm with reflux during the Valsalva maneuver |
| Grade 3: venous dilation and tortuosity >3.5 mm with reflux during the Valsalva maneuver |
| Classification by Hirsh et al. [16] |
| Grade 1: absence of spontaneous reflux, but reflux is inducible by the Valsalva maneuver |
| Grade 2: intermittent spontaneous reflux |
| Grade 3: continuous spontaneous reflux |
| Classification by Oyen [17] |
| Grade 1: mild reflux (<2 s) during the Valsalva maneuver |
| Grade 2: reflux (>2 s) during the Valsalva maneuver, but not continuous |
| Grade 3: reflux at rest and continuous during the entire Valsalva maneuver |
| Classification by Iosa ed al [14] |
| Grade 1: reflux >1 s during the Valsalva maneuver |
| Grade 2: intermittent spontaneous reflux, which is not intensified during the Valsalva maneuver |
| Grade 3: intermittent spontaneous reflux, which is intensified during the Valsalva maneuver |
| Grade 4A: continuous spontaneous reflux, which is not intensified during the Valsalva maneuver |
| Grade 4B: continuous spontaneous reflux, which is intensified during the Valsalva maneuver |
The different characteristics of the classification systems and the absence of a universally shared classification are mainly linked to the lack of a diagnostic gold standard, but also to the lack of correlation between the degree of varicocele and impaired fertility and to the choice of treatment aimed at preventing spermatogenetic damage. All the proposed classification systems present a low predictive value in terms of impaired spermatogenesis, which is the indication for any therapeutic choice.
US diagnosis
US imaging has become a routine investigation tool in the diagnosis of varicocele, even though there are no studies reporting on correctly performed methodological research projects on comparing palpation and US imaging [18]. Scientific societies, such as the American Institute of Ultrasound in Medicine (AIUM) and Italian Society for Vascular Investigation (SIDV-GIUV) [19, 20], have issued similar but not identical recommendations on how to perform US examination in varicocele and the parameters to be evaluated.
The US equipment currently on the market, but also older versions, has probes for the study of soft tissues with frequencies ranging from 7.5 MHz and upwards as well as color-Doppler US. For the study of the testicles, a linear probe is required with a field of view of 5 cm if the operator chooses to measure the longitudinal testicular diameter to calculate the testicular volume. Probes with a more restricted field of view, e.g., used in the study of the vascular system of the neck, are not suitable unless there is the possibility to add trapezoidal imaging, which increases the field of view at the cost of providing fewer structural details (Fig. 1).
Fig. 1.
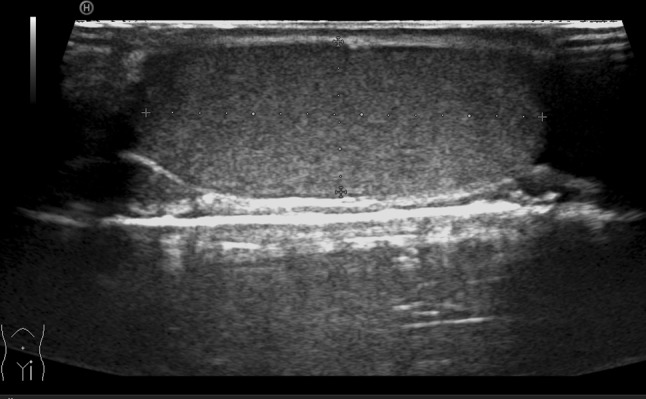
Longitudinal scan of the testicle. Trapezoidal imaging allows evaluation of the entire testicle in only one scan
The US study starts with the patient in the supine position. This permits a preliminary bimanual palpation, which is essential for the subsequent interpretation of US findings. US imaging permits measurement of the three basic didymal diameters and calculation of the testicular volume. The structure of the testicular tissue is carefully studied as well as the morphology of the spermatic cord. The diameters of the spermatic veins at rest and changes during the Valsalva maneuver should be evaluated. The study is completed with the patient in the standing position with evaluation of changes in the vessel calibers, but particularly the changes on color-Doppler images at rest and during the Valsalva maneuver (Fig. 2).
Fig. 2.
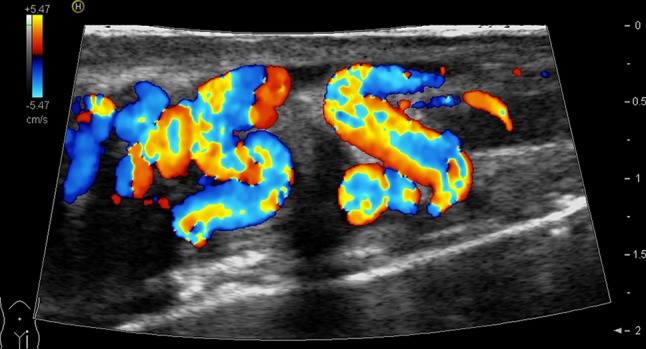
Longitudinal scan of the supratesticular region. Color-Doppler US shows varicocele grade 2 (Sarteschi classification)
Essential methodological recommendations include the need to perform the examination in a suitably heated environment and particularly the use of gel heated to body temperature to prevent contraction of the muscle fibers of the scrotum and the cremaster muscle. It is also necessary to avoid excessive probe compression of the veins. The patient should be instructed to perform the Valsalva maneuver correctly, more or less in the same way each time, and especially to move as little as possible in order not to affect the diagnostic data and create motion artifacts. These methodological recommendations seem trivial, but they are important to avoid diagnostic errors.
The following US parameters must be evaluated and the outcome reported:
Unilateral or bilateral varicocele;
Volume of the two testicles and percentage difference in volume;
Extension of varicocele (spermatic cord, scrotal, interscrotal or intratesticular area) [21];
Greatest diameter of the venous vessels at rest and under load;
Extension of venous reflux assessed semiquantitatively at color-Doppler US, at rest and during the Valsalva maneuver (continuous or intermittent, only during the Valsalva maneuver, only in the standing position, restricted to the spermatic cord or extended to the entire scrotum)
Spectral analysis performed in the recumbent and standing positions, at rest and during the Valsalva maneuver; duration of reflux, which must be >2 s to acquire diagnostic significance
Possible measurement of the maximum velocity of reflux during the Valsalva maneuver, if a rectilinear venous vessel is identified within the spermatic cord; it must be suitable for a correct positioning of the color-Doppler US angle which in any case must be <60°. This measurement is difficult to perform correctly also because the mere compression exerted by the probe on the venous vessels causes an increase in blood flow velocity. At present, this assessment should be considered optional and it should be performed only in centers where the patients are monitored after treatment.
Clinical conditions
In his/her daily clinical practice, the physician may be faced with three situations, which require an adequate therapeutic response:
A prepubescent boy or adolescent with varicocele and reflux, in most cases with unknown parameters of fertility;
An adult male with varicocele and the left testicle smaller than the contralateral testicle, a volume difference exceeding 10–15 % and abnormal outcome of semen analysis;
An adult male with varicocele but the testicles of the same size, with normal parameters of fertility
Varicocele in prepubescent boys or adolescents
Varicocele is diagnosed in about 6 % of 10-year-old males, and the prevalence of this disorder increases to 15 % in males aged 13 or older. A series of longitudinal studies have confirmed that varicocele may be responsible for future impaired spermatogenesis and, therefore, be the cause of impaired fertility in adult males. This is more likely when varicocele is associated with growth arrest affecting the left testicle. After treatment of varicocele, growth arrest is reversed in 70–80 % of adolescents, as the smaller left testicle starts growing and tends to reach the volume of the right testicle [22]. In the rare cases where it was possible to evaluate spermatogenesis before and after treatment, there was a clear improvement after vein ligation [23].
The clinical situation is particularly complex when varicocele is diagnosed in adolescents at the time of puberty, just before or shortly after, as future fertility behavior is not predictable in these patients [24].
The statistics clearly show that 80 % of adult males with varicocele are fertile. Preventive treatment of all cases of varicocele diagnosed in adolescents would, therefore, be overtreatment and ethically questionable, particularly in view of the complication rates [25]. In the past, indication for surgery was linked to clinical classification of varicocele, response to stimulation through administration of the follicle-stimulating hormone, gonadotropin, difference in size between the two testicles and particularly the outcome of semen analysis. As evaluation of the latter is neither feasible nor repeatable in adolescents, the diagnostic criterion that was most commonly applied in clinical practice was assessment of the volume difference between the two testicles [25, 26].
Pediatricians and urologists agree that measurement of testicular volume performed at US imaging is more accurate and repeatable than measurement made with an orchidometer. US, is, therefore the method of choice in most centers dealing with this clinical problem.
US measuring of testicular volume has become a routine procedure, but there is still no consensus on two main points: what is the threshold value of testicular volume asymmetry requiring surgical treatment and when is the best time to intervene in an adolescent? [27, 28]. Several methods have been employed to calculate testicular volume, but the most accurate method is based on measurements made at US imaging. The volume is calculated using the ellipsoid formula by multiplying the three principal diameters with the constant 0.52 (Fig. 2). In a prospective study, Diamond et al. [29] observed that abnormal outcome of semen analysis occurred already when volume disparity was >10 %, and abnormal outcome became significant when the volume disparity was more than 20 %. In contrast, there was no statistical correlation between the degree of varicocele and impaired semen analysis. However, difference in size between the testicles of 10–15 % may also be observed in adolescents without varicocele and be expression of an asymmetrical development of the two testicles, which will be normalized over time. Only when this asymmetry persists after 1 year of observation, surgery is indicated in patients with varicocele, which should be carefully assessed.
In the clinical assessment of adolescents with varicocele, Kozakowski et al. [30] have recently proposed the use of a new US parameter in addition to the volume. They propose evaluation of peak reflux velocity measured on the spermatic cord with a cut off value of 38 cm/s. Evaluation of this velocity is an integration of color-Doppler US imaging, which is commonly performed in the investigation of varicocele with venous reflux to assess the severity of the condition on US. Peak reflux velocity is calculated by placing the sample volume on one of the largest veins of the plexus of the spermatic cord with the patient in the supine position and during the Valsalva maneuver. The reported results are interesting as they show that none of the patients with a volume difference >20 % and a venous peak velocity >38 cm/s presented a significant growth of the hypotrophic testicle during the period of follow-up. In these patients, surgery should be performed early to avoid irreversible damage to the testicles and impaired fertility. In contrast, patients who presented a volume difference <20 % and a venous peak velocity <38 cm/s at the time of diagnosis did not develop significant testicular hypotrophy. In these patients, a “wait and see” management and careful monitoring are correct. This study and the resulting prognostic evaluations are interesting, but the method presents some problems that require careful evaluation before it is adopted in clinical practice. The factors to consider are:
Reproducibility of Doppler measurements which is often carried out on tortuous vessels and with an unfavorable Doppler angle >60°.
Probe compression of the dilated spermatic vein can significantly change venous flow velocity with oscillations similar to those observed at the femoral vein.
It is difficult for the patient to carry out the Valsalva maneuver in the same way each time, and this leads to changes in intensity and duration of reflux;
The authors place the patients in the supine position, which makes it easier to carry out the examination; however, venous reflux is more accurately detected, when the patient is in the standing position, and the duration and amplitude may be different;
Last but not least, it must be kept in mind that there is a group of patients with a volumetric asymmetry >20 %, but a peak reflux velocity <38 cm/s, who are at risk of spermatogenetic damage during the period of observation.
Surgical results reported in the literature show accelerated growth of the affected testicle after varicocele surgery, also when surgery is performed in older adolescents, when semen analysis can provide more accurate information on the state of fertility [30].
In adolescents with varicocele, but no volume asymmetry between the two testicles, annual examinations are recommended up to the age of 22–24 years, as growth arrest may also occur later than normal [31].
Varicocele in adult males
In adult males, most cases of varicocele are revealed in the course of investigation related to infertility and/or due to an unfavorable outcome of semen analysis (reduced number and impaired motility) [32, 33]. Numerous data have shown a gradual decline in fertility from adolescence to adulthood documented by histological examinations showing alterations in the seminiferous tubules. Varicocele has a depressing effect on the total number of sperm, their motility and normal forms. Improvement of spermatogenesis is found in adults after surgery if the left testicle presents increased volume, whereas improvement of spermatogenesis is not found in surgically treated patients whose left testicle does not present increased volume and in those patients whose abnormal outcome of semen analysis remains unchanged [34, 35].
In the presence of varicocele confirmed by clinical and/or US diagnosis and an abnormal outcome of semen analysis, adequate therapy is necessary to obtain permanent reversal of venous reflux. If varicocele is diagnosed in an adult male, who has had no children but might want to reproduce, presenting with normal outcome of semen analysis, surgery is advisable instead of careful monitoring. If there is no difference in volume between the two testicles at the time of diagnosis, this difference will very rarely develop during the observation period, whereas the outcome of semen analysis may deteriorate.
To assess the functional status of the testicles in patients with varicocele, some authors have evaluated resistance index (RI) measured on the intratesticular arteries. RI is an expression of the intratesticular microcirculation and should allegedly increase in patients with clinically significant varicocele and reduced testicle volume [36]. Similarly, some authors have reported a direct correlation between follicle-stimulating hormone values and testicular vasculature and an inverse correlation with the testicular volume. US and color-Doppler US evaluation of the testicle may, therefore, be used to evaluate the gonadal function when semen analysis is not available, e.g., in adolescents. Studies involving RI measurements in patients with abnormal semen analysis have shown increased RI values compared to the threshold value of 0.60, and color-Doppler studies performed in azoospermic patients have allegedly shown that the quality and quantity of the ejaculate depend on the degree of blood circulation in the testicles. Reduced arterial perfusion was observed in patients with varicocele and impaired spermatogenesis, probably as a result of a metabolic deficiency affecting the microcirculation [37].
Also quantitative elastography of the testicles may prove useful in the study of infertile patients with or without varicocele, but there are still no clear data in the literature in this regard [38, 39].
Symptomatic varicocele
In adults, varicocele rarely causes scrotal pain necessitating treatment unrelated to fertility problems. Also surgery performed to overcome esthetic problems or discomfort due to the presence of a particularly bulky and cumbersome scrotum is rare.
Varicocele occurring in adults––especially if the patient’s clinical history is negative for varicocele in youth––makes it advisable to perform US imaging of the kidneys to rule out a clinically silent malignancy that may have caused neoplastic thrombosis of the left renal vein and, therefore, also of the spermatic vein (secondary varicocele).
Therapy
The aim of all types of treatment is to arrest venous reflux, which is, according to the most widely accepted theory, considered responsible for parenchymal damage and, therefore, impaired fertility. After treatment of varicocele, a significant improvement of semen analysis results in a spontaneous pregnancy rate of 29.7 % after varicocelectomy only; 72 % over 2 years if surgery is associated with in vitro fertilization (IVF).
Numerous surgical and non-surgical techniques [40] have been proposed and used in the treatment of clinically significant varicocele, but there is no consensus on what might be termed as the “gold standard” leading to the best clinical outcome, the lowest complication rate and the best cost/benefit ratio.
There is still no certain evidence of the best procedure; the following techniques are employed:
Retroperitoneal ligation of the spermatic veins (Palomo)
Inguinal ligation (Ivanissevich)
Subinguinal binding
Laparoscopic subinguinal ligation
Subinguinal microsurgery
Inguinal microsurgery [41]
Embolization of the spermatic veins using antegrade sclerotherapy (inguinal incision) or retrograde sclerotherapy (through transfemoral catheterization of the left renal and spermatic vein) [42].
Pediatric surgeons and urologists tend to prefer the Palomo technique and laparoscopy, whereas subinguinal microsurgery is generally preferred in adult males.
Analysis of the data reported in surgical series show that not all patients respond favorably to treatment, thus presenting a failure rate ranging between 20 and 37 %. In a comparative study, outcome of semen analysis improved in 42 % of patients after surgery using the Palomo technique and in 51 % after laparoscopy. Much better results were obtained after subinguinal microsurgery resulting in improved spermatogenesis in 80–83 % of patients. In 2009, Zampieri et al. [43] reported normalization of semen analysis in 40.6 % of patients treated laparoscopically and in 58.3 % of patients after microsurgery [44]. Few studies have analyzed the changes in testosterone and follicle-stimulating hormone levels before and after varicocelectomy [45], and also changes in testicle volume after surgery have not been properly studied.
Complications
All the above-mentioned surgical techniques may lead to complications, such as hydrocele and particularly testicular atrophy [46]. However, there are no adequate follow-up studies analyzing postoperative functional and clinical outcome over time.
Hydrocele is the most common complication after varicocele repair ranging between 0.29 and 7.58 % [47], but not after embolization. Onset of hydrocele is linked to the type of surgery and is a result of defective lymphatic drainage. The lymphatic vessels are located posterior to the vascular bundle at the spermatic cord and they are easily damaged if they are not isolated and preserved during ligation of the venous vessels. Another complication occurring in patients with damage to the lymphatic system is pseudohypertrophy of the testicle due to interstitial edema caused by lymphatic obstruction. In these patients, testicular affliction may be evidenced by increased RI values measured on the intratesticular artery and compared with the contralateral healthy testicle.
Atrophy of the testis is rare, but this possibility should be kept in mind in adolescent patients who undergo surgery to reverse growth arrest affecting a hypoplastic testicle [48].
Conclusion
Varicocele is a disorder which has been known for numerous years, but diagnosis and treatment is still complex as diagnostic criteria and a universally accepted classification of varicocele are yet to be defined. There are furthermore still no specific and sufficiently reliable criteria permitting identification of those patients who should be submitted to therapy to avoid impaired spermatogenesis, which occurs only in 20–25 % of patients with varicocele. Surgical treatment of all adolescents or young adults with varicocele would result in overtreatment and consequent complications as well as elevated health care costs. In addition to this, physicians still have to agree on the most appropriate therapeutic procedure for achieving the best therapeutic results with the least possible risk. It can, therefore, be concluded that there are more open questions on varicocele than answers provided in the literature.
Conflict of interest
Massimo Valentino, Michele Bertolotto, Lorenzo Derchi, Pietro Pavlica declare that they have no conflict of interest related to this paper.
Human and animal studies
The study described in this article does not include any procedures involving humans or animals.
References
- 1.Dubin L, Amelar RD. Etiologic factors in 1294 consecutive cases of male infertility. Fertil Steril. 1971;22:469–474. doi: 10.1016/s0015-0282(16)38400-x. [DOI] [PubMed] [Google Scholar]
- 2.Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, Schlegel PN, Howards SS, Nehra A, Damewood MD, Overstreet JW, Sadovsky R. Best practice policies for male infertility. Fertil Steril. 2002;77:873–882. doi: 10.1016/S0015-0282(02)03105-9. [DOI] [PubMed] [Google Scholar]
- 3.Sarteschi ML, Menchini Fabris GM. Ecografia andrologica. Modena: Ed Athena; 2003. [Google Scholar]
- 4.Royal College of Obstetricians and Gynaecologists (2004) National evidence-based clinical guidelines. Fertility: assessment and treatment for people with fertility problems. National Collaborating centre for Women’s and Children’s Health, pp 54–55
- 5.Chen SS, Huang WJ, Chang LS, et al. Attenuation of oxidative stress after varicocelectomy in subfertile patients with varicocele. J Urol. 2008;179:639–642. doi: 10.1016/j.juro.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 6.Cocuzza M, Athayde KS, Agarwal A, et al. Impact of clinical varicocele and testis size on seminal reactive oxygen species levels in a fertile population: a prospective controlled study. Fertil Steril. 2008;90:1103–1108. doi: 10.1016/j.fertnstert.2007.07.1377. [DOI] [PubMed] [Google Scholar]
- 7.Lee RK, Goldstein M. Simultaneous vasectomy and varicocelectomy: indications and technique. Urology. 2007;70:362–365. doi: 10.1016/j.urology.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Alukal JP, Zurakowski D, Atala A, Bauer SB, Borer JG, Cilento BG, Jr, Mandell J, Peters CA, Paltiel HJ, Retik AB, Diamond DA. Testicular hypotrophy does not correlate with grade of adolescent varicocele. J Urol. 2005;174:2367–2370. doi: 10.1097/01.ju.0000180418.23208.1d. [DOI] [PubMed] [Google Scholar]
- 9.Shafik A. The physiology of testicular thermoregulation in the light of new anatomical and pathological aspects. In: Zorgniotti AW, editor. Temperature and environmental effects on the testis. New York, London: Plenum Press; 1991. pp. 153–172. [DOI] [PubMed] [Google Scholar]
- 10.Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;21:606–609. doi: 10.1016/s0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 11.Liguori G, Trombetta C, Garaffa G, Bucci S, Gattuccio I, Salamè L, Belgrano E. Color Doppler ultrasound investigation of varicocele. World J Urol. 2004;22:378–381. doi: 10.1007/s00345-004-0421-0. [DOI] [PubMed] [Google Scholar]
- 12.Sarteschi M, Paoli R, Bianchini M, Menchini Fabris GF. Lo studio del varicocele con eco-color-Doppler. Giornale Italiano di Ultrasonologia. 1993;4:43–49. [Google Scholar]
- 13.Chiou RK, Anderson JC, Wobig RK, Rosinsky DE, Matamoros A, Jr, Taylor RJ. Color-Doppler ultrasound criteria to diagnose varicoceles: correlation of a new scoring system with physical examination. Urology. 1997;50:953–956. doi: 10.1016/S0090-4295(97)00452-4. [DOI] [PubMed] [Google Scholar]
- 14.Iosa G, Lazzarini D. Hemodynamic classification of varicoceles in men: our experience. J Ultrasound. 2013;16:57–63. doi: 10.1007/s40477-013-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoekstra T, Witt MA. The correlation of internal spermatic vein palpability with ultrasonographic diameter and reversal of venous flow. J Urol. 1995;153:82–84. doi: 10.1097/00005392-199501000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Hirsh AV, Cameron KM, Tyler JP, Simpson J, Pryor JP. The Doppler assessment of varicoceles and internal spermatic vein reflux. Br J Urol. 1980;52:50–56. doi: 10.1111/j.1464-410X.1980.tb02919.x. [DOI] [PubMed] [Google Scholar]
- 17.Oyen RH. Scrotal ultrasound. Eur Radiol. 2002;12:19–24. doi: 10.1007/s00330-001-1224-y. [DOI] [PubMed] [Google Scholar]
- 18.Bertolotto M, Trombetta C. Scrotal pathology. Berlin: Springer-Verlag; 2012. [Google Scholar]
- 19.ACR-AIUM-SIU (2010) Practice guideline for the performance of scrotal ultrasound examination. Resolution 33. http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/US_Scrotal.pdf
- 20.Società Italiana di Diagnostica vascolare SIDV-GIUV (2012) Procedure operative per indagini diagnostiche vascolari. http://www.sidv.net/file_doc/GIUV%2023-24.pdf
- 21.Das KM, Prasad K, Szmigielski W, Noorani N. Intratesticular varicocele: evaluation using conventional and Doppler sonography. AJR. 1999;173:1079–1083. doi: 10.2214/ajr.173.4.10511183. [DOI] [PubMed] [Google Scholar]
- 22.DeCastro GJ, Shabsigh A, Poon SA, Laor L, Glassberg KI. Adolescent varicocelectomy––is the potential for catch-up growth related to age and/or tanner stage? J Urol. 2009;181:322–327. doi: 10.1016/j.juro.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Diamond D, Zurakowski D, Bauer SB, Borer JG, Peters CA, Cilento BG, Jr, Paltiel HJ, Rosoklija I, Retik AB. Relationship of varicocele grade and testicular hypotrophy to semen parameters in adolescents. J Urol. 2007;178:1584–1588. doi: 10.1016/j.juro.2007.03.169. [DOI] [PubMed] [Google Scholar]
- 24.Diamond D. Adolescent versus adult varicoceles––how do evaluation and management differ? J Urol. 2009;181:2410–2419. doi: 10.1016/j.juro.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Spinelli C, Di Giacomo M, Lo Piccolo R, Martin A, Messineo A. The role of testicular volume in adolescents with varicocele: the better way and time of surgical treatment. J Urol. 2010;184:1722–1726. doi: 10.1016/j.juro.2010.06.057. [DOI] [PubMed] [Google Scholar]
- 26.Sigman M, Jarow JP. Ipsilateral testicular hypotrophy is associated with decreased sperm counts in infertile men with varicocele. J Urol. 1997;158:605–607. doi: 10.1016/S0022-5347(01)64567-1. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto H, Ogawa Y, Yoshida H. Relationship between testicular volume and varicocele in patients with infertility. Urology. 2008;71:104–109. doi: 10.1016/j.urology.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Zampieri N, Mantovani A, Ottolenghi A, Camoglio FS. Testicular catch-up growth: does surgical techniques make a difference? Urology. 2009;73:289–292. doi: 10.1016/j.urology.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 29.Diamond DA. Adolescent varicocele: emerging understanding. BJU Int. 2003;92(Suppl 1):48–51. doi: 10.1046/j.1464-410X.92.s1.9.x. [DOI] [PubMed] [Google Scholar]
- 30.Kozakowski KA, Gjertson CK, Decastro JD, Poon S, Gasalberti A, Glassberg KI. Peak retrograde flow: a novel predictor of persistent, progressive and new onset asymmetry in adolescent varicocele. J Urol. 2009;181:2717–2723. doi: 10.1016/j.juro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 31.Kolon TF, Clement MR, Cartwright L, Bellah R, Carr MC, Canning DA. Transient asynchronous testicular growth in adolescent males with a varicocele. J Urol. 2008;180:1111–1114. doi: 10.1016/j.juro.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 32.Greenfield SP, Seville P, Wan J. Experience with varicoceles in children and young adults. J Urol. 2002;168:1684–1688. doi: 10.1016/S0022-5347(05)64388-1. [DOI] [PubMed] [Google Scholar]
- 33.Kim HH, Goldstein M. Adult varicocele. Curr Opin Urol. 2008;18:608–612. doi: 10.1097/MOU.0b013e3283136493. [DOI] [PubMed] [Google Scholar]
- 34.Zini A, Boman J, Jarvi K, et al. Varicocelectomy for infertile couples with advanced paternal age. Urology. 2008;72:109–113. doi: 10.1016/j.urology.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Papanikolaou F, Chow V, Jarvi K, Fong B, Ho M, Zini A. Effect of adult microsurgical varicocelectomy on testicular volume. Urology. 2000;56:136–139. doi: 10.1016/S0090-4295(00)00535-5. [DOI] [PubMed] [Google Scholar]
- 36.Balci A, Karazincir S, Gornur S, Sunbas H, Egilmez E, Inandi T. Long-term effect of varicocele on intratesticular arterial resistive index. J Clin Ultrasound. 2008;36:148–152. doi: 10.1002/jcu.20439. [DOI] [PubMed] [Google Scholar]
- 37.Tarhan S, Ucer DO, Sakin MO, Gums B. Long-term effect of microsurgical inguinal varicelectomy on testicular blood flow. J Androl. 2013;32:33–39. doi: 10.2164/jandrol.109.009977. [DOI] [PubMed] [Google Scholar]
- 38.Garra BS. Elastography: current status, future prospects, and making it work for you. Ultrasound Q. 2011;27:177–186. doi: 10.1097/RUQ.0b013e31822a2138. [DOI] [PubMed] [Google Scholar]
- 39.Schurich M, Aigner F, Frauscher F, Pallwein L. The role of ultrasound in assessment of male fertility. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S192–S198. doi: 10.1016/j.ejogrb.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 40.Diegidio P, Jhaveri JK, Ghannam S, Pinkhasov R, Shabsigh R, Fisch H. Review of current varicocelectomy techniques and their outcomes. BJU Int. 2011;108:1157–1172. doi: 10.1111/j.1464-410X.2010.09959.x. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein M, Gilbert BR, Dicker AP, Dwosjh J, Gnecco C. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol. 1992;148:1808–1811. doi: 10.1016/s0022-5347(17)37035-0. [DOI] [PubMed] [Google Scholar]
- 42.Bigot JM, Caretta MF, Boudghene F, Lebreton C (1989) La Flebografia gonadica nella diagnosi e nel trattamento non chirurgico del varicocele. In: Pistolesi GF (ed) L’imaging Diagnostico del piccolo bacino funzionale. Cortina Ed, Verona
- 43.Zampieri N, Zuin V, Corroppolo M, Chironi C, Cervellione RM, Camoglio FS. Varicocele and adolescents: semen quality after 2 different laparoscopic procedures. J Androl. 2007;28:727–800. doi: 10.2164/jandrol.107.002600. [DOI] [PubMed] [Google Scholar]
- 44.Al-Said S, Al-Naimi A, Al-Ansari A, Younis N, Shamsodini A, A-sadiq K, Shokeir AA. Varicocelectomy for male infertility: a comparative study of open, laparoscopic and microsurgical approaches. J Urol. 2008;180:266–270. doi: 10.1016/j.juro.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 45.Lee JS, Park HJ, Seo JT. What is the indication of varicocelectomy in men with nonobstructive azoospermia? Urology. 2007;69:352–355. doi: 10.1016/j.urology.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Chan PT, Wright EJ, Goldstein M. Incidence and postoperative outcomes of accidental ligation of the testicular artery during microsurgical varicelectomy. J Urol. 2005;173:482–484. doi: 10.1097/01.ju.0000148942.61914.2e. [DOI] [PubMed] [Google Scholar]
- 47.Nees SN, Glassberg KI. Observations on hydroceles following adolescent varicocelectomy. J Urol. 2011;186:2402–2407. doi: 10.1016/j.juro.2011.07.116. [DOI] [PubMed] [Google Scholar]
- 48.Wegner HE, Meier T, Miller K. Testicular necrosis after antegrade sclerotherapy of varicocele. Int Urol Nephrol. 1996;28:357–358. doi: 10.1007/BF02550498. [DOI] [PubMed] [Google Scholar]


