Abstract
Purpose
Intraoperative ultrasound (ioUS) has become increasingly widespread in brain tumor surgery but it is not yet a standard procedure in spinal surgery. We analyzed intraoperative ultrasonographic findings of different spinal tumors and their influence on the surgical strategy.
Methods
We evaluated patients who underwent surgery for spinal tumor (extradural, intradural extramedullary, intradural intramedullary) removal, with ultrasound (US) guidance. Intraoperative standard B-mode images were acquired using a 3–11 MHz linear US probe. Before tumor removal the lesion was identified on the two axes and measured and defined as hyperechoic, isoechoic or hypoechoic. Other characteristics of the lesions were considered: the presence of calcifications, cystic/necrotic areas, diffuse or circumscribed appearance, and the relationships with the surrounding anatomical structures.
Results
In all 34 cases it was possible to visualize the lesion, as well as the surrounding neural structures (like dura mater, dentate ligament, arachnoid membranes) and vascular structures. In 9 out of 34 cases, ioUS showed that the surgical approach was not wide enough: therefore it was necessary to enlarge the bony approach before dural opening. In 8 intramedullary cases, ioUS was used to correctly tailor the myelotomy.
Conclusions
We present our ioUS series findings along with some pictorial essays of different spinal tumors treated at our institution. IoUS is a valuable tool to detect spinal lesions, evaluate the surgical approach and plan the surgical strategy considering the position and relationships of the lesion with bony, neural and vascular structures.
Keywords: Intraoperative ultrasound, Spinal tumor, Intradural, Extradural, Intramedullary, Extramedullary
Riassunto
Obiettivi
L’ecografia intraoperatoria rappresenta una modalità sempre più utilizzata in neurochirurgia per il trattamento dei tumori cerebrali; le applicazioni nella chirurgia dei tumori spinali sono tuttavia ancora in fase di definizione. Abbiamo analizzato le caratteristiche ultrasonografiche di diversi tumori spinali sottoposti ad asportazione presso il nostro istituto, nonché l’influenza dell’ecografia intraoperatoria sulla strategia chirurgica.
Materiali e metodi
Abbiamo applicato l’ecografia intraoperatoria in 34 pazienti con sospetta diagnosi di tumori spinali. Dopo una laminectomia o laminotomia mirata mediante reperi radiologici, sono state acquisite immagini intraoperatorie in B-mode standard attraverso sonde lineari con frequenza di 3–11 MHz. Tutte le lesioni sono state identificate, misurate e definite come iper-, ipo- o isoecogene. Sono state considerate anche calcificazioni, aree necrotico-cistiche ed aspetto diffuso o circoscritto.
Risultati
In tutti i casi, è stato possibile identificare il tumore ed il midollo spinale, nonché le strutture circostanti quali dura madre, legamenti dentati, radici anteriori e posteriori, membrane aracnoidee e la presenza di eventuali aree cistiche, nonché le strutture vascolari. In 9 casi su 34, tale metodica ha determinato l’ampliamento dell’accesso chirurgico prima dell’incisione durale, in quanto non sufficiente ad esporre l’intera lesione. In 8 casi di tumori intramidollari, abbiamo utilizzato l’ecografia intraoperatoria per selezionare il sito della mielotomia.
Conclusioni
L’ecografia ultrasonografica rappresenta uno strumento dinamico, economico e sicuro durante la chirurgia dei tumori spinali. Oltre a costituire una procedura real-time, consente di acquisire informazioni riguardo la localizzazione delle lesioni ed i rapporti anatomici che intercorrono con le strutture nervose e vascolari circostanti. Consente inoltre di adattare l’estensione dell’approccio neurochirurgico alle reali dimensioni delle lesioni stesse.
Introduction
Primary spinal tumors are relatively rare lesions, representing 4–8 % of all central nervous system tumors [1, 2]. They can arise from the spinal cord, nerve roots, meninges or cauda equina. More than 2/3 of spinal tumors are nonmalignant lesions. In regard of their origin and relationship with neural structures, spinal tumors could be divided into extradural, intradural extramedullary or intradural intramedullary. Meningiomas, nerve sheet tumors and ependymomas are the most common frequent histotypes [1, 2].
Surgical removal represents the standard treatment option, still yielding a considerable risk of neurological deficits. This is particularly true for surgery of intramedullary tumors, where a myelotomy is performed [3, 4]. Recently, the introduction of new surgical tools along with intraoperative electrophysiological monitoring, has contributed to significantly reduce the surgical risks of postoperative neurological deficits [5, 6]. However, still, 5–40 % of patients undergoing surgical resection of an intradural tumor will develop new neurologic deficits [3].
Traditional preoperative radiological investigations are not always reliable in allowing the surgeon to perform an adequate surgical approach to the real extension of the lesion. A dynamic, real-time, intraoperative imaging tool for spinal tumor surgery could help improve this aspect of surgery, in order to reduce neural structures manipulation leading to worsening of neurological symptoms [3].
The use of ultrasound (US) during neurosurgical procedures is becoming more widespread and standardized in brain tumor surgery [7]. Furthermore, ultrasonography has dramatically improved in recent years, both in terms of image quality and information availability, offering new dataset of information, thanks to the use of new technologies. Intraoperative ultrasound (ioUS) has become an indispensable tool for localizing and controlling the resection of cerebral tumors and vascular anomalies [8, 9].
The appearance of spinal tumors on US is described only in few studies and the role of ioUS is not yet well defined and standardized for this surgical field. In this study we analyze the ioUS characteristics of different spinal lesions, its potential role in surgical planning and during spinal tumor resection.
Materials and methods
We evaluated all the patients harboring a spinal tumor who underwent ioUS-assisted surgery at our institution. After a standard laminectomy/laminotomy was performed, patients were intraoperatively examined with transdural and direct sonography, using a last generation US device (MyLab, Esaote, Italy) with a multifrequency (3–11 MHz) linear US probe. The probe was placed in a transparent plastic surgical sterile sheath (Civco, USA), after placing a US-specific transducing gel into it. The probe was then placed on the surgical cavity after filling it with saline solution. Standard B-mode imaging was acquired, in order to identify and measure the lesion on the two axes. An online intraoperative qualitative analysis was performed in order to assess tumor US characteristics, boundaries, and specific anatomical landmarks. All lesions were evaluated with B-mode imaging: they were defined as hyperechoic, isoechoic or hypoechoic compared to the surrounding tissues. Other lesion characteristics were taken into account, namely their diffuse or circumscribed appearance, homogeneity versus heterogeneity, presence of calcification and/or cystic/necrotic areas as well as their relationships with the surrounding structures (Figs. 1, 2).
Fig. 1.
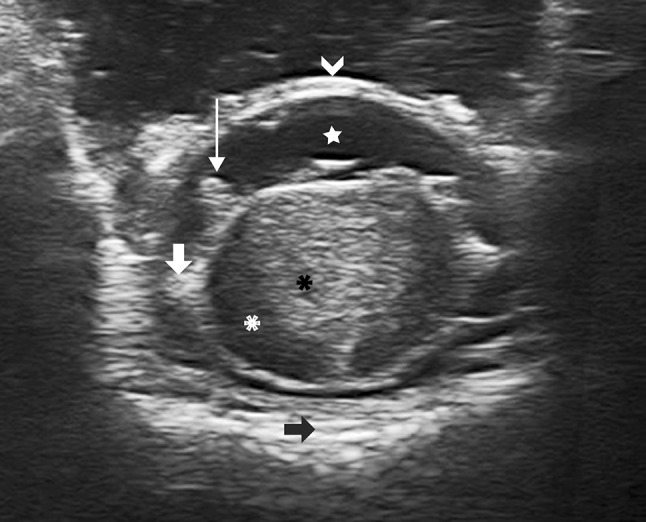
IoUS axial B-mode transdural imaging of an intramedullary tumor (cavernoma). It is possible to appreciate how ioUS is able to show the lesion with its features as well as the surrounding structures: cavernoma (black asterisk) embedded in the central-posterior portion of the spinal cord (white asterisk). The surrounding nervous structures are also visible: the dura mater (white arrowhead), cerebrospinal fluid space (white star), posterior rootlets (thin arrow), the dentate ligament (thick arrow) and the vertebral body (black arrow)
Fig. 2.
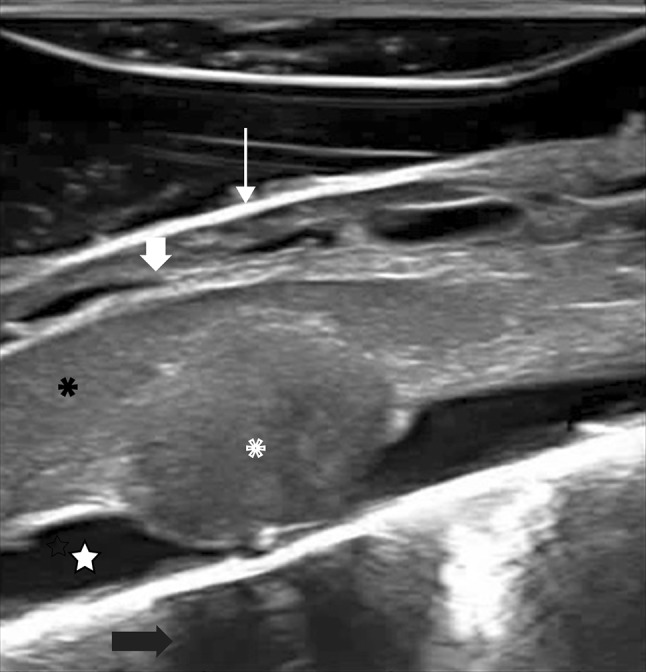
IoUS sagittal B-mode transdural imaging showing a dorsal intradural extramedullary tumor (neurinoma): ioUS scan identifies the tumor (asterisks) compressing the spinal cord (black asterisks), which appears slightly enlarged and hyperechoic due to spinal cord edema. Also the surrounding structures are depicted: dura mater (thin arrow), cerebrospinal fluid space (star), posterior rootlets (thick arrow) and the vertebral body (black arrow)
All data obtained were later correlated with the histopathological diagnosis.
Results
Thirty-four patients (age range 18–76 years; mean 50 years), harboring different spinal lesions (12 meningiomas, 11 neurinomas, 4 ependymomas, 3 hemangioblastomas, 3 cavernomas, 1 astrocytoma) were evaluated. All lesions were intraoperatively visualized on B-mode imaging. Standard US B-mode imaging features were related to the histopathological diagnosis after removing the bone (laminectomy/laminotomy); transdural US showed that the tumor was not fully exposed in eight cases. Further bone removal was then performed according to the ioUS findings, obtaining a satisfactory surgical exposure for all lesions. All intramedullary lesions were further evaluated with ioUS after dural opening, in order to correctly place the myelotomy and to adjust its length so to fully visualize and remove the lesion without unnecessary neural tissue manipulation. All ioUS findings for all the above lesions are summarized below:
Extradural tumors
Two cervical nerve sheath schwannomas were completely visible after hemi-laminectomy, with arthrectomy in one case. Such lesions appeared well circumscribed, with a hyperechoic capsule and a hypoechoic homogeneous texture. In all cases there was bone scalloping that allowed room for a good visualization of the distal portion of the lesion (Fig. 3). Vertebral artery (VA) was displaced in all cases and was evaluated using Doppler US.
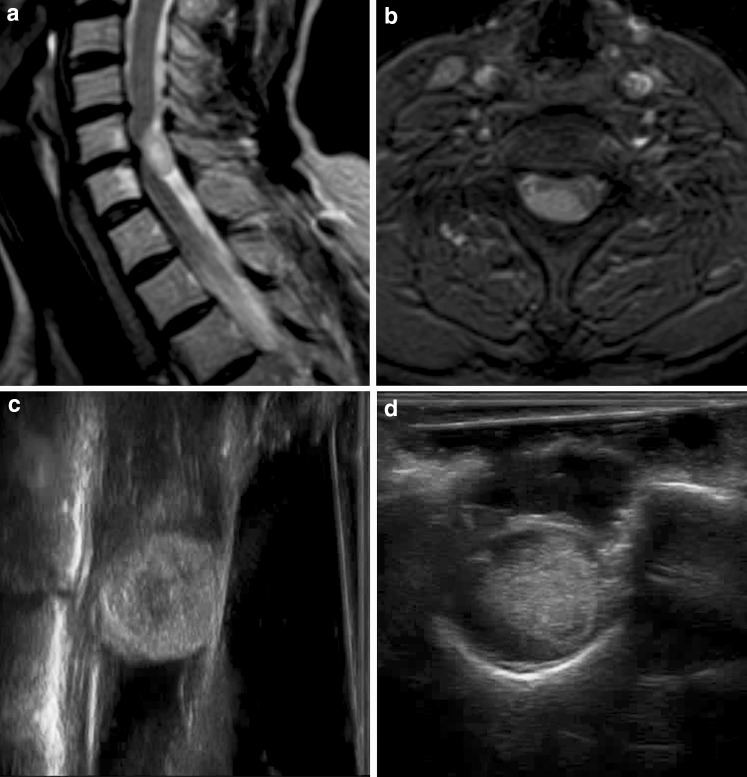
Fig. 3.
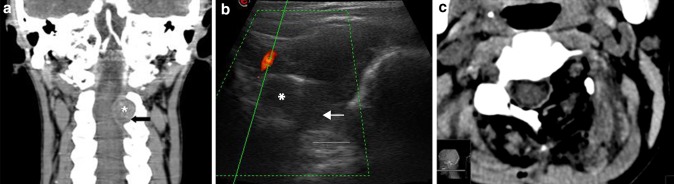
Extradural tumor: angio-CT coronal scan (a) showing a dumbbell extradural left C2–C3 neurinoma (asterisks) with bone scalloping (black arrow). IoUS B-mode imaging (b) with tumor (asterisks) evaluation also showing bone remodeling (arrow). Doppler imaging is used to evaluate vascular structures close to the lesion such as the vertebral artery. Postoperative CT scan (c) showing the complete tumor removal and bone work
Intradural extramedullary tumors
Meningiomas (12 cases) appeared as discrete, ovular, well-circumscribed, mildly hyperechoic lesions, compared to normal spinal cord and cerebrospinal fluid (CSF), with finely granular, homogeneous appearance (Fig. 4). The dural attachment was not visible since it was posterolateral (with some calcifications) in all cases but one, where it appeared hyperechoic as compared to the lesion. In all cases the spinal cord and the rootlets were visible and displaced contralaterally.
Fig. 4.
Intradural extramedullary tumor: preoperative T1 weighted Gadolinium enhanced sagittal (a) and axial (b) MRI scans of an extramedullary meningioma. Sagittal (a) and axial (b) B-mode scans of a meningioma which appears roundly shaped, homogeneous, capsulated. The spinal cord is progressively pushed anteriorly and laterally, with distortion of the arachnoidal structure, not recognizable in the presence of the lesion
Intradural schwannomas (9 cases) presented as ovular, circumscribed, mildly hyperechoic lesions, though being less homogeneous when compared to meningiomas (due to the presence of some microcystic areas). In these lesions the spinal cord was also displaced contralaterally, but in some cases the nerve rootlets appeared stretched posteriorly to the lesion (Fig. 5).
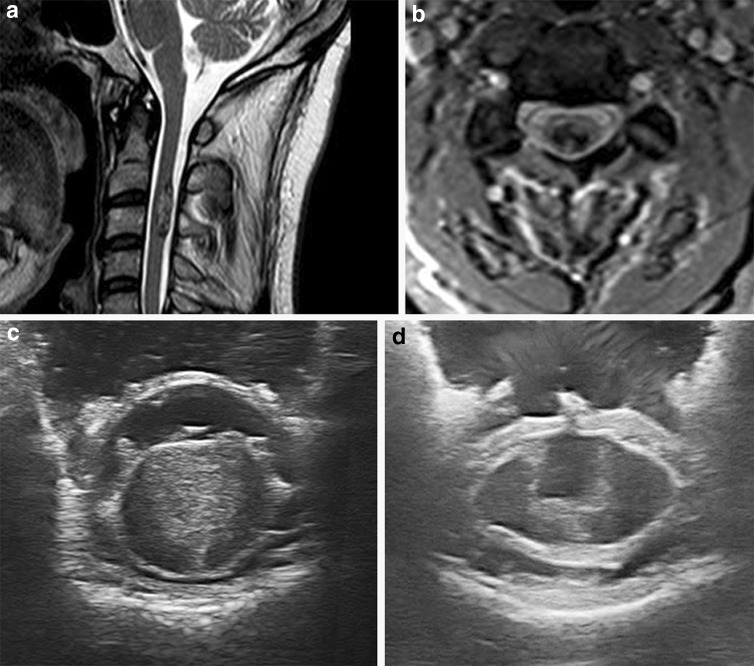
Fig. 5.
Intradural intramedullary tumor: preoperative T2 weighted sagittal (a) and T1-Gadolinium enhanced axial (b) MRI scans of an intramedullary cervical cavernoma. Using ioUS the tumor is identified and located before dural opening (c) so as to evaluate the tumor features (more homogeneous on US than on MRI) and its relationships with surrounding structures. The direct visualization allows also to correctly place the myelotomy. The surgical field is also checked after tumor removal (d)
The arachnoid interface between the tumor and the spinal cord was intact and visible as a bright hyperechoic thin line in all cases but two (one large schwannoma and one melanotic schwannoma), where the arachnoid plane was disrupted in both cases.
Intramedullary tumors
Intramedullary tumors are easily distinguishable from their extramedullary counterparts. They usually appear variably hyperechoic when compared to the spinal cord, with a circumscribed and homogeneous aspect, sometimes with intralesional or perilesional cysts or dilation of the central ependymal canal. The spinal cord usually appears hypoechoic and homogeneous. In one case of an intramedullary lesion it appeared enlarged, with mild hyperechogenicity due to spinal cord edema (Fig. 6). We evaluated four intramedullary ependymomas, which appeared hyperechoic, with a circumscribed homogeneous aspect, associated with some small/microcystic polar areas and a syrinx.
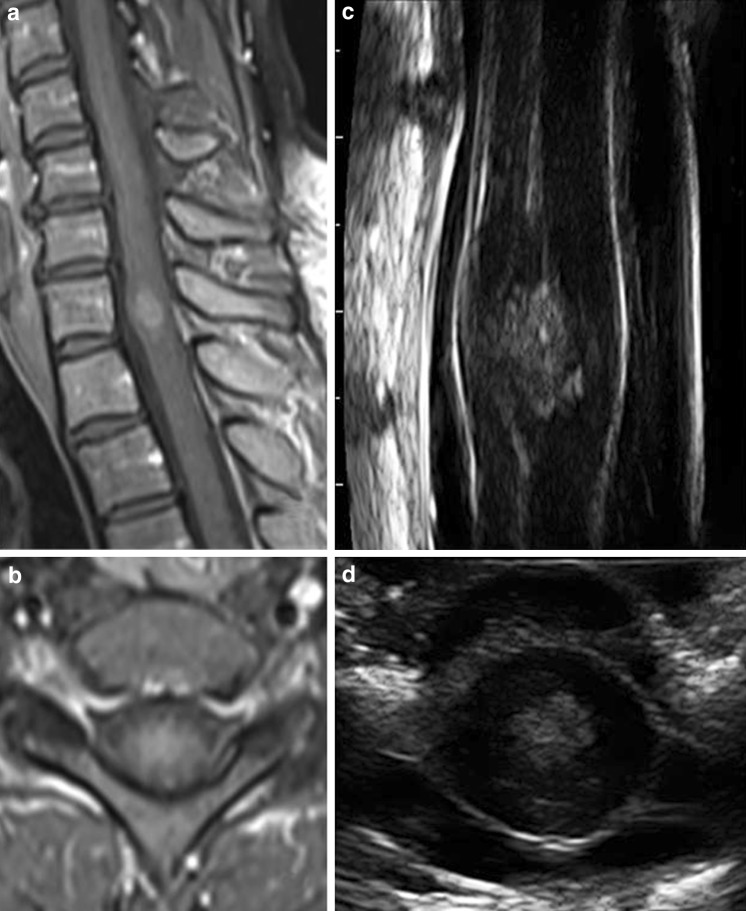
Fig. 6.
Intradural intramedullary tumor: preoperative T1 weighted Gadolinium enhanced sagittal (a) and axial (b) MRI scans of an intramedullary ependymoma. ioUS findings are similar to those obtained with the MRI, showing the enlarged spinal cord without apparent cord edema. The central ependymal canal is also well demonstrated with ioUS
Hemangioblastomas (3 cases) appeared hyperechoic with a nodular, homogeneous aspect, often with perilesional cyst and macrocystic appearance. In one case the lesion was superficial, while in two other cases they were deeply embedded within the spinal cord.
Cavernomas (3 cases) have a highly hyperechoic presentation, with microcystic and microcalcification areas. They appear less circumscribed with a more nodular aspect; in all cases they were superficial, right on the surface of the spinal cord.
In one case of intramedullary astrocytoma the spinal cord was enlarged; the lesion appeared with fine, granular hyperechogenicity and blurred margins, due to its infiltrative attitude. In fact, astrocytomas are highly infiltrative and sometimes hypervascular tumors. Due to their origin from the glia, it can be very difficult to distinguish them from the surrounding spinal cord. Usually the border between intact myelin and tumor is not sonographically evident.
Discussion
In our experience we were able to fully expose and visualize the lesion in all 34 cases with ioUS B-mode imaging and to conduct the surgical resection under direct visualization.
We clearly obtained ioUS findings and characteristics of all lesions and identified surrounding neural structures, as well as other structures such as dura mater, dentate ligament, anterior and posterior rootlets, arachnoid membranes and vascular structures.
After standard bone removal and dural exposure, we employed ioUS to visualize the real cranio-caudal and lateral extension of the tumor, to adapt and target the laminectomy before dural opening.
Bony exposure was adjusted prior to dural opening based on US findings in nine cases; this was very helpful because it prevented further bone removal after dural opening, thus reducing the risk of direct injury of the exposed neural structures and also avoided venous bleeding inside the intradural compartment, due to decompressed epidural venous plexuses.
In all cases ioUS allowed the surgeon to analyze the surgical field with different sections prior to tumor removal, overcoming the limitation of the mere direct microscopic intraoperative view, allowing the evaluation of the anatomy underneath the surface of the surgical field, thus improving the surgical strategy. The integration of ioUS and direct view allows for a three-dimensional visualization of the surgical field, facilitating neural and vascular structures manipulation and ultimately the surgical removal of the lesion.
The ability of ioUS to highlight the lesion and the surrounding anatomical structures is also helpful during tumor resection. As a matter of fact, the relationship with the surrounding neural structures and the blood vessels represents another challenge during surgery; the use of color-mode Doppler US helped us to identify major vessels as in two cases of cervical dumbbell-shaped schwannoma, which displaced the vertebral artery.
IoUS also allowed to visualize nerve rootlets and dentate ligaments, thus facilitating neural structures mobilization especially for those cases that had a predominant anterior location.
Intramedullary tumors represent a challenge in spinal tumor surgery, because of the risk of neurological morbidity due to their location and the surgical approach. Usually the spinal cord appears enlarged due to the lesion which is partially or completely embedded within. Using ioUS we were able to visualize the true extent of the tumor in all 11 cases. In 8 cases, the lesion did not appear on the spinal cord surface and it was necessary to perform a myelotomy along the posterior sagittal sulcus which was not always discernible. In these intramedullary cases, ioUS were used to correctly place the myelotomy, thus minimizing spinal cord manipulation.
IoUS could also help the surgeon guide surgical strategy in cases of highly infiltrative lesions: when a tumor appears isoechoic to the spinal cord and the surface between tumor and myelin is not clearly distinguished, the surgical procedure could be limited to performing an internal decompression and obtaining an definitive histological diagnosis, provided there is an intraoperative histopathological examination that supports such findings.
In the last years, the use of ioUS-guidance has become progressively more widespread during neurosurgical procedures especially for brain tumor removal. On the other hand ioUS does not yet represent a standard technique in spinal tumors surgery, as demonstrated by the few previous reported studies [10–18].
In fact, in 1978 Reid reported the first case of ultrasonographic visualization of a cervical spine astrocytoma with an arc scanner [10]. In 1991, Epstein presented a retrospective study about echographic characteristics of a very large series of intramedullary lesions [11]. Obviously, these studies presented some limitations due to the technical characteristics of the machines that were available at that time. More recently, Regelsberger reported a large series of 78 patients, in which ioUS was used to localize spinal tumors [12]. In 2012, Bozinov focused on the use of ioUS for localization and resection of intramedullary cavernomas [13]. A recent paper from Shamov tried to assess the impact of ioUS on the extent of resection, in particular in case of extramedullary tumor [19].
The goal of all these studies was to validate the intraoperative ultrasound-guided surgery of spinal tumors, which is not yet a routine procedure.
A few technical limitations have to be considered: the size of the tip of the currently used US probe (1 × 3.5 cm) is at times larger than the actual surgical field. Furthermore it is difficult to visualize the parenchyma in case of diffuse bleeding or excessive use of haemostatic material, which is highly hyperechoic [20].
As a matter of fact, most neurosurgeons are not familiar with US imaging because it is not a standard diagnostic tool for neurosurgical pathologies and they do not receive a specific training on US. Therefore it is not easy to interpret ioUS imaging and to set the correct parameters of the machine: both factors could lead to a difficult imaging interpretation.
Conclusions
IoUS appears to be a fast, safe, economic tool and represents a real-time dynamic procedure that can be performed during spinal tumor surgery.
This technique allows full evaluation of the lesion before dural opening and adds valuable real-time information in regard to the anatomic relationships between the tumor and the surrounding neural and vascular tissues.
The evaluation of different parameters will possibly lead in the future to the identification of specific US patterns for different spinal lesions. This will favor and contribute not only to enhance our intraoperative knowledge in regard to tumor biology, but will also lead us to characterize spinal tumors through ioUS, as it already happens within the radiological community for other organs.
IoUS also allows to Taylor the extension of the neurosurgical approach to the true extent of the tumor. This avoids further bone removal while the dura is already opened with the edematous spinal cord protruding through the opening, thus avoiding possible surgical complications. ioUS could also be helpful in adapting and maybe reducing the extent of myelotomy in cases of intramedullary tumors, reducing unnecessary spinal cord manipulation.
Our experience confirms that ioUS might be used as a standard intraoperative tool in these high-risk surgical entities.
Acknowledgments
The authors would like to thank Mrs. Caroline King for her kind advice in revising the manuscript and Mr. Luca Lodigiani for his technical support.
Conflict of interest
Francesco Prada, Ignazio G. Vetrano, Assunta Filippini, Massimiliano Del Bene, Alessandro Perin, Cecilia Casali, Federico Legnani, Marco Saini, Francesco DiMeco declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). All patients provided written informed consent to enrolment in the study and to the inclusion in this article of information that could potentially lead to their identification.
Human and animal studies
The study was conducted in accordance with all institutional and national guidelines for the care and use of laboratory animals.
Footnotes
F. Prada and I. G. Vetrano equally contributed in all aspects of this study.
References
- 1.Duong LM, McCarthy BJ, McLendon RE, Dolecek TA, Kruchko C, Douglas LL, Ajani UA. Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004–2007. Cancer. 2012;118:4220–4227. doi: 10.1002/cncr.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008;87:173–179. doi: 10.1007/s11060-007-9507-z. [DOI] [PubMed] [Google Scholar]
- 3.Sandalcioglu IE, Gasser T, Asgari S, Lazorisak A, Engelhorn T, Egelhof T, Stolke D, Wiedemayer H. Functional outcome after surgical treatment of intramedullary spinal cord tumors: experience with 78 patients. Spinal Cord. 2005;43:34–41. doi: 10.1038/sj.sc.3101668. [DOI] [PubMed] [Google Scholar]
- 4.Jenkinson MD, Simpson C, Nicholas RS, Miles J, Findlay GF, Pigott TJ. Outcome predictors and complications in the management of intradural spinal tumours. Eur Spine J. 2006;15:203–210. doi: 10.1007/s00586-005-0902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avila EK, Elder JB, Singh P, Chen X, Bilsky MH. Intraoperative neurophysiologic monitoring and neurologic outcomes in patients with epidural spine tumors. Clin Neurol Neurosurg. 2013;115:2147–2152. doi: 10.1016/j.clineuro.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Costa P, Peretta P, Faccani G. Relevance of intraoperative D wave in spine and spinal cord surgeries. Eur Spine J. 2013;22:840–848. doi: 10.1007/s00586-012-2576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen IA, Lindseth F, Rygh OM, Berntsen EM, Selbekk T, Xu J, NagelhusHernes TA, Harg E, Håberg A, Unsgaard G. Functional neuronavigation combined with intra-operative 3D ultrasound: initial experiences during surgical resections close to eloquent brain areas and future directions in automatic brain shift compensation of preoperative data. Acta Neurochir (Wien) 2007;149:365–378. doi: 10.1007/s00701-006-1110-0. [DOI] [PubMed] [Google Scholar]
- 8.NagelhusHernes TA, Lindseth F, Selbekk T, Wollf A, Solberg OV, Harg E, Rygh OM, Tangen GA, Rasmussen I, Augdal S, Couweleers F, Unsgaard G. Computer-assisted 3D ultrasound-guided neurosurgery: technological contributions, including multimodal registration and advanced display, demonstrating future perspectives. Int J Med Robot. 2006;2:45–59. doi: 10.1002/rcs.68. [DOI] [PubMed] [Google Scholar]
- 9.Bozinov O, Burkhardt JK, Fischer CM, Kockro RA, Bernays RL, Bertalanffy H. Advantages and limitations of intraoperative 3D ultrasound in neurosurgery. Technical note. Acta Neurochir Suppl. 2011;109:191–196. doi: 10.1007/978-3-211-99651-5_30. [DOI] [PubMed] [Google Scholar]
- 10.Reid MH. Ultrasonic visualization of a cervical cord cystic astrocytoma. AJR Am J Roentgenol. 1978;131:907–908. doi: 10.2214/ajr.131.5.907. [DOI] [PubMed] [Google Scholar]
- 11.Epstein FJ, Farmer JP, Schneider SJ. Intraoperative ultrasonography: an important surgical adjunct for intramedullary tumors. J Neurosurg. 1991;74:729–733. doi: 10.3171/jns.1991.74.5.0729. [DOI] [PubMed] [Google Scholar]
- 12.Regelsberger J, Fritzsche E, Langer N, Westphal M. Intraoperative sonography of intra- and extramedullary tumors. Ultrasound Med Biol. 2005;31:593–598. doi: 10.1016/j.ultrasmedbio.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Bozinov O, Burkhardt JK, Woernle CM, Hagel V, Ulrich NH, Krayenbühl N, Bertalanffy H. Intra-operative high frequency ultrasound improves surgery of intramedullary cavernous malformations. Neurosurg Rev. 2012;35:269–275. doi: 10.1007/s10143-011-0364-z. [DOI] [PubMed] [Google Scholar]
- 14.Friedman JA, Atkinson JL, Lane JI. Migration of an intraspinal schwannoma documented by intraoperative ultrasound: case report. Surg Neurol. 2000;54:455–457. doi: 10.1016/S0090-3019(00)00314-1. [DOI] [PubMed] [Google Scholar]
- 15.Kolstad F, Rygh OM, Selbekk T, Unsgaard G, Nygaard OP. Three-dimensional ultrasonography navigation in spinal cord tumor surgery. Technical note. J Neurosurg Spine. 2006;5:264–270. doi: 10.3171/spi.2006.5.3.264. [DOI] [PubMed] [Google Scholar]
- 16.Maiuri F, Iaconetta G, de Divitiis O. The role of intraoperative sonography in reducing invasiveness during surgery for spinal tumors. Minim Invasive Neurosurg. 1997;40:8–12. doi: 10.1055/s-2008-1053405. [DOI] [PubMed] [Google Scholar]
- 17.Maiuri F, Iaconetta G, Gallicchio B, Stella L. Intraoperative sonography for spinal tumors. Correlations with MR findings and surgery. J Neurosurg Sci. 2000;44:115–122. [PubMed] [Google Scholar]
- 18.Zhou H, Miller D, Schulte DM, Benes L, Bozinov O, Sure U, Bertalanffy H. Intraoperative ultrasound assistance in treatment of intradural spinal tumours. Clin Neurol Neurosurg. 2011;113:531–537. doi: 10.1016/j.clineuro.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Shamov T, Eftimov T, Kaprelyan A, Enchev Y. Ultrasound-based neuronavigation and spinal cord tumour surgery––marriage of convenience or notified incompatibility? Turk Neurosurg. 2013;23:329–335. doi: 10.5137/1019-5149.JTN.6639-12.2. [DOI] [PubMed] [Google Scholar]
- 20.Selbekk T, Jakola AS, Solheim O, Johansen TF, Lindseth F, Reinertsen I, Unsgård G. Ultrasound imaging in neurosurgery: approaches to minimize surgically induced image artefacts for improved resection control. Acta Neurochir (Wien) 2013;155:973–980. doi: 10.1007/s00701-013-1647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]