Abstract
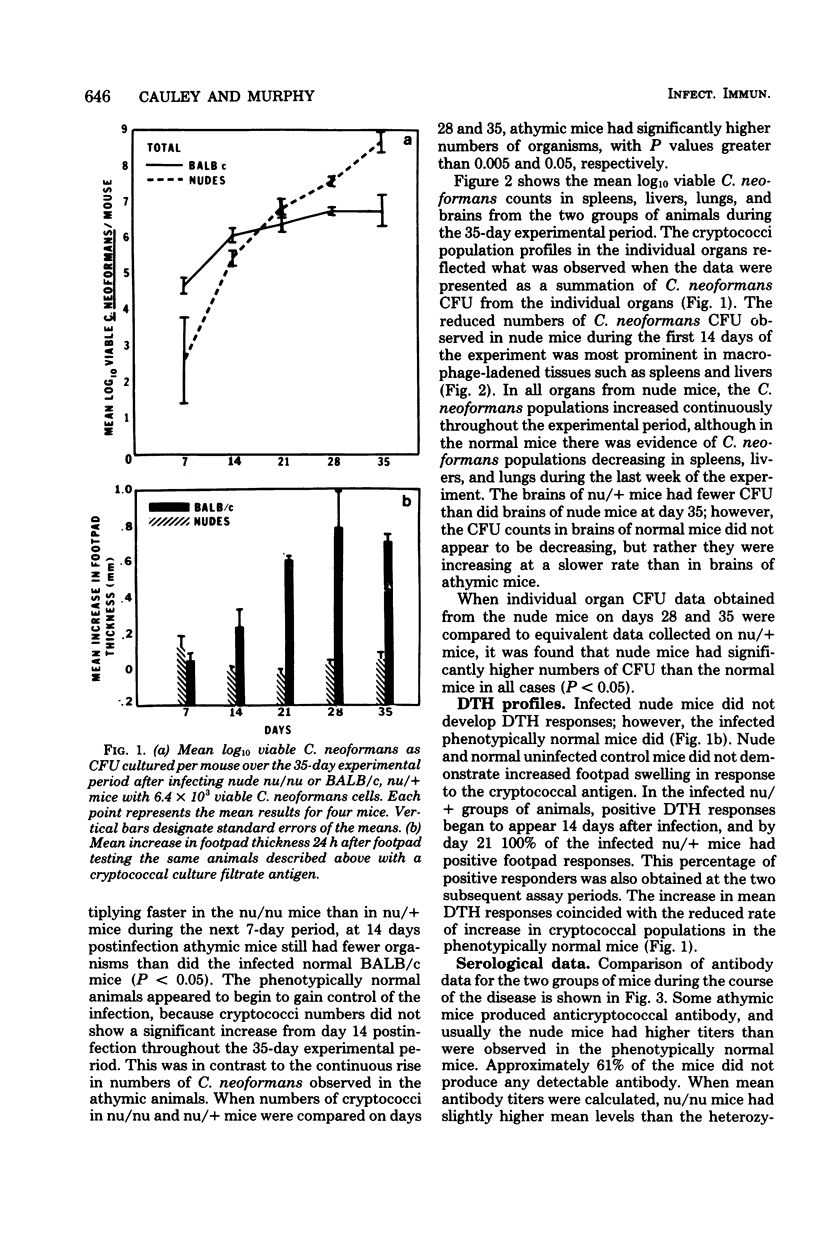
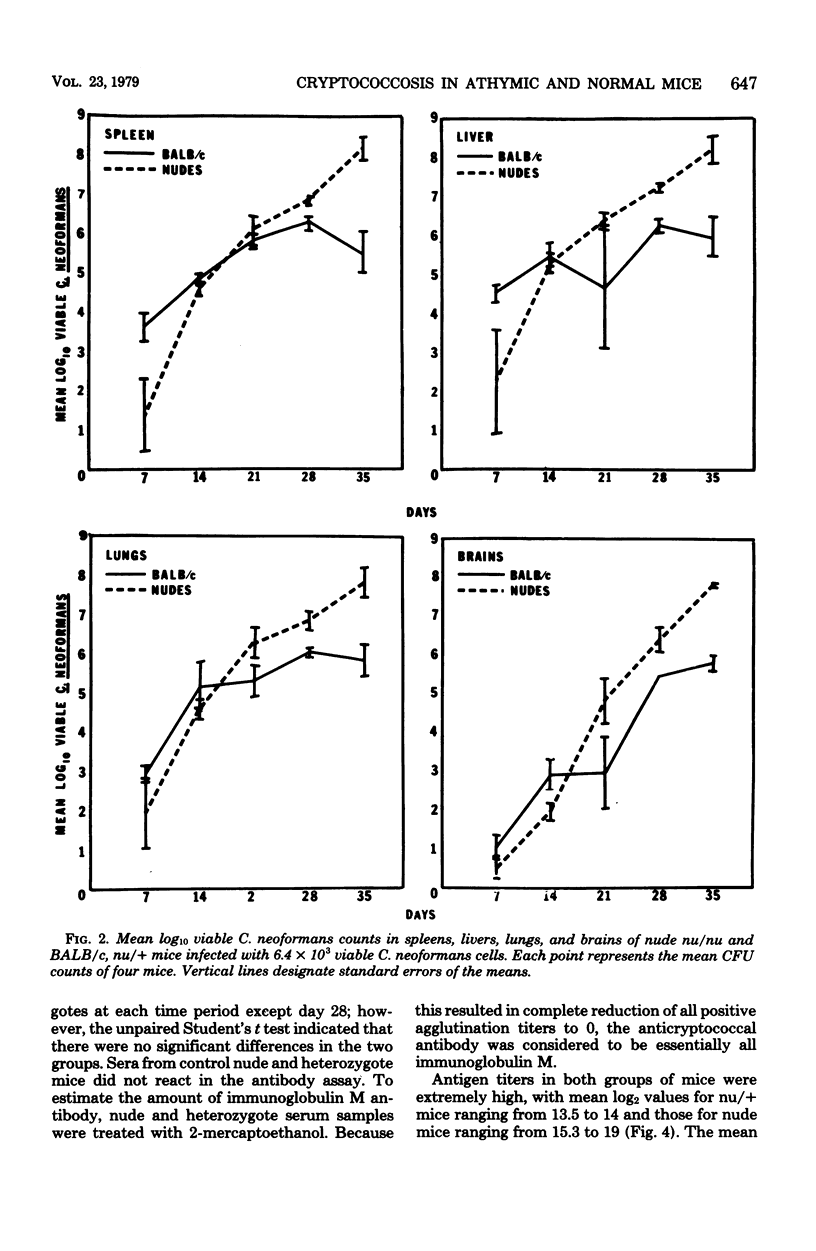
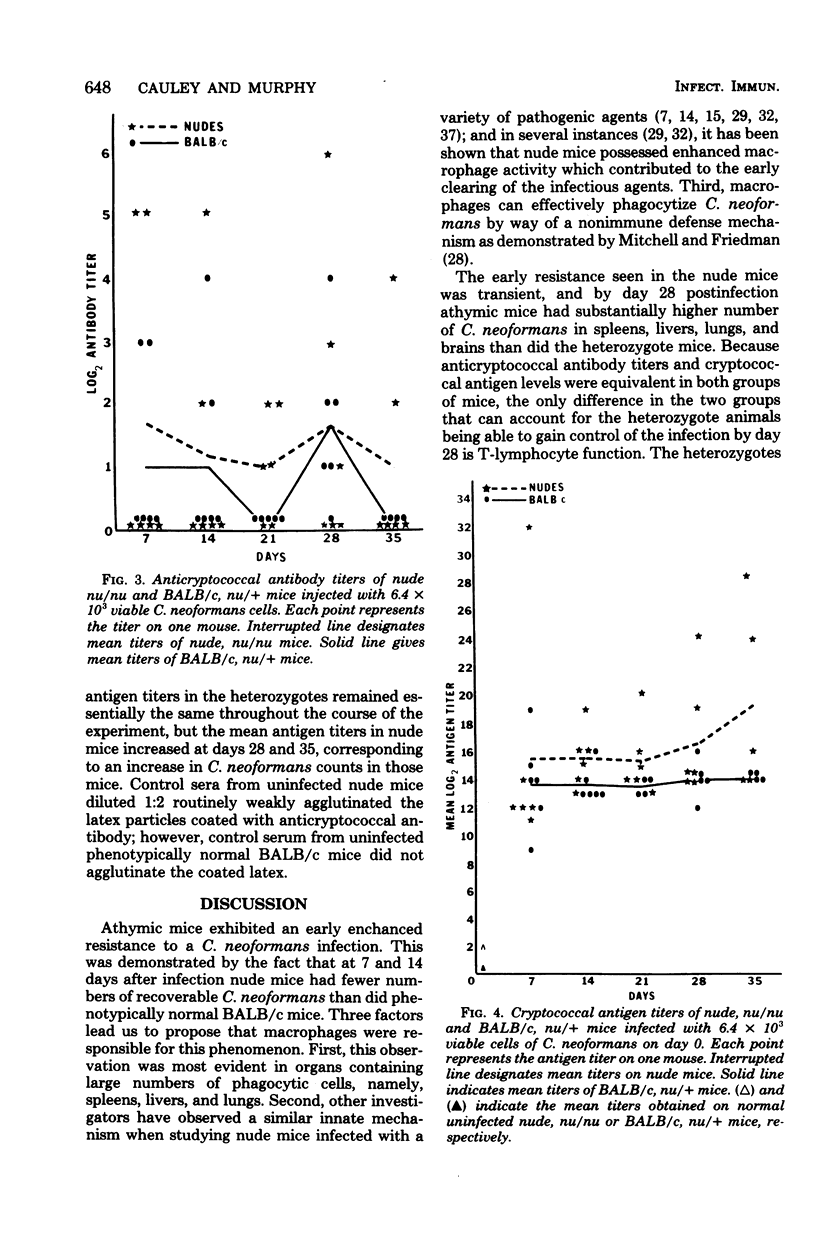
A Cryptococcus neoformans infection in congenitally athymic (nude) mice and phenotypically normal heterozygote BALB/c mice was used to determine how T lymphocyte-deficient mice compared with normal mice in restricting proliferation of C. neoformans and to determine whether a correlation exists between delayed-type hypersensitivity and resistance to C. neoformans. Although nude mice displayed the ability to maintain cryptococcal population levels lower than did the phenotypically normal animals during the first 14 days of infection, the resistance was not sufficient to control the infection during the remainder of the 35-day experimental period. Heterozygote mice began to demonstrate positive delayed-type hypersensitivity responses by day 14 postinfection; however, nude mice were unable to mount delayed-type hypersensitivity responses. The appearance of the delayed-type hypersensitivity response in the heterozygote mice was concomitant with the reduced rate of proliferation of C. neoformans observed in those animals from days 14 to 35. Because anticryptococcal antibody titers and cryptococcal antigen levels were equivalent in both groups of mice, T-lymphocyte function was considered to be responsible for the resistance observed in the heterozygote mice. The mechanism by which cryptococcal populations were reduced was not addressed; however, the mouse model system used in these studies would be an ideal tool for studying those mechanisms. Nude mice were able to produce antibodies against cryptococcal cells, indicating that at least one component of C. neoformans is a T-independent antigen. The antibody response was predominantly immunoglobulin M in nude and heterozygote mice. Cryptococcal antigen levels were extremely high in both groups of animals and appeared to increase as C. neoformans cell numbers increased.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams I. Further studies on acquired resistance to murine cryptococcosis: enhancing effect of Bordetella pertussis. J Immunol. 1966 Mar;96(3):525–529. [PubMed] [Google Scholar]
- Adamson D. M., Cozad G. C. Effect of antilymphocyte serum on animals experimentally infected with Histoplasma capsulatum or Cryptococcus neoformans. J Bacteriol. 1969 Dec;100(3):1271–1276. doi: 10.1128/jb.100.3.1271-1276.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Pappagianis D., Benjamini E. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect Immun. 1977 Sep;17(3):580–585. doi: 10.1128/iai.17.3.580-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Diamond R. D., Allison A. C. Nature of the effector cells responsible for antibody-dependent cell-mediated killing of Cryptococcus neoformans. Infect Immun. 1976 Sep;14(3):716–720. doi: 10.1128/iai.14.3.716-720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J Infect Dis. 1973 Jun;127(6):694–697. doi: 10.1093/infdis/127.6.694. [DOI] [PubMed] [Google Scholar]
- Diamond R. D. Effects of stimulation and suppression of cell-mediated immunity on experimental cryptococcosis. Infect Immun. 1977 Jul;17(1):187–194. doi: 10.1128/iai.17.1.187-194.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauve R. M., Hevin B. Résistance paradoxale des souris thymoprives à l'infection par Listeria monocytogenes et Salmonella typhimurium et action immunostimulante d'un extrait bactérien phospholipidique (EBP). C R Acad Sci Hebd Seances Acad Sci D. 1974 Nov 4;279(19):1603–1605. [PubMed] [Google Scholar]
- Gentry L. O., Remington J. S. Resistance against Cryptococcus conferred by intracellular bacteria and protozoa. J Infect Dis. 1971 Jan;123(1):22–31. doi: 10.1093/infdis/123.1.22. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Experimental murine cryptococcosis: effect of hyperimmunization to capsular polysaccharide. J Immunol. 1967 May;98(5):914–922. [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Cell-mediated immunity in Cryptococcosis. Cell Immunol. 1974 Oct;14(1):12–21. doi: 10.1016/0008-8749(74)90164-6. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Taylor R. L. Host defense in cryptococcosis. I. An in vivo model for evaluating immune response. Int Arch Allergy Appl Immunol. 1978;57(2):101–113. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. G., Friedman L. In vitro phagocytosis and intracellular fate of variously encapsulated strains of Cryptococcus neoformans. Infect Immun. 1972 Apr;5(4):491–498. doi: 10.1128/iai.5.4.491-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Role of activated macrophages in resistance of congenitally athymic nude mice to hepatitis induced by herpes simplex virus type 2. Infect Immun. 1978 Mar;19(3):792–798. doi: 10.1128/iai.19.3.792-798.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Gregory J. A., Larsh H. W. Skin testing of guinea pigs and footpad testing of mice with a new antigen for detecting delayed hypersensitivity to Cryptococcus neoformans. Infect Immun. 1974 Feb;9(2):404–409. doi: 10.1128/iai.9.2.404-409.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Anomalous high native resistance to athymic mice to bacterial pathogens. Infect Immun. 1977 Dec;18(3):636–645. doi: 10.1128/iai.18.3.636-645.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- PERCEVAL A. K. EXPERIMENTAL CRYPTOCOCCOSIS: HYPERSENSITIVITY AND IMMUNITY. J Pathol Bacteriol. 1965 Apr;89:645–655. doi: 10.1002/path.1700890225. [DOI] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Schimpff S. C., Bennett J. E. Abnormalities in cell-mediated immunity in patients with Cryptococcus neoformans infection. J Allergy Clin Immunol. 1975 Jun;55(6):430–441. doi: 10.1016/0091-6749(75)90082-2. [DOI] [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Experimental mycobacterial infection in congenitally athymic "nude" mice. J Reticuloendothel Soc. 1976 Feb;19(2):77–90. [PubMed] [Google Scholar]
- Walter J. E., Jones R. D. Serodiagnosis of clinical cryptococcosis. Am Rev Respir Dis. 1968 Feb;97(2):275–282. doi: 10.1164/arrd.1968.97.2.275. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V. Macrophage activation in mice lacking thymus-derived (T) cells. Experientia. 1975 May 15;31(5):591–593. doi: 10.1007/BF01932477. [DOI] [PubMed] [Google Scholar]



