Abstract
Chinese herbal medicines such as hawthorn, salvia, etc., are frequently combined with statins so as to treat cardiovascular diseases more effectively. Chinese herbal medicines contain many kinds of active components, which may have drug–drug interactions with statins. This study aims to explore the effect and mechanism by which ursolic acid affects OATP1B1-mediated transport of rosuvastatin. This study will explore the effect of ursolic acid on OAPT1B1-mediated transport of rosuvastatin in the different cell systems. Given the genetic polymorphisms of OATP1B1, simultaneously, this study will further explore the effect of ursolic acid on OATP1B1 (521T>C)-mediated transport of rosuvastatin. When the concentration of ursolic acid was 1.8 and 18 µM, it showed that ursolic acid significantly inhibits the uptake of rosuvastatin in both OATP1B1*1a-HEK 293T cells and OATP1B1*5-HEK 293T cells. The reduction of OATP1B1*1a transport of rosuvastatin were 34.60 ± 2.99 and 66.08 ± 1.83 %, and for OATP1B1*5 were 34.27 ± 7.08 % and 66.95 ± 1.14 %. Inhibitory parameters of IC50 were 6.25 ± 0.42 and 6.07 ± 0.57 µM, respectively. This study suggests that ursolic acid can affect the uptake of rosuvastatin in hepatocytes by inhibiting the transport of OATP1B1, and gene mutation of OATP1B1 may cause different effects on its transport of rosuvastatin.
Keywords: Rosuvastatin, Ursolic acid, OATP1B1, Transporter
Introduction
With the development of economy and the improvement of living standards, Chinese people’s lifestyle and dietary structure have been tremendously changed. The incidence of cardiovascular diseases such as hyperlipidemia showed a rising trend year by year. Statins, as inhibitor of HMG-GoA reductase, can significantly improve the blood lipid level and be widely applied for the treatment of cardiovascular diseases. Although lipid-lowering effect of statins is undoubted, the side effects especially severe rhabdomyolysis should be considered. Simultaneously, the action target point of statins is too single. Chinese herbal medicines with fewer adverse reactions and with multiple action target points to regulate the hyperlipidemia. Therefore, Chinese herbal medicines such as salvia, hawthorn, gardenia, glossy privet fruit, etc., with effect of lipid-lowering, were frequently combined with statins to treat the cardiovascular disease. However, Chinese herbal medicines have many kinds of bioactive components that may cause drug–drug interaction and lead to the pharmacokinetic change of statins.
Our previous study found that, compared to the control group given rosuvastatin alone, the concurrent use of ursolic acid (80 mg/kg) prior to the oral administration of rosuvastatin (100 mg/kg) increased the systemic exposure of rosuvastatin more than 163.02 % (Wen and Xiong 2011). However, the mechanism by which ursolic acid affected the pharmacokinetics of rosuvastatin has not been explored. Ursolic acid was proved to have sedation, anti-inflammatory, antibacterial, antiulcer and anti-cancer activities. Ursolic acid is a ubiquitous pentacyclic triterpenoid compound obtained from many herbal plants such as hawthorn, Salvia miltiorrhiza, gardenia, Ligustrum lucidum, prunella, plantain, licorice, forsythia and Kuding tea, etc., (Wen and Xiong 2011). These herbal medicines are frequently combined with statins to treat the cardiovascular diseases. Therefore, it is necessary to pay close attention to the effect of ursolic acid on the pharmacokinetics of rosuvastatin. However, the reason that ursolic acid significantly affected the pharmacokinetics of rosuvastatin is not clear. Exploring the mechanism could not only provide valuable reference for the development of ursolic acid, but also reduce the adverse reactions and treatment failure.
Modern pharmacokinetics studies showed that transporters played an important role in the absorption, distribution, excretion and metabolism of both endogenous and xenobiotic compounds. Transporters may act as physiological “gatekeepers” in the regulation of the pharmacological and/or toxicological effects of drugs by limiting distribution to tissues responsible for their effect and/or toxicity (Liu et al. 2011).
Rosuvastatin, a novel statin, has more powerful effects on the treatment of hyperlipidemia. The study showed that organic anion transporting polypeptide 1B1 (formally known as OATP-C or OATP2, gene SLCO1B1/OATP1B1) played an important role in the uptake of rosuvastatin in liver (Wen et al. 2012). Rosuvastatin was mainly transported by OATP1B1 in liver (Funk 2008). OATP1B1 is mainly expressed on the sinusoidal membrane of human hepatocytes, and the homologous gene Oatp1b2 is also expressed in rats (Wen and Xiong 2011).
OATP1B1 had genetic polymorphisms and some studies showed that OATP1B1 (521T>C) had significantly effect on the pharmacokinetic of rosuvastatin (Sui et al. 2011). It suggests that genetic variation associated with changes in protein expression or function of OATP1B1 may have a substantial impact on systemic drug exposure and toxicity. OATP1B1 act as main transporter in uptake of many drugs in liver.
For a long time, drug–drug interaction in pharmacokinetic based on metabolizing enzymes have been reported in many studies. But now, as more and more drugs were found as the substrates of transporters, drug–drug interactions may be involved membrane transport by competition for the substrate binding sites of the transporters or by a change in the expression level of the transporters (Mizuna et al. 2003). At present, the hepatic drug–drug interaction via OATP1B1 has been reported in some study (Muck et al. 1999; Shitara et al. 2003).
In China, herbal medicines are frequently used in combination with conventional drugs and interactions are likely to be more common than those which manifest clinically. Pharmacokinetic interactions mediated by transporters may be involved in many herb–drug interactions (Liu et al. 2011). Therefore, the study that ursolic acid significantly affected pharmacokinetics of rosuvastatin in rats should be focused.
In another study, result showed that ursolic acid act as a selective OATP1B1 inhibitor (IC50 = 12.5 μM) in OATP1B1-mediated fluorescein-methotrexate uptake (Gui et al. 2010). Therefore, there may be drug–drug interaction between ursolic acid and rosuvastatin based on OATP1B1. This study will explore the effect of ursolic acid on OAPT1B1-mediated transport of rosuvastatin by establishing rat primary hepatocytes model and transgenic cell model. Simultaneously, the study will further explore the effect of ursolic acid on OATP1B1 (521T>C)-mediated transport of rosuvastatin. This study aims to explore the effect and mechanism that ursolic acid affect OATP1B1-mediated transport of rosuvastatin in hepatobiliary at cell and molecular level, and elucidate the mechanism that ursolic acid affected the pharmacokinetics of rosuvastatin in the previous study (Wen and Xiong 2011).
Materials and methods
Materials
Ursolic acid (UA, 3b-hydroxy-urs-12-en-28-oic-acid, chemical structure shown in Fig. 1) was purchased from the bioengineering development center of Yi Chun Academy of Jiangxi Province, which was obtained from hawthorn fruits by homogenate and alcohol and purity degree was 98.2 %. Rosuvastatin was kindly offered by Lunan Beite pharmaceutical Co. Ltd; DMSO was purchased from Amresco Chemical Co. Heparin sodium was purchased from Shimizu Pharmaceutical Co., Ltd. (Shizuoka, Japan). Trypan blue was purchased from ICN Biomedica, Inc. (Aurora, OH, USA). Heat-inactivated fetal bovine serum (FBS) was purchased from Shanghai Al-Amin Bio-tech Co., Ltd. (Shanghai, China). Hank’s balanced salt solution (HBSS), trypsin–EDTA, Dulbecco’s modified Eagle’s medium (DMEM), non-essential amino acid solution (NEAA), and penicillin–streptomycin solution were purchased from Gibco Laboratories (Invitrogen Co, Grand Island, NY, USA). Methanol and ethyl acetate were obtained from Merck Co. (Darmstadt, Germany). All other chemicals were of analytical grade and all solvents were of HPLC grade. The polyclonal antibodies pESL was raised in rabbits against human OATP1B1. Horseradish peroxidase-conjugated goat anti-rabbit IgG was obtained from Amersham (GE Healthcare Europe GmbH, Munich, Germany).
Fig. 1.

Structural of ursolic acid
Preparation of standard solutions
Standard stock solutions of rosuvastatin and pitavastatin (IS) were prepared separately in methanol at the concentrations of 0.860 and 0.783 mg/ml. These stock solutions were used in spectrometry analysis. Stock solutions of rosuvastatin and UA were prepared separately in DMSO at the concentrations of 320 and 45.1 mmol/l. These stock solutions were used in hepatocytes and transgenic cells uptake experiments.
Animals
Male rats (210 ± 30) served as liver donors and were purchased from animal center of Nanchang University (Jiangxi, China). All rats were maintained under standard conditions with a reverse dark–light cycle and were treated humanely. Food and water were available ad libitum. Rats were used in accordance with ethical procedures following the guidelines for the care and use of laboratory animals issued by the Chinese government and Nanchang University.
Isolation of hepatocytes
In this paper, a modified version of the method reported by Seglen PO (Seglen 1976) was used to isolate rat hepatocytes. Rats were injected 5 % pentobarbital sodium (100 µl/100 g) to tail vein, then were administered heparin (1,000 units/kg) intravenously. The liver was perfused with 0.02 % calcium-free EDTA-PBS solution (20–40 ml/min for 5–10 min) at 37 °C. The liver was further perfused with Hanks solution containing 0.04 % IV collagenase and CaCl2·H2O4 mM (Hanks-II buffer) at 37 °C (30 ml/min for 3 min), and the liver was removed from the animal abdominal wall. The digested liver was excised and shaken so that hepatocytes were released into 30 ml ice-cold Dulbecco’s modified Eagle’s medium (DMEM). Then, cells were filtered through a nylon mesh and centrifuged at 167 g/min for 30 s. This process was done repeatedly for 3 times to discard the supernatant fraction. Finally, the hepatocytes were resuspended in Krebs–Henseleit buffer [KHB, including 118 NaCl, 23.8 NaHCO2, 4.8 KCl, 1.0 KH2PO4, 1.2MgSO4, 12.5 2-(4-(2-hydroxyethyl)-1-piperazinyl) ethanesulfonic acid (HEPES), 5 glucose and 1.5 CaCl2 adjusted to pH 7.4]. All experiments were completed within 2 h after cell preparation. Trypan blue exclusion test was used to measure the viability of hepatic cells. Cells with a viability of greater than 90 % were used for further study. The number of hepatocytes were counted using a Bürker–Türk cell-counting plate.
Construction of OATP1B1*1a and *5 Cells
Taking target gene OATP1B1*5 from the OATP1Bl*5 plasmid (PeDNA3.l(−)/Zeo:Invitrogen, kindly provided by Chiba University), and using the target gene as a template to clone another gene OATP1B1*la by site-directed mutagenesis, DNA sequencing was performed on both. The pGC-FU GFP lentiviral vector transfer system was used as gene transmit medium to construct the recombinant lentiviral vector of OATPIBI*la–GFP fusion gene and OATPIB1*5–GFP fusion gene. The titer of virus was tested by real-time quantitative PCR (RT-PCR). HEK293T were transfected by the OATPIB1-GFP fusion gene lentivirus (Lenti-OATPIB1*la, Lenti- OATPIB1*5) and GFP lentivirus (Lenti-GFP), respectively. After being transduced with lentivirus, the green fluorescent protein (GFP) in HEK293T was observed using fluorescence microscope, and the expression of OATP1B1*1a and *5 (GFP tagged fusion protein) was detected by Western Blot. The primary antibodies pESL was diluted 1:5,000 in TPBS (Dulbecco’s phosphate-buffered saline, pH 7.4, and 0.1 % Tween 20). The secondary antibody was a horseradish peroxidase-conjugated goat anti-rabbit IgG from Amersham (GE Healthcare Europe GmbH) used at a 1:10,000 dilution. Vector-transfected HEK293T cells served as negative controls, respectively.
Cell culture
Human embryonic kidney (HEK293T) cells and transgenic cells were cultured in minimum essential medium, containing 10 % heat-inactivated fetal bovine serum and 1 % antibiotic–antimycotic solution (100 U/ml penicillin, and 100 µg/ml streptomycin) at 37 °C under 5 % CO2 and 95 % humidity (Gui et al. 2010).
Uptake experiments
Hepatocytes uptake experiments
Hepatocytes were diluted to 1 × 106/ml with KHB and were prewarmed for 5 min at 37 °C, then cells were added to 24-well culture plate. Time-course of uptake of rosuvastain (20 µM) by isolated rat hepatocytes were studied in 10, 40, 60, 80, 120, 240, 360 s. Concentration dependency of initial uptake rate of rosuvastatin by isolated rat hepatocytes were studied in series concentration of 5, 10, 20, 30, 40, 60, 100 µM, and all were incubated for a scheduled period of 40 s. The effect of ursolic acid on the uptake of rosuvastatin in rat hepatocytes was explored. Experiment was designed as blank group, rosuvastatin control group, rosuvastatin combined with a series concentrations of ursolic acid. Series concentration of ursolic acid were 4, 8 and 16 µM. Cells were placed in incubator at 37 °C for 40 s. At the end of the incubation period, uptake was terminated by removing the medium, and the cells were immediately washed with ice-cold Krebs–Henseleit buffer four times and then 0.5 ml sterile water was added. The sample was placed at ultra-low temperature freezer at −80 °C, repeated freezing solution 3 times. Then vortexing briefly, a part of the supernatant (300 µl) was transferred to a fresh tube and 10 µl 10 µg/ml pitavastatin (internal standard) was added. Then 1.5 ml acetic ether was added and vortexed about 3 min. Afterwards, the mixture was centrifuged for 10 min at 12,000×g. The organic layer was evaporated under vacuum drying at 37 °C. Samples were reconstituted with 150 μl of mobile phase, of which 10 μl was injected to determine the concentration of rosuvastatin. The protein concentration was determined according to the method of Bradford (1976), a Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA) was used.
Transgenic cells uptake experiments
Before the start of uptake experiments, the cells were washed with prewarmed (37 °C) uptake buffer (142 mM NaCl, 5 mM KCl, 1 mM K2HPO4, 1.2 mM MgSO4, 1.5 mM CaCl2, 5 mM glucose, and 12.5 mM HEPES, pH 7.3). The rosuvastatin was dissolved in uptake buffer and was added to the final concentration of 100, 50, 20, 10, 5 and 1 µM BSP (bromosulfophthalein) for studies the uptake character of rosuvastatin in HEK-OATP1B1*1a and HEK-OATP1B1*5 cells, respectively. To characterize the ursolic acid as inhibitors, they were added in increasing concentrations (0.18, 1.8 and 18 µM). The cells were incubated with the test solution at 37 °C for 10 min. Subsequently, the cells were washed three times with ice-cold uptake buffer. After the cells were lysed with 0.2 % SDS, the intracellular accumulation of rosuvastatin was determined by LC/MS and the appropriate protein concentration was determined by bicinchonic acid assay (BCA Protein Assay Kit).
Liquid chromatography tandem mass spectrometry analysis
Rosuvastatin concentrations of cell sample were determined by liquid chromatography–mass spectrometry (LC/MS). A Shim-pack GVP-ODS C18 guard column (5.0 mm 9 2.0 mm, Shimadzu, Kyoto, Japan) and a mobile phase consisting of 0.02 % triethylamine in 0.5 mM ammonium acetate (A) and methanol (B) at a flow rate of 0.2 ml/min were applied (A:B = 40:60). Gradient elution was applied as follow: 0–0.2 min, B:60 %; 0.2–4.50 min, B:60–5 %; 4.50–6.0 min, B:5 %; 6.0–6.50 min, B:5–60 %; 6.50–12.00 min, B: 60 %. The analysis was performed in selective ion monitoring mode at m/z 420.0 for pitavastatin (internal standard, IS) and m/z 480.0 for rosuvastatin. SIM chromatograms of the analytes and IS are shown in Fig. 2.
Fig. 2.
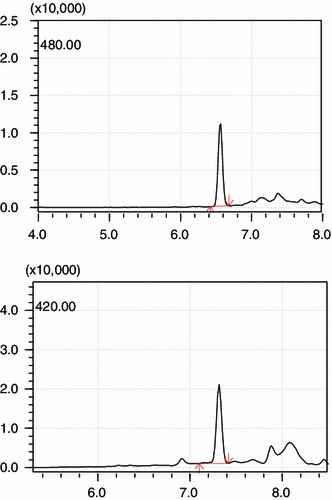
SIM chromatograms of the rosuvastatin (10 µg/ml) and IS (10 µg/ml) ([M-H]−1 m/z 420.0 for IS and m/z 480.0 for rosuvastatin)
Determination of the kinetic parameters and statistical analysis
The kinetic parameters K m (Michaelis constant) and V max (maximum uptake rate) were calculated using the competitive-inhibition equation. Statistical analysis was conducted using SSPS12.0. P value less than 0.05 was considered as statistical significance. Calculation of the inhibition constant (K i). The K i of ursolic acid for inhibiting the initial uptake of rosuvastatin was calculated using Win Nonlin Professional Version 3.3 (Pharsight Corporation, Mountain View, CA, USA) according to the following equation:
V 0 = V max. S/{[K m (1 + i/K i) + S] + S}, where i is the ursolic acid concentration (mM) and K i is the inhibition constant (mM). The data were fitted using the Gauss–Newton (Levenberg and Hartley) method.
Results
Morphology of hepatic cells
The morphology of hepatics cells were observed by inverted microscope. It presented that isolated rat hepatocytes were cord-like or round block. The cells were translucent and boundary was clear. The cytoplasm of cells was abundant and many small particles were shown in the cytoplasm. The nucleus of hepatics cells was round or oval shape, and position was more deviated. The nucleus of hepatocytes showed dual-core or multi-core. Morphology of hepatocytes was showed in Fig. 3. Functionality and purity of hepatics all were well.
Fig. 3.
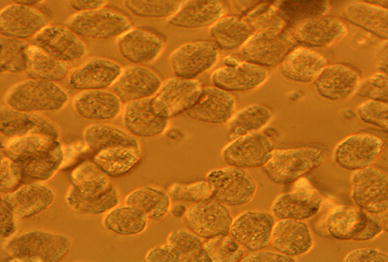
The morphology of hepatics cells in microscope (100 × 40)
HEK293T cells were transfected with pGC-FU-OATP1B1*1a and pGC-FU-OATP1B1*5, and the fluorescent photos of HEK293T cells after being transfected were showed in Fig. 4. The protein expression of the selected cell clones has been analyzed using the OATP1B1-specific antibody pESL. This analysis demonstrated high protein expression in the HEK-OATP1B1 was showed in Fig. 5.
Fig. 4.
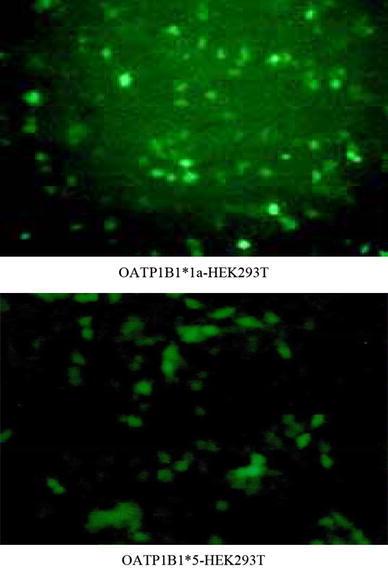
The fluorescent photos of HEK293T cells after being transfected with pGC-FU-OATP1B1*1a and pGC-FU-OATP1B1*5 (100 × 10)
Fig. 5.
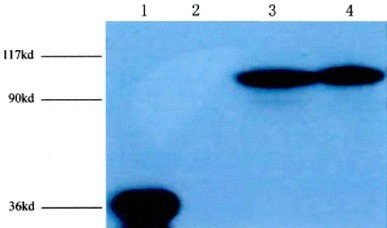
The expression of OATP1B1 in transgenic cells, cell homogenates (20 µg) were separated by SDS-polyacrylamide gel electrophoresis, detected by the polyclonal antibody pESL (diluted 1:5,000) and goat anti-rabbit IgG. (1 expression of GFP, 2 blank controls, 3 expression of OATP1B1*5 in OATP1B1*5-HEK293T cell, 4 expression of OATP1B1*1a in OATP1B1*1a-HEK293Tcell. Characterization of stably transfected HEK293 cells. A, immunoblot analysis of HEK-OATP1B1 cell)
Uptake characteristics studies of rosuvastatin in hepatic cells
The uptake of rosuvastatin increased linearly over a period of 40 s. After 80 s, the uptake of rosuvastatin showed alleviation and no increase. Time-course of uptake of rosuvastain was showed in Fig. 6. The concentration-dependence uptake of rosuvastain was determined as in Fig. 7. The result indicated that the uptake of rosuvastatin was not saturated up to 60 µM and increase linearly in concentration range of 5–20 μM. When concentration was 100 µM, the uptake of rosuvastian presents saturation. Rosuvastatin uptake was concentration-dependent with a K m value of 25.11 ± 8.49 µM and a V max value of 16.97 ± 2.19 nmol/min/mg protein. The intrinsic transport capacity (V max/K m) was 0.69 ± 0.10.
Fig. 6.
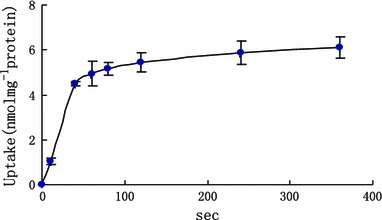
The effect of time on the uptake of rosuvastatin in primary hepatocytes [x axis was the time (s), y axis was the uptake of rosuvastatin in hepatocytes]
Fig. 7.
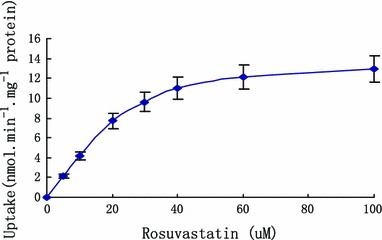
Concentration-dependent uptake of rosuvastain in hepatocytes (x axis was the concentration of rosuvastatin, y axis was the uptake of rosuvastatin in hepatocytes)
The inhibiting of ursolic acid on uptake of rosuvastatin in hepatic cells
The inhibitory effect of ursolic acid on uptake of rosuvastatin in hepatic cells was evaluated at appropriate concentrations (Fig. 8). When the concentrations of ursolic acid were 4, 8 and 16 µM, the uptake of rosuvastain was reduced, respectively, about 1.70 ± 0.94, 47.58 ± 1.80 and 71.16 ± 0.19 %. The K i of ursolic acid for inhibiting the initial uptake of rosuvastatin was as 10.88 ± 0.29 µM. The result showed that significantly inhibitory effect of ursolic acid on uptake of rosuvastatin was observed when concentrations of ursolic acid were 8 and 16 µM.
Fig. 8.
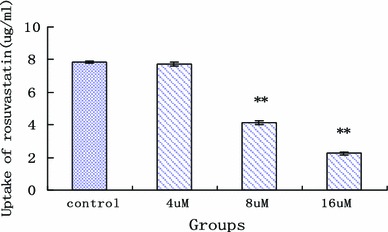
The effect of ursolic acid on rosuvastatin uptake in rat primary hepatocytes (x axis was the concentration of ursolic acid, y axis was the uptake of rosuvastatin in hepatocytes; **statistically different, P < 0.05, n = 5)
Uptake characteristics studies of rosuvastatin in OATP1B1-HEK293Tcells
Uptake experiments of rosuvastatin have been carried out as described with addition of different concentrations of rosuvastatin. The result indicated that the uptake of rosuvastatin was not saturated up to 20 µM and increased linearly in concentration range of 5–20 μM. When concentration was 50 µM, the uptake of rosuvastian presents saturation. The result suggests that the uptake of rosuvastain in OATP1B1-HEK293Tcells display concentration-dependence. Simultaneously, the result of our study showed that transport of rosuvastatin by OATP1B1 was related with genotypes. Compared with OATP1B1*1a, mutant OATP1B1*5 significantly decreased the transport capacity. The uptake of rosuvastatin in OATP1B1*5-HEK293T cells was significantly less than OATP1B1*1a-HEK293T (P < 0.05). The uptake kinetic parameters K m and V max of OATP1B1*1a-HEK293T on rosuvastatin were 19.87 ± 5.96 µM and 6.63 ± 0.70 pmol min−1 mg−1 protein, while the uptake kinetic parameters K m and V max of OATP1B1*5-HEK293T on rosuvastatin were 10.05 ± 4.02 µM and 2.58 ± 0.30 pmol min−1 mg−1 protein. The transport difference of rosuvastatin in OATP1B1*1a-HEK293T cells and OATP1B1*5-HEK293T cells was showed in Fig. 9. This suggests that rosuvastatin may have a higher affinity to the OATP1B1*1a.
Fig. 9.

The uptake of rosuvastatin between OATP1B1*1a-HEK293T cells and OATP1B1*5-HEK293T cells (0 served as blank vector-HEK293T, 1a served as uptake of rosuvastatin in OATP1B1*1a-HEK293T cells, 5 served as uptake of rosuvastatin in OATP1B1*5-HEK293T cells. *Statistically different from OATP1B1*5)
Inhibition of OATP1B1-mediated rosuvastatin uptake by ursolic acid
Uptake experiments have been carried out as described with addition of different concentrations of the respective ursolic acid. Interestingly, we found that ursolic acid showed a clear dose dependent inhibition of OATP1B1-mediated rosuvastatin uptake into OATP1B1-HEK cells. Ursolic acid has been analyzed up to a concentration of 18 µM and when the concentration was 1.8 µM a significant decrease in rosuvastatin uptake was observed. When the concentration of ursolic acid was 1.8 and 18 µM, it showed that ursolic acid significantly inhibit the uptake of rosuvastatin in both OATP1B1*1a-HEK 293T cells and OATP1B1*5-HEK 293T cells (showed in Figs. 10, 11). The reducing of OATP1B1*1a transport on rosuvastatin were 34.60 ± 2.99 % and 66.08 ± 1.83 %, and for OATP1B1*5 were 34.27 ± 7.08 % and 66.95 ± 1.14 %. Inhibitory parameters of IC50 were 6.25 ± 0.42 and 6.07 ± 0.57 µM, respectively. All the date are showed in Tables 1 and 2.
Fig. 10.
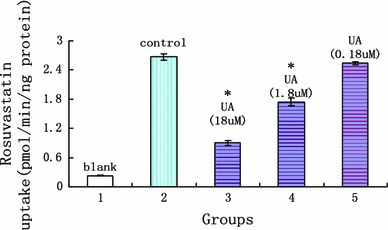
The effect of ursolic acid on rosuvastatin uptake in OATP1B1*1a-HEK 293T (x axis was the experimental groups, 1 blank vector, 2 rosuvastatin, 3 rosuvastatin + 18 µM UA, 4 rosuvastatin + 1.8 µM UA, 5 Rosuvastatin + 0.18 µM UA, y axis was the uptake of rosuvastatin in cells; *statistically different, P < 0.05, n = 5)
Fig. 11.
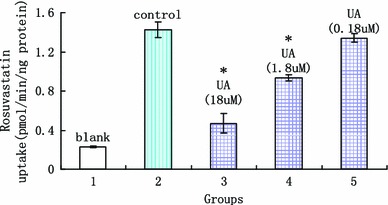
The effect of ursolic acid on rosuvastatin uptake in OATP1B1*5-HEK 293T (x axis was the experimental groups, 1 blank vector, 2 rosuvastatin, 3 rosuvastatin + 18 µM UA, 4 rosuvastatin + 1.8 µM UA, 5 rosuvastatin + 0.18 µM UA, y axis was the uptake of rosuvastatin in cells; *statistically different, P < 0.05, n = 5)
Table 1.
The effect of ursolic acid a on rosuvastatin transport by OATP1B1*1a (n = 5)
| Uptake of ROS | Groups | ||||
|---|---|---|---|---|---|
| Blank | Control | ROS + UA | |||
| 18 µM UA | 1.8 µM UA | 0.18 µM UA | |||
| (pmol/min/mg protein) | 0.23 | 2.67 | 0.90 | 1.74 | 2.54 |
| SD | 0.01 | 0.07 | 0.05 | 0.08 | 0.03 |
| IC50 (µM) | 6.25 ± 0.42 | ||||
ROS rosuvastatin, UA ursolic acid
Table 2.
The effect of ursolic acid on rosuvastatin transport by OATP1B1*5 (n = 5)
| Uptake of ROS | Groups | ||||
|---|---|---|---|---|---|
| Blank | Control | ROS + UA | |||
| 18 µM UA | 1.8 µM UA | 0.18 µM UA | |||
| (pmol/min/mg protein) | 0.23 | 1.426 | 0.471 | 0.937 | 1.342 |
| SD | 0.01 | 0.080 | 0.101 | 0.031 | 0.044 |
| IC50 (µM) | 6.07 ± 0.57 | ||||
ROS rosuvastatin, UA ursolic acid
Discussion
The liver is the target organ of HMG-CoA reductase inhibitors to reduce level of lipid, therefore liver-selective uptake of these drugs is a desirable property. A previous study has reported that ursolic acid was highly distributed in liver and has hypolipidemic effects on hepatocytes (Jia et al. 2011). In the present study, we investigated the uptake characteristics of rosuvastatin and inhibitory effect of ursolic acid on uptake of rosuvastatin using isolated rat hepatocytes. The uptake of rosuvastatin in isolated hepatocytes reached steady state after about 80 s. When concentration was 100 µM the uptake of rosuvastatin presented saturation. Inhibitory effect of ursolic acid on uptake of rosuvastain was found and K i was 10.88 ± 0.29 µM. However, the mechanism of inhibitory effect is not clear. As we already know, multiple drug transporters expressed in hepatocyte membrane may take part in the transport of drugs. At present, series studies have confirmed that multiple transporters such as OATPs/Oatps, NTCP and BCRP were involved in the hepatic uptake and efflux of rosuvastain (Satoshi et al. 2008). Among these transporters, OATP1B1/Oatp1b2 contributes predominantly to the hepatic uptake of rosuvastatin.
OATP1B1 is mainly expressed on the sinusoidal membrane of human hepatocytes, where it mediates the uptake of its substrates from blood into the liver. The homologous gene Oatp1b2 is expressed in rats (Wen and Xiong 2011). A number of drugs such as statins, endothelin receptor antagonists atrasentan and bosentan, the antibiotics benzylpenicillin and rifampicin, the antifungal agent caspofungin, the angiotensin converting enzyme inhibitors enalapril and temocapril, the angiotensin II receptor antagonists olmesartan and valsartan, etc., were all the substrates of OATP1B1 (Wen et al. 2012; Seithel et al. 2007; Kalliokoski and Niemi 2009; Nakanishi and Tamai 2012). Rosuvastatin was also the substrate of OATP1B1 (Wen et al. 2012).
A large number of SNPs and other sequence variations have been described in the gene region of SLCO1B1 (Tirona et al. 2001). Furthermore, the SNPs were different at different races. The c.521T>C (p.Val174Ala) SNP and c.388A>G (p.Asn130Asp) SNP were the common mutation in Asians, and incidence rate of these two SNPs were about 66 and 15.8 %, respectively (Abe et al. 1999).
The effect of OATP1B1 SNPs on the pharmacokinetics of drugs were found in more and more studies. Specially, the c.521T>C (p.Val174Ala) SNP has been associated with a markedly reduced transport activity in vitro using several OATP1B1 substrates, including estrone-3-sulfate, estradiol-17β-d-glucuronide, atorvastatin, cerivastatin, pravastatin, SN-38, the active metabolite of irinotecan, and rifampicin (Niemi 2007).
The pharmacokinetic exposure of rosuvastatin was higher in the OATP1B1*15/*15 subjects than the others, suggesting that OATP1B1 genetic polymorphisms can alter rosuvastatin pharmacokinetics in Korean populations (Choi et al. 2008). In one of our studies, we also found that there were significant differences between OATP1B1 mutation in the 521T>C and wild-type homozygote for rosuvastatin pharmacokinetic process in Chinese people. In contrast to OATP1B1 wild-type group, OATP1B1 mutation group’s absorption degree increased and elimination process decreased (Sui et al. 2011).
Although OATP1B1 521T>C has significant effect on the pharmacokinetic of rosuvastatin in vivo, the result has not been confirmed in vitro yet. As we know, other transporters such as BCRP, NTCP and MRP all are involved the transport of rosuvastatin in hepatocytes, and these transporters all have genetic polymorphism (Wen et al. 2012; Choi et al. 2011; Jemnitz et al. 2010).
Simultaneously, contradicting these findings, however, Satoshi et al. (2008) reported that SLCO1B1*5 did not alter the transporting activity of OATP1B1 for estrone-3-sulfate when it was expressed in HEK 293cells (Nozawa et al. 2002).
Therefore, controversy remains regarding the effects of this variant allele on the activity of OATP1B1. Whether the same result could be presented that OATP1B1 521T>C had significant effect on the transport of rosuvastatin in vivo and vitro?
At present, there have been no reports on the effect of SLCO1B1*5 on the transporting activity of rosuvastatin by using a cDNA expression system. In this paper, we successfully established OATP1B1*1a-GFP and OATP1B1*5-GFP fusion gene recombinant lentiviral vector, then infected the HEK-203T cells. The cell models of Lenti-OATP1B1*1a-HEK293T and Lenti-OATP1B1*5-HEK293T were successfully established. Both OATP1B1 *1a and OATP1B1*5 were expressed in HEK239T cells, respectively. Then we study the transport capacity of OATP1B1*1a and OATP1B1*5 on rosuvastatin. As a result, compared with OATP1B1*1a, mutant OATP1B1*5 significantly decreased the transport capacity. The uptake of rosuvastatin in OATP1B1*5-HEK293T cells was significantly less than OATP1B1*1a-HEK293T (P < 0.05). Gene mutation of OATP1B1 (521 T>C) may decrease its transport capacity. However, the difference in rosuvastatin transport between *1a and *5 cells may be simply a result of different amounts of transport-competent protein in the cellular membrane. Therefore, further study should be provided to explore the images of higher quality and analyze membrane localization of the OATP1B1 variants in these infected HEK cells by using their OATP1B1 antibody.
The c.388A>G and the c.521T>C variants occur in alleles of OATP1B1*15. It also seems that OATP1B1*15 show significantly reduced transport activity of some substrates in vitro (Kameyama et al. 2005). Whether OATP1B1*15 has significant effect on the transport of rosuvastatin in vitro needs further studies.
Ursolic acid, a ubiquitous pentacyclic triterpenoid compound, can be widely obtained from many plants such as hawthorn, Salvia miltiorrhiza, gardenia, Ligustrum lucidum, prunella, plantain, licorice, forsythia and Kuding tea, etc. (Wen and Xiong 2011). These herbal medicines are frequently combined with statins to treat the cardiovascular diseases.
For ursolic acid, the uptake process and whether transporters are involved in the hepatic uptake is not known. In a study, the result showed that ursolic acid was the most selective OATP1B3 inhibitor (IC50 = 2.3 μM vs. 12.5 μM for OATP1B1) (Gui et al. 2010). Therefore, ursolic acid may be the substrate and or inhibitor of OATPs/Oatps. In this paper, we confirmed that ursolic acid can significantly inhibit the transport of OATP1B1*1a and OATP1B1*5. The inhibitory parameters of IC50 were 6.25 ± 0.42 and 6.07 ± 0.57 µM, respectively. The result suggests that when the concentration of ursolic acid is about 2.85 µg/ml, half of the rosuvastatin transported by OATP1B1 may be inhibited in hepatocytes. A study showed that the C max of ursolic acid was about 3.40 ± 0.75 µg/ml after intravenous infusion at dose of 98 mg/m2 to volunteers (Xia et al. 2011). The study suggested that ursolic acid in human body could achieve the concentration that may significantly inhibit the transport of rosuvastatin in hepatocytes by OATP1B1. Therefore, one must be cautious when herbal medicines with ursolic acid as the main component are combined with drugs that are the substrates of OATP1B1 to treat diseases. They may cause serious drug–drug interaction when these drugs are combined together.
At present, as more and more Chinese herbal medicines were found as the substrates of transporters, drug–drug interactions may involve membrane transport by a change in the expression level of the transporters (Mizuna et al. 2003; Lin et al. 2012; Amanda et al. 2012). Whether ursolic acid affects the expression level of OATP1B1 or other transporters in hepatocytes?
Simultaneously, other transporters such as NTCP, MRP and BCRP are also involved in the transport of rosuvastatin in hepatocytes (Liu et al. 2011; Zhang et al. 2006). Although whether they play major or minor role in pharmacokinetics of rosuvastatin is not very clear, it is necessary to further explore whether ursolic acid could inhibit other transporters involved the hepatic uptake or efflux of rosuvastatin. Furthermore, rosuvastatin is slightly metabolized by cytochrome P450 (CYP)2C family (CYPs2C9 and 2C19) (Toth et al. 2011). Ursolic acid has been demonstrated to be an inhibitor of CYP2C19 in vitro (Kim et al. 2004). It is the likelihood of inhibition in vivo of CYP2C9/19 by ursolic acid which can contribute to the observed increase in rosuvastatin plasma concentrations? However, the study has showed that pravastatin, rosuvastatin and pitavastatin were not susceptible to any CYP inhibition (Neuvonen et al. 2010).
Conclusions
In a word, this study suggests that ursolic acid can inhibit the uptake of rosuvastatin in hepatocytes. Then we successfully established the HEK293T cell model that can stably express gene of OATP1B1*1a and OATP1B1*5. Using the model, we studied the transport character of rosuvastatin and explored whether ursolic acid inhibits the transport of rosuvastatin. The result showed that transport of rosuvastatin by OATP1B1 was related to genotypes. Compared with OATP1B1*1a, mutant OATP1B1*5 significantly decreased the transport capacity. When the concentrations of ursolic acid were 1.8 and 18 µM, they both significantly inhibited the uptake of rosuvastatin in OATP1B1*1a-HEK 293T cells and OATP1B1*5-HEK 293T cells. Inhibitory parameters of IC50 were 6.25 ± 0.42 and 6.07 ± 0.57 µM, respectively.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81202583), Education Department Fund of Jiangxi Province (GJJ12145) and Research Fund Project of Traditional Chinese Medicine of the Health Department of Jiangxi Province (2012A137).
References
- Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, et al. Identification of a novel gene family encoding human liver specific organic anion transporter LST21 [J] J Biol Chem. 1999;274(24):17159–17163. doi: 10.1074/jbc.274.24.17159. [DOI] [PubMed] [Google Scholar]
- Amanda O, Megan R, Bruno H. The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu Rev Pharmacol Toxicol. 2012;52:135–151. doi: 10.1146/annurev-pharmtox-010510-100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Choi JH, Lee MG, Cho JY, Lee JE, Kim KH, Park K. Influence of OATP1B1 genotype on the pharmacokinetics of rosuvastatin in Koreans. Clin Pharmacol Ther. 2008;83(2):251–257. doi: 10.1038/sj.clpt.6100267. [DOI] [PubMed] [Google Scholar]
- Choi MK, Shin HJ, Choi YL, Deng JW, Shin JG, Song IS. Differential effect of genetic variants of Na(+)-taurocholate co-transporting polypeptide (NTCP) and organic anion-transporting polypeptide 1B1 (OATP1B1) on the uptake of HMG-CoA reductase inhibitors. Xenobiotica. 2011;41(1):24–34. doi: 10.3109/00498254.2010.523736. [DOI] [PubMed] [Google Scholar]
- Funk C. The role of hepatic transporters in drug elimination. Expert Opin. Drug Metab Toxicol. 2008;4(4):363–379. doi: 10.1517/17425255.4.4.363. [DOI] [PubMed] [Google Scholar]
- Gui C, Obaidat A, Chaguturu R, Hagenbuch B. Development of a cell-based high-throughput assay to screen for inhibitors of organic anion transporting polypeptides 1B1 and 1B3. Curr Chem Genomics. 2010;4:1–8. doi: 10.2174/1875397301004010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemnitz K, Veres Z, Tugyi R, Vereczkey L. Biliary efflux transporters involved in the clearance of rosuvastatin in sandwich culture of primary rat hepatocytes. Toxicol In Vitro. 2010;24(2):605–610. doi: 10.1016/j.tiv.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Jia Y, Bhuiyan MJH, Jun HJ, Lee JH, Hoang MH, Lee HJ, et al. Ursolic acid is a PPAR-a agonist that regulates hepatic lipid metabolism. Bioorg Med Chem Lett. 2011;21(19):5876–5880. doi: 10.1016/j.bmcl.2011.07.095. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15 + C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics. 2005;7:513–522. doi: 10.1097/01.fpc.0000170913.73780.5f. [DOI] [PubMed] [Google Scholar]
- Kim KA, Lee JS, Park HJ, Kim JW, Kim CJ, Shim IS, et al. Inhibition of cytochrome P450 activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci. 2004;74(22):2769–2779. doi: 10.1016/j.lfs.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Lin CC, Fan HY, Kuo CW, Pao LH. Evaluation of Chinese-herbal-medicine-induced herb–drug interactions: focusing on organic anion transporter 1. Evid Based Complement Altern Med. 2012;2012:967182. doi: 10.1155/2012/967182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CX, Yi XL, Si DY, Xiao XF, He X, Li YZ. Herb-drug interactions involving drug metabolizing enzymes and transporters. Curr Drug Metab. 2011;12:835–849. doi: 10.2174/138920011797470083. [DOI] [PubMed] [Google Scholar]
- Mizuna N, Niwa T, Yotsumoto Y, Sugiyama Y. Impact of drug transporter studies on drug discovery and development. Pharmacol Rev. 2003;55:425–461. doi: 10.1124/pr.55.3.1. [DOI] [PubMed] [Google Scholar]
- Muck W, Mai I, Fritsche L, Ochmann K, Rohde G, Unger S, et al. Increase in cerivastatin systemic exposure after single and multiple dosing in cyclosporine-treated kidney transplant recipients. Clin Pharmacol Ther. 1999;65:251–261. doi: 10.1016/S0009-9236(99)70104-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Tamai I. Genetic polymorphisms of OATP transporters and their impact on intestinal absorption and hepatic disposition of drugs. Drug Metab Pharmacokinet. 2012;27(1):106–121. doi: 10.2133/dmpk.DMPK-11-RV-099. [DOI] [PubMed] [Google Scholar]
- Neuvonen PJ. Drug interactions with HMG-CoA reductase inhibitors (statins): the importance of CYP enzymes, transporters and pharmacogenetics. Curr Opin Investig Drugs. 2010;11(3):323–332. [PubMed] [Google Scholar]
- Niemi M. Role of OATP transporters in the disposition of drugs. Pharmacogenomics. 2007;8(7):787–802. doi: 10.2217/14622416.8.7.787. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Nakajima M, Tamai I, Noda K, Nezu J, Sai Y, et al. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther. 2002;302:804–813. doi: 10.1124/jpet.302.2.804. [DOI] [PubMed] [Google Scholar]
- Satoshi K, Kazuya M, Yi W, Yuichi S. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Metab Dispos. 2008;36:2014–2023. doi: 10.1124/dmd.108.021410. [DOI] [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat live cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/S0091-679X(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Seithel A, Eberl S, Singer K, Auge D, Heinkele G, Wolf NB, Dörje F, Fromm MF, König J. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab Drug Dispos. 2007;35:779–786. doi: 10.1124/dmd.106.014407. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Itoh T, Sato H, Li AP, Sugiyama Y. Inhibition of transporter mediated hepatic uptake as a mechanism for drug–drug interaction between cerivastatin and cyclosporin A. J Pharmacol Exp Ther. 2003;304:610–616. doi: 10.1124/jpet.102.041921. [DOI] [PubMed] [Google Scholar]
- Sui SM, Wen JH, Li XH, Xiong YQ. Effect of OATP1B1 521T>C heterogenesis on pharmacokinetic characteristics of rosuvastatin in Chinese volunteers. Yao Xue Xue Bao. 2011;46(6):695–700. [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem. 2001;276:35669–35675. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- Toth PP, Dayspring TD. Drug safety evaluation of rosuvastatin[J] Expert Opin Drug Saf. 2011;10(6):969–986. doi: 10.1517/14740338.2012.626764. [DOI] [PubMed] [Google Scholar]
- Wen JH, Xiong YQ. The effect of herbal medicine danshensu and ursolic acid on pharmacokinetics of rosuvastatin in rats. Eur J Drug Metab Pharmacokinet. 2011;36:205–211. doi: 10.1007/s13318-011-0048-7. [DOI] [PubMed] [Google Scholar]
- Wen JH, Wei XH, Hu JF. The role of OATP1B1 and BCRP in pharmacokinetics and DDI of novel statins. Cardiovasc Ther. 2012;30:e234–e241. doi: 10.1111/j.1755-5922.2011.00290.x. [DOI] [PubMed] [Google Scholar]
- Xia YY, Wei GL, Si DY, Liu CG. Quantitation of ursolic acid in human plasma by ultra performance liquid chromatography tandem mass spectrometry and its pharmacokinetic study. J Chromatogr B. 2011;879:219–224. doi: 10.1016/j.jchromb.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yu BN, He YJ, Fan L, Li Q, Liu ZQ. Role of BCRP 421C>A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males[J] Clin Chim Acta. 2006;373:99–103. doi: 10.1016/j.cca.2006.05.010. [DOI] [PubMed] [Google Scholar]


