Abstract
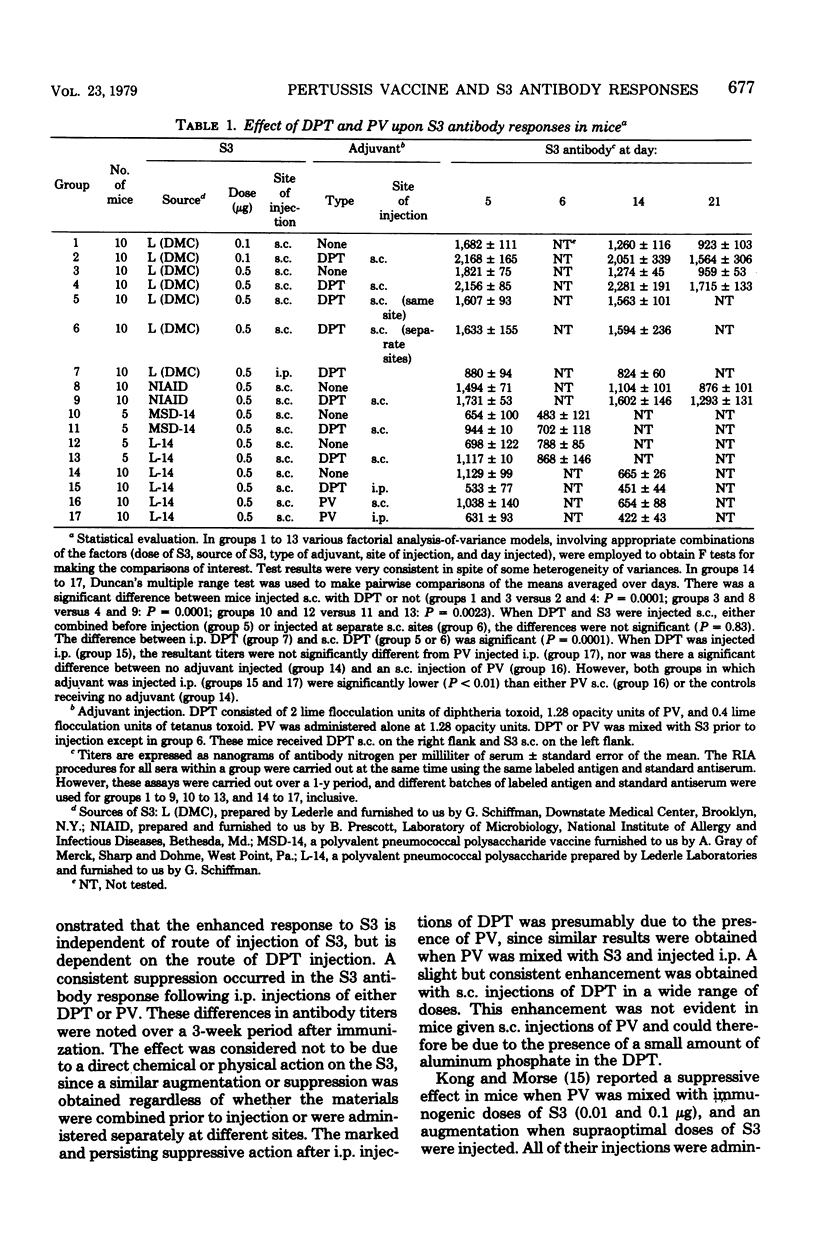
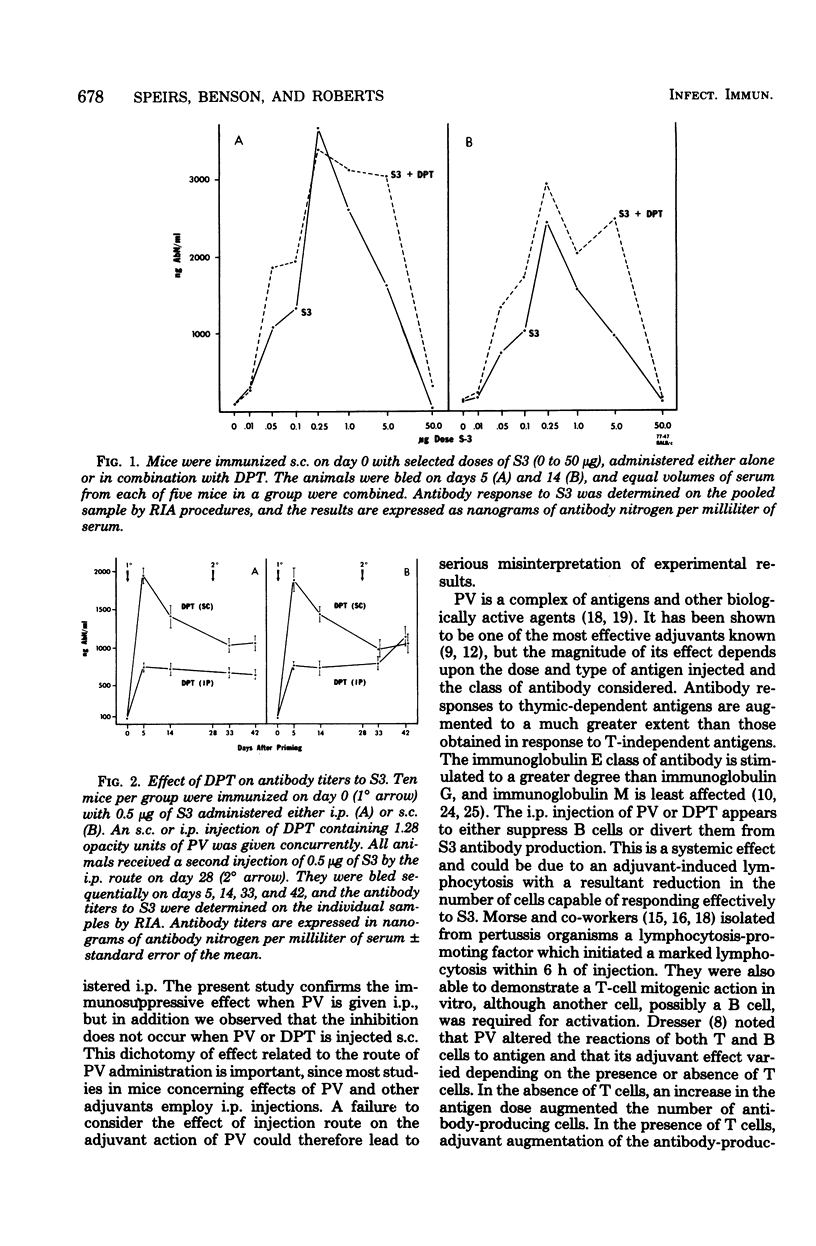
Pertussis vaccine (PV) or diphtheria toxoid-PV-tetanus toxoid (DPT) altered the antibody response of BALB/c female mice to type III pneumococcal polysaccharide antigen (S3). The key factor affecting the magnitude of the response to S3 was the route of injection of PV or DPT, whereas the route of injection of S3 was not crucial. Subcutaneous injections of DPT augmented the antibody response to low, optimal, and tolerogenic (high) doses of S3 injected either subcutaneously or intraperitoneally. This enhancement was persistent and was observed both when S3 and DPT were mixed and injected subcutaneously and when S3 and DPT were injected concurrently at separate subcutaneous sites. When either PV or DPT was injected intraperitoneally, the antibody response to subcutaneously or intraperitoneally injected S3 was significantly decreased. These experiments demonstrate a dichotomy of effect dependent on the route of administration of PV. Most studies in mice utilizing PV employ the intraperitoneal injection route, and it is important to consider whether PV treatment by this route may have unique effects. Our data suggest that intraperitoneal injection of PV suppresses B cells, possibly by influencing regulatory cell function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Athanassiades T. J. Adjuvant effect of Bordetella pertussis vaccine to sheep erythrocytes in mice: enhancement of cell-mediated immunity by subcutaneous administration of adjuvant and antigen. Infect Immun. 1977 Nov;18(2):416–423. doi: 10.1128/iai.18.2.416-423.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Barth R. F., Stashak P. W., Amsbaugh D. F. Enhancement of the antibody response to type 3 pneumococcal polysaccharide in mice treated with antilymphocyte serum. J Immunol. 1970 May;104(5):1313–1315. [PubMed] [Google Scholar]
- Baker P. J. Homeostatic control of antibody responses: a model based on the recognition of cell-associated antibody by regulatory T cells. Transplant Rev. 1975;26:3–20. doi: 10.1111/j.1600-065x.1975.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Landy M. Brevity of the inductive phase in the immune response of mice to capsular polysaccharide antigens. J Immunol. 1967 Oct;99(4):687–694. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. II. Mode of action of thymic-derived suppressor cells. J Immunol. 1974 Jan;112(1):404–409. [PubMed] [Google Scholar]
- Barth R. F., Singla O., Liu C. Suppressor T cells and host resistance to tye 111 pneumococcus after treatment with antilymphocyte serum. Infect Immun. 1975 Dec;12(6):1307–1312. doi: 10.1128/iai.12.6.1307-1312.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Johnson P. T cell-dependent suppression of an anti-hapten antibody response. Transplant Rev. 1975;26:130–169. doi: 10.1111/j.1600-065x.1975.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Dresser D. W. The role of T cells and adjuvant in the immune response of mice to foreign erythrocytes. Eur J Immunol. 1972 Feb;2(1):50–57. doi: 10.1002/eji.1830020111. [DOI] [PubMed] [Google Scholar]
- Finger H., Emmerling P., Plager L. Time relationships between injection of antigen and adjuvant. 3. Adjuvancy of Bordetella pertussis given at various times after the primary antigenic stimulus. Infect Immun. 1972 May;5(5):783–791. doi: 10.1128/iai.5.5.783-791.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M., Kariyone A. Incidental appearance of suppressor T cells in the induction of immunological tolerance. Immunology. 1978 Jan;34(1):51–56. [PMC free article] [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Stashak P. W., Prescott B., Baker P. J., Alling D. W. Kinetics of the antibody response to type III pneumococcal polysaccharide. I. Evidence that suppressor cells function by inhibiting the recruitment and proliferation of antibody-producing cells. J Immunol. 1976 Mar;116(3):647–656. [PubMed] [Google Scholar]
- Kong A. S., Morse S. I. The effect of Bordetella pertussis on the antibody response in mice to type III pneumococcal polysaccharide. J Immunol. 1976 Apr;116(4):989–993. [PubMed] [Google Scholar]
- Kong A. S., Morse S. I. The in vitro effects of Bordetella pertussis lymphocytosis-promoting factor on murine lymphocytes. I. Proliferative response. J Exp Med. 1977 Jan 1;145(1):151–162. doi: 10.1084/jem.145.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A. S., Morse S. I. The in vitro effects of Bordetella pertussis lymphocytosis-promoting factor on murine lymphocytes: II. Nature of the responding cells. J Exp Med. 1977 Jan 1;145(1):163–174. doi: 10.1084/jem.145.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNOZ J. J. SYMPOSIUM ON RELATIONSHIP OF STRUCTURE OF MICROORGANISMS TO THEIR IMMUNOLOGICAL PROPERTIES. I. IMMUNOLOGICAL AND OTHER BIOLOGICAL ACTIVITIES OF BORDETELLA PERTUSSIS ANTIGENS. Bacteriol Rev. 1963 Dec;27:325–340. doi: 10.1128/br.27.4.325-340.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. I., Bray K. K. The occurrence and properties of leukocytosis and lymphocytosis-stimulating material in the supernatant fluids of Bordetella pertussis cultures. J Exp Med. 1969 Mar 1;129(3):523–550. doi: 10.1084/jem.129.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S. D., Athanassiades T. J., Speirs R. S. A quantitative procedure for the study of cells in experimentally induced granulomas. Proc Soc Exp Biol Med. 1972 Apr;139(4):1090–1095. doi: 10.3181/00379727-139-36305. [DOI] [PubMed] [Google Scholar]
- Speirs R. S., Benson R. W., Knowles B. J., Roberts D. Models for assessing the effect of toxicants on immunocompetence in mice. II. Effect of cyclophosphamide on the antibody responses to type III pneumococcal polysaccharide and tetanus toxoid in BALB/c female mice. J Environ Pathol Toxicol. 1978 Jul-Aug;1(6):791–812. [PubMed] [Google Scholar]
- Speirs R., Benson R. W., Schiffman G. Models for assessing the effect of toxicants on immunocompetence in mice. Part I: The effect of diphtheria, pertussis, and tetanus vaccine on antibody response to type III pneumococcal polysaccharide. J Environ Pathol Toxicol. 1978 May-Jun;1(5):689–699. [PubMed] [Google Scholar]
- Suko M., Ogita T., Okudaira H., Horiuchi Y. Preferential enhancement of IgE antibody formation by Bordetella pertussis. Int Arch Allergy Appl Immunol. 1977;54(4):329–337. doi: 10.1159/000231845. [DOI] [PubMed] [Google Scholar]
- Torrigiani G. Quantitative estimation of antibodies in the immunoglobulin classes of the mouse. I. Effect of adjuvants on the antibody response to human serum albumin and keyhole limpet haemocyanin. Clin Exp Immunol. 1972 May;11(1):125–135. [PMC free article] [PubMed] [Google Scholar]
- Turner M. X., Speirs R. S., McLaughlin J. A. Effect of primary injection site upon cellular and antitoxin responses to subsequent challenging injection. Proc Soc Exp Biol Med. 1968 Dec;129(3):738–743. doi: 10.3181/00379727-129-33413. [DOI] [PubMed] [Google Scholar]



