Abstract
Background
There has been a trend toward limiting perioperative prophylactic antibiotics, based on research not conducted in plastic surgery patients. The authors’ university hospital instituted antibiotic prescribing guidelines based on the Surgical Care Improvement Project. An increased rate of surgical-site infections was noted in breast reconstruction patients. The authors sought to determine whether the change in antibiotic prophylaxis regimen affected rates of surgical-site infections.
Methods
A retrospective study compared patients undergoing breast reconstruction who received preoperative and postoperative prophylactic antibiotics with a group who received only a single dose of preoperative antibiotic. Type of reconstruction and known risk factors for implant infection were noted.
Results
Two hundred fifty patients were included: 116 in the pre–Surgical Care Improvement Project group and 134 in the Surgical Care Improvement Project group. The overall rate of surgical-site infections increased from 18.1 percent to 34.3 percent (p = 0.004). Infections requiring reoperation increased from 4.3 percent to 16.4 percent (p = 0.002). Multivariate logistic regression demonstrated that patients in the Surgical Care Improvement group were 4.74 times more likely to develop a surgical-site infection requiring reoperation (95 percent CI, 1.69 to 13.80). Obesity, history of radiation therapy, and reconstruction with tissue expanders were associated with increased rates of surgical-site infection requiring reoperation.
Conclusions
Withholding postoperative prophylactic antibiotics in prosthetic breast reconstruction is associated with an increased risk of surgical-site infection, reoperation, and thus reconstructive failure. The optimal duration of postoperative prophylactic antibiotic use is the subject of future study.
Surgical-site infection is a potential complication for all surgical procedures and is one of the leading causes of morbidity for postmastectomy breast reconstruction.1–3 Surgical-site infections have been reported in approximately 2 percent of patients undergoing postmastectomy implant breast reconstruction.2 Although this rate has varied widely in a number of different studies,4 it likely represents infections that required implant removal. Infections can range from mild cellulitis requiring oral antibiotics to more severe cellulitis resolving with inpatient intravenous antibiotics to frank abscess formation requiring implant removal, and thus the actual rates of infection, if defined more broadly, may be much higher.
Most plastic surgeons prescribe preoperative prophylactic antibiotics for patients undergoing breast reconstruction and continue perioperative prophylactic antibiotics administered intravenously during hospitalization and orally on discharge until a set time (e.g., 7 days) or until drains are removed.5 This practice has been prone to criticism by infection control officers and others who quote data from studies6–11 suggesting that, despite the increasing use of prophylactic antibiotics, there has been no corresponding decrease in rates of infection postoperatively. However, Hawn and colleagues12 recently examined the efficacy of improved adherence to the Surgical Care Improvement Project guidelines and noted stable rates of surgical-site infection at the patient and hospital levels despite increased use of preoperative prophylactic antibiotics and decreased use of postoperative prophylactic antibiotics. These studies would suggest that prolonged (>24 hours) postoperative prophylactic antibiotics are not indicated for routine clean surgical procedures.
At our university-affiliated tertiary care center, guidelines governing the use of perioperative antibiotic prophylaxis were implemented in October of 2008 based on pay-for-performance incentives and the Surgical Care Improvement Project. Our Division made a decision to adopt evidence-based perioperative antibiotic prophylaxis guidelines and in January of 2009 began prescribing a single pre-operative dose for all patients undergoing breast reconstruction. Patients undergoing lengthy operations (e.g., perforator flap) were given additional intraoperative doses as indicated. Although the Surgical Care Improvement Project protocol permitted up to 24 hours of postoperative antibiotics, our group made a decision not to give any postoperative prophylactic antibiotics in an effort to engage in strictly evidence-based medicine.
In the ensuing year, an increase in the rate of postoperative surgical-site infection was noted in patients undergoing breast reconstruction, particularly those undergoing prosthetic reconstruction. This prompted a review of our experience before and after the Surgical Care Improvement Project protocol was instituted to determine whether or not the change in perioperative antibiotic prophylaxis regimen was associated with an increased risk of surgical-site infection. We hypothesized that patients receiving postoperative prophylactic antibiotics would have decreased postoperative infection rates compared with patients who received only perioperative antibiotics according to the Surgical Care Improvement Project protocol.
PATIENTS AND METHODS
This institutional review board–approved, retrospective cohort study was conducted at a tertiary academic medical center between October of 2007 and January of 2010. Patients who underwent post-mastectomy breast reconstruction before implementation of the Surgical Care Improvement Project protocol between October of 2007 and October of 2008 received both preoperative and postoperative antibiotics (until drains were removed). This group was compared with similar patients who underwent postmastectomy implant or autologous reconstructions performed between January of 2009 and January of 2010. These patients received only preoperative and possibly intraoperative antibiotics in accordance with the Surgical Care Improvement Project guidelines. We report the rates of surgical-site infection in both groups using a much broader definition of infection than has been reported in the plastic surgery literature, which has tended to report only the rate of infections requiring implant removal. We categorized surgical-site infection according to the way it was treated: infections requiring oral antibiotics only, those treated with intravenous antibiotics, and those requiring reoperation.
Preoperative variables included demographic factors known to be associated with surgical-site infection, including age, body mass index, current smoking status, radiation history, chemotherapy history, tumor stage, and diabetic status. Body mass index was also categorized (≤30 versus >30). Intra-operative variables included technical aspects of the procedure such as single or two-stage implant reconstruction and type of autologous reconstruction. Women undergoing single-stage reconstruction were excluded from all analyses. Women who received tissue expanders or permanent implants were combined for all analyses. Patients who underwent reconstruction using both an autologous flap and an implant were included in the implant group. Postoperative infection rates are reported. Povidone-iodine was the most commonly used preparation solution; however, chlorhexidine was also occasionally used. Antibiotic irrigation was used at the discretion of the attending surgeon and was not reported consistently. Non–penicillin-allergic patients all received cefazolin preoperatively. Penicillin-allergic patients received either clindamycin or vancomycin; however, this information was not specifically captured in our database. After the implementation of the Surgical Care Improvement Project guidelines, we began capturing the distribution profile of organisms isolated from women who experienced postoperative infection. Bacterial isolates for women requiring removal of the implant are reported.
The majority of patients undergoing breast reconstruction had mastectomy for curative resection. Other patients underwent prophylactic mastectomy after positive genetic testing for the BRCAI and BRCAII genes or because of a strong familial history of breast cancer. In subset analyses, we also examined the use of AlloDerm (LifeCell Corp., Branchburg, N.J.) and axillary lymph node dissection, either before or concurrent with the mastectomy.
Sample Size Estimation
Although the published rate of infection after implant reconstruction is approximately 2 percent, this rate likely reflects only cases requiring removal of the implant. In a pilot study at our institution, patients undergoing implant reconstruction experienced an increase in the baseline infection rate from 5.4 percent to 18.2 percent with the implementation of the Surgical Care Improvement Project protocol. This study was adequately powered to detect a 20 percent difference in the rate of infection between the two groups studied. A type I error probability of 5 percent (alpha) and a type II error probability of 10 percent (beta) were used for this calculation. We estimated an event rate (proportion) of 10 percent in the group treated with preoperative antibiotics only. The required sample size in each of the two study groups was 47 patients, or a total sample size of 94 patients.
Statistical Analyses
Descriptive statistics, including frequencies for the independent variables and unadjusted rates of infection, are reported. Pearson’s chi-square and Fisher’s exact tests were used for categorical variables and t tests were used for continuous independent variables. Multivariate logistic regression using a backward, conditional modeling technique was used to investigate predictors of surgical-site infection requiring reoperation. Values of p < 0.05 were considered statistically significant. SPSS version 19 (SPSS, Inc., Chicago, Ill.) was used for all analyses.
RESULTS
A total of 250 women were included for analyses (116 in the pre–Surgical Care Improvement Project group and 134 in the Surgical Care Improvement Project group). There were no statistically significant differences in the mean age of patients, diabetic status, current smoking status, radiation history, chemotherapy history, or tumor stage between the two groups (Table 1). The women in the Surgical Care Improvement Project group had a slightly higher body mass index (27.2 versus 28.8; p = 0.052). The overall rate of surgical-site infection (treated with any modality) increased from 18.1 percent in the pre–Surgical Care Improvement Project group to 34.3 percent after the adoption of the Surgical Care Improvement Project treatment protocol (p = 0.004) (Table 2). No statistically significant differences were noted in the rates of surgical-site infection successfully treated with oral or intravenous antibiotics alone, but the rate of infection requiring reoperation increased from 4.3 percent to 16.4 percent (p = 0.002). Regarding the different types of reconstruction, the number of tissue expander surgical-site infections increased from 18.5 percent to 34.3 percent in the pre–Surgical Care Improvement Project group versus the Surgical Care Improvement Project group (p = 0.013). The number of tissue expanders requiring removal increased from 5.4 percent to 18.2 percent (p = 0.007). Among women undergoing autologous reconstruction, we did note an overall increase in surgical-site infection rates from 16.7 percent in the pre–Surgical Care Improvement Project to 34.3 percent in the Surgical Care Improvement Project group; however, this difference was not statistically significant (p = 0.135). We also noted an increase in the number of autologous flap reconstruction surgical-site infections requiring reoperation (0 percent versus 11.4 percent); however, this difference was also not statistically significant (p = 0.115).
Table 1.
Patient Demographic and Clinical Characteristics
| Pre-SCIP (%) | SCIP (%) | p | |
|---|---|---|---|
| No. of patients | 116 | 134 | |
| Mean age, yr | 48.5 ± 10.1 | 49.5 ± 11.5 | 0.502 |
| Mean BMI | 27.2 ± 5.7 | 28.8 ± 7.2 | 0.052 |
| Diabetic | 4 (3.4) | 7 (5.2) | 0.495 |
| Currently smoking status | 17 (14.7) | 14 (10.4) | 0.314 |
| Radiation therapy | 32 (27.6) | 46 (34.3) | 0.251 |
| Neoadjuvant chemotherapy | 37 (31.9) | 54 (40.3) | 0.189 |
| Concurrent chemotherapy | 9 (7.7) | 14 (10.4) | 0.516 |
| Adjuvant chemotherapy | 3 (2.6) | 5 (3.7) | 0.728 |
| Average tumor stage | 1 | 1 | 0.953 |
SCIP, Surgical Care Improvement Project; BMI, body mass index.
Table 2.
Surgical Site Infection, by Type of Reconstruction
| Pre-SCIP (%) | SCIP (%) | p* | |
|---|---|---|---|
| No. of patients | 116 | 134 | |
| Total patients with SSI | 21 (18.1) | 46 (34.3) | 0.004 |
| SSI treated with oral antibiotics | 10 (8.6) | 15 (11.2) | 0.499 |
| SSI treated with IV antibiotics | 6 (5.2) | 9 (6.7) | 0.608 |
| SSI requiring reoperation | 5 (4.3) | 22 (16.4) | 0.002 |
| Reconstruction with tissue expander | 92 (79.3) | 99 (73.9) | 0.313 |
| Infected tissue expander | 17 (18.5) | 34 (34.3) | 0.013 |
| Infected tissue expander requiring reoperation | 5 (5.4) | 18 (18.2) | 0.007 |
| Reconstruction with autologous flap | 24 (20.7) | 35 (26.1) | 0.313 |
| Infected autologous flap | 4 (16.7) | 12 (34.3) | 0.135 |
| Infected autologous flap requiring reoperation | 0 (0) | 4 (11.4) | 0.115 |
SCIP, Surgical Care Improvement Project; SSI, surgical-site infection; IV, intravenous.
Italicized values represent significant findings.
In multivariate analyses, after adjusting for history of radiation therapy, body mass index (categorized as ≤30 versus >30), treatment group (pre–Surgical Care Improvement Project versus Surgical Care Improvement Project), and type of reconstruction performed (autologous versus tissue expander/implant), patients in the Surgical Care Improvement Project group were 4.74 times (95 percent confidence interval, 1.69 to 13.80) more likely to develop a surgical-site infection requiring reoperation than patients treated in the pre–Surgical Care Improvement Project group. Furthermore, patients with a history of radiation therapy were 4.50 times (95 percent confidence interval, 1.80 to 11.29) more likely to develop a surgical-site infection requiring reoperation than patients not treated with radiation. Obese women (body mass index >30) were 4.99 times (95 percent confidence interval, 2.03 to 12.31) more likely to develop a surgical-site infection requiring reoperation, and women who underwent reconstruction with tissue expanders/implants were 3.77 times (95 percent confidence interval, 1.11 to 12.83) more likely to develop a surgical-site infection requiring reoperation and removal of the tissue expander/implant after adjusting for the above covariates (Table 3).
Table 3.
Multivariate Logistic Regression Results Predicting Surgical-Site Infection Requiring Reoperation*
| Adjusted Odds Ratio (95% CI) | p† | |
|---|---|---|
| Pre-SCIP group | 1.00 | |
| SCIP group | 4.74 (1.69–13.80) | 0.004 |
| Radiation therapy | ||
| No | 1.00 | |
| Yes | 4.50 (1.80–11.29) | 0.001 |
| Body mass index | ||
| ≤30 | 1.00 | |
| >30 | 4.99 (2.03–12.31) | <0.001 |
| Tissue expander/implant | ||
| No | 1.00 | |
| Yes | 3.77 (1.11–12.83) | 0.033 |
CI, confidence interval; SCIP, Surgical Care Improvement Project.
N = 250 patients.
All p values represent significant findings.
In subset analyses of the women who developed a surgical-site infection requiring reoperation (n = 27), history of axillary lymph node dissection and use of AlloDerm were not associated with a surgical-site infection requiring reoperation (p = 0.334 and p = 0.819, respectively). Use of AlloDerm was less common (43.8 percent) in the Surgical Care Improvement Project group versus the pre–Surgical Care Improvement Project group (56.3 percent); however, the difference was not statistically significant (p = 0.128). We also examined the time interval between initial surgery and the development of a surgical-site infection requiring implant removal, and compared results between the two treatment groups. We found that women in the pre–Surgical Care Improvement Project group developed a surgical-site infection a mean of 256 ± 182 days after surgery compared with women in the Surgical Care Improvement Project group, who developed a surgical-site infection a mean of 90 ± 93 days (p = 0.011) after surgery. Regarding early infections requiring explantation (≤30 days), fewer women in the pre–Surgical Care Improvement Project group (20 percent) developed such an infection compared with women in the Surgical Care Improvement Project group (35 percent) (p = 0.477). Bacterial isolates before and after implementation of the Surgical Care Improvement Project guidelines are reported in Figures 1 and 2. After implementation of the Surgical Care Improvement Project protocol, the bacterial isolates became more diverse, with a much higher incidence of Gram-negative bacteria (Fig. 2).
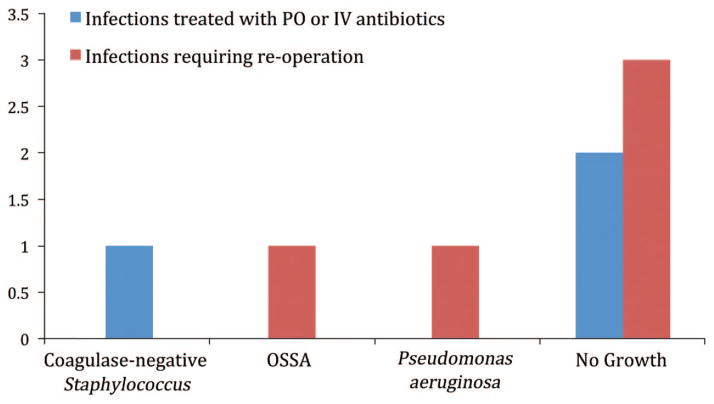
Fig. 1.
Bacteria isolated before implementation of the Surgical Care Improvement Project protocol. PO, oral; IV, intravenous; OSSA, oxacillin-sensitive Staphylococcus aureus.
Fig. 2.
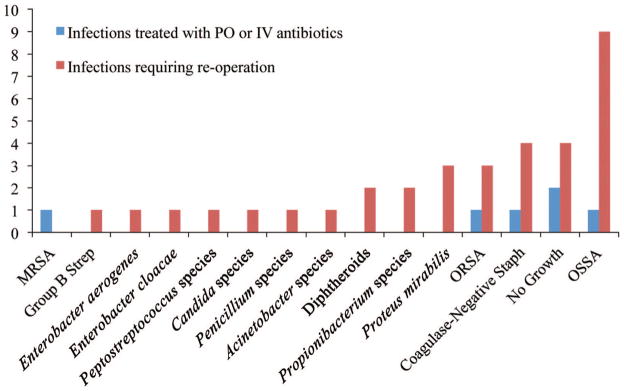
Bacteria isolated after implementation of the Surgical Care Improvement Project protocol. PO, oral; IV, intravenous; MRSA, methicillin-resistant Staphylococcus aureus; ORSA, oxacillin-resistant Staphylococcus aureus; OSSA, oxacillin-sensitive Staphylococcus aureus.
DISCUSSION
Women undergo mastectomies for both prophylactic and therapeutic reasons. Whether performed to treat breast cancer or as a preventative measure for women with a genetic predisposition to cancer or strong familial indicators, breast reconstruction following a mastectomy can be performed by placement of an implant or by means of autologous techniques. Surgical-site infection following breast reconstruction can necessitate oral or intravenous antibiotic therapy, lengthen the duration of the hospital stay, or lead to loss of the implant or flap. An evidence-based universal protocol governing the use of prophylactic antibiotics to prevent postoperative surgical-site infections following breast reconstruction does not currently exist.1,2
The current standard of care is that a preoperative dose of prophylactic antibiotic should be given to patients undergoing postmastectomy breast reconstruction.4,13 In an advisory statement from the National Surgical Infection Prevention Project, which is based on published evidence, infusion of the first antimicrobial dose should begin no sooner than 60 minutes before the incision.12 Based on published evidence, the same workgroup endorsed the national performance measure (Surgical Care Improvement Project guidelines) that prophylactic antimicrobial agents should be discontinued within 24 hours of the end of surgery.12 However, specific recommendations for plastic surgery were not included. Many surgeons prescribe postoperative antibiotics for up to 1 week following postmastectomy breast reconstruction, whereas others routinely continue postoperative antibiotics until all drains are removed, which can be as long as 2 weeks.5
According to the published Guideline for Prevention of Surgical-Site Infection,14 three categories of variables have proven to be reliable predictors of surgical-site infection risk: (1) those that estimate the intrinsic degree of microbial contamination of the surgical site, (2) those that measure the duration of an operation, and (3) those that serve as markers for host susceptibility. Patient-related factors possibly associated with an increased risk of surgical-site infection include remote site infection or colonization, diabetes, cigarette smoking, obesity, extremes of age, and poor nutritional and immunocompromised status.15–22 Many of these characteristics are present in the plastic surgery patient population, including overweight and obese patients, current smoking status and, to a lesser extent, diabetes and immunosuppression.
There have been a number of excellent studies from the general surgery and surgical oncology literature examining surgical-site infection rates among women undergoing surgery for breast cancer. A review of this literature by Penel et al.23 and others have documented surgical-site infection rates ranging from 1.9 to 50 percent.24–37 In the prospective study by Penel et al. comparing surgical-site infection rates before and after the implementation of prophylactic antibiotics, the authors conclude that the antibiotic prophylaxis reduced the risk of surgical-site infection in breast cancer surgery by 81 percent.
A recent systematic review of the literature examining preoperative and perioperative prophylactic antibiotic use in breast surgery included seven articles with a total of 1924 participants in a meta-analysis.38 No eligible studies evaluating prophylactic antibiotics used during reconstructive surgery (with or without implants) were identified in this study. From this review, pooling of the results demonstrated that prophylactic antibiotics significantly reduce the incidence of surgical-site infection for patients undergoing breast cancer surgery without reconstruction (pooled relative risk, 0.66; 95 percent confidence interval, 0.48 to 0.89). No studies presented separate data for patients who underwent reconstructive surgery at the time of removal of the breast tumor.
Other studies have examined the overall incidence of periprosthetic infection following tissue expander insertion for breast cancer reconstruction. In a review by Francis et al.,39 the authors noted infection rates ranging from 2.5 to 24 percent.40–45 Other authors have reported infection rates after expander-based reconstructions ranging between 1 and 24 percent.41,44,46,47 However, no consensus regarding the use of postoperative antibiotics after implant reconstruction exists.
There have been only a small number of randomized clinical trials examining antibiotic prophylaxis following breast reconstruction. In the only prospective, double-blinded, placebo-controlled trial of a single dose of azithromycin on postoperative wound infections in plastic surgery patients conducted by Amland et al.,48 the authors reported significantly fewer wound infections (5 percent versus 20 percent) in patients undergoing breast reconstruction who received prophylactic antibiotics. There was a significant reduction in postoperative complications with the additional use of antibiotics postoperatively in the prophylaxis group.48
Recent findings published by the National Surgical Infection Prevention Project suggest that the administration of prophylactic antibiotics should be discontinued within 24 hours of the completion of surgery.2 These recommendations, however, are based on studies outside of the practice of plastic surgery and are not based on studies conducted with women undergoing implant-based reconstructions. Reconstructive breast surgery differs from other types of surgery because of a greater surface area of undermined tissue, nearly universal ischemia to the skin flaps from the mastectomy, breast duct bacteria, and the possible presence of an implant.
We have noted an increase in the rate of surgical-site infections requiring treatment with oral and intravenous antibiotics and a statistically significant increase in the rate of surgical-site infection requiring reoperation since the adoption of the Surgical Care Improvement Project guidelines at our institution. Before the adoption of the Surgical Care Improvement Project guidelines, our rate of surgical-site infection requiring reoperation was similar (4.3 percent) to the rates reported in the literature (approximately 2 to 15 percent); however, we currently have an unacceptably high rate of surgical-site infection requiring reoperation (16.4 percent). In our patient population, single-dose prophylactic intravenous antibiotic use has not been associated with a decreased risk of surgical-site infection, resulting in a greater number of reconstructive failures in patients undergoing prosthetic breast reconstruction. This risk is 4.74 times higher than in patients receiving postoperative prophylactic antibiotics. We have also noted a higher proportion of Gram-negative bacteria from the wound culture isolates of women requiring implant removal after the implementation of the Surgical Care Improvement Project guidelines (Fig. 2). Bacterial isolates from women treated with postoperative antibiotics who required removal of the implant before the implementation of the Surgical Care Improvement Project guidelines were more likely to grow Staphylococcus (Fig. 1). The use of postoperative prophylactic antibiotics in this group may have prevented Gram-negative infections and selected for more common and/or resistant Staphylococcus species, although we were unable to demonstrate this statistically.
We acknowledge several limitations of this study, with the foremost being its retrospective nature. Patients were not randomized and the duration of antibiotic therapy in the pre–Surgical Care Improvement Project group was not controlled for. Antibiotics were stopped after drain removal, which was performed when output was less than 30 ml/day. Although this was consistent, we did not record the mean duration of postoperative antibiotic use. Surgeons at our institution adopted the use of AlloDerm at approximately the same time; however, it was not used in all implant-based reconstructions. We did not record the rate of AlloDerm use for the entire study population, as the purpose of this study was not to examine risk associated with this product and it was used selectively and infrequently. Our group has been using this product for a number of years before the study and therefore we do not feel a learning curve effect was present. We are currently undertaking a prospective randomized study of the duration of antibiotic use at our institution and its impact on the development of surgical-site infection in patients undergoing prosthetic breast reconstruction. We will compare the efficacy of postoperative antibiotic prophylaxis in patients receiving the maximum allowed by current Surgical Care Improvement Project protocol (24 hours) to those receiving an experimental protocol (7 days).
The optimal duration of postoperative prophylactic antibiotic therapy has not been well established in the plastic surgery literature, and we believe the current recommendations are inadequate. We believe that a single dose of preoperative prophylactic antibiotics is not enough and has resulted in higher rates of surgical-site infection requiring reoperation in patients undergoing prosthetic breast reconstruction.
Footnotes
Presented at the 79th Annual Meeting of the American Society of Plastic Surgeons, in Toronto, Ontario, Canada, October 1 through 5, 2010, and the Annual Meeting of the North Carolina Society of Plastic Surgeons, in White Sulfur Springs, West Virginia, October 14 through 17, 2010.
Disclosure: The authors report no financial associations or funds received in the preparation of this article.
CLINICAL QUESTION/LEVEL OF EVIDENCE: Therapeutic, III.
References
- 1.Mukhtar RA, Throckmorton AD, Alvarado MD, et al. Bacteriologic features of surgical site infections following breast surgery. Am J Surg. 2009;198:529–531. doi: 10.1016/j.amjsurg.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Olsen MA, Lefta M, Dietz JR, et al. Risk factors for surgical site infection after major breast operation. J Am Coll Surg. 2008;207:326–335. doi: 10.1016/j.jamcollsurg.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landes G, Harris PG, Lemaine V, et al. Prevention of surgical site infection and appropriateness of antibiotic prescribing habits in plastic surgery. J Plast Reconstr Aesthet Surg. 2008;61:1347–1356. doi: 10.1016/j.bjps.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Pittet B, Montandon D, Pittet D. Infection in breast implants. Lancet Infect Dis. 2005;5:94–106. doi: 10.1016/S1473-3099(05)01281-8. [DOI] [PubMed] [Google Scholar]
- 5.Phillips BT, Wang ED, Mirrer J, et al. Current practice among plastic surgeons of antibiotic prophylaxis and closed-suction drains in breast reconstruction: Experience, evidence, and implications for postoperative care. Ann Plast Surg. 2011;66:460–465. doi: 10.1097/SAP.0b013e31820c0593. [DOI] [PubMed] [Google Scholar]
- 6.Lyle WG, Outlaw K, Krizek TJ, Koss N, Payne WG, Robson MC. Prophylactic antibiotics in plastic surgery: Trends of use over 25 years of an evolving specialty. Aesthetic Surg J. 2003;23:177–183. doi: 10.1067/maj.2003.39. [DOI] [PubMed] [Google Scholar]
- 7.Hunter JG. Appropriate prophylactic antibiotic use in plastic surgery: The time has come. Plast Reconstr Surg. 2007;120:1732–1734. doi: 10.1097/01.prs.0000280567.18162.12. [DOI] [PubMed] [Google Scholar]
- 8.Grunebaum LD, Reiter D. Perioperative antibiotic usage by facial plastic surgeons: National survey results and comparison with evidence-based guidelines. Arch Facial Plast Surg. 2006;8:88–91. doi: 10.1001/archfaci.8.2.88. [DOI] [PubMed] [Google Scholar]
- 9.Perrotti JA, Castor SA, Perez PC, Zins JE. Antibiotic use in aesthetic surgery: A national survey and literature review. Plast Reconstr Surg. 2002;109:1685–1693. doi: 10.1097/00006534-200204150-00033. discussion 1694–1695. [DOI] [PubMed] [Google Scholar]
- 10.Peled IJ, Gur D, Berger J, Ramon I, Ullmann Y, Nachlieli T. Prophylactic antibiotics in aesthetic and reconstructive surgery. Aesthetic Plast Surg. 2000;24:299–302. doi: 10.1007/s002660010050. [DOI] [PubMed] [Google Scholar]
- 11.Rohrich RJ, Rios JL. The role of prophylactic antibiotics in plastic surgery: Whom are we treating? Plast Reconstr Surg. 2003;112:617–618. doi: 10.1097/01.PRS.0000067440.39522.17. [DOI] [PubMed] [Google Scholar]
- 12.Hawn MT, Vick CC, Richman J, et al. Surgical site infection prevention: Time to move beyond the surgical care improvement program. Ann Surg. 2011;254:494–499. doi: 10.1097/SLA.0b013e31822c6929. discussion 499–501. [DOI] [PubMed] [Google Scholar]
- 13.Bratzler DW, Houck PM Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: An advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189:395–404. doi: 10.1016/j.amjsurg.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 15.Gordon SM. Antibiotic prophylaxis against postoperative wound infections. Cleve Clin J Med. 2006;73(Suppl 1):S42–S45. doi: 10.3949/ccjm.73.suppl_1.s42. [DOI] [PubMed] [Google Scholar]
- 16.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. [PubMed] [Google Scholar]
- 17.Greenhalgh DG. Wound healing and diabetes mellitus. Clin Plast Surg. 2003;30:37–45. doi: 10.1016/s0094-1298(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 18.Haley RW, Culver DH, White JW, Morgan WM, Emori TG. The nationwide nosocomial infection rate: A new need for vital statistics. Am J Epidemiol. 1985;121:159–167. doi: 10.1093/oxfordjournals.aje.a113988. [DOI] [PubMed] [Google Scholar]
- 19.Wilson AP, Weavill C, Burridge J, Kelsey MC. The use of the wound scoring method ‘ASEPSIS’ in postoperative wound surveillance. J Hosp Infect. 1990;16:297–309. doi: 10.1016/0195-6701(90)90002-6. [DOI] [PubMed] [Google Scholar]
- 20.Wilson AP, Gibbons C, Reeves BC, et al. Surgical wound infection as a performance indicator: Agreement of common definitions of wound infection in 4773 patients. BMJ. 2004;329:720. doi: 10.1136/bmj.38232.646227.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson AP, Hodgson B, Liu M, et al. Reduction in wound infection rates by wound surveillance with postdischarge follow-up and feedback. Br J Surg. 2006;93:630–638. doi: 10.1002/bjs.5303. [DOI] [PubMed] [Google Scholar]
- 22.Bertin ML, Crowe J, Gordon SM. Determinants of surgical site infection after breast surgery. Am J Infect Control. 1998;26:61–65. doi: 10.1016/s0196-6553(98)70062-8. [DOI] [PubMed] [Google Scholar]
- 23.Penel N, Yazdanpanah Y, Chauvet MP, et al. Prevention of surgical site infection after breast cancer surgery by targeted prophylaxis antibiotic in patients at high risk of surgical site infection. J Surg Oncol. 2007;96:124–129. doi: 10.1002/jso.20796. [DOI] [PubMed] [Google Scholar]
- 24.Rotstein C, Ferguson R, Cummings KM, Piedmonte MR, Lucey J, Banish A. Determinants of clean surgical wound infections for breast procedures at an oncologic center. Infect Control Hosp Epidemiol. 1992;13:207–214. doi: 10.1086/646511. [DOI] [PubMed] [Google Scholar]
- 25.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20:271–274. doi: 10.1016/s0196-6553(05)80201-9. [DOI] [PubMed] [Google Scholar]
- 26.Wedgwood KR, Benson EA. Non-tumour morbidity and mortality after modified radical mastectomy. Am R Coll Surg Engl. 1992;79:314–317. [PMC free article] [PubMed] [Google Scholar]
- 27.Barber GR, Miransky J, Brown AE, et al. Direct observations of surgical wound infections at a comprehensive cancer centre. Arch Surg. 1995;130:1042–1047. doi: 10.1001/archsurg.1995.01430100020005. [DOI] [PubMed] [Google Scholar]
- 28.Morimoto K, Kinoshita H. Once-daily use of ofloxacin for prophylaxis in breast cancer surgery. Chemotherapy. 1998;44:135–141. doi: 10.1159/000007105. [DOI] [PubMed] [Google Scholar]
- 29.Gupta R, Sinnett D, Carpenter R, Preece PE, Royle GT. Antibiotic prophylaxis for postoperative wound infection in clean elective breast surgery. Eur J Surg Oncol. 2000;26:363–366. doi: 10.1053/ejso.1999.0899. [DOI] [PubMed] [Google Scholar]
- 30.Danforth DN, Jr, Lippman ME, McDonald H, et al. Effect of preoperative chemotherapy on mastectomy for locally advanced breast cancer. Am Surg. 1990;56:6–11. [PubMed] [Google Scholar]
- 31.Badr el Din A, Coibion M, Guenier C, et al. Local postoperative morbidity following pre-operative irradiation in locally advanced breast cancer. Eur J Surg Oncol. 1989;15:486–489. [PubMed] [Google Scholar]
- 32.Thomas R, Alvino P, Cortino GR, et al. Long-acting versus short-acting cephalosporins for preoperative prophylaxis in breast surgery: A randomized double-blind trial involving 1,766 patients. Chemotherapy. 1999;45:217–223. doi: 10.1159/000007186. [DOI] [PubMed] [Google Scholar]
- 33.Canavese G, Catturich A, Vecchio C, et al. Surgical complications related to peri-operative adjuvant chemotherapy in breast cancer: Results of a prospective controlled, randomized clinical trial. Eur J Surg Oncol. 1997;23:10–12. doi: 10.1016/s0748-7983(97)80135-7. [DOI] [PubMed] [Google Scholar]
- 34.Platt R, Zaleznik DF, Hopkins CC, et al. Perioperative antibiotic prophylaxis for herniorrhaphy and breast surgery. N Engl J Med. 1990;18:153–160. doi: 10.1056/NEJM199001183220303. [DOI] [PubMed] [Google Scholar]
- 35.Vilar-Compte D, Jacquemin B, Robles-Vidal C, Volkow P. Surgical site infections in breast surgery: Case-control study. World J Surg. 2004;28:242–246. doi: 10.1007/s00268-003-7193-3. [DOI] [PubMed] [Google Scholar]
- 36.Rey JE, Gardner SM, Cushing RD. Determinants of surgical site infection after breast biopsy. Am J Infect Control. 2005;33:126–129. doi: 10.1016/j.ajic.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Kompatscher P, von Planta A, Spicher I, et al. Comparison of the incidence and predicted risk of early surgical site infections after breast reduction. Aesthetic Plast Surg. 2003;27:308–314. doi: 10.1007/s00266-003-3010-5. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham ME, Bunn F, Handscomb K. Prophylactic antibiotics to prevent surgical site infection after breast cancer surgery. Cochrane Database Syst Rev. 2006;(2):CD005360. doi: 10.1002/14651858.CD005360.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Francis SH, Ruberg RL, Stevenson KB, et al. Independent risk factors for infection in tissue expander breast reconstruction. Plast Reconstr Surg. 2009;124:1790–1796. doi: 10.1097/PRS.0b013e3181bf80aa. [DOI] [PubMed] [Google Scholar]
- 40.Cordeiro PG, McCarthy CM. A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: Part I. A prospective analysis of early complications. Plast Reconstr Surg. 2006;118:825–831. doi: 10.1097/01.prs.0000232362.82402.e8. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong RW, Berkowitz RL, Bolding F. Infection following breast reconstruction. Ann Plast Surg. 1989;23:284–288. doi: 10.1097/00000637-198910000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Pinsolle V, Grinfeder C, Mathoulin-Pelissier S, Faucher A. Complications analysis of 266 immediate breast reconstructions. J Plast Reconstr Aesthet Surg. 2006;59:1017–1024. doi: 10.1016/j.bjps.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 43.Nahabedian MY, Tsangaris T, Momen B, Manson P. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg. 2003;112:467–476. doi: 10.1097/01.PRS.0000070727.02992.54. [DOI] [PubMed] [Google Scholar]
- 44.Handel N, Jensen JA, Black Q, Waisman JR, Silverstein MJ. The fate of breast implants: A critical analysis of complications and outcomes. Plast Reconstr Surg. 1995;96:1521–1533. doi: 10.1097/00006534-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Spear SL, Majidian A. Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants: A retrospective review of 171 consecutive breast reconstructions from 1989 to 1996. Plast Reconstr Surg. 1998;101:53–63. doi: 10.1097/00006534-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Disa JJ, Ad-El DD, Cohen SM, Cordiero PG, Hidalgo DA. The premature removal of tissue expanders in breast reconstruction. Plast Reconstr Surg. 1999;104:1662–1665. doi: 10.1097/00006534-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Brown SL, Heflin B, Woo EK, Parmentier CM. Infections related to breast implants reported to the Food and Drug Administration, 1977–1997. J Long Term Eff Med Implants. 2001;11:1–12. [PubMed] [Google Scholar]
- 48.Amland PF, Andenaes K, Samdal F, et al. A prospective, double-blind, placebo-controlled trial of a single dose of azithromycin on postoperative wound infections in plastic surgery. Plast Reconstr Surg. 1995;96:1378–1383. doi: 10.1097/00006534-199511000-00022. [DOI] [PubMed] [Google Scholar]