Abstract
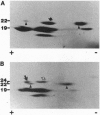
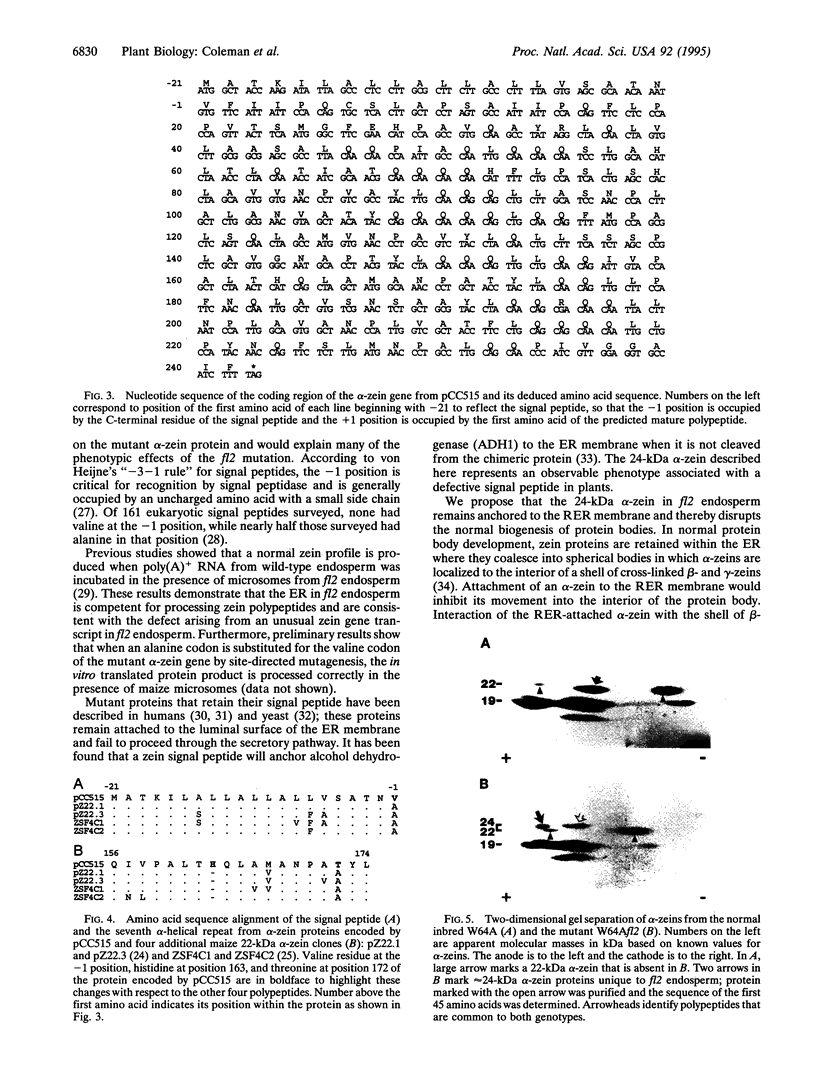
The maize floury 2 (fl2) mutation enhances the lysine content of the grain, but the soft texture of the endosperm makes it unsuitable for commercial production. The mutant phenotype is linked with the appearance of a 24-kDa alpha-zein protein and increased synthesis of binding protein, both of which are associated with irregularly shaped protein bodies. We have cloned the gene encoding the 24-kDa protein and show that it is expressed as a 22-kDa alpha-zein with an uncleaved signal peptide. Comparison of the deduced N-terminal amino acid sequence of the 24-kDa alpha-zein protein with other alpha-zeins revealed an alanine to valine substitution at the C-terminal position of the signal peptide, a histidine insertion within the seventh alpha-helical repeat, and an alanine to threonine substitution with the same alpha-helical repeat of the protein. Structural defects associated with this alpha-zein explain many of the phenotypic effects of the fl2 mutation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Pedersen K., Marks M. D., Larkins B. A. A structural model for maize zein proteins. J Biol Chem. 1982 Sep 10;257(17):9984–9990. [PubMed] [Google Scholar]
- Boston R. S., Fontes E. B., Shank B. B., Wrobel R. L. Increased expression of the maize immunoglobulin binding protein homolog b-70 in three zein regulatory mutants. Plant Cell. 1991 May;3(5):497–505. doi: 10.1105/tpc.3.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr F. A., Burr B. Three mutations in Zea mays affecting zein accumulation: a comparison of zein polypeptides, in vitro synthesis and processing, mRNA levels, and genomic organization. J Cell Biol. 1982 Jul;94(1):201–206. doi: 10.1083/jcb.94.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M., Bruce D., Perry D. J., Price J., Harper P. L., O'Meara A., Carrell R. W. Antithrombin Dublin (-3 Val----Glu): an N-terminal variant which has an aberrant signal peptidase cleavage site. FEBS Lett. 1990 Oct 29;273(1-2):87–90. doi: 10.1016/0014-5793(90)81057-u. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Fontes E. B., Shank B. B., Wrobel R. L., Moose S. P., OBrian G. R., Wurtzel E. T., Boston R. S. Characterization of an immunoglobulin binding protein homolog in the maize floury-2 endosperm mutant. Plant Cell. 1991 May;3(5):483–496. doi: 10.1105/tpc.3.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman J., Nimmesgern E., Ohtsuka K., Hartl F. U. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994 Jul 14;370(6485):111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- Garratt R., Oliva G., Caracelli I., Leite A., Arruda P. Studies of the zein-like alpha-prolamins based on an analysis of amino acid sequences: implications for their evolution and three-dimensional structure. Proteins. 1993 Jan;15(1):88–99. doi: 10.1002/prot.340150111. [DOI] [PubMed] [Google Scholar]
- Habben J. E., Kirleis A. W., Larkins B. A. The origin of lysine-containing proteins in opaque-2 maize endosperm. Plant Mol Biol. 1993 Nov;23(4):825–838. doi: 10.1007/BF00021537. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Martin J., Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Jones R. A. Effects of floury-2 locus on zein accumulation and RNA metabolism during maize endosperm development. Biochem Genet. 1978 Feb;16(1-2):27–38. doi: 10.1007/BF00484382. [DOI] [PubMed] [Google Scholar]
- Kodrzycki R., Boston R. S., Larkins B. A. The opaque-2 mutation of maize differentially reduces zein gene transcription. Plant Cell. 1989 Jan;1(1):105–114. doi: 10.1105/tpc.1.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Hurkman W. J. Synthesis and deposition of zein in protein bodies of maize endosperm. Plant Physiol. 1978 Aug;62(2):256–263. doi: 10.1104/pp.62.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H., Jones R. A., Dalby A., Tsai C. Y. Genetic regulation of storaage protein content in maize endosperm. Biochem Genet. 1976 Aug;14(7-8):641–650. doi: 10.1007/BF00485842. [DOI] [PubMed] [Google Scholar]
- Lending C. R., Larkins B. A. Changes in the zein composition of protein bodies during maize endosperm development. Plant Cell. 1989 Oct;1(10):1011–1023. doi: 10.1105/tpc.1.10.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. N., Rubenstein I. Molecular characterization of two types of 22 kilodalton alpha-zein genes in a gene cluster in maize. Mol Gen Genet. 1992 Aug;234(2):244–253. doi: 10.1007/BF00283845. [DOI] [PubMed] [Google Scholar]
- Lopes M. A., Coleman C. E., Kodrzycki R., Lending C. R., Larkins B. A. Synthesis of an unusual alpha-zein protein is correlated with the phenotypic effects of the floury2 mutation in maize. Mol Gen Genet. 1994 Dec 1;245(5):537–547. doi: 10.1007/BF00282216. [DOI] [PubMed] [Google Scholar]
- MERTZ E. T., BATES L. S., NELSON O. E. MUTANT GENE THAT CHANGES PROTEIN COMPOSITION AND INCREASES LYSINE CONTENT OF MAIZE ENDOSPERM. Science. 1964 Jul 17;145(3629):279–280. doi: 10.1126/science.145.3629.279. [DOI] [PubMed] [Google Scholar]
- Marks M. D., Larkins B. A. Analysis of sequence microheterogeneity among zein messenger RNAs. J Biol Chem. 1982 Sep 10;257(17):9976–9983. [PubMed] [Google Scholar]
- Marocco A., Santucci A., Cerioli S., Motto M., Di Fonzo N., Thompson R., Salamini F. Three high-lysine mutations control the level of ATP-binding HSP70-like proteins in the maize endosperm. Plant Cell. 1991 May;3(5):507–515. doi: 10.1105/tpc.3.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson O. E., Mertz E. T., Bates L. S. Second Mutant Gene Affecting the Amino Acid Pattern of Maize Endosperm Proteins. Science. 1965 Dec 10;150(3702):1469–1470. doi: 10.1126/science.150.3702.1469. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Racchi M., Watzke H. H., High K. A., Lively M. O. Human coagulation factor X deficiency caused by a mutant signal peptide that blocks cleavage by signal peptidase but not targeting and translocation to the endoplasmic reticulum. J Biol Chem. 1993 Mar 15;268(8):5735–5740. [PubMed] [Google Scholar]
- Saghai-Maroof M. A., Soliman K. M., Jorgensen R. A., Allard R. W. Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci U S A. 1984 Dec;81(24):8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer I., Emr S., Gross C., Schekman R. Invertase signal and mature sequence substitutions that delay intercompartmental transport of active enzyme. J Cell Biol. 1985 May;100(5):1664–1675. doi: 10.1083/jcb.100.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. J., Burr F. A., Burr B. Transposon tagging and molecular analysis of the maize regulatory locus opaque-2. Science. 1987 Nov 13;238(4829):960–963. doi: 10.1126/science.2823388. [DOI] [PubMed] [Google Scholar]
- Schmidt R. J., Ketudat M., Aukerman M. J., Hoschek G. Opaque-2 is a transcriptional activator that recognizes a specific target site in 22-kD zein genes. Plant Cell. 1992 Jun;4(6):689–700. doi: 10.1105/tpc.4.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatters R. G., Jr, Miernyk J. A. A zein signal sequence functions as a signal-anchor when fused to maize alcohol dehydrogenase. Biochim Biophys Acta. 1991 Sep 30;1068(2):179–188. doi: 10.1016/0005-2736(91)90208-p. [DOI] [PubMed] [Google Scholar]
- Tatham A. S., Field J. M., Morris V. J., I'Anson K. J., Cardle L., Dufton M. J., Shewry P. R. Solution conformational analysis of the alpha-zein proteins of maize. J Biol Chem. 1993 Dec 15;268(35):26253–26259. [PubMed] [Google Scholar]
- Vogel J. P., Misra L. M., Rose M. D. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990 Jun;110(6):1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M. Multiple zeins from maize endosperms characterized by reversed-phase high performance liquid chromatography. Plant Physiol. 1991 Mar;95(3):777–786. doi: 10.1104/pp.95.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]