Abstract
Current clinical CT contrast agents are mainly small molecular iodinated compounds, which often suffer from short blood pool retention for more comprehensive cardiovascular CT imaging and may cause contrast-induced nephropathy. In this work, we prepared polydisulfides containing a traditional iodinated CT contrast agent in order to optimize the pharmacokinetics of the agent and improve its safety. Initially acting as a macromolecular agent and achieving sharp blood vessel delineation, the polydisulfides can be reduced by endogenous thiols via disulfide-thiol exchange reaction to oligomers that can be readily excreted via renal filtration. Short polyethylene glycol (PEG) chain was also introduced to the polymers to further modify the in vivo properties of the agents. Strong and prolonged vascular enhancement has been generated with two new agents in mice (5–10 times higher blood pool enhancement than iodinaxol). The polydisulfide agents gradually degraded and excreted via renal filtration. The gradual excretion process could prevent contrast induced nephropathy. These results suggest that the biodegradable macromolecular CT contrast agents are promising safe and effective blood contrast agents for CT angiography and image-guided interventions.
1. Introduction
X-ray computed tomography (CT) is one of the most commonly used clinical diagnostic imaging technologies [1–6]. CT is effective to visualize hard tissues due to the inherent density differences between hard and soft tissues. With the assistance of proper contrast agents, CT can also provide high resolution three-dimensional images of soft tissues. Current clinical CT contrast agents are mainly small molecular compounds containing heavy elements, such as iodine (i.v. injection) [5, 7–10] and barium (oral route) [11–13]. Small molecular iodinated organic compounds are the most commonly used clinical contrast agents for cardiovascular CT imaging, including angiography and myocardial perfusion, image-guided intravascular intervention and cancer diagnosis. There have been significant advancements in CT technology. Multidetector CT and dual-source CT have been developed and used to reduce the radiation dose and to improve both temporal and spatial resolution. In contrast, little process has been made to address the limitations of CT contrast agents. Current intravascular CT contrast agents are based on highly functionalized water-soluble triiodobenzene derivatives. These small molecular contrast agents do not have favorable pharmacokinetics and are rapidly cleared from the blood circulation [14–16]. Perfect timing of contrast injection is required to catch the first pass for CT angiography. High doses or multiple doses are often used to generate sufficient contrast enhancement for accurate diagnostic imaging. However, the use of the agents at high doses and multiple doses often induce dose-related toxic side effects, including contrast-induced nephropathy. Their rapid clearance at high concentrations from blood circulation may cause acute kidney injury or renal failure. There is an unmet clinical need for safe and effective CT contrast agents that can provide effective CT contrast enhancement at reduced doses and minimize dose related toxic side effects.
The search for safer and effective X-ray contrast agents has been continued for over a century since the first clinical use of X-ray for medical imaging. Besides the small molecular iodinated contrast agents, various polymeric or nanoparticulate contrast agents containing heavy elements have been reported to improve the pharmacokinetics and effectiveness of the iodinated small molecular contrast agents [10]. Iodinated contrast agents have been incorporated into polymers, polymeric nanoparticles [6, 17–22], liposomes [23–26], dendrimers [27–30] and nano-emulsions [31–33] to prolong the blood circulation of the agents for effective blood pool imaging. Colloids or nanoparticles containing other heavy elements, including thorium, bismuth, gold, erbium, manganese and etc., have also been reported as effective CT contrast agents [34–39]. These agents have prolonged vascular circulation and provide sharp blood vessel delineation essential for CT angiography. For example, colloidal thorium oxide (1–1,000 nm in size) was first used for clinical X-ray imaging in 1930s. However, the agent was terminated for intravenous use in 1950s because of its slow excretion from the body and consequent toxic sides, e.g. cancer and liver fibrosis and cirrhosis. Despite the advantages of the polymeric or nanosized contrast agents for CT blood pool imaging, it would be ideal if these agents could be readily excreted after imaging to alleviate any safety concern associated with their incomplete elimination.
Rational design of CT contrast agents with controlled pharmacokinetics and excretion rates is critical to address the drawbacks of both iodinated small molecular CT contrast agents and nanosized contrast agents. We have recently designed and developed biodegradable macromolecular MRI contrast agents based on polydisulfides [40, 41]. These agents initially behave as macromolecular agents in the body and produce superior contrast enhancement in the blood pool and soft tissues. The disulfide bonds in the polymer backbone are then gradually reduced by endogenous thiols in plasma via disulfide-thiol exchange reaction to give oligomeric or smaller molecules, which are readily excreted via renal filtration after the imaging. As a result, the polydisulfide based MRI contrast agents produce prolonged blood pool enhancement as macromolecular contrast agents and excrete from the body as small molecular agents with minimal tissue retention. Thus, we hypothesize that iodinated polydisulfides can also be safer and effective biodegradable macromolecular CT contrast agents. They will have prolonged blood circulation and limited vascular extravasation to produce strong blood pool enhancement at substantially reduced doses. At the same time, the iodinated polydisulfides can be gradually reduced in the plasma by endogenous thiols to small iodinated oligomers, which can be gradually excreted via renal filtration. The gradual degradation of the agents can significantly slow down their renal excretion rate, reduce the concentration of the iodinated agents in the kidneys and minimize the acute assault to the kidneys, which is often caused by the conventional small molecular iodinated contrast agents. Consequently, these agents will have substantially lower toxic side effects to the kidneys than the small molecular clinical CT agents. The iodinated polydisulfide based biodegradable macromolecular CT contrast agents will have controlled pharmacokinetics and better safety for effective contrast enhanced in vivo CT imaging.
Here we described the synthesis and evaluation of iodinated polydisulfides as biodegradable CT blood pool contrast agents. A clinical iodinated CT contrast agent, 5-amino-2, 4, 6-triiodoisophthalic acid (ATIPA), was used as a monomer to copolymerize with cystamine to give iodinated polydisulfides, polyATIPA. The polydisulfides was further modified with a polymer, polyethylene glycol (PEG550), by pegylation at the amino group of ATIPA to optimize their pharmacokinetics and to prevent non-specific interaction with the reticuloendothelial system (RES) [42, 43]. The degradability of the polydisulfides was investigated in vitro with the presence of cysteine and in vivo by analyzing the urine samples with mass spectrometry. The dynamic contrast enhanced CT imaging of the iodinated polydisulfides was assessed in mice on a micro-CT scanner with a clinical agent as a control. The renal safety of the pegylated iodinated polydisulfides was preliminarily assessed by histological analysis of the major organs after exposure to the agent.
2. Experimental
2.1. Materials
Poly(ethylene glycol) methyl ether (Mn = 550, PEG550) 5-Amino-2, 4, 6-triiodoisophthalic acid (ATIPA), Maleic anhydride, thionyl chloride (SOCl2), cystamine were purchased from Aldrich (St. Louis, MO). N,N-Dimethylformamide (DMF), triethylamine (TEA), Dimethyl sulfoxide (DMSO) and dichloromethane (DCM) were purchased from Fisher (St. Louis, MO) and dried over 4 Å molecular sieves.
2.2. Synthesis
2.2.1. Synthesis of PEG550-Acid
PEG550, (11 g, 20 mmol) was dissolved in 30 mL of anhydrous THF. Succinic anhydride (4 g, 40 mmol) was added into the solution. The mixture was stirred at room temperature for 48 h. THF was removed under vacuum and the rude product was redissolved in DCM and washed with water for 3 times. The solution was dried and pure product was obtained after remove the DCM under vacuum (10.5 g, yield 81%). 1H-NMR (400 MHz, CDCl3): δ (ppm): 4.26 (t), 3.67–3.62 (br), 3.37 (s), 2.64 (t).
2.2.2. Synthesis of PEG550-Chloride
PEG550-acid, (6.5 g, 10 mmol) was dissolved into 30 mL of anhydrous DCM. SOCl2 (5 mL) and a drop of DMF were added into the solution and the mixture was stirred at room temperature for 20 h. The solvent and excess of SOCl2 were removed under vacuum to obtain the chloride (6.5 g, yield 98%). 1H-NMR (400 MHz, CDCl3): δ (ppm): 4.25 (t), 3.68–3.61 (br), 3.37 (s), 3.20 (t), 2.68 (t).
2.2.3. Synthesis of PEG550-ATIPA
ATIPA (5.84 g, 10 mmol) was suspended in 20 mL of DI water. NaOH (1 g, 25 mmol) was added into the solution to make the solution clear. At 0 °C, PEG550-chloride (6.5 g, 12 mmol) was added to the solution dropwise and NaOH (2 M) solution was added at the same time to keep the pH value at around 10. The mixture was stirred at 0 °C for 2 h then RT overnight. Water was removed under vacuum and pure product was purified through silicone gel column (DCM : methanol = 5 : 1) (9.8 g, yield 81%). 1H-NMR (400 MHz, d-Acetone): δ (ppm): 4.26–4.23 (br), 3.68–3.61 (br), 3.37 (s), 2.68 (br).
2.2.4. Synthesis of PEG550-ATIPC
PEG550-ATIPA (12.3 g, 10 mmol) was dissolved in 20 mL of anhydrous DCM. 10 mL of thionyl chloride was added into the mixture and 2 drops of DMF was added as catalyst. The mixture was refluxed at 80 °C overnight. The solvent was removed under vacuum to obtain the final product (11.5g, yield 92%). 1H-NMR (400 MHz, d-Acetone): δ (ppm): 4.26–4.23 (br), 3.68–3.61 (br), 3.37 (s), 2.80–2.68 (br).
2.2.5. Synthesis of the ATIPC chloride
5-Amino-2,4,6-triiodoisophthalic acid (ATIPA, 28 g, 50 mmol) was added into 50 mL of anhydrous toluene. 30 mL of thionyl chloride was added into the mixture and 2 drops of DMF was added as catalyst. The mixture was refluxed at 80 °C for 12 h and TLC showed that there is no acid left. The solvent was removed under vacuum to obtain the crude product as yellow solid powder. The solid was recrystallized with anhydrous ethyl acetate twice to obtain the pure chloride (25g, yield 83%). Characterized with MALDI TOF and HPLC.
2.2.6. Synthesis of the polyATIPA
At 16 °C, cystamine (160 mg, 1.05 mmol) was dissolved in the mixture solvents of 1 mL of anhydrous DMSO and 0.5 mL of TEA. ATIPC powder (630 mg, 1 mmol) was added portion by portion into the mixture during 1.5 h. The mixture was stirred at this temperature for 6 h and put into 60 °C oil bath for another 12 h. After that, the mixture was dissolved into 20 mL of deionized water and put into a dialysis bag (MWO 6000–8000), dialyzed against deionized water, and then lyophilized (480 mg, yield 63%). 1H-NMR (400 MHz, d-DMSO): δ (ppm): 3.5–3.3 (br). PolyPEG550ATIPA was obtained with the same method.
2.3. FPLC and reverse-phase HPLC
All the polymers were characterized by size exclusion chromatography (SEC) on an AKTA FPLC system (Amersham Biosciences Corp., Piscataway, NJ) equipped with a Superose 12 column and a refractive index detector. Molecular weights were calibrated with standard poly[N-(2-hydroxypropyl) methacrylamide]. Ion-pairing reverse-phase HPLC (Agilent 1100, Santa Clara, CA) was performed with a RP-C18 HPLC column (4.6 × 250 mm2, 5 μm particle size) and UV detector. The mobile phase was a gradient of 10–50% of acetonitrile aqueous solution containing 0.5% TFA at a total flow rate of 1 mL/min. An aliquot of 200 μL sample at approximately 0.1 mg/mL was injected, of which 20 μL went into the column at 25 °C. The UV absorption peaked at 210 nm of the elution was recorded for analysis.
2.4. Evaluation of the in vitro CT values
All the samples were scanned on a small-animal PET/SPECT/CT system (Inveon, Siemens) with x-ray energy 0 – 80 kVp and a detector with the field of view 2048 mm × 2048 mm. The images were obtained at an x-ray voltage of 80 kVp, an anode current of 500 μA and an exposure time of 200 ms for every rotational steps (total rotation degree is 360 and rotation steps are 180). The field of view was 2048 mm × 2048 mm. Images were reconstructed on a 512 pixel × 512 pixel grid with a pixel size of 66.7 μm × 66.7 μm. The polymeric contrast agents were scanned at the concentration of 37.5, 75, 150 and 300 mg-I / mL compared with iodixanol based on a standard UV concentration curve at 240 nm. Deionized water was used as the control (0 HU).
2.5. Degradation of polymers
The degradability of the polymer was investigated by the incubation of polymer with DL-homocysteine under physiological conditions [44]. The DL-homocysteine concentration was 15 μM, mimicking the thiol concentration in the plasma. The concentration of polymers was 9.19 mM representing the iodine concentration 27.56 mM (350 mg-I / Kg) based on an average Balb/c mouse weighing 20 g. The polymer solution was put into a dialysis bag (MWO 6000) and incubated with of 15 μM DL-homocysteine solution (1 L X 3) at 37 °C. The UV absorption was detected at 240 nm. The molecular change of the polymer was traced by FPLC.
2.6. In vivo blood pool CT imaging
The polymeric contrast agents and the control contrast agent, iodixanol, were evaluated with BALB/c mice (18 – 22 g body weight, 6 mice per agent). All the mice were scanned on the same machine as the in vitro detection. The images were obtained at an x-ray voltage of 80 kVp, an anode current of 500 μA and an exposure time of 200 ms for every rotational steps (total rotation degree is 360 and rotation steps are 180). The field of view was 1024 mm × 2048 mm. Images were reconstructed in a 1024 pixel × 1024 pixel grid with a pixel size of 18 μm × 18 μm. Each group was anesthetized by intramuscular injection of ketamine (35 mg/kg) and xylazine (8 mg/kg); anesthesia will be maintained by isoflurane inhalation. The injection volume of contrast agent for each mouse was 150 μL with the dose of 350 mg-I / kg. The acquisition times were pre-injection and at 10, 20, 30, 45, and 60 min post-injection.
2.7. In vivo elimination
BALB/c mice were injected the contrast agent (PolyATIPA) at the concentration 27.56 mM (350 mg-I / Kg). Their urine was collected at 10, 20, 30, 45, 60, 90 and 120 min postinjection by pushing bladders. The in vivo elimination was analyzed through MALDI-TOF mass spectrometry.
2.8. CT image analysis
CT values are expressed in Hounsfield Units (HU) and were obtained per organ by drawing volumetric regions of interest (RIOs) [45]. The average ∆HU values and the standard deviation were calculated from the data with 3 ROIs per organ. The CT data was analyzed to determine the contrast enhancement of different agents at various time points post-injection. In vivo CT values of the regions of interest, such as heart, blood, liver and bladder, were measured according to the equation ∆HU = HU (at time t) – HU (before injection) to study the dynamic enhancement of different organs. The dynamic enhancement of the blood showed the efficiency of the polymers used as blood pool contrast agents. The dynamic enhancement of the bladder indicated how fast the contrast agents will degrade and be excluded from the body.
2.9. Histochemical analysis
After CT imaging, the mice were sacrificed under anesthetic conditions, and tissues of interest (lung, kidney, liver, spleen and heart) were excised and fixed in 10% neutral buffered formalin (10% NBF) [46]. For haematoxylin and eosin (H&E) staining, formalin fixed tissues from each organ were embedded into paraffin and paraffin-embedded tissues were sectioned into 4 μm thickness. Each histological section was documented by a dissecting microscope. The results were analyzed to evaluate the safety of the contrast agents to the organs.
2.10. Statistical analysis
Statistical calculations will be performed using Prism (Graphpad Software, Inc). The data will be analyzed by repeated-measures two-way ANOVA using Bonferroni’s post-test, assuming statistical significance at p < 0.05.
2.11. Ethical aspects of proposed research
BALB/c mice are a well-established model for blood pool CT imaging. In-vivo animal studies are critical for this project. There are no non-animal models available for this type of research. There are no alternatives in the literature that we can use to evaluate the degradable macromolecular contrast agents before proceeding to clinical trials. Mice are selected for this project because their physiology closely matches that of the human beings. In addition, all the injection doses were proved safe to the mice. All animals will be cared by veterinarians on the campus in Case Western Reserve University. All animals will be treated under the guidelines of a protocol approved by the Case Western Reserve University, Institutional Animal Care and Use Committee.
3. Results and discussion
3.1. Synthesis
Synthesis of two biodegradable polymeric iodinated contrast agents, polyATIPA and pegylated polyATIPA (poly-PEG550ATIPA), is described in Scheme 2 and detailed in the Supporting Information. First, two carboxylic groups of ATIPA were converted to acyl chlorides by reacting with thionyl chloride. The ATIPA acyl chloride was then polymerized with cystamine to produce the iodinated polydisulfides (Scheme 2.2). For the synthesis of the pegylated contrast agent, PEG550 was first conjugated to the amino group of ATIPA to produce a pegylated ATIPA (Scheme 2.1). MethoxyPEG550 was reacted to succinic anhydride to convert the hydroxyl group to a carboxylic group at the end. This carboxylic group was converted to acyl chloride, and then reacted with ATIPA to form the pegylated monomer PEG550ATIPA. PEG550ATIPA was similarly converted to acyl chloride and was finally polymerized with cystamine to give polyPEG550ATIPA. After polymerization, the products were dialyzed with a membrane (MWCO = 8,000 Da) against deionized water to remove the small molecular weight compounds and oligomers. The polymers were further purified with size exclusion chromatography using G50 Sephadex column eluted with deionized water. The polymer fractions were collected and lyophilized to give off-white solid products. The final polymers were characterized with NMR and size exclusion chromatography (Fig. 1A and B). The number and weight averaged molecular weights of polyATIPA were 21 and 23 KDa, and 22 and 23 KDa for polyPEG550ATIPA. Both iodinated polydisulfides had good water solubility.
Figure 1.
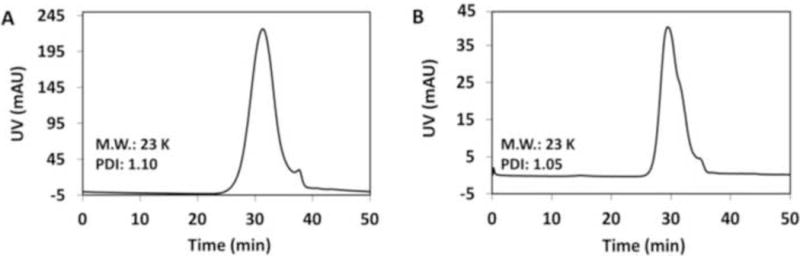
Size exclusion chromatograms of polyATIPA (A) and polyPEG550ATIPA (B).
3.2. In vitro CT values
Figure 2A shows the CT images of iodixanol, polyATIPA and polyPEG550ATIPA at the equivalent iodine concentrations ranging from 37.5 to 300 mg-I/mL. All agents showed concentration dependent X-ray attenuation. The CT values of different concentrations were measured to compare the effectiveness of the contrast agents for X-ray attenuation. Figure 2B shows the X-ray attenuation as a linear function of iodine concentration of the agents. PolyATIPA had higher HU values than the clinical contrast agent iodixanol, especially at high iodine concentration. High iodine content per molecule in polyATIPA could contribute to increase X-ray attenuation of the agent. In contrast, polyPEG550ATIPA exhibited lower HU values than iodixanol possibly due to pegylation. PEG was reported as a negative CT contrast agent. The presence of PEG in polyPEG550ATIPA may diminish the X-ray attenuation ability of the agent.
Figure 2.
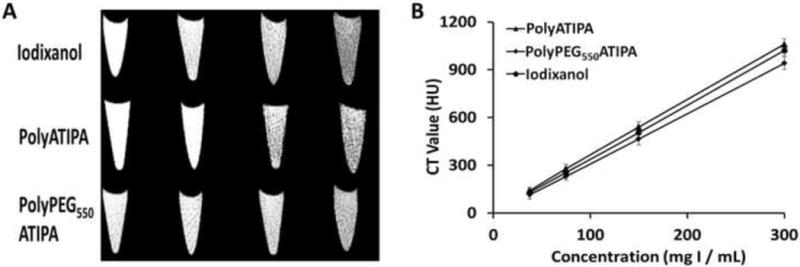
The in vitro CT images of iodixanol, polyATIPA and polyPEG550ATIPA in the equivalent iodine concentrations of 37.5, 75, 150 and 300 mg-I/mL (A) and X-ray attenuation of iodixanol, poly-ATIPA and polyPEG550ATIPA as a function of the concentration (B).
3.3. In vitro degradation
The degradability of the iodinated polydisulfides was investigated by incubating the polymers with cysteine under physiological conditions. The polymer solutions were put into a dialysis bag with MWCO of 6,000 Da and incubated with 15 μM cysteine solution at 37 °C. The cysteine concentration was used to mimic the thiol concentration in the plasma. The concentration of the polymers was 27.56 mM of iodine mimicking the initial plasma iodine concentration of the agents in an average Balb/c mouse weighing 20 g at a dose of 350 mg-I/Kg. Samples were taken at 0.5, 2, 5, 10, 20 and 48 hr and analyzed by SEC on an AKTA FPLC system equipped with a Superose 12 column and a UV detector. Figure 3A and 3B shows the gradual disappearance of the polymer peaks in the presence of cysteine due to the reduction of the disulfide bonds in polymer chains. Because of the dialysis method used here, the low molecular weight fractions could not be detected in the polymer samples. However, the decrease of the height of polymer peaks indicated the degradation of the polymers. Figure 3C shows the degradation kinetics of contrast agents determined by UV spectrometry at 240 nm (according to standard sample). It appears that degradation of polyPEG550ATIPA was slightly slower than polyATIPA possibly due to the steric effect of PEG.
Figure 3.
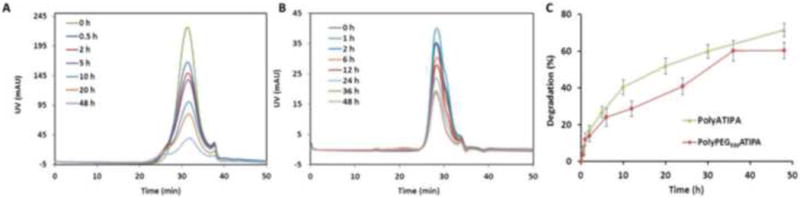
In vitro degradation of the polymers. (A) The molecular weight distribution of polyATIPA (27.56 mM-I) in the incubation with 15 μM cysteine in PBS buffer (pH 7.4) at 37 °C. (B) The molecular weight distribution of polyPEG550ATIPA (27.56 mM-I) in the incubation with 15 μM cysteine in PBS buffer (pH 7.4) at 37 °C. (C) The degradation kinetics curves of contrast agents in PBS buffer (pH 7.4) at 37 °C (determined by UV at 240 nm).
3.4. In vivo CT imaging
The effectiveness of the iodinated polydisulfides for in vivo contrast enhanced CT imaging was assessed in BALB/c mice (18 – 22 g body weight, 6 mice per agent) on a micro-CT scanner with iodixanol as a control agent. The iodinated polydisulfides produced strong vascular enhancement and sharp vascular delineation at a dose of 350 mg-I/kg, while the control agent did not produce any enhancement at the same dose and 10 minute after injection due to rapid extravasation and excretion of the agent (Fig. 4). The pegylated agent polyPEG550ATIPA resulted in better vascular delineation and less background enhancement than polyATIPA, despite the fact that it had a relatively low in vitro X-ray attenuation at the same dose. PEG in polyPEG550ATIPA might decrease non-specific tissue accumulation of the polymers and, consequently, resulted in higher blood concentration for better vascular enhancement than the unpegylated iodinated polydisulfides. Much less non-specific tissue enhancement was also observed for polyPEG550ATIPA than polyATIPA.
Figure 4.
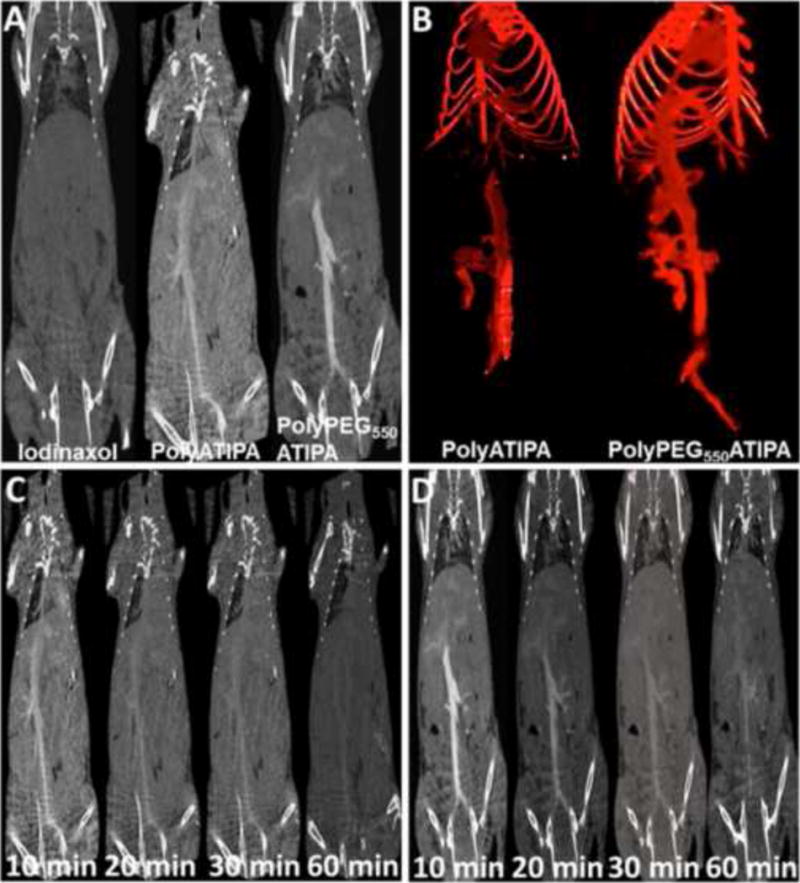
Comparison of in vivo contrast enhanced blood pool CT imaging of iodinaxol, polyATIPA and poly-PEG550ATIPA in Balb/c mice. A, coronal images of mice obtained 10 min after i.v. injection of the agents at a dose of 350 mg-I/kg; B, three-dimensional CT angiogram of the mice contrast enhanced by the polydisulfides at 10 min after i.v. injection. Dynamic contrast enhanced coronal CT images of the mice acquired at 10, 20, 30 and 60 minutes (from left to right) after i.v. injection of polyATIPA (C) and poly-PEG550ATIPA (D) (350 mg-I/kg).
Dynamic CT imaging revealed that the iodinated poydisulfides showed prolonged blood circulation and vascular enhancement. Signal enhancement was still visible in the mouse aorta at least 30 minutes after the intravascular injection of both agents (Fig. 4C and D). PolyPEG550ATIPA exhibited stronger prolonged vascular enhancement than polyATIPA. Significant vascular enhancement was still visible for polyPEG550ATIPA at 60 minutes after the injection. The blood signal increased from 27 ± 30 HU to 526 ± 89 HU at its peak (Fig. 5B). In comparison, the values for polyATIPA and iodinaxol were 226 ± 67 and 56 ± 26, respectively. PolyPEG550ATIPA showed 10 times higher blood pool enhancement than iodinaxol and 2 times higher than polyATIPA. This high enhancement result was comparable to the polymer-coated bismuth contrast agent [37] and polymer-coated gold contrast agent [47].
Figure 5.
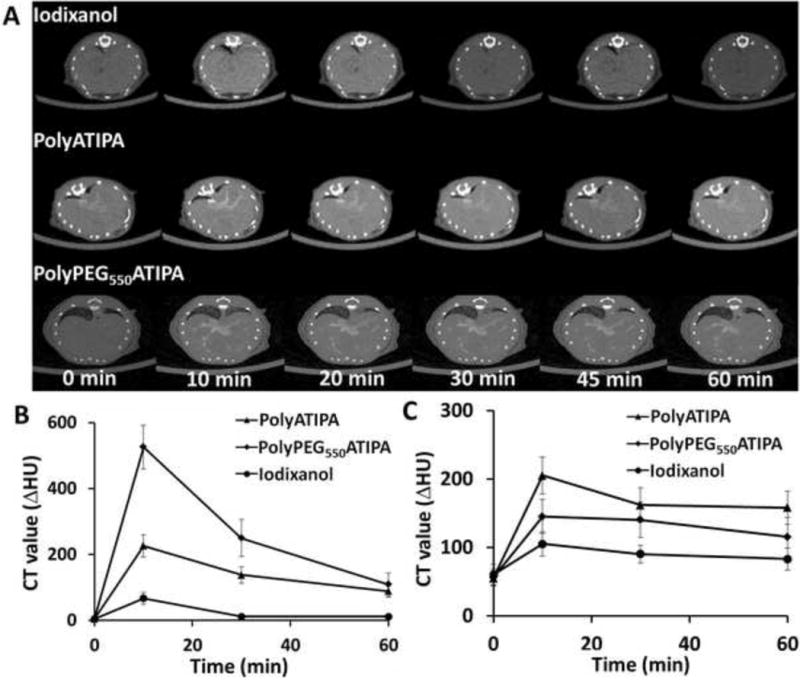
Dynamic contrast enhanced axial CT images (A) of the liver of the mice acquired before and at 10, 20, 30 and 60 minutes after i.v. injection of Iodixanol, polyATIPA and polyPEG550ATIPA (350 mg-I/kg). Increase in HU after injection of different contrast agents were also measured in Blood (B) and Liver (C).
Figure 5A shows the representative CT axial images of the liver before and at different time points after contrast with iodinaxol, polyATIPA and polyPEG550ATIPA in Balb/c mice. It appears that polyPEG550ATIPA resulted in fewer enhancements in the liver tissue than iodinaxol and polyATIPA in Balb/c mice. PolyATIPA had more prolonged liver retention and produced stronger liver enhancement than iodinaxol and polyPEG550ATIPA (Fig. 5B). PEG in polyPEG550ATIPA prevented non-specific tissue accumulation of the agent and resulted in minimal liver enhancement. Consequently, the pegylated agent had a prolonged blood circulation and significant enhancement was still visible in the intrahepatic vasculature. The results indicate that the iodinated polydisulfides, especially the pegylated agent, are advantageous for CT blood pool imaging over the clinical agent and polyATIPA. The prolonged blood pool enhancement with minimal background signal is particularly valuable for CT image-guided interventions. The iodinated polydisulfides of the prolonged blood circulation can also have several other clinical applications such as cancer detection and characterizing tumor angiogenesis.
3.5. In vivo elimination
The in vivo elimination of the iodinated polydisulfides was also investigated using polyATIPA, because the elimination products of polyATIPA excreted in the urine sample were relatively simple and easily to be detected by MALDI-TOF mass spectrometry. Serial CT images of the urinary bladder (red arrow, Fig. 6A) showed significant bladder enhancement from 10 to 120 min postinjection of polyATIPA. The images revealed that the contrast agent started clearing out from the body at least 10 minutes after the injection. The dynamic change of the CT values in the bladder was shown in Fig. 6B. It seems that a significant amount of the contrast agent, possibly small molecular weight oligomers, was released in the first 10 minutes postinjection. CT value reduction at 30 minutes was possibly due to urine discharge. The results strongly indicate that the polydisulfide based CT contrast agent was gradually degraded and excreted via renal filtration. The elimination and renal excretion of the iodinated polydisulfides validated the decrease of the blood contrast showed in Fig. 4. Figure 6C showed the MALDI-TOF mass spectra of the urine samples collected at different time points. The molecular weight of the monomeric units from the reduction of polyATIPA (Fig. 8C) was 676. The 698 peak in the spectra corresponded to the unit with a sodium ion. The increasing intensity of the monomer peak over time strongly suggested the contrast agent was eventually degraded into small molecules. These results clearly demonstrated the feasibility of the iodinated polydisulfides as the effective biodegradable macromolecular CT contrast agents.
Figure 6.
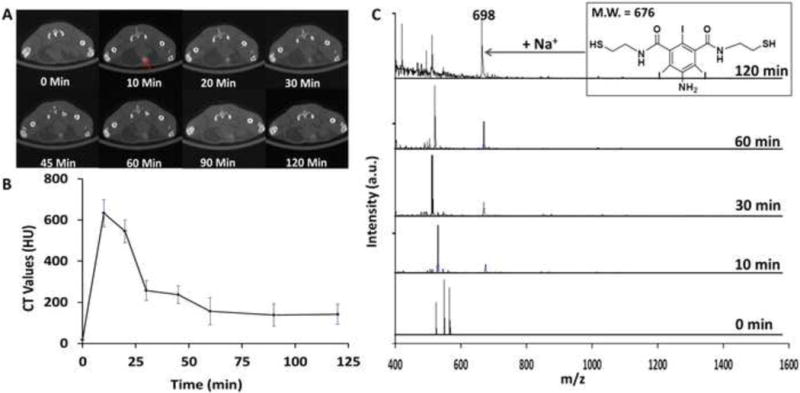
Detection of in vivo degradation. (A) Serial CT imaging of the bladder (red arrow) of a BALB/c mouse after tail-vein injection with polyATIPA (350 mg-I/kg). (B) Increase of HU in bladder after injection of polyATIPA (350 mg-I/kg). The kinetic curve indicates that the in vivo degradation of polyATIPA is relatively fast (less than 10 min). (C) MALDI-TOF MS traces degradation of in vivo degradation of polyATIPA with the urine of a Balb/c mouse collected at different time points. The increasing intensity of the monomer peak (M.W. 676) suggests polydisulfides can be reduced to oligomers and can be readily excreted via renal filtration.
3.6. Histochemical analysis
Iodinated contrast agents are generally safe in clinical applications. However, exposure of major organs to high doses of iodinated agents may cause tissue damages in some cases. Safety of iodinated polydisulfides is a critical parameter to determine their potential for further translational development. It is appears that poly-PEG550ATIPA is a promising biodegradable macro-molecular CT contrast agent because of its superior blood pool contrast enhancement and minimal non-specific tissue accumulation. The safety effect of the agent on the major organs was preliminarily assessed in mice with histochemical analysis. One week after injection of the agent at a dose of 350 mg-I/kg, the mice were sacrificed, and organs of interest (the kidney, liver, spleen, and heart) were collected and fixed in 10% neutral buffered formalin (10% NBF) for haematoxylin and eosin (H&E) staining. The histological images of the organs from the mice injected with the contrast agent were compared with those from the mice injected with PBS (Fig. 7). No significant difference was observed between the mice received with polyPEG550ATIPA and PBS, possibly be-cause of minimal non-specific tissue interaction and gradual excretion of the agent via renal filtration. The result suggests that this contrast agent has a good safety profile to these organs.
Figure 7.
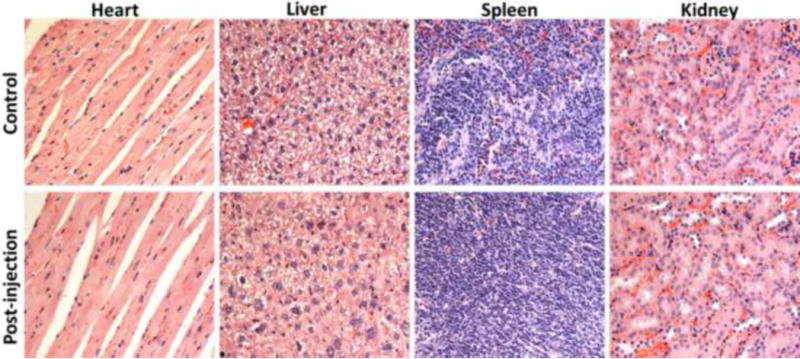
Histological section of the heart, liver, spleen and kidney of the mouse 1 week after intravenous injection of a single dose (350 mg/Kg) of PolyPEG550ATIPA solution. Sections are stained with H&E and observed under a light microscope at 20 × magnification.
3.7. Conclusion
We have validated the hypothesis that iodinated polydisulfides are effective bio-degradable macromolecular contrast agents for X-ray CT imaging. The iodinated polydisulfides had prolonged blood circulation and limited vascular extravasation and produced sharp and prolonged vascular delineation. They were more effective for cardiovascular imaging than the clinical agent iodinaxol. Pegylation of the iodinated polydisulfides further improved the blood pool enhancement by reducing non-specific tissue interaction. The high performance of the agents was comparable to the reported heavy metal based agents, e.g. the polymer-coated bismuth contrast agent and polymer-coated gold contrast agent. The agents degraded in vivo and gradually excreted via renal filtration. The gradual excretion of the agents could obviate the acute contrast assault to the kidneys, resulting in better safety profile. The pegylated iodinated polydisulfides did not show any observable side effects to the major tissues and organs based on histochemical analysis. The biodegradability, gradual clearance after the imaging and good safety profile are clearly the advantageous features of the iodinated polydisulfides for further clinical development of the agents. Thus, the iodinated polydisulfides are promising biodegradable macromolecular CT contrast agents for cardiovascular, cancer CT imaging and image-guided interventions.
Supplementary Material
Scheme 1.
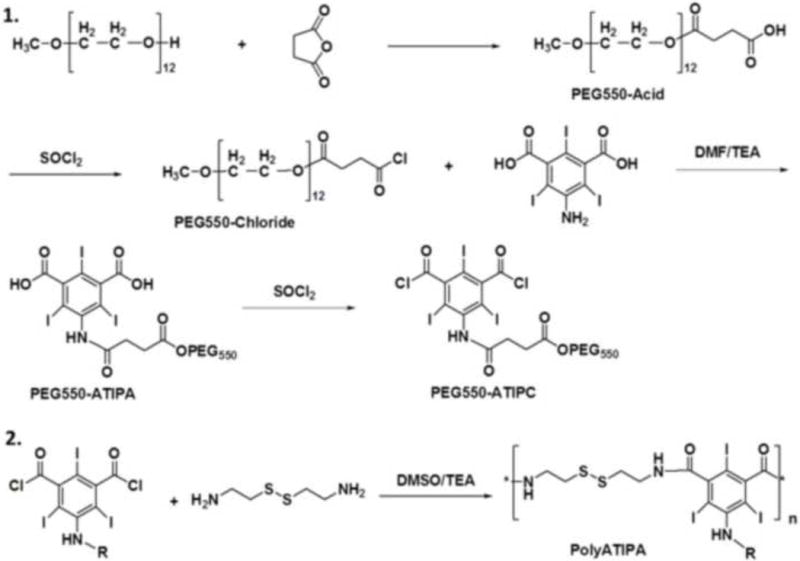
Synthesis of biodegradable polymeric contrast agents. 1, synthesis of the monomer of polyPEG550ATIPA; 2, polymerize the iodinated monomers with cystamine. PolyATIPA, R = H; polyPEG550ATIPA, R = PEG550.
Acknowledgments
This work was supported in part by the NIH grant R01 EB000489.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bogdanov A, Weissleder R, Brady TJ. Long-Circulating Blood-Pool Imaging Agents. Adv Drug Deliv Rev. 1995;16:335–48. [Google Scholar]
- 2.Runge VM. A review of contrast media research in 1999–2000 (vol 36, pg 123, 2000) Invest Radiol. 2001;36:122–30. doi: 10.1097/00004424-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Chou SW, Shau YH, Wu PC, Yang YS, Shieh DB, Chen CC. In vitro and in vivo studies of FePt nanoparticles for dual modal CT/MRI molecular imaging. J Am Chem Soc. 2010;132(38):13270–13278. doi: 10.1021/ja1035013. [DOI] [PubMed] [Google Scholar]
- 4.Torchilin VP. PEG-based micelles as carriers of contrast agents for different imaging modalities. Adv Drug Deliv Rev. 2002;54:235–52. doi: 10.1016/s0169-409x(02)00019-4. [DOI] [PubMed] [Google Scholar]
- 5.Hallouard F, Anton N, Choquet P, Constantinesco A, Vandamme T. Iodinated blood pool contrast media for preclinical X-ray imaging applications – A review. Biomaterials. 2010;31:6249–68. doi: 10.1016/j.biomaterials.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 6.Shilo M, Reuveni T, Motiei M, Popovtzer R. Nanoparticles as computed tomography contrast agents: current status and future perspectives. Nanomedicine-Uk. 2012;7:257–69. doi: 10.2217/nnm.11.190. [DOI] [PubMed] [Google Scholar]
- 7.Yin Q, Yap FY, Yin LC, Ma L, Zhou Q, Dobrucki LW, Fan TM, Gaba RC, Chen jJ. Poly(iohexol) nanoparticles as contrast agents for in vivo X-ray computed tomography imaging. J Am Chem Soc. 2013;135(37):13620–13623. doi: 10.1021/ja405196f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pathe C, Eble K, Schmitz-Beuting D, Keil B, Kaestner B, Voelker M, et al. The presence of iodinated contrast agents amplifies DNA radiation damage in computed tomography. Contrast Media Mol Imaging. 2011;6:507–13. doi: 10.1002/cmmi.453. [DOI] [PubMed] [Google Scholar]
- 9.Hallouard F, Anton N, Zuber G, Choquet P, Li X, Arntz Y, et al. Radiopaque iodinated nano-emulsions for preclinical X-ray imaging. RSC Adv. 2011;1:792–801. [Google Scholar]
- 10.de Vries A, Custers E, Lub J, van den Bosch S, Nicolay K, Grull H. Block-copolymer-stabilized iodinated emulsions for use as CT contrast agents. Biomaterials. 2010;31:6537–44. doi: 10.1016/j.biomaterials.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 11.Anderson NG, Butler AP, Scott NJA, Cook NJ, Butzer JS, Schleich N, et al. Spectroscopic (multi-energy) CT distinguishes iodine and barium contrast material in MICE. Eur Radiol. 2010;20:2126–34. doi: 10.1007/s00330-010-1768-9. [DOI] [PubMed] [Google Scholar]
- 12.Nagata K, Singh AK, Sangwaiya MJ, Nappi J, Zalis ME, Cai WL, et al. Comparative Evaluation of the Fecal-Tagging Quality in CT Colonography: Barium vs. Iodinated Oral Contrast Agent. Acad Radiol. 2009;16:1393–9. doi: 10.1016/j.acra.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Gulati K, Shah ZK, Sainani N, Uppot R, Sahani DV. Gastrointestinal tract labeling for MDCT of abdomen: Comparison of low density barium and low density barium in combination with water. Eur Radiol. 2008;18:868–73. doi: 10.1007/s00330-007-0841-5. [DOI] [PubMed] [Google Scholar]
- 14.Speck U. Preclinical Findings with Iotrolan – a Short Review. Eur Radiol. 1995;5:S8–S13. [Google Scholar]
- 15.Rigsby CK, Gasber E, Seshadri R, Sullivan C, Wyers M, Ben-Ami T. Safety and efficacy of pressure-limited power injection of iodinated contrast medium through central lines in children. Am J Roentgenol. 2007;188:726–32. doi: 10.2214/AJR.06.0104. [DOI] [PubMed] [Google Scholar]
- 16.Heusner TA, Kuehl H, Veit P, Forsting M, Bockisch A, Antoch G. Highly iodinated intravenous contrast agent for use in PET/CT: Feasible? Useful? Advisable? Eur J Nucl Med Mol Imaging. 2007;34:S294–S. [Google Scholar]
- 17.Trubetskoy VS, Gazelle GS, Wolf GL, Torchilin VP. Block-copolymer of polyethylene glycol and polylysine as a carrier of organic iodine: Design of long-circulating particulate contrast medium for X-ray computed tomography. J Drug Target. 1997;4:381–8. doi: 10.3109/10611869709017895. [DOI] [PubMed] [Google Scholar]
- 18.Torchilin VP, Frank-Kamenetsky MD, Wolf GL. CT visualization of blood pool in rats by using long-circulating, iodine-containing micelles. Acad Radiol. 1999;6:61–5. doi: 10.1016/s1076-6332(99)80063-4. [DOI] [PubMed] [Google Scholar]
- 19.Torchilin V, Babich J, Weissig V. Liposomes and micelles to target the blood pool for imaging purposes. J Liposome Res. 2000;10:483–99. [Google Scholar]
- 20.Aryal S, Pilla S, Gong SQ. Multifunctional Nano-Micelles Formed by Amphiphilic Gold-Polycaprolactone-Methoxy Poly(ethylene glycol) (Au-PCL-MPEG) Nanoparticles for Potential Drug Delivery Applications. J Nanosci Nanotechnol. 2009;9:5701–8. doi: 10.1166/jnn.2009.1227. [DOI] [PubMed] [Google Scholar]
- 21.Tang RBA, Chai WM, Ying WH, Yang GY, Xie HL, Liu HQ, Chen KM. Anti-VEGFR2-conjugated PLGA microspheres as an x-ray phase contrast agent for assessing the VEGFR2 expression. Phys Med Biol. 2012;57:3051–3063. doi: 10.1088/0031-9155/57/10/3051. [DOI] [PubMed] [Google Scholar]
- 22.Aviv H, Bartling S, Kieslling F, Margel S. Radiopaque iodinated copolymeric nanoparticles for X-ray imaging applications. Biomaterials. 2009;30:5610–5616. doi: 10.1016/j.biomaterials.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 23.Krause W, Schonborn A, Rupp K. CT imaging with iopromide liposomes in a rabbit model. J Liposome Res. 2011;21:229–36. doi: 10.3109/08982104.2010.527852. [DOI] [PubMed] [Google Scholar]
- 24.Zheng JZ, Allen C, Serra S, Vines D, Charron M, Jaffray DA. Liposome contrast agent for CT-based detection and localization of neoplastic and inflammatory lesions in rabbits: validation with FDG-PET and histology. Contrast Media Mol Imaging. 2010;5:147–54. doi: 10.1002/cmmi.378. [DOI] [PubMed] [Google Scholar]
- 25.Kweon S, Lee HJ, Hyung WJ, Suh J, Lim JS, Lim SJ. Liposomes Coloaded with Iopamidol/Lipiodol as a RES-Targeted Contrast Agent for Computed Tomography Imaging. Pharm Res-Dordr. 2010;27:1408–15. doi: 10.1007/s11095-010-0135-5. [DOI] [PubMed] [Google Scholar]
- 26.Erdogan S. Liposomal Nanocarriers for Tumor Imaging. J Biomed Nanotechnol. 2009;5:141–50. doi: 10.1166/jbn.2009.1016. [DOI] [PubMed] [Google Scholar]
- 27.Peng C, Zheng LF, Chen Q, Shen MW, Guo R, Wang H, et al. PEGylated dendrimer-entrapped gold nanoparticles for in vivo blood pool and tumor imaging by computed tomography. Biomaterials. 2012;33:1107–19. doi: 10.1016/j.biomaterials.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 28.Criscione JM, Dobrucki LW, Zhuang ZW, Papademetris X, Simons M, Sinusas AJ, et al. Development and Application of a Multimodal Contrast Agent for SPECT/CT Hybrid Imaging. Bioconjugate Chem. 2011;22:1784–92. doi: 10.1021/bc200162r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo R, Wang H, Peng C, Shen MW, Pan MJ, Cao XY, et al. X-ray Attenuation Property of Dendrimer-Entrapped Gold Nanoparticles. J Phys Chem C. 2010;114:50–6. [Google Scholar]
- 30.Fu YJ, Nitecki DE, Maltby D, Simon GH, Berejnoi K, Raatschen HJ, et al. Dendritic iodinated contrast agents with PEG-cores for CT imaging: Synthesis and preliminary characterization. Bioconjugate Chem. 2006;17:1043–56. doi: 10.1021/bc060019c. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Anton N, Zuber G, Zhao MJ, Messaddeq N, Hallouard F, et al. Iodinated alpha-tocopherol nano-emulsions as non-toxic contrast agents for preclinical X-ray imaging. Biomaterials. 2013;34:481–91. doi: 10.1016/j.biomaterials.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Hallouard F, Briancon S, Anton N, Li X, Vandamme T, Fessi H. Iodinated nano-emulsions as contrast agents for preclinical X-ray imaging: Impact of the free surfactants on the pharmacokinetics. Eur J Pharm Biopharm. 2013;83:54–62. doi: 10.1016/j.ejpb.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Lim SJ, Lim JS, Choi J, Choi JY, Hyung WJ, Kim HS, et al. Nanoscaled Iodized Oil Emulsion as a CT Contrast Agent for the Detection of Experimental Liver Tumors in a Rat Model. Acad Radiol. 2010;17:985–91. doi: 10.1016/j.acra.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Kojima C, Cho SH, Higuchi E. Gold nanoparticle-loaded PEGylated dendrimers for theragnosis. Res Chem Intermed. 2012;38:1279–89. [Google Scholar]
- 35.Peng C, Wang H, Guo R, Shen MW, Cao XY, Zhu MF, et al. Acetylation of Dendrimer-Entrapped Gold Nanoparticles: Synthesis, Stability, and X-ray Attenuation Properties. J Appl Polym Sci. 2011;119:1673–82. [Google Scholar]
- 36.Kojima C, Umeda Y, Ogawa M, Harada A, Magata Y, Kono K. X-ray computed tomography contrast agents prepared by seeded growth of gold nanoparticles in PEGylated dendrimer. Nanotechnology. 2010;21(24):245104. doi: 10.1088/0957-4484/21/24/245104. [DOI] [PubMed] [Google Scholar]
- 37.Rabin O, Perez JM, Grimm J, Wojtkiewicz G, Weissleder R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat Mater. 2006;5:118–22. doi: 10.1038/nmat1571. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Li ZH, Liu JH, Gu S, Yuan QH, Ren JS, et al. Long-circulating Er3+-doped Yb2O3 up-conversion nanoparticle as an in vivo X-Ray CT imaging contrast agent. Biomaterials. 2012;33:6748–57. doi: 10.1016/j.biomaterials.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 39.Dong K, Liu Z, Liu JH, Huang S, Li ZH, Yuan QH, et al. Biocompatible and highperformance amino acids-capped MnWO4 nanocasting as a novel non-lanthanide contrast agent for X-ray computed tomography and T-1-weighted magnetic resonance imaging. Nanoscale. 2014;6:2211–7. doi: 10.1039/c3nr05455a. [DOI] [PubMed] [Google Scholar]
- 40.Lu ZR, Wang XH, Parker DL, Goodrich KC, Buswell HR. Poly(L-glutamic acid) Gd(III)-DOTA conjugate with a degradable spacer for magnetic resonance imaging. Bioconjugate Chem. 2003;14:715–9. doi: 10.1021/bc0340464. [DOI] [PubMed] [Google Scholar]
- 41.Lu ZR, Parker DL, Goodrich KC, Wang XH, Dalle JG, Buswell HR. Extracellular biodegradable macromolecular gadolinium(III) complexes for MRI. Magn Reson Med. 2004;51:27–34. doi: 10.1002/mrm.10656. [DOI] [PubMed] [Google Scholar]
- 42.Vera DR, Mattrey RF. A molecular CT blood pool contrast agent. Acad Radiol. 2002;9:784–92. doi: 10.1016/s1076-6332(03)80348-3. [DOI] [PubMed] [Google Scholar]
- 43.Kao CY, Hoffman EA, Beck KC, Bellamkonda RV, Annapragada AV. Long-residence-time nano-scale liposomal lohexol for X-ray-based blood pool imaging. Acad Radiol. 2003;10:475–83. doi: 10.1016/s1076-6332(03)80055-7. [DOI] [PubMed] [Google Scholar]
- 44.Mohs AM, Zong YD, Guo JY, Parker DL, Lu ZR. PEG-g-poly(GdDTPA-co-L-cystine): effect of PEG chain length on in vivo contrast enhancement in MRI. Biomacromolecules. 2005;6:2305–2311. doi: 10.1021/bm050194g. [DOI] [PubMed] [Google Scholar]
- 45.Aviv H, Bartling S, Kieslling F, Margel S. Radiopaque iodinated copolymeric nanoparticles for X-ray imaging applications. Biomaterials. 2009;30:5610–5616. doi: 10.1016/j.biomaterials.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 46.Liu YL, Ai KL, Liu JH, Yuan QH, He YY, Lu LH. A high-performance ytterbium-based nanoparticulate contrast agent for in vivo X-ray computed tomography imaging. Angew Chem Int Ed. 2012;51:1437–1442. doi: 10.1002/anie.201106686. [DOI] [PubMed] [Google Scholar]
- 47.Kim D, Park S, Lee JH, Jeong YY, Jon S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J Am Chem Soc. 2007;129(24):7661–7665. doi: 10.1021/ja071471p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


