Abstract
Despite decades of speculation that inhibiting endogenous insulin degradation might treat type-2 diabetes1, 2, and the identification of IDE (insulin-degrading enzyme) as a diabetes susceptibility gene3, 4, the relationship between the activity of the zinc metalloprotein IDE and glucose homeostasis remains unclear. Although Ide−/− mice have elevated insulin levels, they exhibit impaired, rather than improved, glucose tolerance that may arise from compensatory insulin signalling dysfunction5, 6. IDE inhibitors that are active in vivo are therefore needed to elucidate IDE’s physiological roles and to determine its potential to serve as a target for the treatment of diabetes. Here we report the discovery of a physiologically active IDE inhibitor identified from a DNA-templated macrocycle library. An X-ray structure of the macrocycle bound to IDE reveals that it engages a binding pocket away from the catalytic site, which explains its remarkable selectivity. Treatment of lean and obese mice with this inhibitor shows that IDE regulates the abundance and signalling of glucagon and amylin, in addition to that of insulin. Under physiological conditions that augment insulin and amylin levels, such as oral glucose administration, acute IDE inhibition leads to substantially improved glucose tolerance and slower gastric emptying. These findings demonstrate the feasibility of modulating IDE activity as a new therapeutic strategy to treat type-2 diabetes and expand our understanding of the roles of IDE in glucose and hormone regulation.
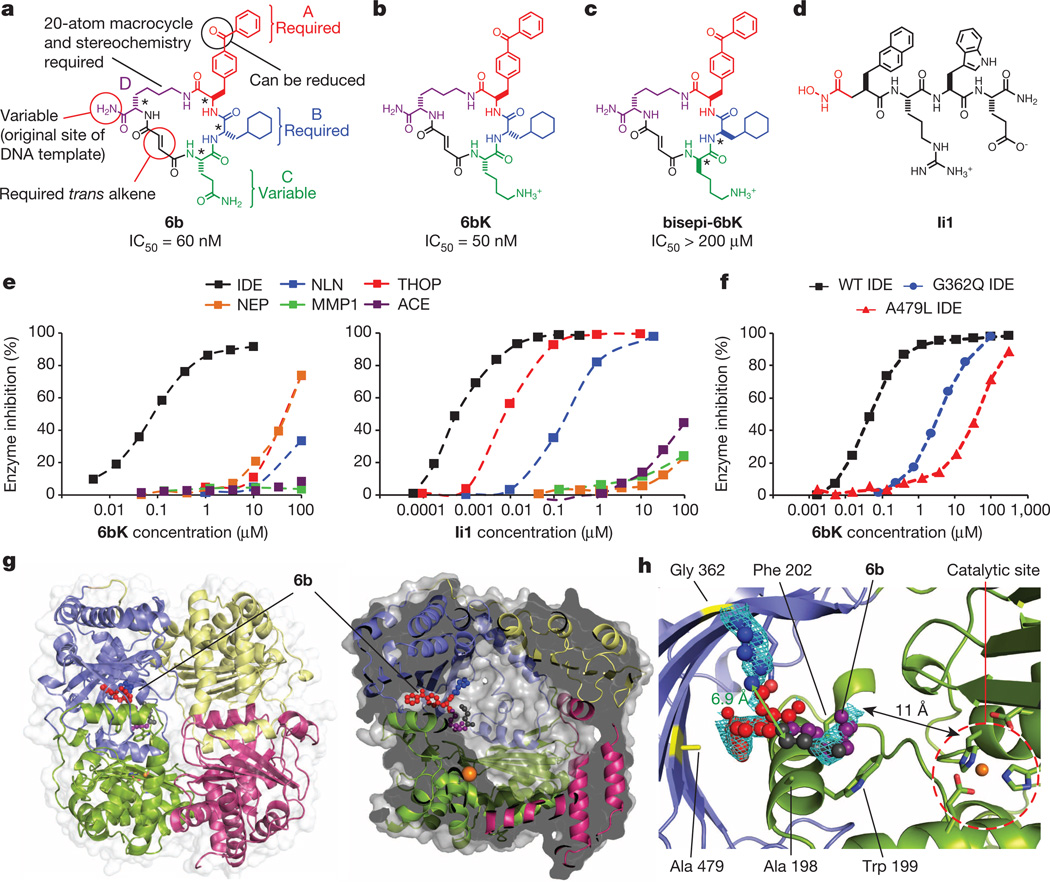
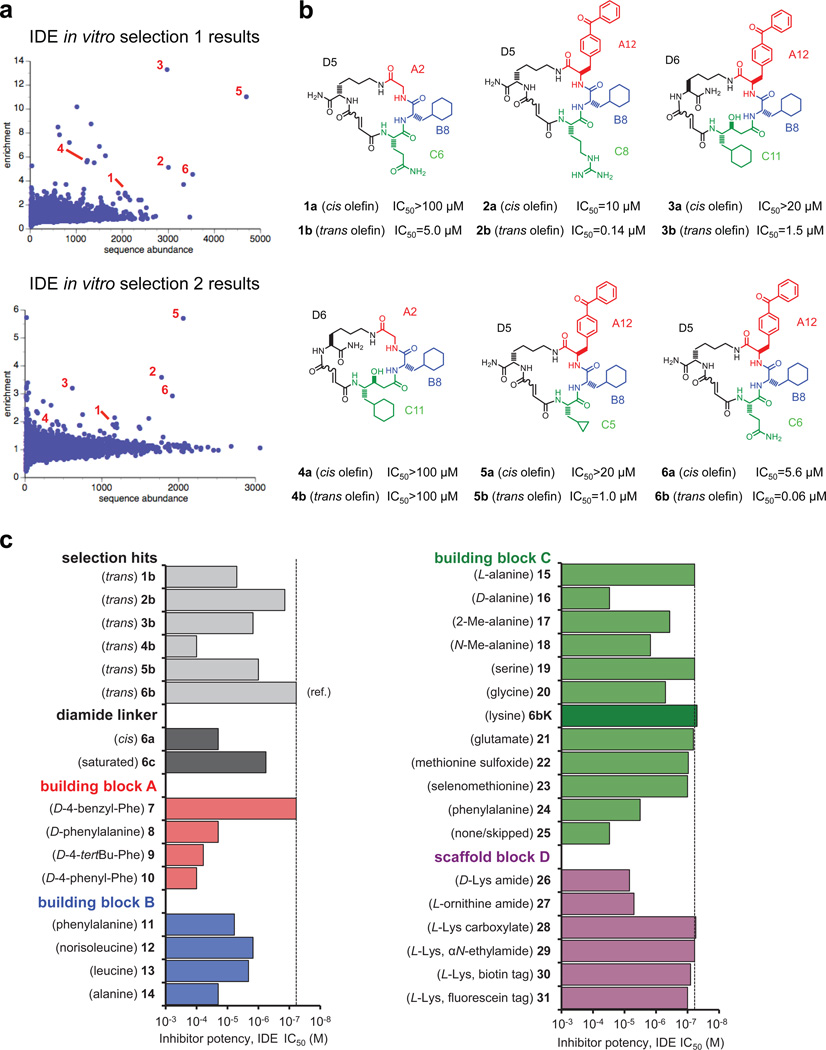
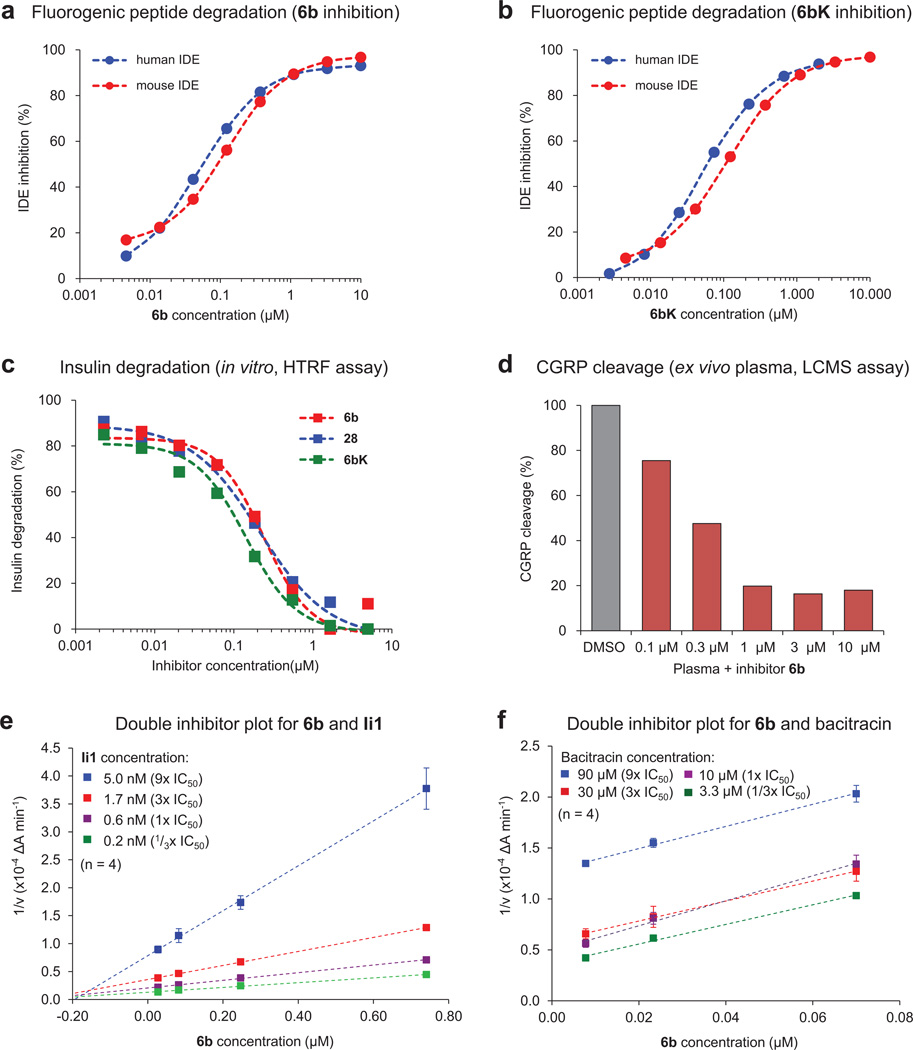
To discover small-molecule modulators of IDE, we performed in vitro selections on a DNA-templated library of 13,824 synthetic macrocycles7, 8 for the ability to bind immobilized mouse IDE, resulting in six candidate IDE-binding molecules (Extended Data Fig. 1).The 20-membered macrocycle 6b (Fig. 1a, half-maximum inhibitory concentration IC50 = 60 nM) potently inhibited IDE activity in three complementary assays (Extended Data Fig. 2)9. We synthesized and biochemically assayed 30 analogues of 6b in which each building block was systematically varied to elucidate the structural and stereochemical requirements (Extended Data Fig. 1), and based on the results we identified the inhibitor 6bK (IC50 = 50 nM, Fig. 1b) as an ideal candidate for in vivo studies.
Figure 1. Potent and highly selective macrocyclic IDE inhibitors from the in vitro selection of a DNA-templated macrocycle library.
a, Structure of 6b and summary of the requirements for IDE inhibition revealed by assaying 6b analogues (Extended Data Fig. 1). b, Physiologically active IDE inhibitor 6bK. c, Inactive diastereomer bisepi-6bK. d, Previously reported substrate-mimetic hydroxamic acid Ii110. e, Selectivity analysis of macrocycle 6bK reveals >1,000-fold selectivity for IDE (IC50 = 50 nM) over all other metalloproteases tested. In contrast, inhibitor Ii110 inhibits IDE (IC50 = 0.6 nM), thimet oligopeptidase (THOP, IC50 = 6 nM) and neurolysin (NLN, IC50 = 185 nM), but not NEP (neprilysin), MMP1 (matrix metalloproteinase, 1) or ACE (angiotensin-converting enzyme). f, Activity assays for wild-type or mutant human IDE variants in the presence of 6bK. g, X-ray co-crystal structure of IDE bound to macrocyclic inhibitor 6b (2.7 Å resolution, PDB 4LTE). h, Electron density map (composite omit map contoured at 1σ) showing the relative position of macrocycle 6b bound 11 Å from the catalytic zinc atom. The glutamine residue and four atoms of the macrocycle backbone were unresolved. See also Extended Data Figs 2–4.
The selectivity of 6bK in vitro was ≥1,000-fold for inhibition of IDE over all other metalloproteases tested, a substantial improvement over the previously reported substrate mimetic hydroxamic acid inhibitor Ii110 (Fig. 1d, e). The selectivity of 6bK, coupled with its ability to inhibit IDE in a synergistic, rather than competitive, manner with Ii1 (Extended Data Fig. 2), led us to speculate that the macrocycle engages a binding site distinct from the enzyme’s catalytic site (Supplementary Discussion).
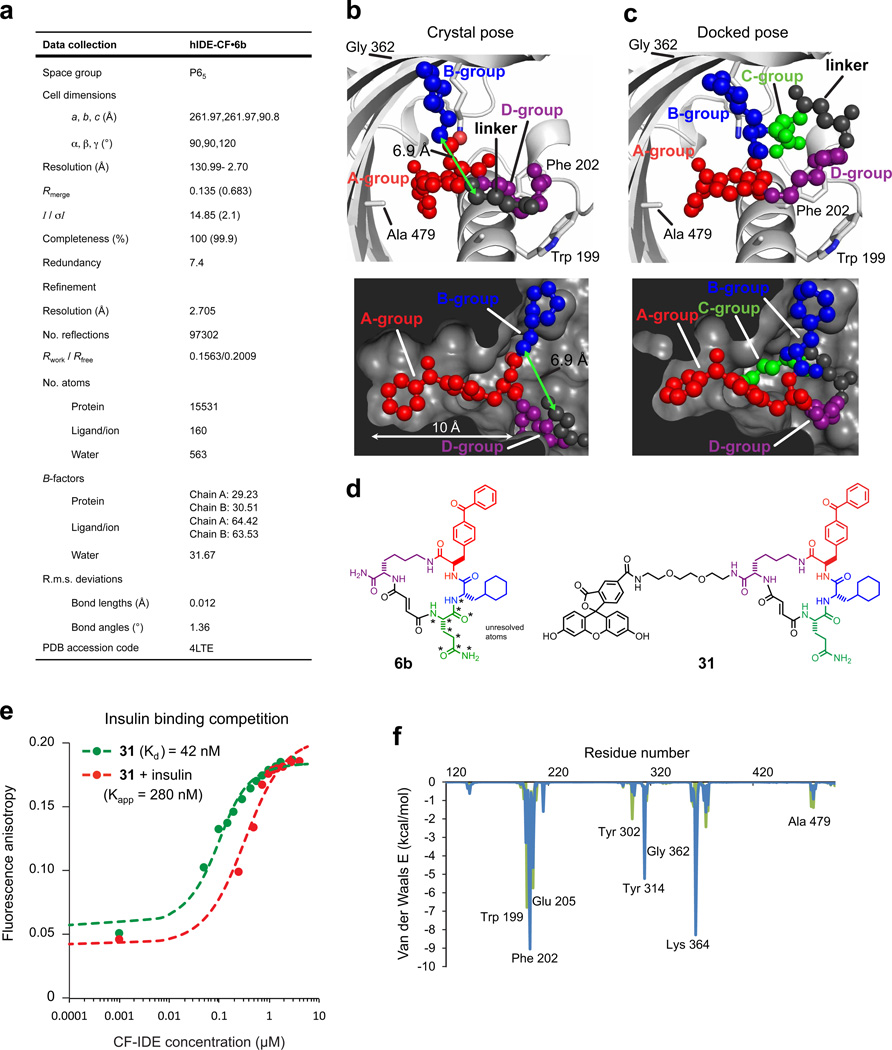
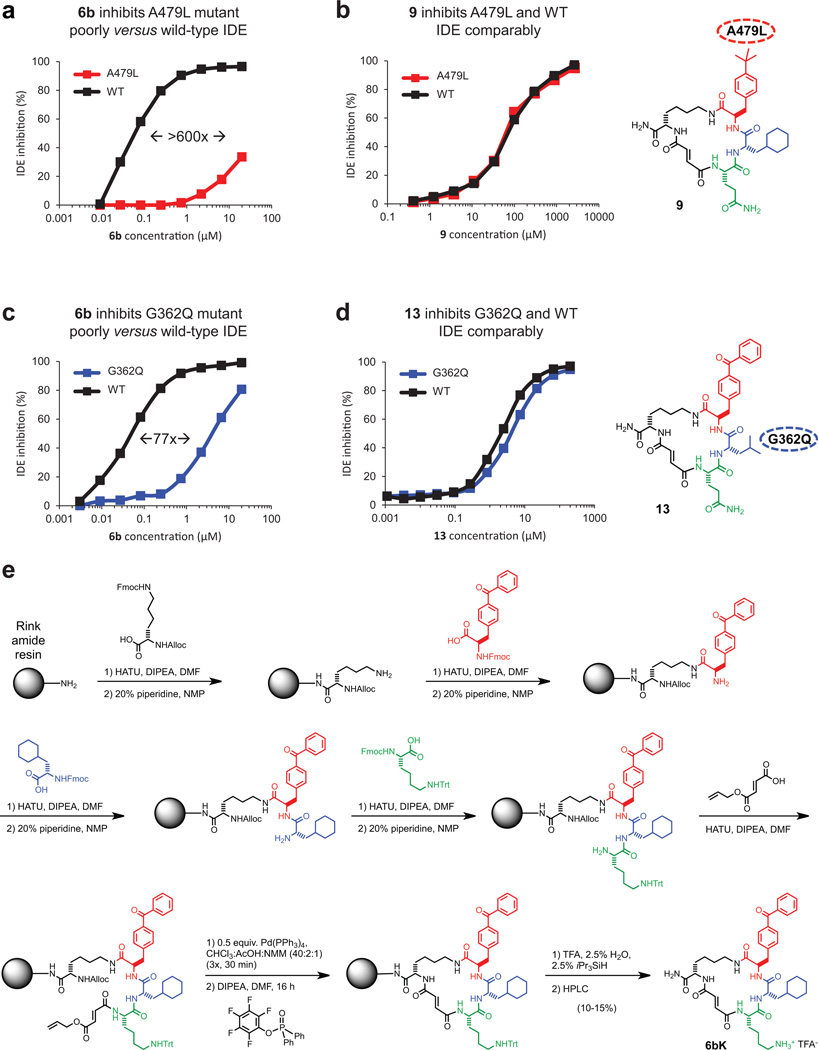
We determined the X-ray crystal structure of catalytically inactive cysteine-free human IDE11 bound to 6b at 2.7 Å resolution (Fig. 1g, Extended Data Fig. 3). Macrocycle 6b occupies a binding pocket at the interface of IDE domains 1 and 2, and is positioned 11 Å away from the catalytic zinc ion (Fig. 1h). This distal binding site is a unique structural feature of IDE compared to related metalloproteases12, and does not overlap with the binding site of Ii110. IDE mutations predicted by the structure to impede macrocycle binding led to losses of 6bK potency (Fig. 1f), and complementary changes in 6b analogues rescued inhibition (Supplementary Discussion, Extended Data Fig. 4). The structure predicts that by engaging this distal site the macrocycle precludes substrate binding and abrogates key interactions that are necessary to unfold peptides for cleavage (Supplementary Video)13, 14.
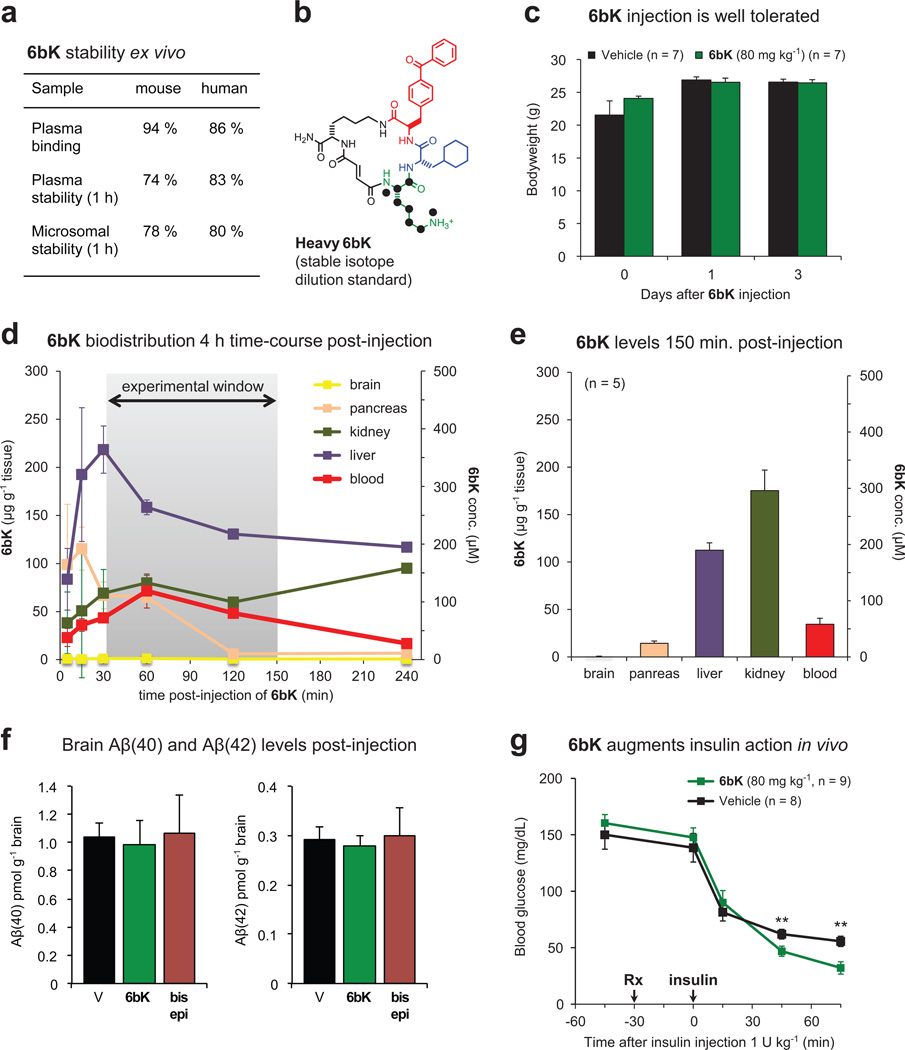
We characterized the stability, and the physicochemical and pharmacokinetic properties, of 6bK formulated in Captisol15, a β-cyclodextrin agent used to improve delivery through intraperitoneal (i.p.) injection at 2 mg 6bK per animal (Supplementary Discussion, Extended Data Fig. 5). The long half-life in mouse plasma (> 2 h) and in circulation (> 1h) of 6bK suggested that it was suitable for in vivo studies (Extended Data Fig. 5). Injection of 6bK resulted in high levels of the inhibitor (> 100-fold IC50) in peripheral circulation and in the liver and kidneys, the main insulin-degrading organs. In contrast, 6bK was undetectable in brain tissue, where IDE is known to degrade amyloid peptides5 (Extended Data Fig. 5), and levels of Aβ(40) and Aβ(42) peptides in mice injected with 6bK were unchanged (Extended Data Fig. 5). Taken together, these findings suggested the viability of 6bK as an in vivo IDE inhibitor.
To evaluate the ability of 6bK to inhibit IDE activity in vivo, we subjected non-fasted mice to insulin tolerance tests 30 min after a single injection with 6bK (2 mg per animal, 80 mg kg−1). Mice treated with 6bK experienced lower hypoglycaemia and higher insulin levels compared to vehicle controls (P < 0.01, see below and Extended Data Fig. 5). These experiments establish that a selective and physiologically stable pharmacological IDE inhibitor can augment the abundance and activity of insulin in vivo.
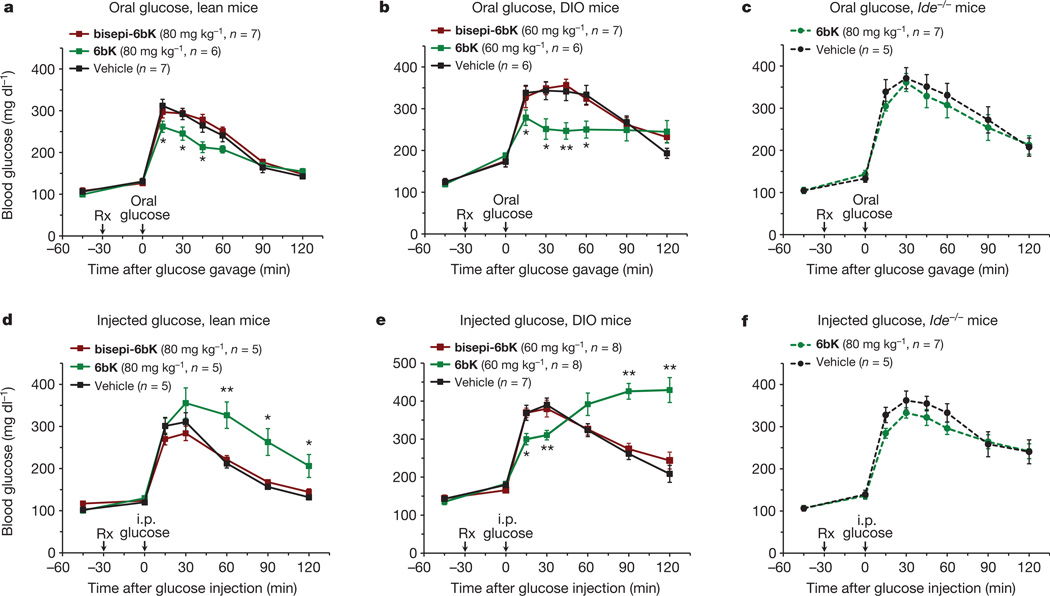
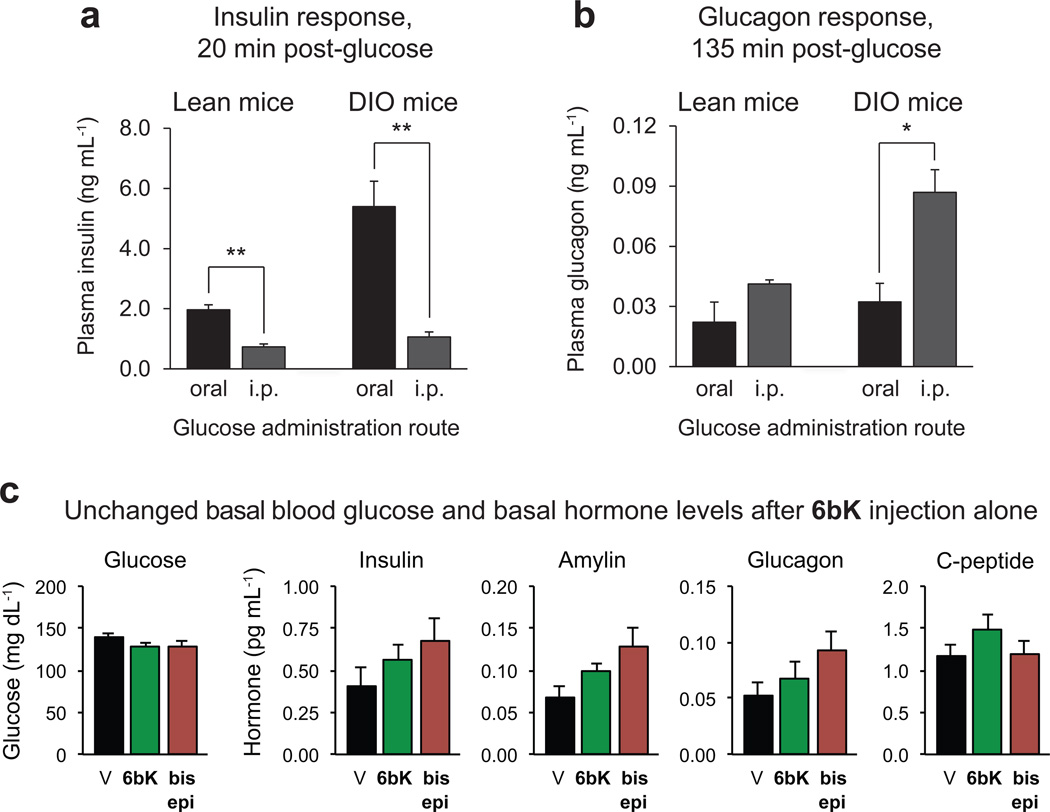
Next we determined the physiological consequences of acute IDE inhibition for glucose tolerance. We used two methods of glucose delivery (oral gavage or i.p. injection16) and two different mouse models (lean or diet-induced obese (DIO) mice17, 18). These four conditions were chosen to survey the role of IDE activity under a broad range of endogenous insulin levels and insulin sensitivities16, 17. Oral glucose administration results in greater insulin secretion compared to injected glucose delivery (Extended Data Fig. 6) that arises from the ‘incretin effect’17, 19. DIO mice display hyperinsulinaemia and insulin resistance, and serve as a model for early type-2 diabetes in humans18.
During oral glucose tolerance tests (OGTTs, Fig. 2a and b), both lean and DIO mice treated with 6bK displayed significantly improved glucose tolerance compared to control groups treated either with vehicle alone, or with the inactive stereoisomer bisepi-6bK (Figs 1c, 2a and b; Extended Data Fig. 7). Effects of similar magnitude on oral glucose tolerance in mice have been observed from several human diabetes therapeutics (Extended Data Fig. 8)19–21. The vehicle and bisepi-6bK control groups exhibited similar blood glucose profiles, indicating that that the effects of 6bK on glucose tolerance are lost when the stereochemistry of 6bK is altered in a way that abolishes IDE inhibition. Collectively, these observations represent the first demonstration that transient IDE inhibition improves blood glucose tolerance1.
Figure 2. Physiological consequences of acute IDE inhibition by 6bK on glucose tolerance in lean, DIO and Ide−/− mice.
a, b, Oral glucose tolerance during acute IDE inhibition. a, Male C57BL/6J lean (25 g) mice were treated (at time Rx) with a single i.p. injection of IDE inhibitor 6bK, inactive control bisepi-6bK, or vehicle alone, 30 min before glucose gavage (3.0 g kg−1). b, DIO mice (35–45 g) were treated with 6bK, and inactive control bisepi-6bK or vehicle alone 30 min before glucose gavage (3.0 g kg−1). c, Mice lacking the target (Ide−/−) treated with 6bK followed by oral glucose (3.0 g kg−1) produce a response comparable to that of vehicle-treated Ide−/− mice. d, e, Glucose tolerance phenotypes after i.p. injection of glucose (1.5 g kg−1) in, respectively, lean (d) and DIO (e) male mice treated with 6bK, inactive bisepi-6bK, or vehicle alone. f, Mice lacking IDE treated with 6bK followed by IPGTT (1.5 g kg−1) produce a response comparable to that of vehicle-treated Ide−/− mice. Area under the curve (AUC) calculations are shown in Extended Data Fig. 9. Data: mean ± s.e.m.; *P < 0.05, **P < 0.01 in two-tail Student’s t-test. Data shown in a, b, d and e are representative of two or more independent studies. Control studies using knockout mice were performed once.
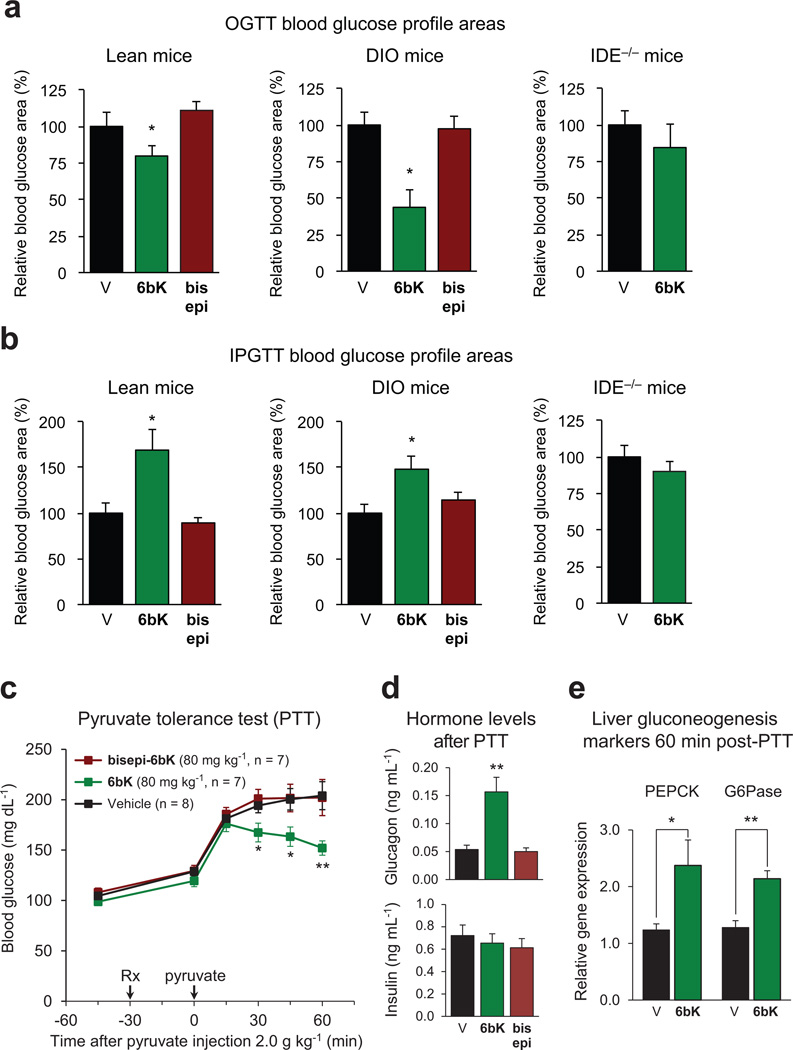
We repeated the above experiments with 6bK using i.p.-injected glucose tolerance tests (IPGTTs). In contrast to the observed improvement in oral glucose tolerance on 6bK treatment, IDE inhibition with 6bK followed by a glucose injection (1.5 g kg−1 i.p.) surprisingly resulted in impaired glucose tolerance in both lean and obese mice compared to vehicle alone or bisepi-6bK-treated controls (Fig. 2d and e). DIO mice treated with 6bK followed by glucose injection displayed a biphasic response: glucose levels were lower over the initial 30 min of the IPGTT, followed by a hyperglycaemic ‘rebound’ starting 1 h after glucose injection (Fig. 2e and Extended Data Fig. 7).To further test if the effects of 6bK are specific to its interaction with IDE, we repeated these experiments using Ide−/− knockout mice5, 6. Mice lacking IDE were not affected by 6bK treatment and exhibited OGTT and IPGTT blood glucose responses comparable to that of vehicle-treated cohorts (Fig. 2c and f), suggesting that blood glucose profile improvement during OGTT and impairment during IPGTT of 6bK-treated wild-type mice are mediated by IDE.
The dependence of the physiological response to 6bK on the route of glucose administration cannot be easily explained by a simple model in which IDE only degrades insulin. Instead, these results strongly suggest a role for IDE in modulating other glucose-regulating hormones in vivo beyond insulin. The biochemical properties of IDE and its substrate recognition mechanism12, 13 enable this enzyme to cleave a wide range of peptide substrates in vitro for which experimental validation in vivo has not been previously possible (Supplementary Table 1). Two glucose-regulating hormones, beyond insulin, that are potential candidates for physiological regulation by IDE are glucagon and amylin. Whereas purified IDE can cleave these two peptides in vitro22, 23, neither hormone is known to be regulated by IDE in vivo or ex vivo.
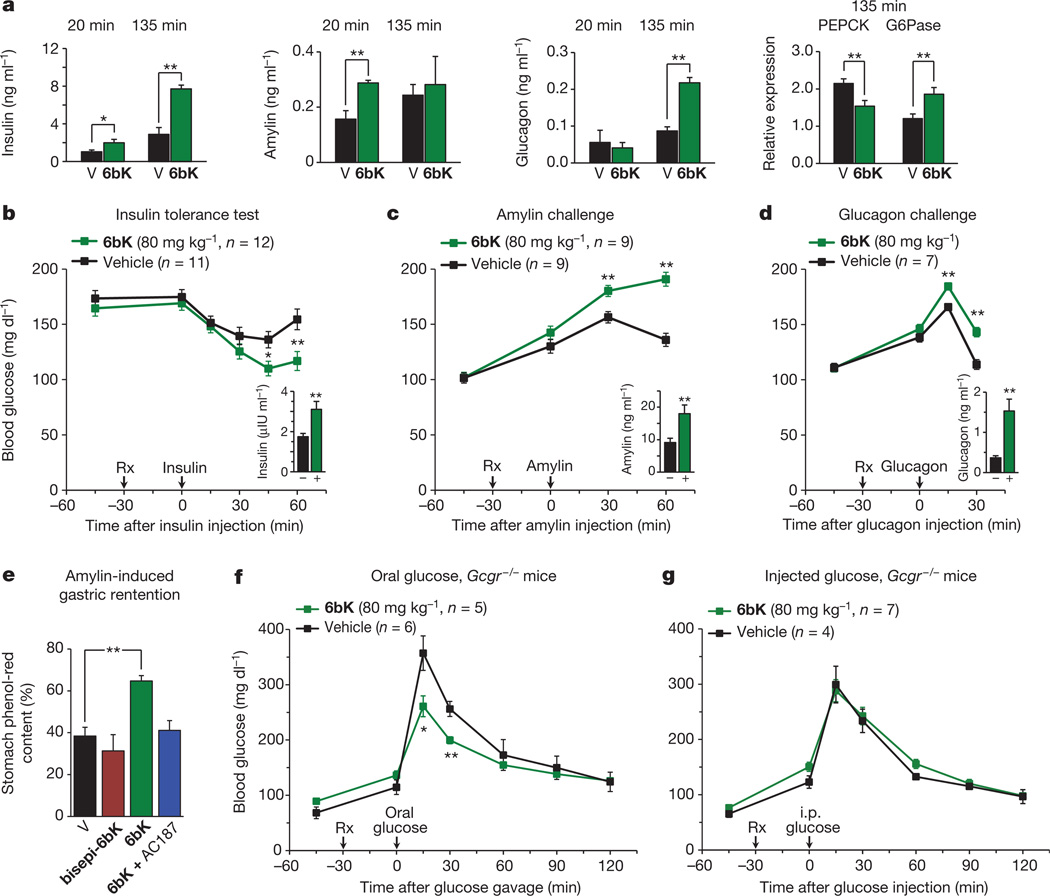
To probe the possibility that glucagon and/or amylin are regulated in vivo by IDE, we measured plasma levels of these hormones in DIO mice treated with 6bK or vehicle alone following IPGTTs (Fig. 3a), and observed substantially higher levels of insulin, glucagon and amylin. Next we injected each of these three putative substrates 30 min after treatment with 6bK or vehicle alone (Fig. 3b–d).The 6bK-treated cohorts exhibited significantly stronger blood glucose responses to each of these hormones; mice treated with 6bK experienced hypoglycaemia during insulin tolerance tests (Fig. 3b) and hyperglycaemia following challenges with either amylin24 (Fig. 3c) or glucagon (Fig. 3d) compared to control animals (via non-physiological activation of the Cori cycle, and gluconeogenesis, respectively)24–26. Moreover, in each case the plasma level of the hormone injected remained elevated at the end of the procedure in 6bK-treated mice relative to control animals (Figs 3b–d insets). Collectively these results reveal that IDE regulates the abundance and physiological effects of glucagon and amylin, in addition to insulin.
Figure 3. Acute IDE inhibition affects the abundance of multiple hormone substrates and their corresponding effects on blood glucose levels.
a, Plasma hormone measurements at 20 min and 135 min after i.p.-injected glucose tolerance tests (IPGTTs) on DIO mice (Fig. 2e) and RT–PCR analysis of DIO liver samples collected at 135 min after IPGTT. RT–PCR reveals 50% higher glucose-6-phosphatease (G6Pase) and 30% lower phosphoenolpyruvate carboxykinase (PEPCK) transcript levels for the 6bK-treated cohort (6bK; n = 7) versus vehicle-only controls (V; n = 7). b–d, Blood glucose responses and injected hormone abundances in lean mice 30 min after treatment with 6bK or vehicle alone. b, Insulin (0.25 U kg−1, subcutaneous) after 5-h fast. c, Amylin (250 µg kg−1, subcutaneous) after overnight fast. d, Glucagon (100 µg kg−1, subcutaneous) after overnight fast. Trunk blood was collected at the last time-points for plasma hormone measurements (insets; IU, international units). e, Acute IDE inhibition slows gastric emptying through amylin signalling. Fasted WT mice were given oral glucose supplemented with 0.1 mg ml−1 phenol red 30 min after treatment with 6bK alone (n = 6), 6bK co-administered with the amylin receptor antagonist25 AC187 (3 mg kg−1 i.p., n = 6), vehicle alone (V; n = 6) or inactive bisepi-6bK (n = 4). f, g, G-protein-coupled glucagon receptor knockout mice (Gcgr−/−) treated with IDE inhibitor 6bK display altered glucose tolerance relative to vehicle-treated mice if challenged with oral glucose (3.0 g kg−1; f) but not i.p. injected glucose (1.5 g kg−1; g). Data: mean ± s.e.m.; *P < 0.05, **P < 0.01 in two-tail Student’s t-test. Data shown in a–e are representative of two or more independent studies. Studies using Gcgr−/− knockout mice were performed once.
Amylin is co-secreted with insulin, and is involved in glycaemic control by inhibiting gastric emptying, promoting satiety and antagonizing glucagon secretion24, 25. Pramlintide (Symlin) is a synthetic amylin derivative used clinically to control post-prandial glucose levels20, 21. To determine the effects of IDE inhibition on endogenous amylin signalling, we measured gastric emptying efficiency, an amylin-mediated process25. Mice treated with 6bK exhibited twofold slower gastric emptying compared to vehicle and bisepi-6bK-treated controls (Fig. 3e). Importantly, co-administration of AC187, a 25-mer peptide amylin receptor antagonist25, blocked the effects of 6bK on gastric emptying (Fig. 3e). Collectively, these data reveal that IDE inhibition can slow post-prandial gastric emptying by modulating amylin signalling in vivo at physiologically relevant levels. Amylin-mediated effects on gastric emptying and satiety have been recognized to have therapeutic relevance20, 21, and our results demonstrate a small-molecule-based strategy to modulate amylin signalling (Extended Data Fig. 10).
Higher glucagon levels on 6bK treatment provide a possible explanation for impaired glucose tolerance during IPGTT experiments. Substrates are processed by IDE at rates dependent on their relative concentrations (Supplementary Discussion). We observed two- to four-fold higher insulin levels during OGTTs than IPGTTs, consistent with the incretin effect16–19 (Extended Data Fig. 6). During an OGTT, IDE inhibition therefore results primarily in an increase in insulin signalling and lower blood glucose levels. In contrast, during an IPGTT, insulin secretion levels are lower16–19, IDE processes proportionally more glucagon, and the loss of IDE activity thus results in higher glucagon and glucose levels (Figs 2d, e and 3a; Supplementary Discussion)16–19.
To test this hypothesis, we repeated the glucose tolerance experiments using Gcgr−/− mice that lack the G-protein-coupled glucagon receptor (Fig. 3f and g)26. Treatment of Gcgr−/− mice with 6bK followed by an OGTT resulted in improved glucose tolerance as expected (Fig. 3f), consistent with a model in which insulin signalling in these mice is intact and regulated by IDE. In contrast, glucose tolerance in Gcgr−/− mice following IPGTT was not impaired by 6bK treatment, consistent with a model in which glucagon signalling is responsible for driving higher glucose levels in wild-type mice on IDE inhibition during IPGTTs (compare Figs 3g and 2d). In addition, 6bK treatment augmented the expression of liver gluconeogenic markers regulated by endogenous glucagon signalling following a pyruvate injection (Supplementary Discussion, Extended Data Fig. 9). These results collectively show that the ability of IDE to regulate glucagon in vivo can account for the impaired glucose tolerance observed during IPGTTs (see the Supplementary Discussion on the hyperglycaemic rebound phase in DIO mice).
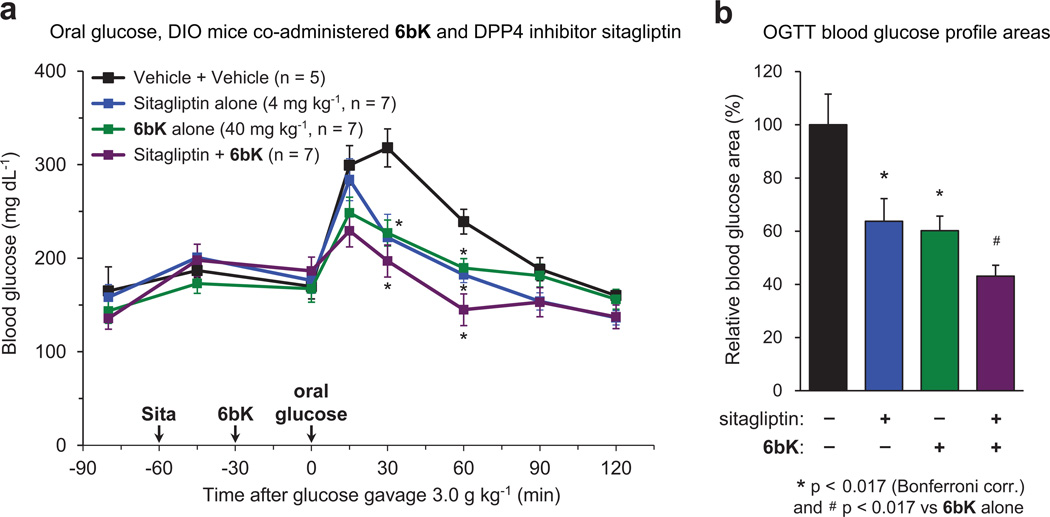
The discovery and application of the physiologically active IDE inhibitor 6bK reveals that transient IDE inhibition can improve glucose tolerance under conditions that mimic the intake of a meal. In the context of recent genetics studies identifying IDE as a diabetes susceptibility gene3, 4, our findings establish the potential of IDE as a target for the treatment of diabetes1, 2. Our study also reveals a new requirement for therapeutic strategies that target IDE—namely, that transient IDE inhibition during meals, rather than chronic treatment, is desirable to minimize elevation of glucagon signalling (Extended Data Fig. 10)21, 27. Plausible pharmacological strategies to circumvent elevation of postprandial glucagon levels include: (1) development of fast- and short-acting pre-meal IDE inhibitors21; (2) combination therapy of an IDE inhibitor with glucagon-lowering incretin therapies20, 21; and (3) co-administration with glucagon receptor antagonists27. Moreover, existing anti-diabetic therapeutics when co-administered with an IDE inhibitor may result in synergistic effects28; indeed 6bK treatment results in stronger improvement in oral glucose tolerance when co-administered with the DPP4 inhibitor sitagliptin (Extended Data Fig. 8)19, 20. In addition, the IDE•6b structure raises the possibility of developing IDE inhibitors that selectively impede insulin degradation without affecting glucagon degradation. These findings collectively inform and motivate additional studies to progress these discoveries towards new diabetes therapeutics.
METHODS SUMMARY
The in vitro selection of the DNA-templated library8 used 20 µg His6-tagged mouse IDE immobilized on cobalt magnetic beads (Invitrogen). IDE inhibition was assayed using the fluorogenic peptide Mca-RPPGFSAFK(Dnp)-OH (R&D), confirmed using an anti-insulin antibody time-resolved FRET assay (Cysbio), and a LCMS assay for CGRP cleavage fragments in plasma9. Macrocyclic inhibitors were synthesized by Fmoc-based solid-phase synthesis and purified by HPLC. LCMS quantitation of 6bK in biological samples was performed using 6bK synthesized with 13C6,15N2 lysine (Sigma-Aldrich).
Wild-type lean and DIO C57BL/6J age-matched male mice (Jackson Laboratories) were used at 14–16, and 24–26 weeks respectively (> 20 weeks of high-fat diet). Gcgr−/− and Ide−/− mice were fully backcrossed to the C57BL/6J line, bred from heterozygous mice, and used between 11 and 21 weeks. Animals were fasted overnight 14 h for all experiments, except for the insulin tolerance test, which required 5 h of fasting during the morning. Blood glucose was measured from tail nicks using AccuCheck (Aviva) meters. Trunk blood was obtained for plasma hormone measurements using the Multiplexed Mouse Metabolic Hormone panel (Milliplex, EMD Millipore) on a Luminex FlexMap 3D instrument.
Extended Data
Extended Data Figure 1.
a, Enrichment plots from two independent in vitro selections against N-His6-mIDE using a 13,824-membered DNA-templated macrocycle library7,8. The numbers highlight compounds enriched at least twofold in both selections. b, Structures of IDE-binding macrocycles 1–6 decoded from DNA library barcodes corresponding to building blocks A, B, C and D (Fig. 1). The cis and trans isomers are indicated by suffices a and b respectively (for example, 1a and 1b). The two isomers were synthesized as previously reported18,19 and separated by HPLC. c, IDE inhibition potency of trans hits 1b to 6b compared to 30 structurally related 6b analogues in which the linker, scaffold and the three building blocks were systematically varied. IDE inhibition activity was assayed by following cleavage of the fluorogenic peptide substrate Mca-RPPGFSAFK(Dnp)-OH.
Extended Data Figure 2. Inhibition of human and mouse IDE activity in multiple assays.
a, b, Human IDE shares 95% sequence homology with mouse IDE13, and cleavage of the fluorogenic substrate peptide Mca-RPPGFSAFK(Dnp)-OH by human and mouse IDE is inhibited with similar potency by 6b (a) and 6bK (b). c, Homogeneous time-resolved fluorescence (HTRF) assay measuring degradation of insulin by IDE in the presence of 6b, 6bK and analogue 28 (Extended Data Fig. 1). d, LC-MS assay for ex vivo degradation of CGRP (10 µM) by endogenous IDE in mouse plasma in the presence of 6b. e, f, Biochemical assays suggesting that 6b binds a site in IDE distinct from the conventional peptide substrate binding site known to bind substrate mimetic Ii1. Yonetani–Theorell double inhibitor plots of IDE activity in the presence of 6b and Ii1 (panel e), or 6b and bacitracin (panel f). Crossing lines indicate synergistic and independent binding of inhibitors, while parallel lines indicate competition for binding to the enzyme.
Extended Data Figure 3. Data collection and refinement statistics (molecular replacement), docking simulation for 6b, and competition test between insulin and fluorescein-labelled macrocycle 31 for binding cysteine-free IDE (CF-IDE).
a, One crystal was used to solve the CF-IDE•6b structure. The highest-resolution shell is shown in parentheses. Structure coordinates are deposited in the Protein Data Bank (accession number 4 LTE). b, Molecular docking simulations are consistent with the placement of building blocks A and B in the structural model (two views shown: top and bottom panels). The structure of 6b in the binding site from crystallographic data with composite omit map contoured at 1.0 σ (p-benzoyl-phenylalanine is shown in red, the cyclohexylalanine in blue, the fumarate linker in grey, and the d-lysine backbone in purple). c, Highest-scoring pose from DOCK simulations (glutamine group is shown in green, see Supplementary Information for docking calculations). d, Structure of macrocycle 6b and fluorescent analogue 31. Stars indicate atoms not resolved in the crystal structure (the Gln building block and four atoms of the flexible macrocycle backbone). e, Competition test between the fluorescein-labelled macrocycle 31 and insulin for binding CF-IDE. Cysteine-free catalytically inactive IDE was titrated against 0.9 nM macrocycle 31 alone (filled green circles) or together with 2.15 µM insulin (red filled circles), producing a shift in apparent dissociation constant for macrocycle 31 according to equation (1) (Supplementary Information). f, Residue-decomposed energy of the crystal (green) and docked (blue) poses of 6b (see Supplementary Information for docking calculations).
Extended Data Figure 4. Small-molecule/enzyme mutant complementation study to confirm the macrocycle binding site and placement of the benzophenone and cyclohexyl building-block groups.
a, IDE mutant A479L is inhibited by 6b > 600-fold less potently compared to wild-type IDE. b, Analogue 9, in which the p-benzoyl ring is substituted for a smaller t-butyl group, inhibits A479L IDE and WT IDE comparably. c, Similarly, IDE mutant G362Q is inhibited 77-fold less potently by 6b compared with WTIDE. d, Analogue 13, in which the L-cyclohexyl alanine side chain was substituted with a smaller L-leucine side chain, inhibits G362Q IDE and WT IDE comparably. The full list of IDE mutants investigated is shown in Supplementary Table 5. e, Synthetic scheme for 6bK, also used for synthesis of 6bK analogues.
Extended Data Figure 5. Pharmacokinetic parameters of 6bK, augmented insulin hypoglycaemic action by 6bK in mice, and effects of 6bK on amyloid peptide levels.
a, Plasma binding, plasma stability, and microsomal stability (1 h incubation) data (S. Johnston and C. Mosher, personal communication). b, Heavy-labelled 6bK was synthesized with 15N, 13C-lysine for stable-isotope dilution LC-MS quantitation. c, Behaviour and post-experiment body weight measurements were not affected for mice treated with 6bK (green; 80 mg kg−1) versus vehicle alone (black). The mice were active, and displayed normal posture, normal grooming and normal response to stimulation. d, Concentration of 6bK in mice tissues and plasma collected over 4 h determined by isotope dilution mass spectrometry (IDMS) with heavy-labelled analogue (n = 2 for all data except the last two time-points, for which n = 1). e, Average biodistribution of 6bK in five lean mice at 150 min post-injection of 6bK 80 mg kg−1 i.p. at the endpoint of an IPGTT experiment. We did not detect 6bK in the brain even using tenfold concentrated samples for LC-MS injection compared to other tissues. f, Treatment of C57BL/6J lean mice with 6bK (green, 80 mg kg−1, n = 6) does not change brain levels of Aβ(40) or Aβ(42) peptides in the brain 2 h post injection compared to treatment with vehicle alone (black, n = 5) or inactive diastereomer bisepi-6bK (brown, 80 mg kg−1, n = 6). g, Mice treated with a single injection of 6bK (green, 80 mg kg−1 i.p.) display increased hypoglycaemic response to insulin injection (Humulin-R, 1.0 U kg−1 i.p., see also Fig. 3b). Data shown in c–e and g are representative of two or more independent studies.
Extended Data Figure 6. Dependence of insulin and glucagon secretion on the route of glucose administration (oral or i.p.) due to both the ‘incretin effect’ and the hyperinsulinaemic phenotype of DIO versus lean mice.
a, The early insulin response to glucose in lean and DIO mice is higher during OGTT than IPGTT. b, Suppression of glucagon secretion post-glucose administration is less effective after IPGTT and in DIO mice. c, Administration of 6bK (green, 80 mg kg−1, n = 7) to lean mice not followed by injection of a nutrient such as glucose or pyruvate (see Figs 2–3) did not significantly alter basal blood glucose or basal hormone levels compared to bisepi-6bK (brown, n = 7) or vehicle controls (black, n = 7) 30 min post-injection. All data points and error bars represent mean ± s.e.m. Statistics were performed using a two-tail Student’s t-test, and significance levels shown in the figures are *P < 0.05 or **P < 0.01 between the labelled groups. Data shown are representative of two or more independent studies.
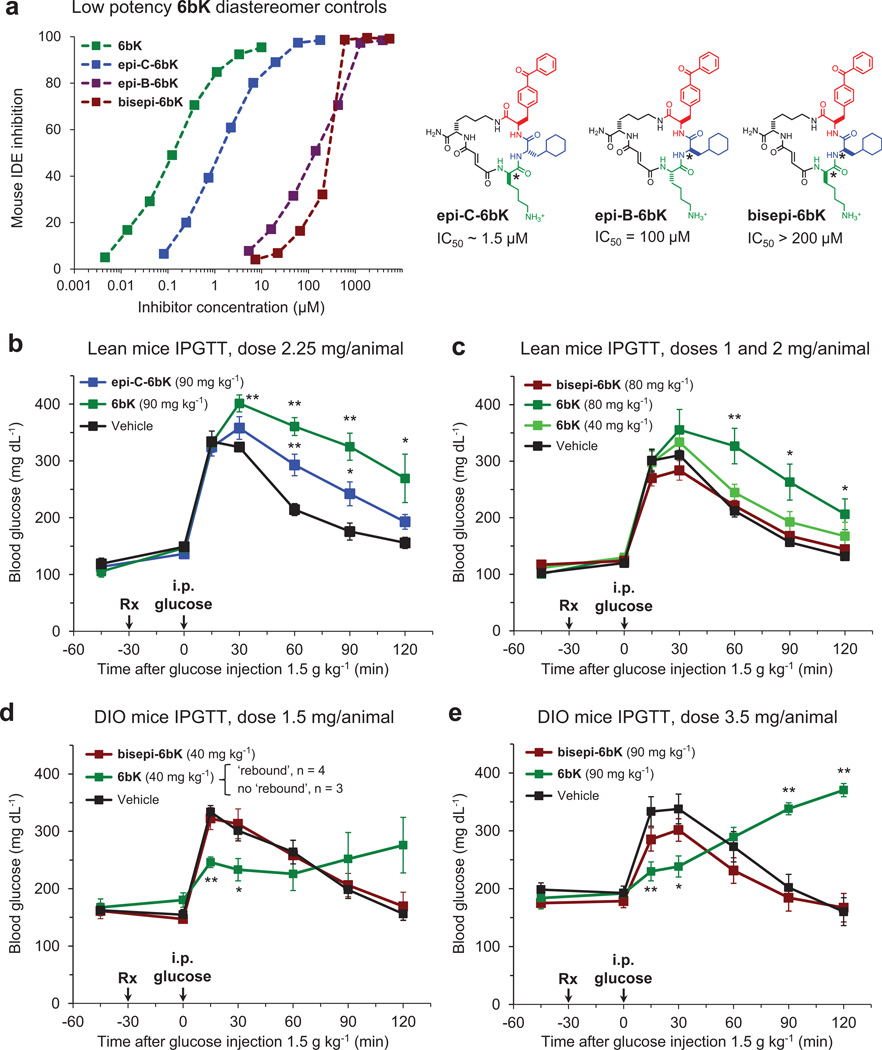
Extended Data Figure 7. Low-potency diastereomers of 6bK used to determine effective dose range and confirm on-target IDE inhibition effects during IPGTTs.
a, Left, inhibition of mouse IDE activity by low potency diastereomers of 6bK (right). The stereocentres altered in each compound relative to those of 6bK are labelled with asterisks. b–e, In dose optimization experiments, the effects of 6bK (40 to 90 mg kg−1, n = 5 and 4, respectively) were compared with equal doses of either weakly active stereoisomer epi-C-6bK (n = 5, panel b) or inactive stereoisomer bisepi-6bK (n = 5, panel c) and vehicle controls (n = 5) in IPGTTs using lean and obese mice. d, DIO mice treated with low doses of 6bK (40 mg kg−1, n = 7) responded to IPGTT in either of two ways: improved glucose tolerance throughout the experiment (n = 3) or a hyperglycaemic ‘rebound’ as described in the main text (n = 4), suggesting this dose is too low to achieve a consistent ‘rebound’ phenotype (note the large error bars). e, DIO mice treated with high doses of 6bK (3.5 mg per animal, 90 mg kg−1, n = 5) respond similarly to Fig. 2e (2 mg per animal, 60 mg kg−1), but the weak activity observed for bisepi-6bK (IC50 > 200 µM) using a matching dose (90 mg kg−1, n = 5) compared to vehicle alone (n = 5) suggests that 60 mg kg−1 (2 mg per animal) is the appropriate dose for DIO mice experiments. All data points and error bars represent mean ± s.e.m. Statistics were performed using a two-tail Student’s t-test, and significance levels shown in the figures are *P < 0.05 versus vehicle control group; **P < 0.01 versus vehicle control group. Data shown in b–e are representative of two or more independent studies.
Extended Data Figure 8. Effects of co-administration of 6bK and dipeptidyl peptidase 4 (DPP4) inhibitor sitagliptin followed by oral glucose challenge.
a, DIO mice were first treated with either oral gavage of sitagliptin (4 mg kg−1, 5 ml kg−1 in sterile saline, n = 14) or saline alone (n = 12). After 30 min, each group of mice were treated either with a low dose of 6bK (40 mg kg−1, n = 7) or vehicle alone (n = 5), and after an additional 30 min all mice were given a bolus of glucose by gavage (3.0 g kg−1, 10 ml kg−1). Mice treated with the combination of sitagliptin and 6bK displayed glucose levels lower than baseline (t = 0) after 60 min. b, Blood glucose profile areas of sitagliptin and 6bk were similarly reduced by 60–64% compared to vehicle alone, and further 15% lower when sitagliptin and 6bK were co-administered. Symbol and bar colour-coding: black, vehicle alone; blue, sitagliptin alone; green, 6bK alone; purple, sitagliptin + 6bK. All data points and error bars represent mean ± s.e.m. Statistics were performed using a one-tail Student’s t-test. Significance levels shown in the figures are: *P < 0.017 (Bonferroni correction) versus vehicle control group, #P < 0.017 versus the 6bK cohort. See Supplementary Methods for a description of the AUC calculation. The data shown are from a study performed once.
Extended Data Figure 9. Glucose tolerance test AUC(area under the curve) calculations, and pyruvate tolerance test for gluconeogenesis during IDE inhibition.
a, AUC calculations for Fig. 2 data show that during OGTT, lean and DIO mice treated with 6bK display improved glucose tolerance, compared to vehicle controls and inactive bisepi-6bK. b, In contrast, during IPGTT both lean and DIO mice treated with 6bK display impaired glucose tolerance compared to vehicle or bisepi-6bK controls. The AUCs for 6bK versus vehicle treatments using Ide−/− mice are similar for both OGTT and IPGTT. c, Wild-type mice fasted overnight were injected i.p. with pyruvate (2.0 g kg−1) 30 min after treatment with 6bK, inactive analogue bisepi-6bK, or vehicle alone. d, Plasma hormone measurements 60 min post-PTT reveal elevated glucagon (top) but similar insulin (bottom) levels for the 6bK-treated cohort relative to bisepi-6bK or vehicle controls. e, RT–PCR analysis of liver samples 60-min post-PTT revealed elevated gluconeogenesis transcriptional markers for the 6bK-treated group relative to vehicle controls. All data points and error bars represent mean ± s.e.m. Statistics were performed using a two-tail Student’s t-test, and significance levels shown in the figures are *P < 0.05 versus vehicle control group; **P < 0.01 versus vehicle control group. See Supplementary Methods for a description of the AUC calculation. Data shown not involving knockout mice are representative of two or more independent studies; studies on knockout mice and PTT measurements were performed once.
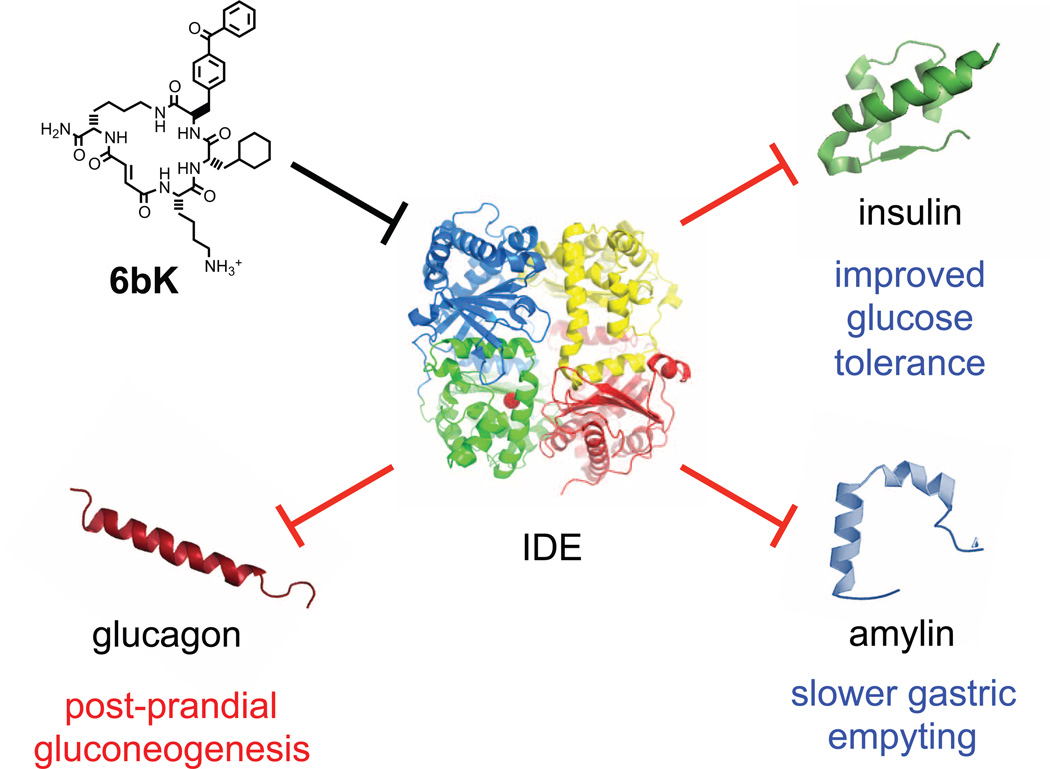
Extended Data Figure 10. Model for the expanded roles of IDE in glucose homeostasis and gastric emptying based on the results of this study.
IDE inhibition increases the abundance and signalling of three key pancreatic peptidic hormones—insulin, amylin and glucagon—with the corresponding physiological effects shown in blue, blue and red, respectively.
Acknowledgements
This research was supported by NIH/NIGMS (R01 GM065865 (D.R.L.), R00 GM080097 (M.A.S.), R01 GM81539 (W.-J.T.), T32 GM008444 (Z.H.F.), F30 CA174152 (Z.H.F.), DP2 OD002374 (A.S.)), Howard Hughes Medical Institute (D.R.L.), Diabetes and Cancer Centers of Albert Einstein College of Medicine (M.J.C.), American Diabetes Association no. 7-11-CD-06 (M.A.L.), Burroughs Wellcome Fund CABS (A.S.), and the Searle Scholars Program(A.S.). The Fonds de Recherche en Santé du Québec (FRSQ) and the Alfred Bader Fund supported J.P.M. We thank C. Russ and H. Spurling (Broad Institute) and C. Daly (FAS Center for Systems Biology) for DNA sequencing assistance. We are grateful to S. Johnston and C. Mosher (Broad Institute) for 6bK stability measurements. W. Nolte provided mouse IDE, L. McCord purified CF-IDE and Y.-G. Kim performed CGRP cleavage assays. We are grateful to A. Badran, E. Homan, A. M. Lone and M. Leidl (Harvard University) for experimental assistance. We thank B. Kahn and N. Gray for discussions.
Footnotes
Online Content Any additional Methods, Extended Data display items and Source Data are available in the online version of the paper; references unique to these sections appear only in the online paper.
Supplementary Information is available in the online version of the paper.
Author Contributions J.P.M., A.M., Z.H.F., R.E.K. and X.Q.D. performed the experiments. M.A.L. provided the Ide−/− mice. W.-J.T. provided IDE protein for structural studies. M.J.C. supervised the Gcgr−/− studies. M.A.S. supervised the IDE•6b structural studies. A.S. supervised the pharmacological validation of 6bK and the in vivo studies. D.R.L. supervised the discovery of IDE inhibitors, the pharmacological studies and the in vivo studies. All authors analysed the data and wrote the manuscript.
Author Information The coordinates and the structure factors of the IDE•6b complex have been deposited in the Protein Data Bank under the accession code 4 LTE. The authors declare competing financial interests: details are available in the online version of the paper. Readers are welcome to comment on the online version of the paper.
References
- 1.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr. Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 2.Mirsky IA, Broh-Kahn RH. The inactivation of insulin by tissue extracts the distribution and properties of insulin inactivating extracts. Arch. Biochem. 1949;20:1–9. [PubMed] [Google Scholar]
- 3.Sladek R, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 4.Zeggini E, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farris W, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc. Natl Acad. Sci. USA. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdul-Hay SO, et al. Deletion of insulin-degrading enzyme elicits antipodal, age-dependent effects on glucose and insulin tolerance. PLoS ONE. 2011;6:e20818. doi: 10.1371/journal.pone.0020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gartner ZJ, et al. DNA-templated organic synthesis and selection of a library of macrocycles. Science. 2004;305:1601–1605. doi: 10.1126/science.1102629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tse BN, Snyder TM, Shen Y, Liu DR. Translation of DNA into a library of 13,000 synthetic small-molecule macrocycles suitable for in vitro selection. J. Am. Chem. Soc. 2008;130:15611–15626. doi: 10.1021/ja805649f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YG, Lone AM, Nolte WM, Saghatelian A. Peptidomics approach to elucidate the proteolytic regulation of bioactive peptides. Proc. Natl Acad. Sci. USA. 2012;109:8523–8527. doi: 10.1073/pnas.1203195109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leissring MA, et al. Designed inhibitors of insulin-degrading enzymeregulate the catabolism and activity of insulin. PLoS ONE. 2010;5:e10504. doi: 10.1371/journal.pone.0010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malito E, et al. Molecular bases for the recognition of short peptide substrates and cysteine-directed modifications of human insulin-degrading enzyme. Biochemistry. 2008;47:12822–12834. doi: 10.1021/bi801192h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malito E, Hulse RE, Tang WJ. Amyloid beta-degrading cryptidases: insulin degrading enzyme, presequence peptidase, and neprilysin. Cell. Mol. Life Sci. 2008;65:2574–2585. doi: 10.1007/s00018-008-8112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen Y, Joachimiak A, Rosner MR, Tang WJ. Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism. Nature. 2006;443:870–874. doi: 10.1038/nature05143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manolopoulou M, Guo Q, Malito E, Schilling AB, Tang WJ. Molecular basis of catalytic chamber-assisted unfolding and cleavage of human insulin by human insulin-degrading enzyme. J. Biol. Chem. 2009;284:14177–14188. doi: 10.1074/jbc.M900068200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stella VJ, He Q. Cyclodextrins. Toxicol. Pathol. 2008;36:30–42. doi: 10.1177/0192623307310945. [DOI] [PubMed] [Google Scholar]
- 16.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 17.Ahrén B, Winzell MS, Pacini G. The augmenting effect on insulin secretion by oral versus intravenous glucose is exaggerated by high-fat diet in mice. J. Endocrinol. 2008;197:181–187. doi: 10.1677/JOE-07-0460. [DOI] [PubMed] [Google Scholar]
- 18.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 19.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Riddle MC, Drucker DJ. Emerging therapies mimicking the effects of amylin and glucagon-like peptide 1. Diabetes Care. 2006;29:435–449. doi: 10.2337/diacare.29.02.06.dc05-1267. [DOI] [PubMed] [Google Scholar]
- 21.Mooradian AD, Thurman JE. Drug therapy of postprandial hyperglycaemia. Drugs. 1999;57:19–29. doi: 10.2165/00003495-199957010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Duckworth WC, Kitabchi AE. Insulin and glucagon degradation by the same enzyme. Diabetes. 1974;23:536–543. doi: 10.2337/diab.23.6.536. [DOI] [PubMed] [Google Scholar]
- 23.Bennett RG, Duckworth WC, Hamel FG. Degradation of amylin by insulin-degrading enzyme. J. Biol. Chem. 2000;275:36621–36625. doi: 10.1074/jbc.M006170200. [DOI] [PubMed] [Google Scholar]
- 24.Young A. Effects on plasma glucose and lactate. Adv. Pharmacol. 2005;52:193–208. doi: 10.1016/S1054-3589(05)52010-6. [DOI] [PubMed] [Google Scholar]
- 25.Gedulin BR, Jodka CM, Herrmann K, Young AA. Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC187. Regul. Pept. 2006;137:121–127. doi: 10.1016/j.regpep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Gelling RW, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc. Natl Acad. Sci. USA. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J. Clin. Invest. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadry SA, Drucker DJ. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nature Rev. Endocrinol. 2013;9:425–433. doi: 10.1038/nrendo.2013.47. [DOI] [PubMed] [Google Scholar]