Abstract
Erythrocyte invasion by Plasmodium falciparum merozoites is an essential step for parasite survival and hence the pathogenesis of malaria. Invasion has been studied intensively, but our cellular understanding has been limited by the fact that it occurs very rapidly: invasion is generally complete within 1 min, and shortly thereafter the merozoites, at least in in vitro culture, lose their invasive capacity. The rapid nature of the process, and hence the narrow time window in which measurements can be taken, have limited the tools available to quantitate invasion. Here we employ optical tweezers to study individual invasion events for what we believe is the first time, showing that newly released P. falciparum merozoites, delivered via optical tweezers to a target erythrocyte, retain their ability to invade. Even spent merozoites, which had lost the ability to invade, retain the ability to adhere to erythrocytes, and furthermore can still induce transient local membrane deformations in the erythrocyte membrane. We use this technology to measure the strength of the adhesive force between merozoites and erythrocytes, and to probe the cellular mode of action of known invasion inhibitory treatments. These data add to our understanding of the erythrocyte-merozoite interactions that occur during invasion, and demonstrate the power of optical tweezers technologies in unraveling the blood-stage biology of malaria.
Background
Most cases of severe and fatal malaria in humans are caused by the malaria parasite Plasmodium falciparum. All the symptoms of the disease are caused by the blood stage of the parasite life cycle, which are initiated when P. falciparum merozoites recognize and invade human red blood cells. Erythrocyte invasion is essential for the development of malaria pathology and for parasite survival, and is therefore an attractive intervention target, whether by vaccines or small molecule inhibitors.
This therapeutic potential has made invasion the subject of intense study for several decades. Pioneering optical (1) and electron microscopy (2) studies showed that invasion is a multistep process, first involving weak, reversible interactions between the merozoite and the red blood cell, which trigger dynamic erythrocyte deformation that helps to reorient the merozoite, resulting in an irreversible tight contact being formed between the cells. This electron-dense junction then splits, and is passed backward around the periphery of the merozoite until the parasite becomes fully internalized.
Significant progress has been made in identifying the protein-protein contacts that allow merozoites to interact with erythrocytes, as well as the intracellular actin-myosin motor that appears to drive the entry process (3). However, we still have a very limited understanding of the physical forces that drive invasion, primarily because of technical limitations in microscopy. As a recent flowering of video microscopy studies has reinforced, P. falciparum erythrocyte invasion is extremely rapid, usually being complete in <1 min, and with merozoites losing their infective viability within 2–3 min (4–6). This makes capturing individual invasion events challenging, requiring large amounts of operator time, and makes it difficult for video studies of invasion events to generate the sample numbers required to approach statistical significance. The recent development of a robotic imaging platform that can automatically identify and record merozoite egress-invasion sequences in live cultures under controlled experimental conditions (7) paves the way for a more-quantitative approach to measuring invasion events. However, as of this writing, even this method does not allow quantitation of the central event in the invasion process—the physical contact between the merozoite and erythrocyte.
In this article, for what we believe is the first time, we describe the use of optical tweezers to quantify the strength of interactions between merozoite and erythrocytes. Laser optical tweezers have become mainstream over the last 30 years, emerging as biophysical tools from the early work of Ashkin et al. (8). They have been applied to study such matters as physiological behavior of red blood cells (9–12), changes occurring during intracellular parasite maturation (13,14), and motility of the Plasmodium sporozoite (15), but have not yet been employed to investigate merozoite-erythrocyte interactions. We used optical tweezers to deliver freshly released merozoites to erythrocytes, and found that successful invasion could result if the tweezers confinement time was carefully controlled to limit stress to the merozoite. After attachment, merozoites could be forcibly detached by increasing the power of the optical tweezers, positioning the beam to apply force in a direction perpendicular to the original delivery vector. The strength of the merozoite-erythrocyte contact was calculated by measuring the extent of erythrocyte elongation, at the instant when under the action of the optical tweezers a merozoite was forcibly detached from the erythrocyte to which it had adhered. This method relies on the fact that erythrocyte mechanics has been well characterized; it offers the key benefit of bypassing the need to calibrate the optical trap force on the erythrocyte itself, which would be difficult and less precise. We applied this same approach to measure the impact of known inhibitors of invasion on merozoite-erythrocyte adhesion, raising what we believe is a new model for why P. falciparum merozoites lose the ability to invade erythrocytes relatively soon after egress.
Methods
Plasmodium falciparum culture
Erythrocytes infected with P. falciparum A4 clone, derived from the ITO4 line, were cultured under a low-oxygen atmosphere by standard methods reported in Crick et al. (7), Esposito et al. (16), and Tiffert et al. (17). The culture medium, changed daily, was RPMI-1640 supplemented with 40 mM HEPES (n-2-hydroxyethylpiperazine-n-2-ethanesulfonic acid), 25 mg/L gentamicin sulfate, 10 mM D-glucose, 2 mM glutamine, and 0.5% vol/vol albumax (Sigma-Aldrich, St. Louis, MO). Synchronization was performed by alternating sorbitol lysis (18) and gelatin flotation (19,20). A final gelatin flotation procedure was carried out immediately before each experiment to separate and use the top, schizont-enriched fraction of parasitized cells. Parasitaemia was set to 10% by the addition of uninfected erythrocytes, to provide the required proportion of uninfected to infected cells. The final hematocrit was reduced to 0.1% for imaging, to prevent overlapping of cells and to provide enough space for released merozoites to move visibly.
Imaging and optical tweezers
Cells were imaged in SecureSeal 200 μL-capacity hybridization chambers (SecureSeal; Grace Bio-Labs, Bend, OR), mounted on glass slides, and maintained at 37°C throughout experiments by using a custom-built temperature-control stage. A TI-Eclipse inverted microscope (Nikon, Melville, NY) with a 60× water objective (Plan Apo VC 60× WI/1.2 N.A.; Nikon) was used for imaging; motorized functions of the microscope were controlled via custom-written software running on a PC under a LINUX operating system. Images were acquired, in bright field unless otherwise stated, using a model No. MQ013MG-E2 camera (Ximea, Golden, CO) at a frame rate of 60 fps, pixel size corresponding to 0.09 μm. Automation software, previously described in Crick et al. (7), accessed live images via shared memory for real time analysis and feedback into microscope and camera controls.
The optical tweezers setup, described in detail in Bruot et al. (21), comprised a solid-state pumped NdYag laser (IRCL-2W-1064; CrystaLaser, Reno, NV) with 2-W optical output at a wavelength of 1064 nm, focused through the objective detailed above, trapping from below. The laser beam was steered via a pair of acousto-optical deflectors (AA Opto-Electronic, Orsay, France) controlled by custom-built electronics, allowing for multiple trap generation with subnanometer position resolution.
Measurement of the adhesive force of postviable merozoite-erythrocyte bonds
Adhesive forces existing at the merozoite-erythrocyte interface were quantified by applying a tweezer-induced force in a normal direction away from the erythrocyte at the point of merozoite attachment, and by analyzing the elastic morphological response of the erythrocyte as it resisted merozoite detachment. We do not pull on the merozoite directly, because this force would be weak and difficult to calibrate. We instead pull on the erythrocyte that adheres to the merozoite. The opposing force, on a second erythrocyte, is given by either a second optical trap or by adhesion to the bottom of the sample chamber.
The mechanical response of the erythrocyte to a dynamic stretching force has been well characterized in Yoon et al. (11) and Dao et al. (22). In previous work from our group, the optical tweezers were used to exert forces of known magnitude along a single axis of the erythrocyte by monitoring the displacement of attached beads in calibrated traps, measuring cell deformation, and cell stiffness (11). Because erythrocyte stiffness was shown to vary with the rate of applied strain (11), the strain rates used in this study were confined to a narrow range in ∼10−1 s−1. For an individual erythrocyte, the relationship between the magnitude of the applied force along a single axis and the elastic elongation of the cell along that axis was shown to be linear. In these rheological conditions the erythrocyte stiffness was estimated from Yoon et al. (11) to be ∼20 pN/μm, in accordance also with earlier studies (22). There is a spread of values even within the cell of the same person, so this stiffness value should be considered to have an uncertainty of ∼20% (11).
Enzyme and inhibitor treatments
Heparin (Sigma) was added to P. falciparum blood cultures at a concentration of 30 IU/mL (∼230 μg/mL) (23). Cytochalasin D (Sigma) was prepared in DMSO at a concentration of 5 mg/mL and added to cultures at a final concentration of 1 μg/mL. For treatment of cultures with chymotrypsin, cells were first washed and resuspended in serum-free medium, before being incubated with 1 mg/mL chymotrypsin for 1 h at 37°C (24). The cells were then washed twice with serum-free medium before being resuspended in growth medium.
Results
Invasion can be induced by delivering merozoites to erythrocytes using optical tweezers
Laser tweezers were incorporated into our recently described automated microscope system for monitoring invasion (7). After schizont egress, newly-released P. falciparum merozoites were manipulated by optical trapping and delivered to the surface of uninfected erythrocytes. Trapping durations were kept short (<10 s) to minimize any possible detrimental effects of local heating (1064 nm radiation is harmless except for heating effects: at full laser power, a few degrees Celsius of heating are expected locally around the laser beam focus). Delivery of merozoites in this manner resulted in successful invasion, of which a typical video is shown in Movie S1 in the Supporting Material, with selected frames shown in Fig. 1 A. Egress in this instance occurred ∼5 s before the commencement of the video. Merozoites are shown before the application of a trapping force (0 s), after delivery onto a target erythrocyte (3 s), and after internalization of one of the merozoites (20 s), triggering erythrocyte echinocytosis (80 s). Similar invasion sequences could be observed by using optical tweezers to deliver uninfected erythrocytes to recently egressed merozoites (not shown).
Figure 1.
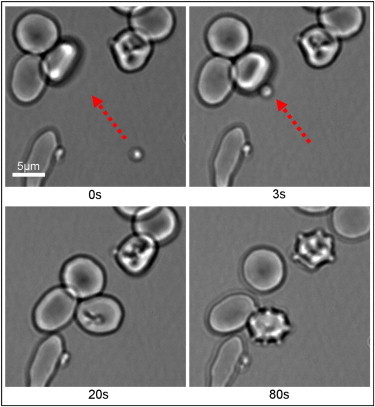
Selected frames taken from Movie S1 in the Supporting Material, showing optical trapping of a freshly released merozoite, with delivery to and invasion of a targeted erythrocyte. (Red arrow) Optical tweezers-directed movement. The merozoite of interest is shown before the application of a trapping force (0 s), upon delivery of the merozoite to a target cell (3 s), during the subsequent invasion process (20 s), and after the postinvasion echinocytosis of the invaded cell (80 s). To see this figure in color, go online.
Laser tweezers delivery does not therefore intrinsically interfere with the normal process of invasion. As well as newly egressed merozoites, we also used tweezers to transfer merozoites that had been released from schizonts >3 min earlier. We, like others, have observed that such merozoites have lost their ability to invade erythrocytes (5,7), but such spent merozoites were still observed to spontaneously adhere to the membranes of neighboring erythrocytes quite strongly, with no spontaneous detachments ever noted. To test the potential of spent merozoites to form cell-cell attachments, merozoites that had egressed >3 min earlier but had not yet adhered to a neighboring cell were delivered to erythrocytes using optical tweezers as described above. A small subpopulation (∼15%) of these spent merozoites would not adhere to erythrocytes even after tweezer-mediated delivery, although it is impossible for us to know whether these had lost the ability to make cellular attachments postegress, or lacked such capability to begin with, perhaps due to being released before their maturation was complete. The majority of spent merozoites did however adhere, and these merozoites could subsequently be forcibly detached and delivered to another erythrocyte, where attachment would occur again (data not shown). Therefore, from this observation, merozoites that have lost the ability to invade are still able to adhere to erythrocytes.
Erythrocyte membrane deformations can be induced by forcibly reorienting merozoites on the erythrocyte surface
The ability to deliver spent merozoites, which adhere to erythrocytes but do not subsequently invade, allowed us to address a key question in invasion—the cause of local deformations of the erythrocyte surface that are seen soon after merozoite attachment. Merozoites are ovoid cells, and for penetration to take place the apex has to be aligned perpendicular to the red cell membrane (2). Initial merozoite-erythrocytes contacts are of random orientation, but these initial interactions somehow trigger vigorous local deformations of the red cell, which we have hypothesized increases invasion efficiency (25,26). To test whether simple movement of merozoites on the erythrocyte surface is sufficient to induce these membrane deformations, erythrocytes were delivered to spent merozoites that had egressed >3 min previously. The point of contact between the merozoite and erythrocyte was then manipulated by passing an optical trap across the erythrocyte, altering its position and orientation in relation to the merozoite, which was kept clear of the path of the trap.
Within a short time (1–2 s) of this perturbation taking place, local temporal membrane deformations were observed around the adhered merozoite, similar in appearance to those seen in typical preinvasion behavior (1,5,25). The laser had been disabled by the time that they occurred (visible in Movie S1, Movie S2, Movie S3, Movie S4, and Movie S5 as “On” and “Off” labels), so the membrane deformations are not caused by the direct action of the laser on the erythrocyte surface. Examples of this observation are shown in Movie S2 and Movie S3, with selected frames given in Fig. 2. These membrane deformations could sometimes be instigated several times for a single spent merozoite, by repeating the process while the merozoite remained adhered to the same erythrocyte (data not shown). Physical attachment, and possibly active movement of merozoites on the erythrocyte surface, are therefore sufficient to trigger erythrocyte deformation.
Figure 2.
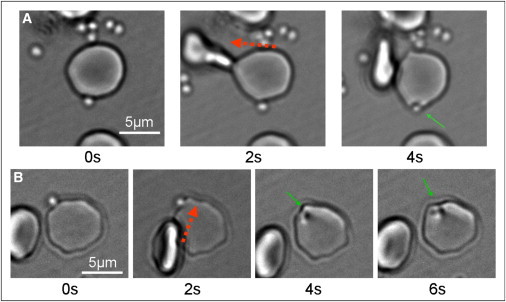
Selected frames taken from Movie S4 (A) and Movie S5 (B), showing transient local deformations of the erythrocyte membrane, after optical tweezers-induced changes to the contact area between the erythrocyte and an adherent merozoite. (Red arrows) Optical tweezers-directed movements; (green arrows) local membrane deformations surrounding the adherent merozoite. The erythrocyte and attached merozoite are shown before an optical tweezers intervention (0 s), during the application of a force intended to shift the position of the erythrocyte-merozoite interface (2 s), and after the cessation of optical forces, as a spontaneous local membrane deformation is observed in the region of the adhered merozoite (4–6 s). To see this figure in color, go online.
Optical tweezers can be used to measure merozoite-erythrocyte attachment force
Having established that laser tweezers could be used to both initiate invasion and trigger erythrocyte deformations, we next attempted to use them to determine the force of interaction between these two cells. The force required for merozoite detachment was determined by using laser tweezers to pull on a merozoite after attachment has taken place, then recording the process, and finally using the recorded images to measure the maximum extent of erythrocyte elongation before merozoite detachment occurred. This procedure was most easily controlled by using two erythrocytes, with a merozoite attached between them. One erythrocyte was pulled away from the point of attachment by optical tweezers, whereas the second was held fixed, either in a separate optical trap or by fortuitous adhesion to the coverslip. The nonstationary optical trap was moved at a constant speed, starting from the erythrocytes in a relaxed state, and steadily increasing the elastic elongation of both erythrocytes until merozoite detachment occurred.
Examples of this procedure are shown in Movie S4 and Movie S5, with selected frames given in Fig. 3. In Fig. 3 A, both erythrocytes are held in separate optical traps, whereas in Fig. 3 B one of the erythrocytes is adhered to the coverslip. The mean erythrocyte end-to-end elongation before detachment was measured to be 2.0 ± 0.4 μm (mean ± SD; N = 12). The stiffness of the erythrocyte membrane has previously been calculated at 20 pN/μm (see Materials and Methods), and because erythrocytes are known to behave mechanically as a linear spring in this regime (11), these maximal elongations were calculated to correspond to a detachment force of 40 ± 8 pN.
Figure 3.
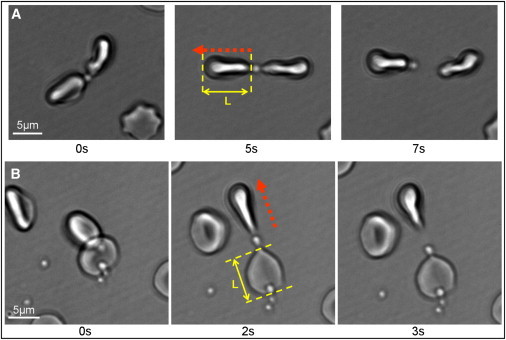
Selected frames taken from Movie S2 (A) and Movie S3 (B), showing optical tweezers-induced detachment of a postviable merozoite adhered to two erythrocytes. (Red arrows) Optical tweezers-directed movement. The cell-merozoite-cell chains are shown in a relaxed state, upon stretching in one direction, and upon detachment of the merozoite from one of the erythrocytes. Both erythrocytes are held in optical traps in panel A. In panel B, one of the erythrocytes is fortuitously attached to the cover glass. L is the cell length, and L = L0 + ΔL, where ΔL is the elongation and L0 is the cell length in the relaxed state. The detachment force F = κΔL, where κ is the cell stiffness used from Yoon et al. (11). To see this figure in color, go online.
Invasion inhibitors can affect either the frequency, or strength, of merozoite-erythrocyte adhesion
The use of optical tweezers therefore offers what we believe to be two completely new measures of merozoite-erythrocyte interactions at an individual cell level: quantitation of the frequency, and force, of merozoite-erythrocyte interactions. Studies over a number of years have identified compounds that are thought to inhibit erythrocyte invasion at different stages and through different mechanisms, but their action on attachment force has never previously been studied. We therefore used these well-established invasion inhibitors to attempt to block merozoite attachment, and compared the data with contemporary models for their mode of action.
Heparin is known to strongly block invasion and video microscopy suggests that it does so at a very early stage, perhaps during attachment (23). Addition of 30 IU/mL heparin to our assay system indeed had a profound effect on merozoite attachment, strongly reducing both the proportion of merozoites that remained attached to erythrocytes after delivery by optical tweezers (from >80% adherences to <20%, P < 0.001; see Fig. 4 A), and on the strength of merozoite-erythrocyte adhesion for the very few merozoites that actually attached (reducing attachment force by >75%, P < 0.001; see Fig. 4 B). Heparin has been proposed to block invasion by specifically preventing MSP1 from binding to a heparin-like proteoglycan receptor (23), although additional heparin binding proteins on merozoites have been observed by mass spectrometry (27). In the context of this specific model for heparin action, it is interesting to note that we found that merozoites in the presence of heparin became almost completely nonadhesive. In standard attachment assays, some proportion of merozoites adhere either to each other, or to the glass slide (data not shown). In the presence of heparin, no such attachments occurred, arguing against this model for a receptor-specific mechanism of heparin action.
Figure 4.
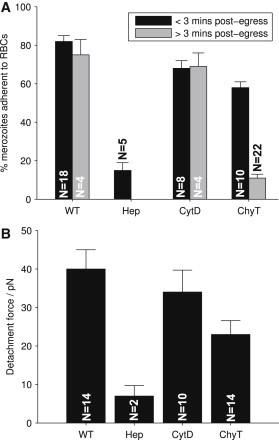
Investigation of the effects of invasion inhibitors heparin (Hep) and cytochalasin D (CytD) and the enzyme treatment of chymotrypsin (ChyT) on merozoite-erythrocyte adhesion (WT = wild-type). (A) Portion of merozoites from each egress adhering to erythrocytes after making contact via optical tweezers delivery, <3 min postegress (black) or >3 min postegress (gray). Values are mean ± SE over egress events. (B) Tweezer-driven merozoite detachment force measured from erythrocyte elongation. No significant correlation was observed between typical detachment forces at <3 min postegress and >3 min postegress (provided merozoites are capable of attachment), so values are mean ± SE over total detachment events.
In contrast to heparin, cytochalasin D is thought to block invasion at a very late stage, after attachment has taken place, and acts by disrupting the actin-myosin motor that drives the physical process of merozoite entry. Inclusion of cytochalasin D at concentrations that potently block invasion (1 μg/mL) had only a minor, albeit statistically significant effect on the proportion of merozoites that were able to remain attached after delivery by optical tweezers (with >80% of wild-type levels of merozoite adherences, P < 0.01; see Fig. 4 A), although this effect was of a completely different scale to that of heparin. There was also no significant difference in merozoite attachment force between cytochalasin-D-treated and control merozoites (P > 0.1; see Fig. 4 B), unlike the effect of heparin. This was somewhat unexpected. Cytochalasin D is thought to block invasion after merozoite reorientation, at the tight-apical contact stage. optical tweezers revealed no difference in attachment force before (control interactions, which may occur at any point on the merozoite surface) or after (cytochalasin-D-treated) apical reorientation.
We were also interested to test the effect of enzyme treatment of erythrocytes on merozoite attachment. P. falciparum strains are known to use multiple alternative receptor-ligand interactions to facilitate invasion, and these pathways can be distinguished at gross level by pretreating erythrocytes with neuraminidase, chymotrypsin, or trypsin to remove a subset of potential invasion receptors from the erythrocyte surface. There have been large numbers of studies of this variable invasion phenotype both in lab strains and in recently adapted field strains (28–31), but the actual impact of these treatments on merozoite-erythrocyte interactions has never been measured at a cellular level. They could conceivably act in any one of a number of ways, from reducing the frequency of attachment, to reducing the strength of attachment, to affecting only some, but not all, merozoites.
Performing standard enzyme-treated invasion assays revealed that invasion by A4, the strain used for these experiments, is inhibited by ∼60% when erythrocytes are pretreated with chymotrypsin. Is this an effect on merozoite attachment, like heparin, or postattachment, like cytochalasin D? Chymotrypsin had no significant effect on the proportion of merozoites that remained attached after delivery by optical tweezers, as long as those merozoites had only recently emerged from a schizont—only 25% fewer newly emerged merozoites remained attached to chymotrypsin-treated erythrocytes as they did to untreated erythrocytes. By contrast, chymotrypsin treatment of erythrocytes did have a very profound effect on the ability of spent merozoites to adhere, reducing adhesion to levels similar to heparin treatment (reduction of >70% compared to wild-type, P < 0.001; Fig. 4 A). Note that this is a much more profound effect than the overall effect of chymotrypsin on invasion rates (see Fig. S1 in the Supporting Material). Chymotrypsin treatment of erythrocytes therefore does not seem to fundamentally alter the ability of merozoites to adhere, but unlike either of the other inhibitors, it shortens the time window in which adhesion can take place. There was also an impact on attachment force, because those merozoites that did adhere to chymotrypsin-treated erythrocytes did so with a nearly 50% reduction in adhesive force (Fig. 4 B). The implications for contemporary models of chymotrypsin inhibition are discussed below.
Discussion
Optical tweezers are shown here to be powerful tools to understand merozoite-erythrocyte interactions at a cellular level. We have shown that individual P. falciparum merozoites may be directly held and manipulated in optical traps, and delivered to target erythrocytes, without inhibiting their invasive viability. Although it cannot be completely ruled out that optical trapping merozoites could affect the invasion machinery in some manner, there is, as of this writing, no indication that this is the case. In fact, manipulating and moving newly-released merozoites may provide a more comparable system to the in vivo environment of blood flow in capillaries than standard in vitro culture conditions. Although there is still much debate regarding the specific flow conditions at the location and time of parasitic egress (32,33) (including the role of blockages caused by adhesive mature schizonts (34)), a static environment with zero flow in which merozoites may only undergo thermal motion is unlikely to be a realistic picture; however, these are the conditions typical of most cell cultures and live imaging so far.
Having developed this seemingly novel technology, we have then used it in three apparently new ways: to probe the viability of merozoites postegress, to measure the physical strength of merozoite-erythrocyte interactions, and to study the action of known invasion inhibitors at the single cell level.
Delivery of merozoites by optical tweezers revealed that merozoites that can no longer invade erythrocytes (>3 min postegress) can still adhere to the erythrocyte membrane, at random orientation, and do so very strongly, with forces of ∼40 pN needed to detach them. This establishes, to our knowledge for the first time, that the well-known rapid loss of the ability of P. falciparum merozoites to invade erythrocytes is not due to a loss in their ability to attach to erythrocytes, and that it is instead later steps in the invasion pathway that are time-restricted. Transient local erythrocyte membrane deformations, which are part of the dynamic morphology that characterizes the preinvasion stage, could also be induced long after the loss of invasive viability. Although the specific role of these membrane deformations and the nature of the merozoite-cell interactions that cause them has yet to be fully elucidated, these results suggest that they may occur after merozoite reorientation, rather than preceding it.
Furthermore, the fact that merozoites which were forcibly reoriented did not invade, despite inducing local erythrocyte deformations, reinforces the suggestion that it is a defect in the later stages of invasion that is the cause of the rapid loss of merozoite invasive viability in vitro. Merozoite maturation and release is known to be accompanied by a number of tightly regulated processes, including proteolytic processing of proteins on the merozoite surface and release of ligands from intracellular organelles. It is possible that spent merozoites are either no longer able to complete this maturation process, or alternatively they may have overmatured in some way. Egress leads to a change in the extracellular environment for the merozoite, which is thought to trigger intracellular ligand release (35). These ligands are released initially onto the merozoite surface, but are subsequently detectable in the extracellular medium, presumably due to proteolytic cleavage. Merozoites are not thought to be translationally active, so an extended period in the extracellular environment may lead to the secretion and loss of the full complement of intracellular invasion ligands, preventing invasion even when a subsequent erythrocyte contact is made.
We have also measured, to our knowledge for the first time, the strength of the attachment force between merozoites and erythrocytes, by using the known stiffness properties of erythrocytes. The detachment force measured here, ∼40 pN, can be compared to typical receptor ligand detachment forces that have been typically measured in single-molecule AFM pulling assays; these range from weak actin-myosin interaction 1.7 pN up to the strongest biotin/streptavidin 160 pN (36). It is worth remarking here again, as also reviewed in Bongrand (36), that these measured detachment forces depend on the protocol of measurement, in particular on the rate of strain, so care should be taken when comparing values in the literature. What we conclude from the merozoite/erythrocyte detachment force value of ∼40 pN is that most likely this arises from a combination of multiple weak ligand binding with an even more generic adhesion force that also promotes the partial physical wrapping of the erythrocyte membrane on the merozoite. This is in keeping with measured interaction forces for known receptor-ligand binding events, such as that between RH5 and Basigin, which are low affinity like most extracellular protein-protein interactions (37). Although as noted above, estimates for the rate of blood flow in the microvasculature vary widely, there is no question that merozoite attachment occurs in a more challenging biophysical environment than that in which most cell-cell interactions occur. Whereas the in vitro force measurements described here are a good first estimate, further exploration of this parameter in multiple conditions will be required. The inherent variation in erythrocyte stiffness (likely to arise from, among other factors, cell age (38,39)) affects its deformability, and the detachment force is presumably also dependent on the number of individual bonds between the merozoite surface coat and erythrocyte membrane, which may have a wide range (40,41). We have assumed here that the merozoites themselves are inextensible, and that erythrocyte elongations are due to cytoskeletal deformations, rather than local detachments of the lipid bilayer. It is important to note that there was no indication of erythrocyte membrane blebbing nor an inelastic response, which would be expected if local detachments of the lipid bilayer had occurred.
Finally, we have used optical tweezers technology in combination with known invasion inhibitors to probe contemporary models for inhibitor mode of action. In the case of heparin and cytochalasin D, previously thought to function at the very beginning and very end of the invasion process, respectively, optical tweezers delivery of merozoites powerfully confirmed previous modes of action. There were, however, two significant and apparently new findings even with these inhibitors:
-
1.
Heparin blocked merozoite attachment to any surface, including glass slides, suggesting a receptor-independent mode of action, in contrast to what has been proposed previously (23).
-
2.
Cytochalasin-D treatment had no effect on attachment force, either positively or negatively.
Previous electron microscopy studies (1) have shown that cytochalasin D blocks invasion after apical reorientation, at the tight-junction stage. These believed-first physical measurements of attachment force showed no difference between early contacts at any point on the merozoite surface, and late contacts between apically reoriented merozoites after cytochalasin-D treatment. The distinction between early/weak and late/strong interactions may not be as clear as widely believed.
In the case of enzyme treatment, two different effects were observed. When A4 merozoites did adhere to chymotrypsin-treated erythrocytes, they did so with a reduction in force of attachment that was similar to the effect of the enzyme on the overall efficiency of invasion. This is perhaps expected: if a subset of potential invasion receptors are removed, the number of protein-protein contacts that can be made is reduced, impacting the strength of attachment. However, surprisingly, merozoites that had been released >3 min previously were no longer able to adhere to chymotrypsin-treated erythrocytes at all, suggesting that as well as affecting the force of merozoite attachment, chymotrypsin also inhibits invasion by restricting the time window in which invasion can occur. This may be related to the time dependency of invasion noted above. Some of the intracellular ligands released on to the merozoite surface, such as Rh2b, bind to chymotrypsin-sensitive receptors (42). If spent merozoites have indeed lost all intracellular ligands, this could preclude binding to erythrocytes where proteolytic treatment has left only a few receptor subclasses remaining.
In summary, the optical tweezers methodology described here provides a powerful proposed new approach to dissect the biophysics of interactions occurring during P. falciparum invasion. Combining this technique with specific antibodies and genetically modified lines will enable us to move our understanding of invasion to the single-cell level, as we believe, for the first time.
Acknowledgments
We thank Mike Blackman and Clemens Kaminski for very useful discussions, and Jurij Kotar for help with optical tweezing.
A.J.C. was funded by an Engineering and Physical Sciences Research Council Doctoral Training Account award. Funding was also provided by the Wellcome Trust (grant No. 098051).
Contributor Information
Pietro Cicuta, Email: pc245@cam.ac.uk.
Julian C. Rayner, Email: jr9@sanger.ac.uk.
Supporting Material
References
- 1.Dvorak J.A., Miller L.H., Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science. 1975;187:748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa M., Miller L.H., Epstein N. Freeze-fracture study on the erythrocyte membrane during malarial parasite invasion. J. Cell Biol. 1981;91:55–62. doi: 10.1083/jcb.91.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowman A.F., Berry D., Baum J. The cellular and molecular basis for malaria parasite invasion of the human red blood cell. J. Cell Biol. 2012;198:961–971. doi: 10.1083/jcb.201206112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glushakova S., Yin D., Zimmerberg J. Membrane transformation during malaria parasite release from human red blood cells. Curr. Biol. 2005;15:1645–1650. doi: 10.1016/j.cub.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 5.Gilson P.R., Crabb B.S. Morphology and kinetics of the three distinct phases of red blood cell invasion by Plasmodium falciparum merozoites. Int. J. Parasitol. 2009;39:91–96. doi: 10.1016/j.ijpara.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Riglar D.T., Richard D., Baum J. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe. 2011;9:9–20. doi: 10.1016/j.chom.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Crick A.J., Tiffert T., Cicuta P. An automated live imaging platform for studying merozoite egress-invasion in malaria cultures. Biophys. J. 2013;104:997–1005. doi: 10.1016/j.bpj.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashkin A., Dziedzic J.M., Chu S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986;11:288. doi: 10.1364/ol.11.000288. [DOI] [PubMed] [Google Scholar]
- 9.Hénon S., Lenormand G., Gallet F. A new determination of the shear modulus of the human erythrocyte membrane using optical tweezers. Biophys. J. 1999;76:1145–1151. doi: 10.1016/S0006-3495(99)77279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sleep J., Wilson D., Gratzer W. Elasticity of the red blood cell membrane and its relation to hemolytic disorders: an optical tweezers study. Biophys. J. 1999;77:3085–3095. doi: 10.1016/S0006-3495(99)77139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon Y.Z., Kotar J., Cicuta P. The nonlinear mechanical response of the red blood cell. Phys. Biol. 2008;5:036007. doi: 10.1088/1478-3975/5/3/036007. [DOI] [PubMed] [Google Scholar]
- 12.Yoon Y.Z., Kotar J., Cicuta P. Red blood cell dynamics: from spontaneous fluctuations to non-linear response. Soft Matter. 2011;7:2042–2051. [Google Scholar]
- 13.Suresh S., Spatz J., Seufferlein T. Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater. 2005;1:15–30. doi: 10.1016/j.actbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Chandramohanadas R., Park Y., Suresh S. Biophysics of malarial parasite exit from infected erythrocytes. PLoS ONE. 2011;6:e20869. doi: 10.1371/journal.pone.0020869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegge S., Uhrig K., Frischknecht F. Direct manipulation of malaria parasites with optical tweezers reveals distinct functions of Plasmodium surface proteins. ACS Nano. 2012;6:4648–4662. doi: 10.1021/nn203616u. [DOI] [PubMed] [Google Scholar]
- 16.Esposito A., Choimet J.B., Tiffert T. Quantitative imaging of human red blood cells infected with Plasmodium falciparum. Biophys. J. 2010;99:953–960. doi: 10.1016/j.bpj.2010.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiffert T., Ginsburg H., Lew V.L. Potent antimalarial activity of clotrimazole in in vitro cultures of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA. 2000;97:331–336. doi: 10.1073/pnas.97.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambros C., Vanderberg J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 19.Jensen J.B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1978;27:1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- 20.Pasvol G., Wilson R.J.M., Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann. Trop. Med. Parasitol. 1978;72:87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- 21.Bruot N., Damet L., Lagomarsino M.C. Noise and synchronization of a single active colloid. Phys. Rev. Lett. 2011;107:094101. doi: 10.1103/PhysRevLett.107.094101. [DOI] [PubMed] [Google Scholar]
- 22.Dao M., Lim C., Suresh S. Mechanics of human red blood cell deformed by optical tweezers. J. Mech. Phys. Solids. 2003;51:2259–2280. [Google Scholar]
- 23.Boyle M.J., Richards J.S., Beeson J.G. Interactions with heparin-like molecules during erythrocyte invasion by Plasmodium falciparum merozoites. Blood. 2010;115:4559–4568. doi: 10.1182/blood-2009-09-243725. [DOI] [PubMed] [Google Scholar]
- 24.Theron M., Hesketh R.L., Rayner J.C. An adaptable two-color flow cytometric assay to quantitate the invasion of erythrocytes by Plasmodium falciparum parasites. Cytometry A. 2010;77:1067–1074. doi: 10.1002/cyto.a.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lew V.L., Tiffert T. Is invasion efficiency in malaria controlled by pre-invasion events? Trends Parasitol. 2007;23:481–484. doi: 10.1016/j.pt.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Tiffert T., Lew V.L., Mohandas N. The hydration state of human red blood cells and their susceptibility to invasion by Plasmodium falciparum. Blood. 2005;105:4853–4860. doi: 10.1182/blood-2004-12-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Jiang N., Chen Q. Proteomic analysis of Plasmodium falciparum schizonts reveals heparin-binding merozoite proteins. J. Proteome Res. 2013;12:2185–2193. doi: 10.1021/pr400038j. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Escobar N., Amambua-Ngwa A., Conway D.J. Erythrocyte invasion and merozoite ligand gene expression in severe and mild Plasmodium falciparum malaria. J. Infect. Dis. 2010;201:444–452. doi: 10.1086/649902. [DOI] [PubMed] [Google Scholar]
- 29.Jennings C.V., Ahouidi A.D., Duraisingh M.T. Molecular analysis of erythrocyte invasion in Plasmodium falciparum isolates from Senegal. Infect. Immun. 2007;75:3531–3538. doi: 10.1128/IAI.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Perez M., Villasis E., Lustigman S. Plasmodium falciparum field isolates from South America use an atypical red blood cell invasion pathway associated with invasion ligand polymorphisms. PLoS ONE. 2012;7:e47913. doi: 10.1371/journal.pone.0047913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okoyeh J.N., Pillai C.R., Chitnis C.E. Plasmodium falciparum field isolates commonly use erythrocyte invasion pathways that are independent of sialic acid residues of glycophorin A. Infect. Immun. 1999;67:5784–5791. doi: 10.1128/iai.67.11.5784-5791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedosov D.A., Caswell B., Karniadakis G.E. Quantifying the biophysical characteristics of Plasmodium-falciparum-parasitized red blood cells in microcirculation. Proc. Natl. Acad. Sci. USA. 2011;108:35–39. doi: 10.1073/pnas.1009492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dondorp A.M., Pongponratn E., White N.J. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop. 2004;89:309–317. doi: 10.1016/j.actatropica.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Shelby J.P., White J., Chiu D.T. A microfluidic model for single-cell capillary obstruction by Plasmodium falciparum-infected erythrocytes. Proc. Natl. Acad. Sci. USA. 2003;100:14618–14622. doi: 10.1073/pnas.2433968100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S., Alam M.M., Chitnis C.E. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 2010;6:e1000746. doi: 10.1371/journal.ppat.1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bongrand P. Ligand-receptor interactions. Rep. Prog. Phys. 1999;62:921–968. [Google Scholar]
- 37.Crosnier C., Bustamante L.Y., Wright G.J. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutera S.P., Gardner R.A., Williamson J.R. Age-related changes in deformability of human erythrocytes. Blood. 1985;65:275–282. [PubMed] [Google Scholar]
- 39.Shiga T., Sekiya M., Okazaki M. Cell age-dependent changes in deformability and calcium accumulation of human erythrocytes. Biochim. Biophys. Acta Biomembr. 1985;814:289–299. doi: 10.1016/0005-2736(85)90447-x. [DOI] [PubMed] [Google Scholar]
- 40.Bannister L.H., Butcher G.A., Mitchell G.H. Studies on the structure and invasive behavior of merozoites of Plasmodium knowlesi. Trans. R. Soc. Trop. Med. Hyg. 1975;69:5. [PubMed] [Google Scholar]
- 41.Bannister L.H., Mitchell G.H., Cohen S. Structure and development of the surface coat of erythrocytic merozoites of Plasmodium knowlesi. Cell Tissue Res. 1986;245:281–290. doi: 10.1007/BF00213933. [DOI] [PubMed] [Google Scholar]
- 42.Duraisingh M.T., Triglia T., Cowman A.F. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 2003;22:1047–1057. doi: 10.1093/emboj/cdg096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


