
Keywords: Capillaria, Eucoleus, Pathology, Rat, Stomach, Wild
Highlights
-
•
We used histology to examine wild rats for stomach pathology.
-
•
Reactive lesions in the stomach of wild rats were associated with Eucoleus sp.
-
•
Sexually mature and heavy rats had increased odds of infection.
-
•
This represents a substantial host response to parasitism.
Abstract
Histological lesions associated with Eucoleus sp. infection of the non-glandular stomach were discovered in a wild, urban population of Norway rats (Rattus norvegicus) trapped over a 1-year period in Vancouver, Canada. Four distinct categories of histological lesions in the non-glandular stomach were identified in association with infection in a sample of 183 rats. The apparent prevalence of Eucoleus sp. in the upper gastrointestinal tract (ventral tongue, oropharynx, esophagus and non-glandular stomach) was 43.1% (79/183). Infection with Eucoleus sp. was significantly associated with hyperkeratosis, mucosal hyperplasia, keratin pustules and submucosal inflammation in the non-glandular stomach (P < 0.05). Eucoleus sp. infection and/or related stomach pathology was present in 135/183 (73.8%) of rats. Statistical analysis showed the odds of being affected by Eucoleus sp. or associated stomach pathology were greater in heavier (OR = 1.06, 95% CI = 1.00–1.12) and sexually mature rats (OR = 4.64, 95% CI = 1.23–17.10). Eucoleus sp. infection is common in wild rats in Vancouver and induces substantial host response. The impact of Eucoleus sp. and associated lesions on the health of individual rats and the population as a whole remains to be investigated.
1. Introduction
Rats (Rattus spp.) are among the most widespread and ubiquitous rodent species (Feng and Himsworth, 2013). They are well-adapted to cohabitation with people, particularly in urban centers, and this adaptation has led to a number of negative consequences for human populations, including destruction of property, consumption and contamination of food sources and spread of zoonotic diseases (Feng and Himsworth, 2013; Himsworth et al., 2013). Despite the fact that humans and rats have coexisted in cities around the world for centuries, very little is known about the natural diseases of wild rats.
Previous studies of Norway rats (Rattus norvegicus) have shown that the expected life-span of this species in urban environments is brief, with few individuals surviving more than one year (Feng and Himsworth, 2013). In contrast, the expected lifespan of conspecific laboratory rats ranges from 2 to 3 years (Nadon, 2006). The factors contributing to this rapid population turn over in wild rats remains unclear. The role of natural disease in wild rat morbidity and mortality has not been well studied.
Few studies have investigated diseases affecting the non-glandular stomach of wild rats. Fibiger (1927) described papillomas in the non-glandular stomach of three wild rats associated with the nematode Gongylonema neoplasticum (formerly Spiroptera carcinoma), which led to the discovery that gastric irritants, including parasites, could induce neoplasia (Fibiger, 1927; Hitchcock and Bell, 1952). Vogel (1929) in Germany and Beatti (1930) in South America described similar hyperplastic changes in the non-glandular stomach of three wild Norway rats associated with a capillarid nematode infection (presumably Eucoleus gastricus; formerly Capillaria gastrica; Hepaticola gastrica). Experimental infections in four laboratory rats produced similar results (Vogel, 1929). While these historical studies provide limited descriptions of the effect of nematode infection in the non-glandular stomach of wild rats, an evaluation of the apparent prevalence of infection, associated pathology and risk factors for infection has never been done.
The objectives of this study were to describe pathology in the non-glandular stomach of wild urban Norway rats, to characterize the association between stomach pathology and Eucoleus sp. infection and to characterize the ecology of this infection in rat populations.
2. Materials and methods
2.1. Rat trapping
Norway and black rats (R. rattus) were trapped, euthanized and examined from 43 contiguous city blocks within an inner city urban neighborhood of Vancouver, Canada (N49°17′/W123°6′) known as the Downtown Eastside (DTES), as well as from one property in an international shipping port which forms the northern border of the study area. Pairs of city blocks and the port site were randomly allocated to one three-week trapping period over the course of one year (September 2011–August 2012).
Briefly, 15–20 Tomahawk Rigid Traps for rats (Tomahawk Live Trap llc., Hazlelhurst, USA) were set out in an alleyway that ran the length of the block behind the buildings. At the port, traps were placed in areas where port staff had observed rats. Traps were pre-baited with bacon fat, peanut butter and oats, with doors fixed open for one week to acclimatize rats to trapping equipment and bait, followed by two weeks of active trapping. Rats were anesthetized using isoflurane prior to blood collection via cardiac puncture and euthanasia by intracardiac injection of pentobarbital. At the port site, rats trapped by a private pest control professional using snap-type lethal traps were also collected. A total of 725 rats was collected over the course of the study.
Data collected in the field included species (based on external morphology), sex, weight and sexual maturity. Females with an open vaginal orifice and males with scrotal testes were considered sexually mature. Rats were stored at −30 °C and sent to the Animal Health Centre, British Columbia Ministry of Agriculture, Abbotsford, British Columbia, for further analysis. This study was approved by the University of British Columbia’s Animal Care Committee (A11-0087).
2.2. Autopsy and tissue collection
Rats were thawed at 4 °C prior to undergoing a standardized autopsy and tissue collection protocol. During the autopsy, the stomach was opened along the greater curvature and contents were noted. Grossly-evident abnormalities were also noted and photographed. For clarity, the background and redundant connective tissues were digitally edited in Fig. 1 using Adobe Photoshop CS5 (12.0.4 × 32). The stomach was sectioned axially into two parts, each containing both non-glandular and glandular stomach. The stomach contents and one half of the stomach were stored at −80 °C for further analysis. The remaining half of the stomach and representative samples of tongue, pharynx and esophagus were immersed in 10% neutral buffered formalin and processed routinely for histopathology. Liver from each rat was also evaluated for the presence of Calodium hepaticum (syn. Capillaria hepatica) infection, which was confirmed histologically in a subset of rats (Rothenburger et al., 2014).
Fig. 1.
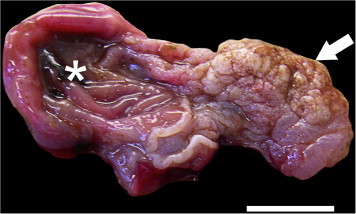
Squamous papilloma (arrow) arising from the non-glandular stomach of a wild Norway rat (Rattus norvegicus) from Vancouver, Canada. Asterisk (∗) indicates the unaffected glandular stomach. Scale bar = 1 cm.
2.3. Histopathology
Tissues from 198 rats were evaluated using light microscopy. These included 91 rats with gross lesions in any organ system and a random selection of 107 rats without gross lesions.
The following formalin-fixed tissues were sectioned: tongue (cross section through the caudal third), larynx (longitudinally to include the epiglottis and oropharynx), esophagus (transverse at the level of the thyroid glands) and stomach (axially including non-glandular and glandular portions). Following routine processing, these tissues were embedded in paraffin and 5 μm tissue sections were stained using hematoxylin and eosin (H & E). Based on initial microscopic findings of H & E-stained slides, additional sections of selected tissues were stained with Grocott’s methenamine silver (GMS) stain.
2.4. Parasite identification
Nematodes in the upper gastrointestinal tract were initially identified using light microscopy. Thawed fresh stomach tissues from five rats that had histological evidence of infection were selected for further examination. Stereoscopic microscope examination (Olympus, SZ16) was used to retrieve adult nematodes and eggs from stomach samples. Subsequently, isolated parasites and eggs were examined using a high-power microscope (Olympus, BX53) for morphologic identification. Specifically, the tail of a male specimen was studied for generic identification based on a published key (Moravec, 1982).
2.5. Histological identification of the nematode and lesion categorization
Rats were considered infected with nematodes if characteristic eggs or adults were observed in ventral tongue, pharynx, esophagus or non-glandular stomach using light microscopy.
The non-glandular stomach of each rat was also examined microscopically in detail for any other microscopic abnormalities and these were recorded. This initial examination, in combination with a review of the literature on laboratory and wild rat stomach pathology (Fibiger, 1927; Vogel, 1929; Hitchcock and Bell, 1952; Brown and Hardisty, 1990; Frantz et al., 1991; Boorman and Everitt, 2006; Greaves, 2012), was then used to develop a system for classifying and categorizing non-glandular stomach lesions (Table 1). Inclusion and exclusion criteria were defined for each lesion to further enhance objectivity. Stringent binary classification schema were used in order to accurately and conservatively evaluate the presence and apparent prevalence of microscopic abnormalities in this sample of rats. Lesion severity or scoring was not assessed. Once the classification scheme was developed, all tissues were re-evaluated and categorized by these criteria. Detailed evaluation of lesions elsewhere in the upper gastrointestinal tract was not done because the most obvious and objectively assessable lesions were found in the non-glandular stomach.
Table 1.
Categories of microscopic changes in the non-glandular stomach of wild urban Norway rats (Rattus norvegicus).
| Change | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Eucoleus sp. infection | Presence of Eucoleus sp. nematodes or eggs in the mucosa of the ventral tongue, oropharynx, esophagus or non-glandular stomach | Absence of inclusion criteria |
| Hyperkeratosis | Superficial keratin layer ⩾ 45 μm thick in sections not adjacent to limiting ridge and where keratin is not artifactually separated | Keratin < 45 μm thick or > 45 μm only in areas immediately adjacent to limiting ridge |
| Keratin pustules | Aggregates of polymorphonuclear cells and cell debris within the superficial keratin | Food debris or superficial bacterial overgrowth |
| Mucosal hyperplasia | Mucosa ⩾ 5 layers thick affecting ⩾ 25% of the section examined | Mucosa < 5 layers thick or < 25% of the section affected |
| Submucosal inflammation | Polymorphonuclear cells infiltrating submucosa in ⩾ 2 foci in sections not adjacent to limiting ridge | <2 foci or immediately adjacent to limiting ridge |
2.6. Statistical evaluation
Bivariate associations between each microscopic change and the presence of nematodes in upper gastrointestinal tract were assessed using simple logistic regression (Table 2). A new variable was then created (stomach pathology present/absent), for which any rat with nematode infection and/or significantly associated microscopic lesions was considered positive. This variable was used as the outcome in logistic regression analyses investigating the relationship between stomach pathology and a variety of potentially explanatory variables. These variables included season (September–November = fall; December–February = winter; March–May = spring; June–August = summer), sex, sexual maturity (immature vs. mature), body condition as assessed by volume of internal fat stores (score of 0–2), weight and presence or absence of grossly-evident C. hepatica in the liver (Table 3). Variables that were significantly associated with stomach nematode infection at an alpha level of ⩽0.10 on bivariate analysis were included in a generalized linear mixed model (GLMM) controlling for clustering by city block of origin. The goal of the model building strategy was to identify the most parsimonious set of explanatory variables that predicted the outcome. The final model was selected using manual backward selection and only statistically significant variables (P ⩽ 0.05) were retained. Individuals for which data were missing for one or more of the variables under study were excluded. All statistical analyses were conducted using R (R Development Core Team, Vienna, Austria).
Table 2.
Microscopic changes and their association with Eucoleus sp. in Norway rats (Rattus norvegicus) trapped in Vancouver, Canada.
| Category | Sub-category | Totala (%) (n = 183) |
Eucoleus infection |
p-valueb | ORc | 95% CI | |
|---|---|---|---|---|---|---|---|
| Presenta (%) (n = 79) |
Absenta (%) (n = 104) |
||||||
| Hyperkeratosis | Yes | 115 (62.8) | 68 (86.1) | 47 (45.2) | <0.001 | 8.20 | 3.83–19.29 |
| No | 60 (32.8) | 9 (11.4) | 51 (49.0) | ||||
| Mucosal hyperplasia | Yes | 108 (59.0) | 63 (79.7) | 45 (43.3) | <0.001 | 5.30 | 2.68–11.01 |
| No | 67 (36.6) | 14 (17.7) | 53 (51.0) | ||||
| Keratin pustules | Yes | 32 (17.5) | 25 (31.6) | 7 (6.7) | <0.001 | 6.25 | 2.64–16.58 |
| No | 143 (78.1) | 52 (65.8) | 91 (87.5) | ||||
| Submucosal inflammation | Yes | 87 (47.5) | 58 (73.4) | 29 (27.9) | <0.001 | 6.80 | 3.54–13.53 |
| No | 88 (48.1) | 20 (25.3) | 68 (65.4) | ||||
Frequencies and percentages may not add to 100% because of exclusion of rats with missing data.
Determined using logistical regression models.
Odds ratio with 95% confidence interval (CI).
Table 3.
Characteristics and associations with Eucoleus sp. or associated lesions in the non-glandular stomach among a group of Norway rats (Rattus norvegicus) trapped in Vancouver, Canada.
| Category | Subcategory | Number of ratsa (%) (n = 183) |
Stomach pathology |
p-valueb | |
|---|---|---|---|---|---|
| Presenta (%) (n = 135) |
Absenta (%) (n = 48) |
||||
| Season | Fall | 89 (48.6) | 74 (54.8) | 15 (31.3) | |
| Winter | 44 (24.0) | 32 (23.7) | 12 (25.0) | 0.163 | |
| Spring | 34 (18.6) | 21 (15.6) | 13 (27.1) | 0.014 | |
| Summer | 16 (8.7) | 8 (5.9) | 8 (16.7) | 0.005 | |
| Sex | Male | 106 (57.9) | 79 (58.5) | 27 (56.3) | 0.785 |
| Female | 77 (42.1) | 56 (41.5) | 21 (43.8) | ||
| Sexual maturity | Mature | 141 (77.0) | 118 (87.4) | 23 (47.9) | <0.001 |
| Immature | 31 (16.9) | 10 (7.4) | 21 (43.8) | ||
| Weight (g) | Median (IQR) | 231 (108–320) | 252 (184–330) | 85 (64–248) | <0.001 |
| Fat score (categorical) | Poor (0) | 46 (25.1) | 23 (17.0) | 23 (47.9) | |
| Moderate (1) | 61 (33.3) | 52 (38.5) | 9 (18.8) | <0.001 | |
| Good (2) | 73 (39.9) | 59 (43.7) | 14 (29.2) | <0.001 | |
| Fat score (continuous) | Median (IQR) | 1 (0–2) | 1 (1–2) | 0.5 (0–2) | <0.001 |
| Liver Capillaria hepatica lesions | Present | 118 (64.5) | 98 (72.6) | 20 (41.7) | 0.005 |
| Absent | 65 (35.5) | 37 (27.4) | 28 (58.3) | ||
IQR = interquartile range.
Frequencies and percentages may not add to 100% because of exclusion of rats with missing data for the variable in question.
Determined using logistical regression models.
2.7. Spatial analysis
The location of each trap within the 43 block area of the DTES, and the number of rats caught in each trap that were infected with the nematode or had associated stomach pathology, and rats with neither the nematode nor stomach pathology were mapped using ArcGIS 10.0 (ESRI, Redlands, USA). This information was imported into SaTScan™ (Boston, USA) for cluster analysis using a purely spatial Bernoulli model and scanning for areas with high and low rates of nematode infection or associated stomach pathology using a circular window with a maximum spatial cluster size of 50% of the population at risk. Clusters identified by SaTScan™ were visualized in ArcGIS. The port site was excluded from this analysis because trapping took place at multiple vertical levels within a single geographic foot-print (which is difficult to represent in a two dimensional map) and because trapping was somewhat more opportunistic (vs. systematic) compared to the blocks.
3. Results
3.1. Stomach gross examination
Of 725 rats trapped, 15 were excluded due to incomplete records or autolysis. The only grossly evident stomach lesion detected was in a mature, female Norway rat (1/710; 0.001%). Filling approximately 75% of the stomach lumen and arising from the non-glandular stomach, there was a rough, firm, 1.5 × 2 × 2 cm mass (Fig. 1). The mass was connected to the non-glandular stomach by a dense, wide stalk. Histologically, this mass was characterized by severe hyperkeratosis, mucosal hyperplasia and submucosal edema with intramucosal capillarid nematodes and eggs (Supplemental Fig. 1). Based on these findings, the mass was determined to be a squamous papilloma. This rat had minimal internal fat stores, consistent with poor body condition, and an empty stomach. There were no grossly-evident lesions detected in the tongue, pharynx or esophagus in any of the rats examined.
3.2. Population demographic characteristics and microscopic examination
Among the 198 rats included in the histological analysis, 183 (91.9%) were Norway rats and 15 (8.1%) were black rats. Adult nematodes and/or eggs were identified in 79/183 (43.1%) of Norway rats (Figs. 2 and 3). Only 1/15 black rat (6.3%) was infected. Given the low number of black rats and the differing ecology and biology of Norway and black rats (Feng and Himsworth, 2013), black rats were excluded from further analysis. The following results pertain to Norway rats only (n = 183). The most frequently observed lesions in the non-glandular stomach included hyperkeratosis, mucosal hyperplasia, keratin pustules and submucosal inflammation (Fig. 3; Table 2). These lesions were strongly associated with the presence of capillarid nematodes or eggs (P < 0.001; Table 2). A total of 135/183 (73.8%) rats had nematode infection and/or associated stomach pathology.
Fig. 2.
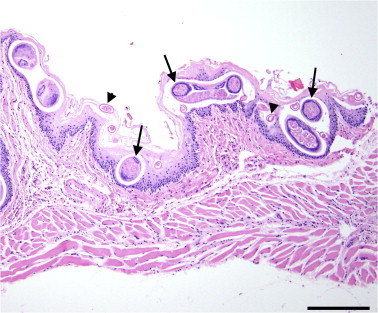
Cross-sections of Eucoleus sp. adults (arrows) and eggs (arrowheads) within the keratin and superficial mucosa of the esophagus in a wild Norway rat (Rattus norvegicus). Scale bar = 100 μm.
Fig. 3.
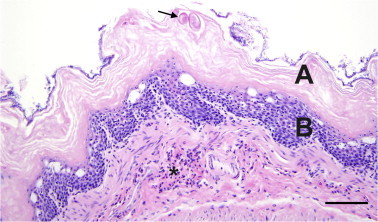
Eucoleus sp. eggs (arrow) embedded within hyperkeratosis (A) in the non-glandular stomach of a wild, Norway rat (Rattus norvegicus). There is mucosal hyperplasia (B) and subcutaneous granulocytic inflammation (∗). Scale bar = 100 μm.
Additional changes included focal ulceration in the non-glandular stomach with associated submucosal edema and suppurative inflammation in one rat, and growth of fungal hyphae and yeast (identified using Grocott’s methenamine silver stain) in the superficial keratin layer of the non-glandular stomach in three rats. Due to the relative rarity of these changes, they were not included in the classification scheme. Hyperkeratosis, mucosal hyperplasia and occasionally inflammation were evident in the esophagus in association with nematodes or eggs; however, these changes were few and not amenable to statistical analysis for association. Lesions associated with nematode or eggs were not appreciated in the ventral tongue or pharynx.
3.3. Microscopic nematode identification
Using microscopic examination of formalin-fixed, embedded tissues, adult nematodes were observed to be approximately 50 μm in diameter with a thin cuticle and hypodermis, coelomyarian musculature, a small digestive tract lined by a single layer of low cuboidal epithelial cells, coiled testes and bacillary bands (Fig. 2). Eggs were non-embryonated and ovoid, with thick shells and bipolar plugs. These features are characteristic of capillarid nematodes (Gardiner and Poynton, 2006).
Among the five samples, nematodes were identified by stereoscopy in the stomach of only one rat. A male and a female specimen were dissected from the stomach tissues of this rat. The physical features of these specimens were poorly preserved due to freezing and thawing. The female was 35.3 mm long and 55–65 μm wide. The male was 28.2 mm long and 55–65 μm wide. The morphology of the male nematode (Fig. 4) was consistent with Eucoleus sp. (Dujardin, 1845; Moravec, 1982). Specifically, it had a rudimentary pseudobursa with two minute, posteriorly-directed lateral lobes, a moderately scelortized slender spicule, a spicular sheath covered with cuticular spines, and it lacked caudal lateral alae (Fig. 5). Nematode eggs were approximately 66 × 35 μm with a dense network of anastomosing ridges on the shell (Fig. 6), which is also consistent with the genus Eucoleus (Traversa et al., 2011; Magi et al., 2012). Species identification was not possible due to a lack of clear morphological keys or distinguishing descriptions.
Fig. 4.
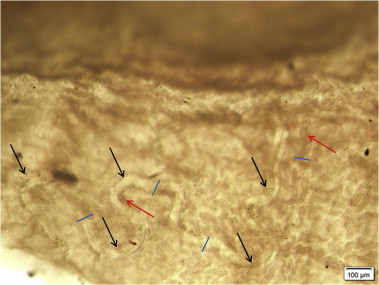
Female Eucoleus sp. embedded in the mucosa of non-glandular stomach of a wild, Norway rat (Rattus norvegicus). Black arrows point to the meandering nematode. Red arrows points to eggs in uterus. Blue lines indicate the width (∼60 μm) of the nematode at various positions. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
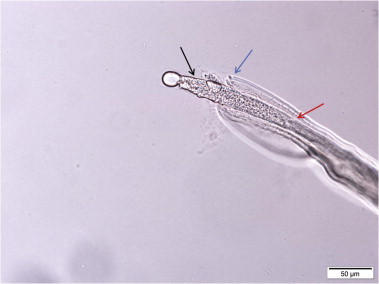
Tail of a male Eucoleus sp. collected from the non-glandular stomach mucosa of a wild, Norway rat (Rattus norvegicus). Note the slender long moderately sclerotized spicule (black arrow) with cuticular spine covered spicular sheath (red arrow). Blue arrow points to posteriorly directed two minute lobes of the rudimentary pseudo bursa. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
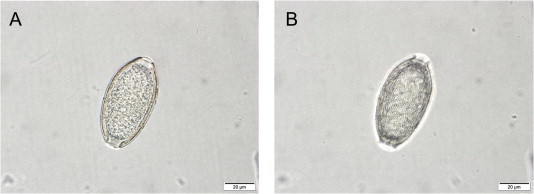
Eucoleus sp. egg collected from a female worm embedded in the non-glandular stomach mucosa of a wild, Norway rat (Rattus norvegicus). (A) Photomicrogaph of an egg taken with correct focus (B) Photomicrogaph of the same egg as in A, but taken in an elevated focus targeting the egg shell. Note the dense network of anastomosing ridges.
3.4. Statistical demographic associations
Demographic and morphometric characteristics of the 183 Norway rats included in the microscopic examination and their bivariate associations with Eucoleus sp. infection are presented in Tables 3 and 4. The final GLMM included only sexual maturity and weight. Specifically, the odds of an individual rat having stomach pathology increased with sexual maturity (4.64, 95% CI = 1.23–17.10) and weight (OR = 1.06, 95% CI = 1.00–1.12 per 10 g; Table 4).
Table 4.
Odds ratios (OR) with 95% confidence intervals (CI) for association between the presence of Eucoleus sp. or its associated lesions with other variables among a group of Norway rats (Rattus norvegicus) trapped in Vancouver, Canada.
| Category | Subcategory | Unadjusted |
Adjusted (GLMMb) |
||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| Season | Fall | Ref.a | – | – | |
| Winter | 0.54 | 0.23–1.30 | – | – | |
| Spring | 0.33 | 0.13–0.80 | – | – | |
| Summer | 0.20 | 0.06–0.63 | – | – | |
| Sex | Female | Ref. | – | – | |
| Male | 1.10 | 0.56–2.13 | – | – | |
| Maturity | Immature | Ref. | – | – | |
| Mature | 10.8 | 4.60–26.8 | 4.64 | 1.23–17.10 | |
| Weight (per 10 g) | 2.46 | 1.75–3.57 | 1.06 | 1.00–1.12 | |
| Fat score (categorical) | Poor (0) | Ref. | |||
| Moderate (1) | 5.78 | 2.39–15.0 | – | – | |
| Good (1) | 4.21 | 1.88–9.78 | – | – | |
| Fat score (continuous) | 2.09 | 1.36–3.28 | – | – | |
| Liver Capillaria hepatica | Negative | Ref. | – | – | |
| Positive | 3.71 | 1.88–7.46 | – | – | |
Reference value for analysis.
GLMM = Generalized linear mixed model (controlled for clustering by block).
The prevalence of stomach pathology and/or Eucoleus sp. parasitism varied from 0% to 81.8% among the different city blocks. In the final GLMM, the variance associated with the random effect of city block was 0.19, which suggests clustering by block. However, within the study area as a whole, there were no specific geographic areas of higher or lower than expected prevalence (Fig. 7).
Fig. 7.
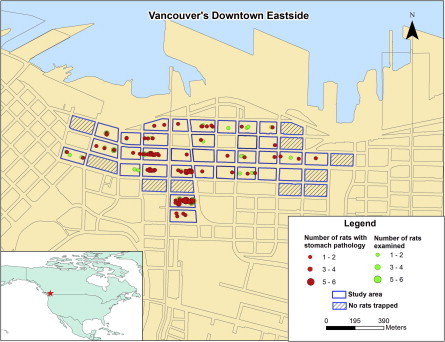
Spatial distribution of Norway rats (Rattus norvegicus) infected with Eucoleus sp. and/or with associated stomach pathology in the Downtown Eastside of Vancouver, Canada. There are no clusters of affected rats.
4. Discussion
The apparent prevalence of infection, 43.1%, while consistent with previous studies in which prevalence has ranged from 30% to 52% (GeGiusti, 1957; Milazzo et al., 2010), likely is an underestimate of true parasite prevalence given that nematodes and/or associated microscopic lesions were present in 73.8% of rats. The strong statistical association between lesions and infection suggest that lesions alone indicate infection. The irregular distribution of the parasite within the stomach and continual sloughing and regeneration of the mucosa may explain the absence of parasites in sections of stomach with associated microscopic lesions (Vogel, 1929). Eucoleus sp. infection and lesions were most prevalent in large, sexually mature rats, suggesting a higher probability of infection with age and that host maturity does not trigger the development of protective immunity (Stojcevic et al., 2004; Milazzo et al., 2010).
A weakness of the current study is the absence of definitive species identification. Esophageal and gastric nematode collection was not specifically included in the autopsy protocol of this study, and well-preserved nematode specimens were not available for identification beyond the genus level. Currently, the taxonomy of capillarid nematodes is in great flux with much debate on classification and a lack of definitive morphological identification criteria (Moravec, 1982). Historically, two species of Eucoleus, E. bacillatus (Eberth, 1863) and E. gastricus (Baylis, 1926), were described from the upper gastrointestinal tract of rats (Hasegawa et al., 1992; Moravec, 2000), but there are no published criteria to distinguish these two species. E. bacillatus was reported in the stomach of Rattus argentiventer, R. rattus and several other rodent species within the families Muridae and Arvicolidae (Hasegawa et al., 1992, 1993; Behnke et al., 1993; Feliu et al., 1997; Moravec, 2000; Fuentes et al., 2004). To our knowledge, E. bacillatus has never reliably been described in Norway rats; therefore E. gastricus is most likely to be the species featured in this paper. First described by Baylis (1926) as a species, E. gastricus has apparent worldwide distribution (Vogel, 1929; Oldham, 1931; Kasai, 1978; Milazzo et al., 2010) and is thought to be transmitted directly (Vogel, 1929).
It is likely that Eucoleus sp. has been under-recognized in previous studies of wild rat parasites. For example, some studies have attributed capillarid eggs found in rat feces to be from Calodium hepaticum (syn. Capillaria hepatica), which is very common but which normally does not liberate eggs into the gastrointestinal tract. Eucoleus sp. is too small to be visible without magnification, fragile, resides within the mucosa, and is easily missed by standard saline rinses to collect gastric helminthes (Behnke et al., 1993) (Fig. 4). In this study, histology proved to be a sensitive method to detect Eucoleus sp. in the upper gastrointestinal tract of rats and also the host response to the parasite.
Microscopic stomach pathology, strongly associated with Eucoleus sp. infection, was common in this population of wild urban Norway rats. Similar gastric lesions were identified in association with E. gastricus (identified based on egg morphology; previously H. gastrica and C. gastrica) in wild Norway and laboratory rats by Vogel (1929). Hitchcock and Bell (1952) found hyperkeratosis and mucosal hyperplasia in the non-glandular stomach associated with a variety of nematode parasites (G. neoplasticum, Trichuris sp. and unknown species) in Norway rats near Minneapolis, USA. Chronic irritation by a variety of irritants, including nematodes, various chemicals, acidified water, vitamin A deficiency and anorexia, can induce hyperkeratosis and mucosal hyperplasia in the non-glandular stomach of laboratory rats (Vogel, 1929; Beatti, 1930; Hitchcock and Bell, 1952; Maeda et al., 1985; Brown and Hardisty, 1990; Greaves, 2012). These changes are also sporadically found in older laboratory rats with no readily apparent cause (Greaves, 2012).
A papilloma in the non-glandular stomach was only detected in one rat, indicating this response to infection is rare. Alternatively, this papilloma may not have been caused by Eucoleus sp. infection. Historical studies of wild rats have attributed stomach papillomas to nematode infection (Fibiger, 1927; Vogel, 1929; Beatti, 1930). Specifically, Fibiger (1927) supports the rarity of these lesions: “investigation of the fundus in nearly 1200 wild rats and laboratory animals gave only negative results”.
Since the days of Fibinger and colleagues, the impact of nematode infection on lesions in the non-glandular stomach of rats has been largely ignored. Since modern rat laboratories exclude all pathogenic rodent nematodes, investigations in these settings is not done. While we describe the pathology associated with Eucoleus sp. infection in the non-glandular stomachs of wild rats, questions persist regarding the ecology of this parasite and its impact on rat health and rat populations.
Acknowledgments
This manuscript is dedicated to the memory of Dr. Robert L. Rausch (1921–2012) for his contribution to the field of wildlife parasitology and for his positive influence on the careers of many veterinarians, wildlife biologists and parasitologists. This study was made possible by the efforts of K. Parsons, A. Feng, V. Chang, D. Rempel, T. Urness, H. Anholt and the Vancouver Area Network of Drug Users (VANDU). I. Shirley, C. Stewart and K. Brown provided invaluable technical assistance. We wish to acknowledge F. Moravec for assistance in confirming the identity of this nematode. This work was supported by the Canadian Institutes of Health Research – Canada (MOP – 119530 and CGV – 104833) and the Western College of Veterinary Medicine Interprovincial Graduate Student Fellowship – Canada. This study was presented in abstract form at the American College of Veterinary Pathologists Annual Meeting, Montreal, Canada in November 2013.
Contributor Information
Jamie L. Rothenburger, Email: jamie.rothenburger@usask.ca.
Chelsea G. Himsworth, Email: cgh050@mail.ubc.ca.
Manigandan Lejeune, Email: mlejeune@ucalgary.ca.
Piper M. Treuting, Email: treuting@uw.edu.
Frederick A. Leighton, Email: ted.leighton@usask.ca.
Appendix A. Supplementary data
Supplementary Figure 1.
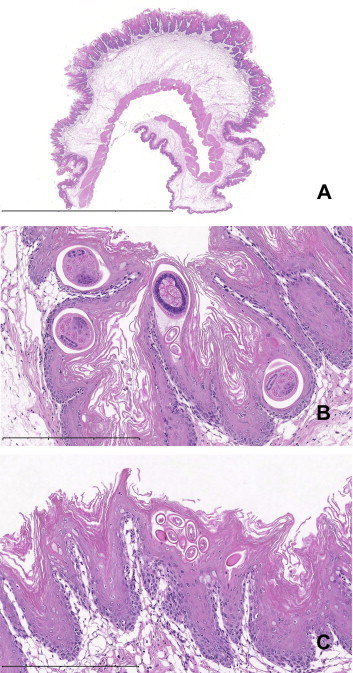
Microscopic image of the squamous papilloma (Fig. 1) arising from the non-glandular stomach of a wild Norway rat (Rattus norvegicus). (A) The mass is comprised of a hyperplastic mucosa overlying a severely edematous submucosa. Scale bar = 6 mm. (B) Cross-sections of Eucoleus sp. adults and eggs within the thickened keratin and superficial mucosa. Scale bar = 300 μm. (C) Eucoleus sp. eggs within the thickened keratin. The underlying mucosa is moderately to severely hyperplasic and the subcutaneous tissues are edematous.
References
- Baylis H.A. A new species of Hepaticola (Nematoda) from the rat’s stomach. J. Trop. Med. Hyg. 1926;29:226–227. [Google Scholar]
- Beatti M. Neue Forschungen über Hepaticola cancerogena. Ihre lokalisation in oesophagus und magen der wildratte (Mus decumanus)—versuchsergebnisse. J. Cancer Res. Clin. Oncol. 1930;32:27–39. [Google Scholar]
- Behnke J.M., Barnard C., Hurst J.L., McGregor P.K., Gilbert F., Lewis J.W. The prevalence and intensity of infection with helminth parasites in Mus spretus from the Setubal Peninsula of Portugal. J. Helminthol. 1993;67:115–122. doi: 10.1017/s0022149x00012992. [DOI] [PubMed] [Google Scholar]
- Boorman G.A., Everitt J.I. Neoplastic disease. In: Suckow M.A., Weisbroth S.H., Franklin C.L., editors. The Laboratory Rat. Elsevier Academic Press; Burlington: 2006. pp. 479–511. [Google Scholar]
- Brown H.R., Hardisty J.F. Oral cavity, esophagus, and stomach. In: Boorman G.A., editor. Pathology of the Fischer Rat. Elsevier Academic Press; San Diego: 1990. pp. 15–30. [Google Scholar]
- Feliu C., Renaud F., Catzeflis F., Hugot J.P., Durand P., Morand S. A comparative analysis of parasite species richness of Iberian rodents. Parasitology. 1997;115:453–466. doi: 10.1017/s0031182097001479. [DOI] [PubMed] [Google Scholar]
- Feng A.Y.T., Himsworth C.G. The secret life of the city rat: a review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus) Urban Ecosyst. 2013 http://dx.doi.org/10.1007/s11252-013-0305-4. [Google Scholar]
- Fibiger J. Investigations on Spiroptera carcinoma and the experimental induction of cancer. Nobel Lecture. 1927 URL: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1926/fibiger-lecture.html Last accessed: 10/02/2014. [Google Scholar]
- Frantz J.D., Betton G., Cartwright M.E., Crissman J.W., Macklin A.W., Maronpot R.R. Guidelines for Toxologic Pathology. S.T.P./A.R.P./A.F.I.P.; Washington, D.C: 1991. Proliferative lesions of the non-glandular and glandular stomach in rats, GI-3. [Google Scholar]
- Fuentes M.V., Sáez S., Trelis M. The helminth community of the wood mouse, Apodemus sylvaticus, in the Sierra Espuña, Murcia, Spain. J. Helminthol. 2004;78:219–223. doi: 10.1079/joh2003226. [DOI] [PubMed] [Google Scholar]
- Gardiner C.H., Poynton S.L. second ed. Armed Forces Institute of Pathology; Washington, D.C: 2006. An Atlas of Metazoan Parasites in Animal Tissues. [Google Scholar]
- GeGiusti D.L. Parasites of rats collected in the city of Detroit. Am. J. Trop. Med. Hyg. 1957;6:375. [Google Scholar]
- Greaves P. Histopathology of Preclinical Toxicity Studies. fourth ed. Elsevier; Slovenia: 2012. Digestive system; pp. 325–432. [Google Scholar]
- Hasegawa H., Shiraishi S., Rochman Tikusnema javaense n. gen., n. sp. (Nematoda: Acuarioidea) and other nematodes from Rattus argentiventer collected in West Java, Indonesia. J. Parasitol. 1992;78:800–804. [PubMed] [Google Scholar]
- Hasegawa H., Arai S., Shiraishi S. Nematodes collected from rodents on Uotsuri Island, Okinawa, Japan. J. Helminthol. Soc. Wash. 1993;60:39–47. [Google Scholar]
- Himsworth C.G., Parsons K.L., Jardine C., Patrick D.M. Rats, cities, people, and pathogens: a systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector Borne Zoonotic Dis. 2013;6:349–359. doi: 10.1089/vbz.2012.1195. [DOI] [PubMed] [Google Scholar]
- Hitchcock C.R., Bell E.T. Studies on the nematode parasite, Gongylonema neoplasticum (Spiroptera neoplasticum), and avitaminosis A in the forestomach of rats: comparison with Fibiger’s results. J. Natl. Cancer Inst. 1952;12:1345–1387. [PubMed] [Google Scholar]
- Kasai Y. Studies on helminth and protozoan parasites of rats in Sapporo. Jpn. J. Vet. Res. 1978;26 31–31. [Google Scholar]
- Maeda H., Gleiser C.A., Masoro E.J., Murata I., McMahan C.A., Yu B.P. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J. Gerontol. 1985;40:671–688. doi: 10.1093/geronj/40.6.671. [DOI] [PubMed] [Google Scholar]
- Magi M., Guardone L., Prati M.C., Torracca B., Macchioni F. First report of Eucoleus boehmi (syn. Capillaria boehmi) in dogs in north-western Italy, with scanning electron microscopy of the eggs. Parasite. 2012;19:433–435. doi: 10.1051/parasite/2012194433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milazzo C., Ribas A., Casanova J.C., Cagnin M., Geraci F., Bella C. Helminths of the brown rat (Rattus norvegicus) (Berkenhout, 1769) in the city of Palermo, Italy. Helminthologia. 2010;47:238–240. [Google Scholar]
- Moravec F. Proposal of a new systematic arrangement of nematodes of the family Capillariidae. Folia. Parasitol. 1982;29:119–132. [PubMed] [Google Scholar]
- Moravec F. Review of capillariid and trichosomoidid nematodes from mammals in the Czech Republic and the Slovak Republic. Acta Soc. Zool. Bohem. 2000;64:271–304. [Google Scholar]
- Nadon N.L. Gerontology and age-associated lesions. In: Suckow M.A., Weisbroth S.H., Franklin C.L., editors. The Laboratory Rat. Elsevier Academic Press; Burlington: 2006. pp. 761–772. [Google Scholar]
- Oldham J.N. The helminth parasites of common rats. J. Helminthol. 1931;9:49–90. [Google Scholar]
- Rothenburger, J.L., Himsworth, C.G., Chang, V., Lejune, M., Leighton, F.A., 2014. Capillaria hepatica in wild Norway Rats (Rattus norvegicus) from Vancouver, Canada. J. Wildl. Dis., http://dx.doi.org/10.7589/2013-09-256. [DOI] [PubMed]
- Stojcevic D., Mihaljevic Z., Marinculic A. Parasitological survey of rats in rural regions of Croatia. Vet. Med. -Czech. 2004;49:70–74. [Google Scholar]
- Traversa D., Di Cesare A., Lia R.P., Castagna G., Meloni S., Heine J., Strube K., Milillo P., Otranto D., Meckes O., Schaper R. New insights into morphological and biological features of Capillaria aerophila (Trichocephalida, Trichuridae) Parasitol. Res. 2011;109:S97–S104. doi: 10.1007/s00436-011-2406-4. [DOI] [PubMed] [Google Scholar]
- Vogel H. Magencarcinom der Ratte nach experimenteller Infektion mit Hepaticola gastrica. J. Cancer Res. Clin. Oncol. 1929;29:351–359. [Google Scholar]


