Keywords: Trypanosoma spp., Marsupial, Rodent, Woylie, Surveillance, Biodiversity
Highlights
-
•
Trypanosomes of Australian marsupials, rodents, bats and monotremes are reviewed.
-
•
22% of the indigenous terrestrial and arboreal mammals have been screened.
-
•
Trypanosomes have been identified from 28 mammal species.
-
•
Eight native trypanosome species have been described from Australian mammals
-
•
Potential pathogenic risks and threatening biosecurity concerns are discussed.
Abstract
Approximately 306 species of terrestrial and arboreal mammals are known to have inhabited the mainland and coastal islands of Australia at the time of European settlement in 1788. The exotic Trypanosoma lewisi was the first mammalian trypanosome identified in Australia in 1888, while the first native species, Trypanosoma pteropi, was taxonomically described in 1913. Since these discoveries, about 22% of the indigenous mammalian fauna have been examined during the surveillance of trypanosome biodiversity in Australia, including 46 species of marsupials, 9 rodents, 9 bats and both monotremes. Of those mammals examined, trypanosomes have been identified from 28 host species, with eight native species of Trypanosoma taxonomically described. These native trypanosomes include T. pteropi, Trypanosoma thylacis, Trypanosoma hipposideri, Trypanosoma binneyi, Trypanosoma irwini, Trypanosoma copemani, Trypanosoma gilletti and Trypanosoma vegrandis. Exotic trypanosomes have also been identified from the introduced mammalian fauna of Australia, and include T. lewisi, Trypanosoma melophagium, Trypanosoma theileri, Trypanosoma nabiasi and Trypanosoma evansi. Fortunately, T. evansi was eradicated soon after its introduction and did not establish in Australia. Of these exotic trypanosomes, T. lewisi is the sole representative that has been reported from indigenous Australian mammals; morphological forms were recorded from two indigenous species of rodents (Hydromys chrysogaster and Rattus fuscipes). Numerous Australian marsupial species are potentially at risk from the native T. copemani, which may be chronically pathogenic, while marsupials, rodents and monotremes appear at risk from exotic species, including T. lewisi, Trypanosoma cruzi and T. evansi. This comprehensive review of trypanosome biodiversity in Australia highlights the negative impact of these parasites upon their mammalian hosts, as well as the threatening biosecurity concerns.
1. Introduction
Parasites from the genus Trypanosoma are ubiquitous haemoprotozoans that infect a wide range of animals, including fish, amphibians, reptiles, aves and mammals, and are the causative agents for some of the most neglected human diseases (Noyes, 1998; Barrett et al., 2003). The basic haematic shape of mammalian trypanosomes are somewhat lanceolate, and oval in transverse section; they contain a dark staining nucleus, as well as a dark staining kinetoplast situated at the base of a single undulating flagellum (Hoare, 1972) (Fig. 1a). The genus Trypanosoma is believed to be a monophyletic group, with the two most important species in people, Trypanosoma brucei and Trypanosoma cruzi, sharing a common ancestor that dates back about 100 million years (Stevens et al., 1999; Barrett et al., 2003). With the exception of Trypanosoma equiperdum, which can be sexually transmitted, and T. cruzi, which can be transmitted vertically and orally, the trypanosomes of mammals are believed to be heteroxenous (Hoare, 1972; Muñoz et al., 2009; Shikanai-Yasuda and Carvalho, 2012).
Fig. 1.
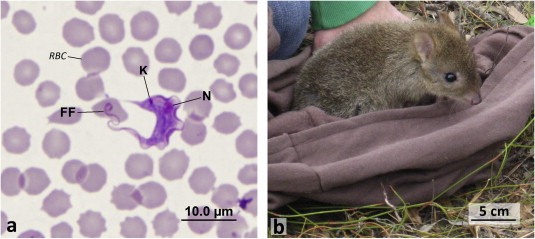
(a) General trypanosome shape (trypomastigote form from the blood of a woylie (Bettongia penicillata)) K = kinetoplast, N = nucleus and FF = free flagellum and RBC = red blood cells, (b) host: woylie (Bettongia penicillata).
There is a growing realisation that trypanosomes infecting mammalian wildlife can adversely affect the health of their hosts, with three documented cases supporting this. The first is the introduction of Trypanosoma lewisi onto Christmas Island, which may have precipitated, or been the sole cause of the extinction of the Maclear’s rat (Rattus macleari) and possibly the bulldog rat (Rattus nativitatis) (Pickering and Norris, 1996; Wyatt et al., 2008; MacPhee and Greenwood, 2013). The second case is the intracellular stage of Trypanosoma copemani and its recent association with changes to the smooth and cardiac muscles of the critically endangered woylie (Bettongia penicillata) (Fig. 1b), with characteristics of infection reportedly similar to Chagas disease in humans (Botero et al., 2013). The third case is the statistical association of Trypanosoma gilletti infections with lower blood packed cell volumes and body condition scores of koalas (Phascolarctos cinereus) with signs of concurrent diseases, including chlamydiosis, bone marrow disease or koala AIDS (McInnes et al., 2011). These documented cases support the need for continued surveillance of trypanosome biodiversity, investigation of the pathogenicity of Australian native trypanosomes and monitoring of the Australian mammals for exotic trypanosome species.
There are growing biosecurity concerns regarding the potential negative impact of exotic trypanosomes if allowed to successfully establish within the indigenous wildlife of Australia (Thompson, 2013). The potential impact of Trypanosoma evansi on the health of Australian marsupials has already been demonstrated (see below) (Reid et al., 2001), but recent analysis of Australian native trypanosomes has also identified shared phylogenetic relationships with exotic trypanosomes, such as the taxonomically undescribed Australian species of Trypanosoma referred to as T. sp. H25 and the pathogenic T. cruzi (Stevens et al., 1999; Hamilton et al., 2005a; Botero et al., 2013) (Fig. 2). This shared genetic relationship could indicate that vectors involved in the transmission of our native trypanosomes could, in theory, transmit the related exotic trypanosome to our wildlife, in much the same way that the day-feeding midge (Diptera: Ceratopogonidae) (and not a phlebotomine sand-fly) has been incriminated with the transmission of Leishmania to the Australian red kangaroo (Macropus rufus) (Dougall et al., 2011). Therefore, the possibility exists for Australian indigenous mammals, such as the brush-tail possum (Trichosurus vulpecula) and short-beaked echidna (Tachyglossus aculeatus) (Backhouse and Bolliger, 1951) to act as reservoirs for the pathogenic T. cruzi if successfully transmitted from an infected Chagas patient, of which there were an estimated 1400–3000 in Australia in 2006 (Gascon et al., 2010; Schmunis and Yadon, 2010). However, further work is required to investigate whether T. sp. H25 and T. cruzi share any biological traits.
Fig. 2.
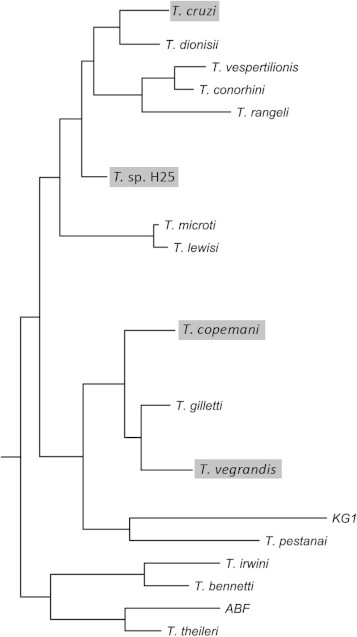
A graphical representation of the phylogenetic relationship shared by some Australian trypanosome isolates based on gGAPDH sequences (≈810 bp) (reproduced with permission from Botero et al. (2013), with modifications highlighted in grey).
Given the growing concerns regarding the health and survival of wildlife, this review outlines the current knowledge of the diversity and distribution of trypanosomes that infect Australian mammals. Here we review the last 100 years of trypanosome research in Australia, identifying geographic ranges of native species, and highlighting where further research is needed and the groups of indigenous mammals that are at highest risk.
2. Mammals of Australia
Current estimates account for approximately 306 species of terrestrial and arboreal mammals that were extant in Australia at the time of European settlement in 1788 (McKenzie et al., 2007). This includes 162 marsupial species, 66 rodents, 76 bats and two monotremes (McKenzie et al., 2007; Van Dyck and Strahan, 2008). In this article, we refer only to “modern” mammals, being those species that were extant and indigenous to Australia at the time of European settlement. We have included species which became extinct soon after European arrival, such as the Nullarbor dwarf bettong (Bettongia pusilla), which has never been recorded alive and is known only from its fossil records (McNamara, 1997; Burbidge, 2008).
The modern indigenous mammals of Australia have not fared well since colonisation; an estimated 7% are extinct, an additional 3% have become restricted to offshore islands, and 14% are currently threatened with extinction (McKenzie et al., 2007). This proportion of mammal extinction exceeds all other continents during the same time period (Short, 1998; Johnson, 2006; McKenzie et al., 2007; Van Dyck and Strahan, 2008).
3. Trypanosomes of Australian mammals
The following describes chronologically the identification of trypanosomes from indigenous Australian mammals: (i) marsupials, (ii) rodents, (iii) bats and (iv) monotremes, as well as (v) introduced mammalian fauna. The geographical identification of trypanosomes from indigenous Australian mammals is illustrated in Fig. 3a. The potential impact of native and exotic trypanosome infections upon their mammalian host species is also discussed.
Fig. 3.
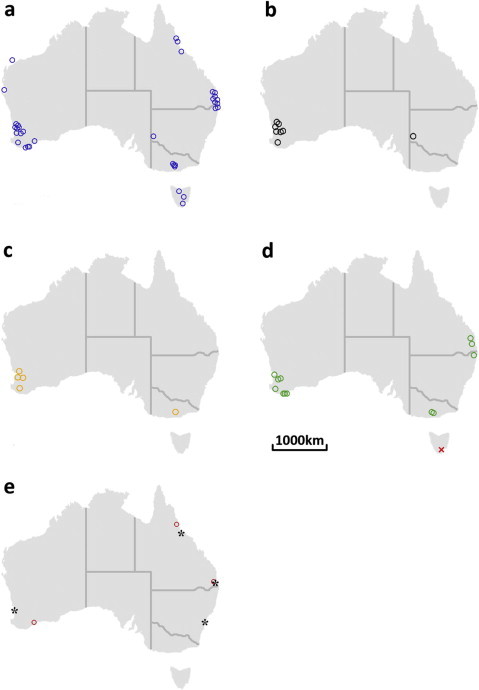
Geographical locations of trypanosomes identified from Australian indigenous mammals- (a) all Trypanosoma spp., (b) Trypanosoma vegrandis only, (c) Trypanosoma sp. H25 only, (d) Trypanosoma copemani only (cross (x) = the possible identification from Tasmania in 1998) and (e) Trypanosoma lewisi only (circle (o) = records from indigenous mammals and asterisk (∗) = records from introduced mammals).
3.1. Trypanosomes of indigenous Australian marsupials
Of the four indigenous mammalian groups included in this review, marsupials have the most number of species (N = 46) examined in terms of trypanosome surveillance in Australia. The first trypanosome identified from an Australian marsupial was from the short-nosed bandicoot (Isoodon macrourus) (nomenclature of 1953: Thylacis obesulus) in 1953; this trypanosome was taxonomically described Trypanosoma thylacis in 1959 (Mackerras, 1958a, 1959). Nine additional marsupial species were examined during this study, all of which were uninfected with trypanosomes (Mackerras, 1959) (Table 1). Trypanosoma thylacis was to remain the only formally described trypanosome species from an Australian marsupial for another 50 years.
Table 1.
Indigenous Australian mammals screened for trypanosomes, number of individuals examined and number of positive infections (∗ indicates a positive trypanosome reference).
| Host: common name | Host: species name | Number of hosts examined | Number of hosts positive | Trypanosoma species | Reference |
|---|---|---|---|---|---|
| Marsupial | |||||
| Kowari | Dasyuroides byrnei | 7 | 0 | D | |
| Eastern quoll | Dasyurus viverrinus | 58 | 0 | D, K | |
| Northern brown bandicoot | Isoodon macrourus | 82 | 12 | T. thylacis | B∗, D* |
| Long-nosed bandicoot | Perameles nasuta | 1 | 0 | D | |
| Sugar glider | Petaurus breviceps | 6 | 0 | D, K | |
| Squirrel glider | Petaurus norfolcensis | 5 | 0 | D, K | |
| Common ringtail possum | Pseudocheirus peregrinus | 4 | 0 | D, K | |
| Long-nosed potoroo | Potorous tridactylus | 3 | 0 | D, F, K | |
| Eastern grey kangaroo | Macropus giganteus | 12 | 1 | T. sp. H25 | D, F*,J*, K |
| Red kangaroo | Macropus rufus | 8 | 0 | D, K, S | |
| Southern brown bandicoot | Isoodon obesulus | >23 | >9 | T. vegrandis, T. copemani | E*, S, T* |
| Eastern barred bandicoot | Perameles gunnii | >7 | >1 | T. sp. | E*, F |
| Common wombat | Vombatus ursinus | 21 | 4 | T. copemani | F*, J*, K* |
| Koala | Phascolarctos cinereus | 604 | 439 | T. irwini, T. copemani, T. gilletti | F, K, O*, Q*, R* |
| Brush-tailed possum | Trichosurus vulpecula | 155 | 40^ + 17 | T. cruzi^ + T. sp. H25, T. copemani | A^,F, K, N*, S*, T* |
| Parma wallaby | Macropus parma | 3 | 0 | F, K | |
| Brush-tailed rock-wallaby | Petrogale penicillata | 2 | 1 | T. sp. | F, K* |
| Swamp wallaby | Wallabia bicolour | 5 | 1 | T. sp. | F, J*, K* |
| Bridled nail-tail wallaby | Onychogalea fraenata | 39 | 0 | G | |
| Agile wallaby | Macropus agilis | 2 | 2^ | T. evansi^ | H^ |
| Dusky antechinus | Antechinus swainsonii | 10 | 0 | K | |
| Brush-tailed phascogale | Phascogale tapoatafa | 4 | 0 | K | |
| Tasmanian devil | Sarcophilus harrisii | 4 | 0 | K | |
| Rufus bettong | Aepyprymnus rufescens | 2 | 0 | K | |
| Red-necked wallaby | Macropus rufogriseus | 3 | 0 | K | |
| Purple-necked rock-wallaby | Petrogale purpureicollis | 2 | 0 | K | |
| Yellow-footed rock-wallaby | Petrogale xanthopus | 2 | 0 | K | |
| Quokka | Setonix brachyurus | 22 | 10 | T. copemani | L*, P*, T* |
| Gilbert’s potoroo | Potorous gilbertii | 8 | 8 | T. copemani | L*, P* |
| Woylie | Bettongia penicillata | 1413 | 535 | T. copemani, T. vegrandis, T. sp. H25 | M*, N*, S*, T*, U* |
| Western quoll | Dasyurus geoffroii | 62 | 3 | T. vegrandis | M*, N*, S, T* |
| Dibbler | Parantechinus apicalis | 2 | 1 | T. sp. | N* |
| Common planigale | Planigale maculata | 6 | 1 | T. sp. | N* |
| Golden bandicoot | Isoodon auratus | 12 | 1 | T. sp. | N* |
| Western barred bandicoot | Perameles bougainville | 11 | 0 | N, S | |
| Greater bilby | Macrotis lagotis | 63 | 0 | N, S | |
| Burrowing bettong | Bettongia lesueur | 86 | 7 | T. sp., T. sp. H25 | N*, S, T* |
| Spectacled hare-wallaby | Lagorchestes conspicillatus | 3 | 0 | N | |
| Banded hare-wallaby | Lagostrophus fasciatus | 10 | 1 | T. sp. H25 | N, T* |
| Black-footed rock-wallaby | Petrogale lateralis | 10 | 0 | N | |
| Western grey kangaroo | Macropus fuliginosus | 45 | 29 | T. vegrandis | S, T* |
| Macropus sp. | Macropus sp. (likely robustus) | 2 | 0 | S | |
| Northern quoll | Dasyurus hallucatus | 6 | 0 | S | |
| Western ringtail possum | Pseudocheirus occidentalis | 13 | 0 | S | |
| Tiger quoll | Dasyurus maculatus | 30 | 17 | T. copemani | T* |
| Tammar wallaby | Macropus eugenii | 7 | 3 | T. vegrandis | T* |
| Rodent | |||||
| Water rat | Hydromys chrysogaster | 39 | 1 | T. lewisi | D*, K, N |
| Grassland melomys | Melomys burtoni | 80 | 0 | D | |
| Spinifex hopping mouse | Notomys alexis | 2 | 0 | N | |
| Western chestnut mouse | Pseudomys nanus | 6 | 0 | N | |
| Bush rat | Rattus fuscipes | 67 | 10 | T. lewisi | C*, D*, N* |
| Dusky field rat | Rattus sordidus | ? | 0 | D | |
| Long-haired rat | Rattus villosissimus | ? | 0 | D | |
| Ash-grey mouse | Pseudomys albocinereus | 2 | 1 | ≈ T. lewisi | N* |
| Shark Bay mouse | Pseudomys fieldi | 18 | 3 | T. sp | N* |
| Bat | |||||
| Black flying fox | Pteropus alecto | >67 | >1 | T. pteropi | C*, D* |
| Spectacled flying fox | Pteropus conspicillatus | 41 | 0 | D | |
| Grey-headed flying fox | Pteropus poliocephalus | >7 | 0 | D | |
| Little red flying fox | Pteropus scapulatus | 13 | 0 | D | |
| Dusky leaf-nosed bat | Hipposideros ater | 1 | 1 | T. hipposideri | D* |
| Semon’s leaf-nosed bat | Hipposideros semoni | 1 | 0 | D | |
| Common bent-wing bat | Miniopterus schreibersii | 23 | 0 | D | |
| Eastern long-eared bat | Nyctophilus bifax | 2 | 0 | D | |
| Eastern forest bat | Vespadelus pumilus | 16 | 0 | D | |
| Monotreme | |||||
| Platypus | Ornithorhynchus anatinus | >11 | >6 | T. binneyi | B*, D*, F*, I*, J*, K* |
| Short-beaked echidna | Tachyglossus aculeatus | 3 | 1^ | T. cruzi^ | A^, F |
References: A = Backhouse and Bolliger (1951), B = Mackerras (1958a), C = Mackerras (1958b), D = Mackerras (1959), E = Bettiol et al. (1998), F = Noyes et al. (1999), G = Turni and Smales (2001), H = Reid et al. (2001), I = Jakes et al. (2001), J = Hamilton et al. (2004), K = Hamilton et al. (2005a,b), L = Clark and Spencer (2006), M = Smith et al. (2008), N = Averis et al. (2009), O = McInnes et al. (2009), P = Austen et al. (2009), Q = Mcinnes et al. (2010), R = McInnes et al. (2011), S = Paparini et al. (2011), T = Botero et al. (2013) and U = Thompson et al. (2013), (^ = host experimentally infected with trypanosomes, ≈ = DNA isolate genetically similar to, * = positive host to trypanosomes)
The first Tasmanian trypanosomes were recorded from the southern brown bandicoot (Isoodon obesulus) and eastern barred bandicoot (Perameles gunnii) in 1998 (Bettiol et al., 1998) (Table 1). The morphological measurements of the trypanosomes observed from I. obesulus were statistically different from those from P. gunnii, indicating two different species of trypanosomes (Bettiol et al., 1998). The morphological measurements of the trypanosome from I. obesulus and I. macrourus were also statistically different from one another, suggesting that the trypanosome from the Tasmanian I. obesulus was not T. thylacis (Mackerras, 1959; Bettiol et al., 1998).
A nested polymerase chain reaction (PCR) was developed in 1999 to detect and identify trypanosomes from blood samples of Australian birds and mammals (Noyes et al., 1999). This approach (with supporting culturing and molecular methodology) was a significant development as it provides a more sensitive technique than conventional parasitological methods, and allows rapid screening of multiple blood samples for the genus Trypanosoma, as well as the identification of previously unknown trypanosome species (Noyes et al., 1999; Adams and Hamilton, 2008). Noyes et al. (1999) examined nine different marsupial species, with six investigated for the first time (Table 1). Trypanosoma sp. H25 was identified from the eastern grey kangaroo (Macropus giganteus) and T. sp. H26 (later identified as T. copemani) from the common wombat (Vombatus ursinus) (Noyes et al., 1999).
The haemoparasites of 39 bridled nail–tail wallabies (Onychogalea fraenata) were examined by microscopy in 2001; no trypanosomes were identified from the 78 blood smears collected (Turni and Smales, 2001). During the same year, the susceptibility of the agile wallaby (Macropus agilis) and the dusky pademelon (Thylogale brunii) to experimental infections with T. evansi was investigated in Papua New Guinea; only the agile wallaby is indigenous to Australia (Reid et al., 2001). Both marsupial species succumbed to infection, suffering from a high level of parasitaemia, morbidity and mortality (Reid et al., 2001). This experiment demonstrated the biosecurity concerns regarding the potential negative impact of T. evansi if it was introduced and successfully established within the wildlife of Australia (Reid et al., 2001; Thompson et al., 2003).
During a phylogenetic analysis in 2004, three different indigenous Australian marsupials were examined for trypanosomes; an undescribed Trypanosoma species was identified (and later cultured (Hamilton et al., 2005a)) from the swamp wallaby (Wallabia bicolour) (Hamilton et al., 2004) (Table 1). During a follow up report in 2005, these authors analysed the blood of 20 indigenous Australian marsupial species, seven of which were investigated for the first time (Hamilton et al., 2005a) (Table 1). An undescribed Trypanosoma species was identified from the brush-tailed rock wallaby (Petrogale penicillata) (Hamilton et al., 2005a). The latter phylogenetic analysis grouped three different trypanosomes from Australian marsupials into three separate clades, and surprisingly, each clade shared a closer genetic relationship with exotic trypanosomes, such as T. cruzi and Trypanosoma theileri, than with one another (Hamilton et al., 2005a).
A haematological study of Western Australian quokkas (Setonix brachyurus) and Gilbert’s potoroos (Potorous gilbertii) in 2006 identified trypanosomes by microscopy (Clark and Spencer, 2006). No morphological measurements were recorded from these parasites.
In 2008, microscopy and molecular methodology were used to identify trypanosomes from the woylie (B. penicillata) and western quoll (Dasyurus geoffroii) in Western Australia (WA) (Smith et al., 2008) (Table 1). At the time, evidence suggested that the trypanosome from the woylie was a new species, as it was morphologically distinct and molecularly novel (Smith et al., 2008). Further, overall prevalence of trypanosomes and individual parasitaemia levels were higher in a declining woylie population in the wild than in a stable, confined population examined concurrently, suggesting that this trypanosome might influence the population size of this host (Smith et al., 2008).
As part of a follow up investigation in 2009, the trypanosomes identified from the quokka and Gilbert’s potoroo in 2006 were recognised as the same species (Clark and Spencer, 2006; Austen et al., 2009). This trypanosome species was named T. copemani (Austen et al., 2009), becoming the second native trypanosome species formally described from an Australian marsupial.
Twelve different species of Australian marsupials were molecularly screened for trypanosomes during the same year, nine of which were analysed for the first time (Averis et al., 2009) (Table 1). Trypanosomes were identified from seven host species, five of which were new host records, including the brush-tailed possum (T. vulpecula), dibbler (Parantechinus apicalis), common planigale (Planigale maculata), golden bandicoot (Isoodon auratus) and the burrowing bettong (Bettongia lesueur) (Averis et al., 2009).
Results from the same study indicated that the trypanosomes from Australian mammals are widespread and appear to be endemic to the continent (Averis et al., 2009). This point was demonstrated by the trypanosomes from the indigenous marsupials on Barrow Island, WA. Barrow Island is believed to have been isolated from mainland Australia about 7500–8000 years ago (Dortch and Morse, 1984). However, despite this lengthy physical separation, trypanosomes of marsupials from Barrow Island share a close genetic relationship with trypanosomes of marsupials from southern WA and from Victoria (Dortch and Morse, 1984; Noyes et al., 1999; Averis et al., 2009; Botero et al., 2013).
The third native trypanosome species from an Australian marsupial was taxonomically described in 2009 (Trypanosoma irwini) from koalas from Queensland and New South Wales (NSW) (McInnes et al., 2009) (Table 1). A year later and using species-specific PCR primers, these authors recognised that koalas could be infected with three different trypanosomes: T. irwini, T. copemani and the newly taxonomically described species, T. gilletti (McInnes et al., 2010) (Table 1). Numerous mixed infections were identified, with a small proportion of koalas simultaneously infected with all three species (McInnes et al., 2011). Also a statistical association was identified between T. gilletti infections and the lower blood packed cell volumes and body condition scores of koalas with signs of concurrent diseases, such as chlamydiosis, bone marrow disease or koala AIDS (McInnes et al., 2011). Trypanosoma gilletti was the fourth native trypanosome formally described from Australian marsupials, and remains the only species of Trypanosoma from an Australian terrestrial and arboreal mammal without a holotype or morphological description (McInnes et al., 2010).
Also in 2011, twelve different species of marsupials, four for the first time, were screened for trypanosomes in WA (Paparini et al., 2011) (Table 1). No new host species were identified in this study (Smith et al., 2008; Averis et al., 2009; Paparini et al., 2011). The brush-tailed possums examined were infected with T. sp. H25, while the woylies were infected with an unidentified trypanosome species (Paparini et al., 2011).
The fifth native trypanosome species from an Australian marsupial was taxonomically described in 2013. It was named Trypanosoma vegrandis and identified from woylies in WA (Thompson et al., 2013). On a global scale, T. vegrandis is believed to be the smallest trypanosome species formally described from mammals, with a minimum length of 6.92 μm and an average length of 8.30 μm (Thompson et al., 2013).
During the same year, ten different species of Western Australian marsupials were screened for trypanosomes using species-specific PCR primers, with two host species examined for the first time (Botero et al., 2013) (Table 1). All ten species were positive to trypanosomes, with trypanosome infections recorded for the first time from the tiger quoll (Dasyurus maculatus), banded hare-wallaby (Lagostrophus fasciatus), tammar wallaby (Macropus eugenii) and western grey kangaroo (Macropus fuliginosus) (Botero et al., 2013). This same study also discovered that the mammalian life cycle of T. copemani transitions through an intracellular stage, with the amastigotes associated with tissue degeneration of the smooth and cardiac muscles of infected woylies (Botero et al., 2013). This intracellular stage (which has also been identified from the related T. caninum (Madeira et al., 2009; Hamilton et al., 2012)) is unusual for other Trypanosoma species apart from the unrelated T. cruzi, and some bat trypanosomes related to T. cruzi (Oliveira et al., 2009).
In addition to the koala, which is host to T. irwini, T. gilletti and T. copemani, the woylie can likewise be infected with three different trypanosomes: T. vegrandis, T. copemani and T. sp. H25 (Botero et al., 2013). The southern brown bandicoot (host to T. copemani and T. vegrandis) (McInnes et al., 2010; Botero et al., 2013) and the brush-tailed possum (host to T. copemani and T. sp. H25) (Paparini et al., 2011; Botero et al., 2013) are the only other marsupials with multiple species infections with native trypanosomes.
The known geographical range of T. vegrandis currently includes WA and NSW and its host range includes the woylie, western grey kangaroo, southern brown bandicoot, tammar wallaby and western quoll (Averis et al., 2009; Paparini et al., 2011; Botero et al., 2013; Thompson et al., 2013) (Fig. 3b). The known geographical range of T. sp. H25 currently includes WA and Victoria and its host range includes the eastern grey kangaroo, woylie, brush-tailed possum, burrowing bettong and banded-hare wallaby (Noyes et al., 1999; Paparini et al., 2011; Botero et al., 2013) (Fig. 3c).
The known geographical range of T. copemani is the most extensive of the native Australian trypanosomes recorded to date. It currently includes Queensland, NSW, Victoria and WA, and infects the common wombat (originally labelled T. sp. H26 in 1999), woylie, Gilbert’s potoroo, quokka, koala, brush-tailed possum, southern brown bandicoot and tiger quoll (Noyes et al., 1999; Clark and Spencer, 2006; Smith et al., 2008; Austen et al., 2009; McInnes et al., 2010, 2011; Botero et al., 2013; Thompson et al., 2013) (Fig. 3d). It is interesting to note that the trypanosomes observed from the Tasmanian I. obesulus in 1998 could have been T. copemani since its morphological measurements were similar to those of T. copemani from the woylie (Thompson et al., 2013) and T. copemani is known to infect the Western Australian I. obesulus (Botero et al., 2013). If this is the case, then the geographical range of T. copemani would be extended to include Tasmania (Fig. 3d).
In total, five native trypanosome species (T. thylacis, T. copemani, T. irwini, T. gilletti and T. vegrandis) have been formally described from Australian marsupials. Host specificity and geographic range of each parasite vary, with T. copemani and T. vegrandis identified in multiple host species. As Fig. 3 illustrates, Australian trypanosomes have largely been identified from hosts that were sampled relatively close to the coast line of the Australia. These are not comprehensive geographical ranges of Australian trypanosomes. As more mammals are sampled from inland habitats and from other states and territories (such as South Australia and Northern Territory), both the host and geographic ranges of these (and other) trypanosomes are likely to increase.
3.2. Trypanosomes of indigenous Australian rodents
To date, only 14% of the Australian native rodent species have been examined for trypanosomes. The first trypanosome identified from a rodent in Australia was by Thomas L. Bancroft in 1888, when he reported the exotic T. lewisi from an introduced Rattus sp. (Johnston, 1916; Mackerras, 1958a). Seventy years later, the first indigenous Australian rodents were screened for trypanosomes by Josephine Mackerras (Mackerras, 1958b). Trypanosoma lewisi was morphologically identified from the bush rat/allied rat (Rattus fuscipes) (nomenclature of 1958: R. assimilis) in southern Queensland (Mackerras, 1958b, 1959), and from the water rat (Hydromys chrysogaster) in northern Queensland (Mackerras, 1959). Three other native rodent species were screened for trypanosomes during this study, but all were uninfected (Mackerras, 1959) (Table 1).
Six different species of indigenous rodents were examined using PCR-based screening in 2009, with four species examined for the first time (Averis et al., 2009) (Table 1). Three host species were infected with trypanosomes, including the Shark Bay mouse (Pseudomys fieldi), which was infected with an unidentified trypanosome, the ash-grey mouse (P. albocinereus), which was infected with an isolate of Trypanosoma genetically similar to T. lewisi, and the bush rat, infected with both an isolate of Trypanosoma genetically similar to T. lewisi and T. sp. H25 (Averis et al., 2009). These findings of T. sp. H25 from the bush rat, the unidentified trypanosome from the Shark Bay mouse and possibly the T. lewisi-like parasite from the ash-grey mouse are the only published reports of native trypanosomes infecting indigenous Australian rodents.
3.3. Trypanosomes of indigenous Australian bats
Of the 76 bat species indigenous to Australia, only 12% have been screened for trypanosomes. The first trypanosome identified from an indigenous Australian bat was reported 46 years before T. thylacis, making it the very first native trypanosome identified from an Australian mammal (Johnston, 1916; Mackerras, 1959). This trypanosome was taxonomically described by Anton Breinl in 1913 and named Trypanosoma pteropi from the Gould’s or Black flying fox (Pteropus alecto) (nomenclature of 1913: P. gouldii) (Johnston, 1916).
The next trypanosome identified from an indigenous Australian bat was in 1959 by Josephine Mackerras, when she examined nine different bat species; eight of which were investigated for the first time (Mackerras, 1959) (Table 1). Of these, only the dusky horseshoe-bat or dusky leaf-nosed bat (Hipposideros ater) (nomenclature of 1913: H. bicolor albanensis) was infected with trypanosomes (Mackerras, 1959). This newly identified trypanosome was taxonomically described, Trypanosoma hipposideri (Mackerras, 1959). As further species of bats are screened for trypanosomes, evidence in support of the ‘bat seeding’ hypothesis may emerge in Australia, with these arboreal trypanosomes species potentially being evolutionary precursors for the terrestrial trypanosome lineage (such as T. sp. H25) within Australian mammals (Hamilton et al., 2012; Lima et al., 2013).
3.4. Trypanosomes of indigenous Australian monotremes
There are only two monotremes indigenous to Australia: the platypus (Ornithorhynchus anatinus) and the short-beaked echidna (T. aculeatus) (Van Dyck and Strahan, 2008). Both of these hosts have been examined for trypanosomes (Table 1).
The first record of a trypanosome from an Australian monotreme was in 1933, when William J. Owen presented his observations from a platypus to the Royal Society of Australia in Canberra (Mackerras, 1959). In 1950, Campbell A. Duncan reported to the Royal Society of Tasmania about another trypanosome identified from a Tasmanian platypus; this trypanosome was later taxonomically described by Josephine Mackerras and named Trypanosoma binneyi in 1959 (Mackerras, 1959). Josephine Mackerras noted in 1959 that the trypanosomes identified by Owen in 1933 appeared distinct and may not be T. binneyi (Mackerras, 1959).
In 1999, a single Victorian platypus was screened for trypanosomes using morphological and molecular techniques; it was found to be infected with T. binneyi (Noyes et al., 1999). The Victorian and Tasmanian platypus has since been re-examined, each time testing positive for T. binneyi (Jakes et al., 2001; Hamilton et al., 2004, 2005a).
In 1999, two short-beaked echidnas were screened for trypanosomes using molecular techniques; both were uninfected (Noyes et al., 1999). As the short-beaked echidna is vulnerable to experimental infection with T. cruzi (Backhouse and Bolliger, 1951), further investigation is required to conclude whether they are susceptible to native Australian trypanosomes.
3.5. Trypanosomes of introduced mammals
Since European settlement, five exotic trypanosomes have been identified in Australia from the various introduced mammals. These include T. lewisi (introduced hosts: house mouse (Mus musculus), brown rat (Rattus norvegicus), and black rat (Rattus rattus)), Trypanosoma melophagium (introduced host: sheep (Ovis aries)), T. theileri (introduced host: cattle (Bos taurus)), Trypanosoma nabiasi (introduced host: European rabbit (Oryctolagus cuniculus)) and T. evansi (introduced host: camel (Camelus dromedarius)) (Johnston, 1909; Mackerras, 1959; O’Donoghue and Adlard, 2000; Hamilton et al., 2005b). A number of introduced mammals are also experimentally susceptible to infection with T. evansi, including the dog (Canis familiaris), guinea pig (Cavia porcellus), horse (Equus caballus) and black rat (R. rattus) (O’Donoghue and Adlard, 2000). A single captive dingo from Healesville, Victoria has been examined for trypanosomes, but was uninfected (Noyes et al., 1999). However, despite this negative result, the dingo, like other canids, is considered to be potentially susceptible to infection with T. evansi, if this exotic trypanosome were to enter Australia (Reid et al., 2001; Reid, 2002).
Since their introduction to Australia, T. lewisi, T. melophagium and T. theileri are each believed to have continental distributions (Mackerras, 1959), while T. nabiasi has been identified only in NSW and Victoria during a single investigation (Hamilton et al., 2005b). Trypanosoma lewisi is the only exotic trypanosome that has been identified, morphologically, from indigenous Australia mammals (Mackerras, 1959). It is interesting to note, that T. lewisi could have diverged since its introduction and adapted to other hosts, as indicated by the T. lewisi-like form from the ash-grey mouse (Averis et al., 2009); however, further research, using updated molecular techniques and larger gene sequences, is needed to confirm this. If pathogenic to indigenous Australian animals, the exotic T. lewisi (and/or T. lewisi-like parasite) could have played a role during the fauna decline in Australia between 1875 and 1925 (Abbott, 2006).
Fortunately, the surra-infected camels that were imported into WA in 1907 were diagnosed quickly and T. evansi was eradicated from Australia before it was allowed to spread (Cleland, 1909; Mackerras, 1959). If T. evansi had been allowed to establish, it too may also have had a continental distribution, with hypothetical hosts including both introduced and indigenous mammals of Australia (O’Donoghue and Adlard, 2000; Reid et al., 2001; Reid, 2002). To date, no native Australian trypanosomes have been identified from an introduced mammal; however, continued surveillance is required to investigate this further.
4. Potential impact of trypanosome infections
The emphasis of trypanosome surveillance in Australia has been on identifying new host records and new species of native trypanosomes. However, the recent association of trypanosomes with the woylie decline (Botero et al., 2013), koala health (McInnes et al., 2011), and potential extinction of the native rats on Christmas Island (Pickering and Norris, 1996; Wyatt et al., 2008) demonstrates the need to continue investigations on the potential impact of these haemoparasites upon their hosts. The surveillance of trypanosomes from Australian wildlife will become of increasing importance, especially as more mammals become endangered and biosecurity concerns grow. However, it will be difficult to associate native trypanosomes with disease, especially when investigating endangered Australian mammals, as exceptional scientific justification and ethical treatment would be needed to translocate individuals from the wild, house them in purpose built enclosures, and experimentally infect and cure them. These studies will also need to correlate the changes of animal health (from several different populations) with pathological results, such as molecular, clinical, haematopathology and histopathology.
Since the identification of trypanosomes from the woylie in 2008, there has been ongoing research investigating the connection between these haemoparasites and the rapid decline of the host (Smith et al., 2008; Botero et al., 2013; Thompson et al., 2013). In 2013, a possible correlation linking T. copemani, its intracellular amastigote stage and the histopathological changes to the smooth and cardiac muscles of infected woylies was identified (Botero et al., 2013). The inflammation and tissue degeneration observed was principally associated with the heart, skeletal muscle, oesophagus and tongue, with histopathological changes similar to those in human patients infected with Chagas disease (Botero et al., 2013). It is currently hypothesised that the chronic intracellular association of T. copemani with these vital organs could be responsible for an overall reduction in fitness and coordination of the woylie, making them more susceptible to predation or to other, as yet unidentified, stressors (Botero et al., 2013; Thompson et al., 2013). However, the chronic effects of T. copemani upon the Gilbert’s potoroo, quokka, koala, the common wombat, southern brown bandicoot, the common brush-tailed possum and tiger quoll require investigation.
In 1899, the shipping vessel “S.S. Hindustan” docked at Christmas Island and as its cargo of hay was offloaded, so too were its trypanosome-infected stowaways (Durham, 1908; Pickering and Norris, 1996). The animals unintentionally introduced onto the island were the black rat (R. rattus), its ectoparasitic fleas, and T. lewisi (Durham, 1908; Wyatt et al., 2008). Over the next three years, the black rat successfully colonised the island while the Maclear’s rat (R. macleari), an indigenous and nocturnal species of rodent, was frequently observed during the day sick or dying (Durham, 1908; Andrews, 1909; Pickering and Norris, 1996). When autopsied, the Maclear’s rats were found to be heavily infected with trypanosomes, which were morphologically similar to T. lewisi (Durham, 1908). The spleen and superficial lymphatic glands of these rodents were also markedly enlarged (Durham, 1908). In 1908, only eight to nine years after the arrival of R. rattus, the Maclear’s rat (once the most abundant mammal on Christmas Island) and the bulldog rat (R. nativitatis) (also an indigenous rodent species on the island) were both reported as extinct (Andrews, 1909).
It is believed that the introduction of T. lewisi onto the island was the causative factor responsible for the extinction of the Maclear’s and bulldog rats (Andrews, 1909; Pickering and Norris, 1996). A century after their extinction, molecular methodology was used on ancient DNA to test this hypothesis (Wyatt et al., 2008). This evidence suggested that native trypanosomes were absent from the indigenous rodents on Christmas Island prior to introduction of the black rat, and that after 1899 both the Maclear’s and bulldog rat were infected with T. lewisi (Wyatt et al., 2008). This well documented series of events provides strong evidence linking T. lewisi to the extinction of the Maclear’s rat and possibly the bulldog rat on the Christmas Island.
As evident from this review, one group of mammals warranting further attention are the rodents of Australia. Given the probable continental distribution of T. lewisi (Mackerras, 1959), its potential to infect other host species (such as Australian rodents (Mackerras, 1959)), monkeys (Maia da Silva et al., 2010) and humans (Johnson, 1933; Howie et al., 2006; Kaur et al., 2007; Sarataphan et al., 2007; Doke and Kar, 2011; Shah et al., 2011; Verma et al., 2011; Truc et al., 2013)), and its postulated association with the extinction of native species (see above) and the death of a person in India (Doke and Kar, 2011), what unreported impact has T. lewisi had on the indigenous mammals of Australia? Also, is this Australian situation unique, or have similar wildlife declines occurred undetected on other continents? Trypanosomes have been demonstrated to vary in virulence when they encounter a new or naїve host species (Maraghi and Molyneux, 1989; Wyatt et al., 2008; MacPhee and Greenwood, 2013), with the exotic T. lewisi possibly playing a role during the fauna decline identified in Australia between 1875 and 1925 (Abbott, 2006). However, to investigate this further, sensitive culturing techniques and better molecular methodology are required to target T. lewisi and document its distribution and host range in Australia.
Ongoing surveillance is required to monitor the potential biosecurity concerns of exotic trypanosomes becoming established in Australian mammals. For example, in 1951, forty brush-tailed possums and a single short-beaked echidna were experimentally infected with T. cruzi, with each individual succumbing to infection; the majority of individuals died between 21 and 35 days after inoculation (Backhouse and Bolliger, 1951). Given the number of host species of T. sp H25, its potential continental distribution (and therefore potential continental distribution of its vector), and its genetic relationship with T. cruzi (Fig. 2), there is a possibility that the vector of T. sp. H25 could transmit T. cruzi from humans (of which there were an estimated 1400–3000 human cases in Australia in 2006 (Gascon et al., 2010; Schmunis and Yadon, 2010)) to indigenous Australian mammals. This may occur in much the same way that an unusual vectorial candidate (day-feeding midge) has been incriminated with the transmission of Leishmania to the Australia red kangaroo (Dougall et al., 2011).
From a biosecurity viewpoint, the transmission of native Australian trypanosomes is another issue that requires urgent attention, as very little is known about their life cycle, vectors (including cyclical and mechanical vectors) and transmission dynamics (stercorarian vs. salivarian). Of the few published reports of the screening of Australian invertebrates for trypanosomes, unidentified trypanosomes have been observed within the gut contents of ticks (Mackerras, 1959) and from the crop of a Haemadipsidae leech (Richardson and Hunt, 1968). Unidentified trypanosomes (related, but not identical to those from the swamp wallaby and brush-tailed rock wallaby) were isolated from terrestrial leeches (Micobdella sp. and Philaemon sp.) (Hamilton et al., 2005a) and T. copemani has been observed in the midgut and faeces of Ixodes ticks (Ixodes australiensis) (Austen et al., 2011). To date, there have been no published reports of experimental transmission demonstrating the complete life cycle of Australian trypanosomes. Therefore, it is very difficult to assess the potential biosecurity risk without knowing what transmits native Australian trypanosomes (Backhouse and Bolliger, 1951; Reid et al., 2001; Dougall et al., 2011). From phylogenetic analyses it is possible to suggest vectorial candidates; however, further work is required as the association between vectors and Trypanosoma clades are never strict.
5. Conclusion
This review shows that a total of 66 indigenous Australian mammal species have been screened for trypanosomes, including 46 of the 162 marsupial species (28%), 9 of the 66 rodent species (14%), 9 of the 76 bat species (12%) and both of the monotreme species (100%). Of the mammals screened, 28 species had trypanosome infections. A total of eight trypanosome species have been taxonomically described from indigenous Australian mammals; these include T. pteropi (1913), T. thylacis (1959), T. hipposideri (1959), T. binneyi (1959), T. irwini (2009), T. copemani (2009), T. gilletti (2010) and T. vegrandis (2013). Others trypanosomes have been investigated, morphologically and molecularly, but not formally named. Judging by the number of recent discoveries, this is unlikely to be an exhaustive list of native trypanosome species in Australia and future surveillance is required, targeting the marsupials, rodents and bats. Of the eight native trypanosome species formally described, only T. copemani and T. vegrandis have been identified in multiple hosts, with the chronic effects of T. copemani infections being associated with the woylie decline. The host range of T. copemani includes the woylie (B. penicillata), Gilbert’s potoroo (P. gilbertii), quokka (S. brachyurus), koala (P. cinereus), the common wombat (V. ursinus), southern brown bandicoot (I. obesulus), the common brush-tailed possum (T. vulpecula) and tiger quoll (D. maculatus). The host range for T. vegrandis includes the woylie (B. penicillata), western grey kangaroo (M. fuliginosus), southern brown bandicoot (I. obesulus), tammar wallaby (M. eugenii) and western quoll (D. geoffroii). Various exotic trypanosomes have been identified in Australia, including T. lewisi, T. melophagium, T. theileri, T. nabiasi and T. evansi. Of these, only T. lewisi has been identified, morphologically, from indigenous Australian rodents. However, it is difficult to assess the biosecurity risks these exotic trypanosomes pose without knowing more about the vectors of the Australian trypanosomes. For example, T. evansi appears a high risk exotic trypanosome due to its non-specific vectorial requirements (with various biting flies capable of transmission), whereas with T. cruzi, we can only make educated guesses. Also, research focusing on whether T. lewisi (and/or T. lewisi-like parasites) are being perpetuated in the native mammals of Australia urgently requires attention. Due to the large number of Australian mammals that are at potential risk, this review highlights the need to continue the surveillance of wildlife, to initiate more intensive surveillance of invertebrate vectorial candidates, and monitor biosecurity concerns.
Acknowledgements
We would like to thank Eileen Salisbury and Judy Dunlop for their comments on earlier drafts. Funding for this review was supplied by Murdoch University Australian Postgraduate Award and was supported by funding from the Western Australian Government’s State NRM Program and by a Discovery Early Career Researcher Award (Australian Research Council).
References
- Abbott I. Mammalian faunal collapse in Western Australia, 1875–1925: the hypothesised role of epizootic disease and a conceptual model of its origin, introduction, transmission, and spread. Aust. Zool. 2006;33:530–561. [Google Scholar]
- Adams E.R., Hamilton P.B. New molecular tools for the identification of trypanosome species. Future Microbiol. 2008:167–176. doi: 10.2217/17460913.3.2.167. [DOI] [PubMed] [Google Scholar]
- Andrews C.W. An account of a visit to Christmas Island in 1908. J. Zool. 1909;1987(1909):101–103. [Google Scholar]
- Austen J.M., Jefferies R., Friend J.A., Ryan U., Adams P., Reid S.A. Morphological and molecular characterization of Trypanosoma copemani n. sp. (Trypanosomatidae) isolated from Gilbert’s potoroo (Potorous gilbertii) and quokka (Setonix brachyurus) Parasitology. 2009;136:783–792. doi: 10.1017/S0031182009005927. [DOI] [PubMed] [Google Scholar]
- Austen J.M., Ryan U.M., Friend J.A., Ditcham W.G.F., Reid S.A. Vector of Trypanosoma copemani identified as Ixodes sp. Parasitology. 2011;138:866–872. doi: 10.1017/S0031182011000497. [DOI] [PubMed] [Google Scholar]
- Averis S., Thompson R.C.A., Lymbery A.J., Wayne A.F., Morris K.D., Smith A. The diversity, distribution and host-parasite associations of trypanosomes in Western Australian wildlife. Parasitology. 2009;136:1269–1279. doi: 10.1017/S0031182009990801. [DOI] [PubMed] [Google Scholar]
- Backhouse T., Bolliger A. Transmission of Chagas disease to the Australian marsupial Trichosurus vulpecula. Trans. R. Soc. Trop. Med. Hyg. 1951;44:521–533. doi: 10.1016/0035-9203(51)90032-6. [DOI] [PubMed] [Google Scholar]
- Barrett M.P., Burchmore R.J.S., Stich A., Lazzari J.O., Frasch A.C., Cazzulo J.J., Krishna S. The trypanosomiases. Lancet. 2003;362:1469–1480. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- Bettiol S.S., Jakes K., Le D.D., Goldsmid J.M., Hocking G. First record of trypanosomes in Tasmanian bandicoots. J. Parasitol. 1998;84:538–541. [PubMed] [Google Scholar]
- Botero A., Thompson C.K., Peacock C., Clode P.L., Nicholls P.K., Wayne A.F., Lymbery A.J., Thompson R.C.A. Trypanosomes genetic diversity, polyparasitism and the population decline of the critically endangered Australian marsupial, the brush tailed bettong or woylie (Bettongia penicillata) Int. J. Parasitol: Parasites Wildl. 2013;2:77–89. doi: 10.1016/j.ijppaw.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbidge, A., 2008. Bettongia pusilla. In: IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2 <www.iucnredlist.org> Downloaded on 12 November 2013.
- Clark P., Spencer P. Haematological characteristics of wild quokka (Setonix brachyurus) Comp. Clin. Pathol. 2006;15:82–86. [Google Scholar]
- Cleland J.B. Department of Agriculture; W.A., Perth, Western Australia: 1909. Trypanosomiasis: and other diseases of camels with experiments in connection with the former. [Google Scholar]
- Doke P.P., Kar A. A fatal case of Trypanosoma lewisi in Maharashtra, India. Ann. Trop. Med. Public Health. 2011;4:91–95. [Google Scholar]
- Dortch C.E., Morse K. Prehistoric stone artefacts on some offshore islands in Western Australia. Aust. Archaeol. 1984;19:31–47. [Google Scholar]
- Dougall A.M., Alexander B., Holt D.C., Harris T., Sultan A.H., Bates P.A., Rose K., Walton S.F. Evidence incriminating midges (Diptera: Ceratopogonidae) as potential vectors of Leishmania in Australia. Int. J. Parasitol. 2011;41:571–579. doi: 10.1016/j.ijpara.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Durham H.E. Notes on Nagana and on some haematozoa observed during my travels. Parasitology. 1908;1:227–235. [Google Scholar]
- Gascon J., Bern C., Pinazo M.J. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010;115:22–27. doi: 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Hamilton P.B., Stevens J.R., Gaunt M.W., Gidley J., Gibson W.C. Trypanosomes are monophyletic: evidence from genes for glyceraldehyde phosphate dehydrogenase and small subunit ribosomal RNA. Int. J. Parasitol. 2004;34:1393–1404. doi: 10.1016/j.ijpara.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Hamilton P.B., Stevens J.R., Gidley J., Holz P., Gibson W.C. A new lineage of trypanosomes from Australian vertebrates and terrestrial bloodsucking leeches (Haemadipsidae) Int. J. Parasitol. 2005;35:431–443. doi: 10.1016/j.ijpara.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Hamilton P.B., Stevens J.R., Holz P., Boag B., Cooke B., Gibson W.C. The inadvertent introduction into Australia of Trypanosoma nabiasi, the trypanosome of the European rabbit (Oryctolagus cuniculus), and its potential for biocontrol. Mol. Ecol. 2005;14:3167–3175. doi: 10.1111/j.1365-294X.2005.02602.x. [DOI] [PubMed] [Google Scholar]
- Hamilton P.B., Teixeira M.M., Stevens J.R. The evolution of Trypanosoma cruzi: the ‘bat seeding’ hypothesis. Trends Parasitol. 2012;28:136–141. doi: 10.1016/j.pt.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Hoare C.A. Blackwell Scientific Publishing; Oxford, England: 1972. The Trypanosomes of Mammals. [Google Scholar]
- Howie S., Guy M., Fleming L., Bailey W., Noyes H., Faye J.A., Pepin J., Greenwood B., Whittle H., Molyneux D., Corrah T. A Gambian infant with fever and an unexpected blood film. PLoS Med. 2006;3:e355. doi: 10.1371/journal.pmed.0030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes K.A., O’Donoghue P.J., Adlard R.D. Phylogenetic relationships of Trypanosoma chelodina and Trypanosoma binneyi from Australian tortoises and platypuses inferred from small subunit rRNA analyses. Parasitology. 2001;123:483–487. doi: 10.1017/s0031182001008721. [DOI] [PubMed] [Google Scholar]
- Johnson P.D. A case of infection by Trypanosoma lewisi in a child. Trans. R. Soc. Trop. Med. Hyg. 1933;26:467–468. [Google Scholar]
- Johnson C. Cambridge University Press; Port Melbourne, Victoria: 2006. Australia’s Mammal Extinctions: A 50,000 year History. [Google Scholar]
- Johnston T.H. Notes on some Australian parasites. Agric. Gaz. N.S.W. 1909;20:581–584. [Google Scholar]
- Johnston T.H. A census of the endoparasites recorded as occurring in Queensland, arranged under their hosts. Proc. R. Soc. Qld. 1916;28:31–79. [Google Scholar]
- Kaur R., Gupta V., Dhariwal A., Jain D., Shiv L. A rare case of trypanosomiasis in a two month old infant in Mumbai, India. J. Commun. Dis. 2007;39:71–74. [PubMed] [Google Scholar]
- Lima L., Espinosa-Álvarez O., Hamilton P.B., Neves L., Takata C.S., Campaner M., Attias M., de Souza W., Camargo E.P., Teixeira M.M. Trypanosoma livingstonei: a new species from African bats supports the bat seeding hypothesis for the Trypanosoma cruzi clade. Parasite Vectors. 2013;6:1–17. doi: 10.1186/1756-3305-6-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackerras M.J. Catalogue of Australian mammals and their recorded internal parasites. I-IV. Part I. Monotremes and marsupials. Proc. Linn. Soc. N.S.W. 1958;83:101–125. [Google Scholar]
- Mackerras M.J. Catalogue of Australian mammals and their recorded internal parasites. I-IV. Part II. Eutheria. Proc. Linn. Soc. N.S.W. 1958;83:126–143. [Google Scholar]
- Mackerras M.J. The haematozoa of Australian mammals. Aust. J. Zool. 1959;7:105–135. [Google Scholar]
- MacPhee R.D., Greenwood A.D. Infectious disease, endangerment, and extinction. Int. J. Evol. Biol. 2013;2013:1–9. doi: 10.1155/2013/571939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira M., Sousa M., Barros J., Figueiredo F., Fagundes A., Schubach A., De Paula C., Faissal B., Fonseca T., Thoma H. Trypanosoma caninum n. sp. (Protozoa: Kinetoplastida) isolated from intact skin of a domestic dog (Canis familiaris) captured in Rio de Janeiro, Brazil. Parasitology. 2009;136:411–423. doi: 10.1017/S003118200900554X. [DOI] [PubMed] [Google Scholar]
- Maia da Silva F., Marcili A., Ortiz P., Epiphanio S., Campaner M., Catao-Dias J., Shaw J., Camargo E., Teixeira M. Phylogenetic, morphological and behavioural analyses support host switching of Trypanosoma (Herpetosoma) lewisi from domestic rats to primates. Infect. Genet. Evol. 2010;10:522–529. doi: 10.1016/j.meegid.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Maraghi S., Molyneux D.H. Studies on cross-immunity in Herpetosoma trypanosomes of Microtus, Clethrionomys and Apodemus. Parasitol. Res. 1989;75:175–177. doi: 10.1007/BF00931270. [DOI] [PubMed] [Google Scholar]
- McInnes L.M., Gillett A., Ryan U.M., Austen J., Campbell R.S., Hanger J., Reid S.A. Trypanosoma irwini n. sp (Sarcomastigophora: Trypanosomatidae) from the koala (Phascolarctos cinereus) Parasitology. 2009;136:875–885. doi: 10.1017/S0031182009006313. [DOI] [PubMed] [Google Scholar]
- McInnes L.M., Hanger J., Simmons G., Reid S.A., Ryan U.M. Novel trypanosome Trypanosoma gilletti sp. (Euglenozoa: Trypanosomatidae) and the extension of the host range of Trypanosoma copemani to include the koala (Phascolarctos cinereus) Parasitology. 2010;138:59–70. doi: 10.1017/S0031182010000971. [DOI] [PubMed] [Google Scholar]
- McInnes L.M., Gillett A., Hanger J., Reid S.A., Ryan U.M. The potential impact of native Australian trypanosome infections on the health of koalas (Phascolarctos cinereus) Parasitology. 2011;138:1–11. doi: 10.1017/S0031182011000369. [DOI] [PubMed] [Google Scholar]
- McKenzie N.L., Burbidge A.A., Baynes A., Brereton R.N., Dickman C.R., Gordon G., Gibson L.A., Menkhorst P.W., Robinson A.C., Williams M.R., Woinarski J.C.Z. Analysis of factors implicated in the recent decline of Australia’s mammal fauna. J. Biogeogr. 2007;34:597–611. [Google Scholar]
- McNamara J.A. Some smaller macropod fossils of South Australia. Proc. Linn. Soc. N.S.W. 1997;117:97–106. [Google Scholar]
- Muñoz J., Coll O., Juncosa T., Vergés M., del Pino M., Fumado V., Bosch J., Posada E.J., Hernandez S., Fisa R. Prevalence and vertical transmission of Trypanosoma cruzi infection among pregnant Latin American women attending two maternity clinics in Barcelona, Spain. Clin. Infect. Dis. 2009;48:1736–1740. doi: 10.1086/599223. [DOI] [PubMed] [Google Scholar]
- Noyes H. Can Trypanosoma trees be trusted? Parasitol. Today. 1998;14:49–50. doi: 10.1016/s0169-4758(97)01159-9. [DOI] [PubMed] [Google Scholar]
- Noyes H.A., Stevens J.R., Teixeira M., Phelan J., Holz P. A nested PCR for the ssrRNA gene detects Trypanosoma binneyi in the platypus and Trypanosoma sp. in wombats and kangaroos in Australia. Int. J. Parasitol. 1999;29:331–339. doi: 10.1016/s0020-7519(98)00167-2. [DOI] [PubMed] [Google Scholar]
- O’Donoghue P., Adlard R. Catalogue of protozoan parasites recorded in Australia. Mem. Qld. Mus. 2000;45:1–163. [Google Scholar]
- Oliveira M.P.d.C., Cortez M., Maeda F.Y., Fernandes M.C., Haapalainen E.F., Yoshida N., Mortara R.A. Unique behavior of Trypanosoma dionisii interacting with mammalian cells: Invasion, intracellular growth, and nuclear localization. Acta Trop. 2009;110:65–74. doi: 10.1016/j.actatropica.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Paparini A., Irwin P.J., Warren K., McInnes L.M., De Tores P., Ryan U.M. Identification of novel trypanosome genotypes in native Australian marsupials. Vet. Parasitol. 2011;183:21–30. doi: 10.1016/j.vetpar.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Pickering J., Norris C.A. New evidence concerning the extinction of the endemic murid Rattus macleari from Christmas Island, Indian Ocean. Aust. Mammal. 1996;19:19–25. [Google Scholar]
- Reid S.A. Trypanosoma evansi control and containment in Australasia. Trends Parasitol. 2002;18:219–224. doi: 10.1016/s1471-4922(02)02250-x. [DOI] [PubMed] [Google Scholar]
- Reid S.A., Husein A., Partoutomo S., Copeman D.B. The susceptibility of two species of wallaby to infection with Trypanosoma evansi. Aust. Vet. J. 2001;79:285–288. doi: 10.1111/j.1751-0813.2001.tb11983.x. [DOI] [PubMed] [Google Scholar]
- Richardson L., Hunt P. Trypanosomes in the crop of an haemadipsid leech. Aust. J. Sci. 1968;30:374–375. [Google Scholar]
- Sarataphan N., Vongpakorn M., Nuansrichay B., Autarkool N., Keowkarnkah T., Rodtian P., Stich R.W., Jittapalapong S. Diagnosis of a Trypanosoma lewisi-like (Herpetosoma) infection in a sick infant from Thailand. J. Med. Microbiol. 2007;56:1118–1121. doi: 10.1099/jmm.0.47222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmunis G.A., Yadon Z.E. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Shah I., Ali U.S., Andanka P., Joshi R.R. Trypanosomiasis in an infant from India. J. Vector Borne Dis. 2011;48:122–123. [PubMed] [Google Scholar]
- Shikanai-Yasuda M.A., Carvalho N.B. Oral transmission of Chagas disease. Clin. Infect. Dis. 2012;54:845–852. doi: 10.1093/cid/cir956. [DOI] [PubMed] [Google Scholar]
- Short J. The extinction of rat-kangaroos (Marsupialia: Potoroidae) in New South Wales, Australia. Biol. Conserv. 1998;86:365–377. [Google Scholar]
- Smith A., Clark P., Averis S., Lymbery A.J., Wayne A.F., Morris K.D., Thompson R.C.A. Trypanosomes in a declining species of threatened Australian marsupial, the brush-tailed bettong Bettongia penicillata (Marsupialia: Potoroidae) Parasitology. 2008;135:1329–1335. doi: 10.1017/S0031182008004824. [DOI] [PubMed] [Google Scholar]
- Stevens J.R., Noyes H.A., Dover G.A., Gibson W.C. The ancient and divergent origins of the human pathogenic trypanosomes Trypanosoma brucei and T. cruzi. Parasitology. 1999;118:107–116. doi: 10.1017/s0031182098003473. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A. Parasite zoonoses and wildlife: one health, spillover and human activity. Int. J. Parasitol. 2013;43:1079–1088. doi: 10.1016/j.ijpara.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.C.A., Owen I.L., Puana I., Banks D., Davis T.M., Reid S.A. Parasites and biosecurity – the example of Australia. Trends Parasitol. 2003;19:410–416. doi: 10.1016/s1471-4922(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Thompson C.K., Botero A., Wayne A.F., Godfrey S.S., Lymbery A.J., Thompson R.C.A. Morphological polymorphism of Trypanosoma copemani and description of the genetically diverse T. vegrandis sp. nov. from the critically endangered Australian potoroid, the brush-tailed bettong (Bettongia penicillata (Gray, 1837)) Parasite Vectors. 2013;6:121. doi: 10.1186/1756-3305-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truc P., Büscher P., Cuny G., Gonzatti M.I., Jannin J., Joshi P., Juyal P., Lun Z.-R., Mattioli R., Pays E., Simarro P.P., Maria M., Teixeira G., Touratier L., Vincendeau P., Desquesnes M. Atypical human infections by animal trypanosomes. PLoS Negl. Trop. Dis. 2013;7:e2256. doi: 10.1371/journal.pntd.0002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turni C., Smales L.R. Parasites of the bridled nailtail wallaby (Onychogalea fraenata)(Marsupialia: Macropodidae) Wildl. Res. 2001;28:403–411. [Google Scholar]
- Van Dyck S., Strahan R. third ed. New Holland/Queensland Museum; Brisbane, Queensland: 2008. The mammals of Australia. [Google Scholar]
- Verma A., Manchanda S., Kumar N., Sharma A., Goel M., Banerjee P.S., Garg R., Singh B.P., Balharbi F., Lejon V., Deborggraeve S., Rana U.V.S., Puliyel J. Case report: Trypanosoma lewisi or T. lewisi-like infection in a 37-day-old Indian infant. Am. J. Trop. Med. Hyg. 2011;85:221–224. doi: 10.4269/ajtmh.2011.11-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt K.B., Campos P.F., Gilbert M.T.P., Kolokotronis S.O., Hynes W.H., DeSalle R., Daszak P., MacPhee R.D.E., Greenwood A.D. Historical mammal extinction on Christmas Island (Indian Ocean) correlates with introduced infectious disease. PLoS One. 2008;3:1–9. doi: 10.1371/journal.pone.0003602. [DOI] [PMC free article] [PubMed] [Google Scholar]


