Graphical abstract
Keywords: Costs, Helminths, Haematology, Juveniles, Macropus giganteus, Parasites
Highlights
-
•
We examine differences between parasitised and non-parasitised juvenile kangaroos.
-
•
Kangaroo helminths affect host albumin levels.
-
•
Kangaroo helminths have surprisingly few effects on host growth and body condition.
-
•
Haematological parameters are more sensitive to parasitism than growth parameters.
-
•
Juvenile kangaroos can possibly compensate for the costs of parasites.
Abstract
Large mammalian herbivores are commonly infected with gastrointestinal helminths. In many host species, these helminths cause clinical disease and may trigger conspicuous mortality events. However, they may also have subclinical impacts, reducing fitness as well as causing complex changes to host growth patterns and body condition. Theoretically, juveniles should experience significantly greater costs from parasites, being immunologically naive and undergoing a significant growth phase. The aims of our study were to quantify the subclinical effects of helminths in juvenile eastern grey kangaroos (Macropus giganteus), which commonly harbour large burdens of gastrointestinal nematodes and are susceptible to associated mass mortality during cold, wet conditions. We conducted a field experiment on a population of free-ranging kangaroos, removing nematodes from one group of juveniles using an anthelmintic treatment. We then compared growth parameters (body condition and growth rates) and haematological parameters of this group with an age-matched, parasitised (untreated) control group. Treated juvenile kangaroos had significantly higher levels of plasma protein (albumin) but, contrary to our predictions, showed negligible changes in all the other parameters measured. Our results suggest that juvenile kangaroos are largely unaffected by their gastrointestinal helminth burdens, and may be able to compensate for the costs of parasites.
1. Introduction
Gastrointestinal helminths commonly infect mammalian herbivores (Sykes, 1987). Helminth infections often have clinical impacts, causing disease and mortality (Holmes, 1987), but can also cause what has been termed ‘subclinical’ disease (Gunn and Irvine, 2003), inducing more subtle effects in the host. Subclinical impacts are well known in livestock: reductions in appetite and food absorption caused by helminth infections can decrease host fecundity and growth (Mejia et al., 1999), body condition (Loyacano et al., 2002) and alter metabolism (O’Kelly et al., 1988). In contrast, the effects of parasitism on wildlife have received far less attention, although there is mounting evidence that parasites can have similar negative impacts, reducing fitness (Watson, 2013) as well as causing complex changes to host physiology (Van Houtert and Sykes, 1996), behaviour (Scantlebury et al., 2007) and population dynamics (Hudson et al., 1992a; Albon et al., 2002; Stien et al., 2002). However, the types of impacts measured in livestock and wildlife often differ, due to the difficulties of studying natural host–parasite relationships and quantifying fitness consequences. In order to thoroughly investigate such effects, experimental manipulation is imperative. Field experimentation allows the actual costs of parasites on hosts to be investigated rigorously, eliminating many of the issues associated with extrapolating laboratory results onto free-living individuals or populations (Seitz and Ratte, 1991). Studies of fitness consequences in the wild typically focus on natural covariation between parasite load and fitness parameters, and so may be confounded by the inherent differences among individuals that can contribute to high parasite burdens. Consequently, it is often unclear whether changes in fitness parameters are due to heavy parasite burdens, or if these burdens result from other pre-existing factors related to fitness. Ecological host–parasite studies in wild animals have been mostly based on correlations, although there have been some field experiments in wild systems (e.g. Svalbard reindeer, Rangifer tarandus platyrhynchus (Stien et al., 2002), Soay sheep, Ovis aries (Gulland, 1992) and red grouse Lagopus lagopus scoticus (Hudson et al., 1992b)).
The subclinical effects of parasites can be extremely difficult to quantify in the wild. Ecologists tend to use body condition as an indicator of an animal’s health and reproductive potential. Body condition essentially reflects available energy reserves (Green, 2001): an animal in good condition is assumed to have more reserves than one in poor condition (Schulte-Hostedde et al., 2005). Energy reserves can be quantified directly by measuring fat stores (e.g. amount of back or kidney fat (Riney, 1955)) or non-invasively using mass/size ratio indices of body condition, which attempt to determine the size of energy stores after correcting for structural body size (Schulte-Hostedde et al., 2005). Alternatively, haematological and serum biochemical parameters can be used to assess an animal’s health. Although less commonly used in ecological studies of wildlife, haematological parameters may provide more sensitive information on the immediate physiological status of a host (Milner et al., 2003; Budischak et al., 2012). That is because parasite infections can alter haematological parameters directly through haematophagy (blood-feeding) and indirectly through activation of host immunity in response to infection or by limiting the digestion and absorption of essential nutrients, such as protein (Colditz, 2008). Red blood cell counts, haemoglobin and plasma protein concentrations can all be used to assess an animal’s health and condition, and have been directly linked to performance and reproductive success (Moore and Hopkins, 2009). The combined application of both haematological and body condition indices may therefore provide greater insight into the subclinical effects of parasite communities on a host.
Juvenile mortality is commonly increased by infection (Schmidt et al., 1979), however evidence from livestock suggests that this age-class can also experience considerable subclinical effects such as reductions in body weight (Chiejina and Sewell, 1974), growth (Loyacano et al., 2002) and appetite (Kyriazakis et al., 1998). Despite the evidence from livestock hosts, it is unclear to what degree subclinical effects occur in juveniles of wildlife species. Theoretically, juveniles should experience significant costs when infected with parasites due to the nutritional deficits they cause and the costs of mounting an immune response (Colditz, 2008), and these effects should be particularly marked during early growth and development. Such effects are important to comprehend as it is well established that conditions early in life can have significant implications for survival and reproductive success as an adult (Metcalfe and Monaghan, 2001).
Most wildlife hosts harbour complex parasite communities (Bordes and Morand, 2011), and kangaroos (Marsupialia: Macropodidae) are known to support more species of parasites than any other group of mammals (Beveridge and Chilton, 2001). The eastern grey kangaroo (Macropus giganteus) carries a diverse fauna of gastrointestinal nematode parasites in its complex, sacculated forestomach (Beveridge and Arundel, 1979), with most species showing seasonal fluctuations, peaking in the winter months (Arundel et al., 1990). Most of these gastrointestinal nematodes are directly transmitted via ingestion (Sykes, 1987). Adult kangaroos do not appear to develop immunity to most of these nematode species (Arundel et al., 1979), and juveniles are susceptible to gastrointestinal parasitism, primarily from high burdens (400–1500) of the intestinal trichostrongylid nematode Globocephaloides trifidospicularis Juveniles can experience high mortality, coupled with declining haematocrit and plasma protein concentrations, in their first winter post-weaning between 14 and 20 months of age (Arundel et al., 1990). Populations of eastern grey kangaroos can reach high densities, and individuals are gregarious, forming mixed-sex, open-membership groups to forage and rest (Coulson, 2009), conditions that favour helminth parasite transmission (Altizer et al., 2003).
Eastern grey kangaroos are capable of breeding throughout the year, but most births occur between September and March, during the austral spring/summer months (Poole, 1983). Following a short gestation period and then an extended period of development in the pouch, young will exit permanently at around 320 days (Poole, 1975). Toward the end of pouch life, juveniles begin to forage on the pasture and are exposed to the infective stages of nematodes. Juveniles will continue to associate with and suckle from their mothers until over 18 months of age (Poole, 1975). The year following permanent pouch exit is the most critical for juvenile kangaroos, as they must undergo substantial growth. During this period, the average monthly weight gain is 1.4 kg for males and 0.9 kg for females (Poole et al., 1982). To sustain this growth, juveniles have around 1.8 times the energy requirement of mature, non-lactating females (Munn and Dawson, 2004). In addition, during this period of growth, individuals are immunologically naive (Arundel et al., 1990), and become infected by gastrointestinal parasites. Individual variability in body size increases following permanent pouch exit (Poole et al., 1982), suggesting that growth rate is a plastic trait that can be influenced by external factors.
We examined the effect of concomitant infection with multiple parasites on the growth, body condition and blood chemistry of one cohort of free-ranging juvenile eastern grey kangaroos, by manipulating parasite loads. We removed gastrointestinal parasites from a group of juveniles using an oral anthelmintic and then compared them with control individuals, with the expectation that control juveniles would show subclinical effects. We predicted that due to an increased availability of nutrients and energy resources, treated juveniles would have a greater growth rate and mass gain, and would increase their body condition relative to controls. We also predicted that there would be changes in haematological parameters, with decreases in red blood cell counts, haemoglobin concentration and haematocrit in control juveniles. Similarly, we expected that serum biochemistry would indicate subclinical effects, with decreased levels of total protein and albumin, and increases in levels of globulin.
2. Materials and methods
2.1. Study site
This study was conducted at the Anglesea Golf Club (38°24′S, 114°10′E) in southern Victoria, Australia, in 2012. The golf course covers an area of 73 ha and contains open, grassy fairways dominated by couch grass (Cynodon dactylon), separated by patches of woodland and shrubland (Inwood et al., 2008). The course is bordered by native heathy woodland to the north and west; kangaroos move freely between the course and native vegetation, as well as through residential properties in the south and east. Population surveys (following Inwood et al., 2008) at the time of the study showed that the population density of kangaroos at the site was approximately 2.0/ha (Cripps and Coulson, unpublished data). Potential predators at the site include the red fox (Vulpes vulpes) and domestic dogs (Canis lupus familiaris).
Post-mortem examinations of three juveniles found dead at the study site during 2010–2011, using standard methods (Beveridge and Arundel, 1979), revealed a diverse gastrointestinal parasite community. The stomach contained the strongyle nematodes Rugopharynx macropodis (intensity 8240–69,000 per kangaroo), Rugopharynx rosemariae (500, n = 1) and Pharyngostrongylus kappa (500–10,000). The small intestine contained G. trifidospicularis (31–73), levels well below those known to cause clinical impacts (Arundel et al., 1990). The large intestine contained two oxyuroid nematodes, Macropoxyuris brevigularis (40–3450) and Macropoxyuris longigularis (500–1050), and one strongyle nematode species Macropostrongyloides baylisi (50–9000). The bile ducts of two juveniles had the cestode Progamotaenia festiva. Examination of seven adults from the site revealed a further ten helminth species: Labiosimplex kungi, Labiosimplex bipapillosus, Cloacina pelops, Cloacina herceus, Cloacina hermes, Cloacina selene, Cloacina artemis, Cloacina expansa, Cloacina obtusa, and Alocostoma clelandi in the stomach (Cripps, unpublished data). Coinfection levels ranged from 5 to 8 helminth species in juveniles kangaroos, and a further 3–5 species in adults (Cripps, unpublished data). Total worm counts in juveniles (12,870–79,853) and adults (1844–84,017) were well within the range recorded for eastern grey kangaroos (Arundel et al., 1990; Beveridge and Arundel, 1979). Ectoparasites (lice, ticks) were not recorded as eastern grey kangaroos have relatively few ectoparasites (Beveridge and Arundel, 1979).
2.2. Animal capture and treatment
Juvenile kangaroos (n = 42) were first captured in March and April 2012. Due to their habituation to humans, kangaroos at this site tolerate close approach on foot. Juveniles were identified primarily based on their size, but also on whether they were closely associated with an adult female. Juveniles were captured using either an extendable pole syringe (1.4 m, 2.4 m or 3.6 m long) (King et al., 2011), or by an injection arrow fired from a band-powered gun (Para-medic; Wildvet, Melbourne, Victoria, Australia). Both methods injected the hind limb musculature with Zoletil® 100 (100 mg/mL of 50:50 tiletamine hydrochloride – zolazepam hydrochloride mixture; Virbac Animal Health Pty Ltd, Milperra, New South Wales, Australia) at a dose of approximately 5 mg/kg body mass.
To identify individuals, they were fitted with a unique combination of coloured, reflective ear tags (Leader, Craigieburn, Victoria, Australia). Standard body measurements (Poole et al., 1982) were collected using a retractable tape measure and Vernier calipers. Leg, pes (foot) and arm lengths were measured to the nearest mm; body mass was measured to the nearest 0.1 kg using 25-kg spring scales (Salter, Melbourne, Victoria, Australia).
The approximate date of birth of each individual was calculated from the mean of three estimates based on leg, foot and arm measurements at the first capture using growth tables provided by Poole et al. (1982). In some cases, one measurement gave an estimate that was >2 months apart from the other two. If this occurred, birthdate was calculated using the mean of the other two estimates. Only individuals born after 1 August 2010 were included in the final analysis to ensure that they were ⩽21 months of age and therefore encountering nematode larvae for the first time over the winter months of June – August 2012.
Individuals were randomly allocated to either a control (n = 20) or a treatment (n = 22) group, stratified by sex to ensure equal numbers of males and females in each group. Treated individuals were given an oral dose of albendazole (Alben® for sheep, lambs and goats, 19 g/L, Virbac Animal Health Pty Ltd, Milperra, New South Wales, Australia) at a rate of 3.8 mg/kg body mass (Cripps et al., 2013), while control individuals were left untreated. No oral control was administered to untreated individuals to avoid indirectly affecting the gastrointestinal fauna. A number of juveniles disappeared (either died or dispersed) during the study, so only 15 controls (8 male, 7 female) and 12 treatment (8 male, 4 female) kangaroos were included in the analysis. The average age of these individuals at first capture was 14.5 months (range 11–19.5 months).
Juvenile kangaroos were recaptured between May and June 2012 to re-administer the anthelmintic to the treated group and to collect body measurements from both groups. The interval of re-treatment was based on the estimated pre-patent period of infection (approximately 3 months) in eastern grey kangaroos (Cripps et al., 2013). Individuals were recaptured in a similar order to their initial captures; the average time between first and second capture was 77 days (range 69–91). Individuals were recaptured again between July and September 2012 (mean recapture interval of 77 days, range 68–114 days) and final body measurements were collected. In total, each individual was captured three times.
2.3. Faecal egg counts
To determine the efficacy of the parasite treatment, egg counts were conducted on faecal samples collected within 33 days of first capture. Samples were collected again at 40–90 days post-treatment, prior to the second re-treatment period. At this point, it is possible that many of the nematodes were larval stages and thus were not reflected in the faecal egg counts. Faecal samples were collected in 2 h blocks at dawn and dusk, as this is when kangaroos are actively foraging and defaecation rates are greatest (Johnson et al., 1987). Marked juveniles were observed until they defaecated, and observers collected faecal samples immediately after they were deposited. It was not possible to collect samples from every individual, so faecal samples were collected for a subset of juveniles in each group. Samples were collected from 13 juveniles (5 treated, 8 controls) days 12–33 post-treatment, and from 20 juveniles (9 treated, 11 controls) days 40–90 post-treatment. Samples were maintained at 4 °C and analysed within 24 h. The number of eggs per gram (epg) was determined by a modified McMaster technique using 2 g of faeces mixed with 60 mL of saturated sodium nitrate solution (Redox Pty Ltd, Minto, New South Wales, Australia). An aliquot of 0.5 mL of homogenised filtrate was transferred into a Whitlock Universal counting chamber then examined under a microscope at 100× magnification. Only typical strongylid eggs were counted, with each egg representing 60 epg of faeces.
2.4. Blood collection and analysis
During the second recapture, blood samples were also collected from the lateral caudal vein. Blood for haematology was transferred into 2 mL vacutainers containing EDTA, and for serum analysis into 5 mL vacutainers containing gel for serum separation but no additives. Vials were immediately placed in a cooler and taken back to the field base, where blood for serum analysis was centrifuged for 15 min. Blood smears were also prepared on glass slides within 4 h of collection. Sera and whole blood samples were refrigerated until transport to a commercial, NATA-accredited, diagnostic veterinary laboratory (IDEXX Laboratories, Mount Waverley, Victoria, Australia). The time between blood collection and delivery to the lab was <24 h. Whole blood was analysed for the number of red blood cells, the haemoglobin concentration and haematocrit. Assessment of haematologic values was performed using a Sysmex XT-2000i haematology analyzer (Sysmex Corportaion, Kobe, Japan). Serum chemistry profiles were obtained with an Olympus AU 400 analyzer (Olympus Diagnostics, Hamburg, Germany) and included total protein, albumin and globulin.
2.5. Statistical analysis
Logistic regression was used to analyse the effects of sex and treatment on the disappearance rate of individuals throughout the study. Faecal egg count reduction calculations were made according to Wood et al. (1995) using the Excel plug-in ‘Reso’ (Cameron, 2003). Analysis of the effects of treatment on kangaroo faecal egg counts was carried out using Genstat, Version 10 (VSN International Ltd., Hemel Hempstead, UK). Faecal egg counts were log (1 + epg) transformed to meet the assumptions of normality and analysed using restricted maximum-likelihood analyses (REML), with time and treatment as fixed factors, and kangaroo identity as a random factor to account for repeated measures.
Differences in body mass and leg lengths of juveniles in the treated and control groups at the initial capture were tested using independent sample t-tests. The Scaled Mass Index (Peig and Green, 2009) was used to measure body condition. This index overcomes several drawbacks of other indices, many of which fail to account for the changing relationship between mass and length as growth occurs. Following Peig and Green’s procedure (2009), the most strongly correlated body measurement with body mass (on a log–log scale) was determined. Initially each sex was tested separately, but there was no difference in the strength of the correlations so sexes were pooled. Both leg and arm length were more highly correlated with body mass (leg: r = 0.92, P < 0.01; arm: r = 0.91, P < 0.01) than pes length (r = 0.81, P < 0.01), so we chose leg length as the length (Li) value for each individual. The population mean (L0) was calculated separately for each of the three capture periods. The Scaled Mass Index of body condition was then calculated as Mi(L0/Li)bSMA, where Mi is the body mass of the individual and bSMA is the standardised major axis regression slope of the ln Mi − ln Li plot (Peig and Green, 2009).
To assess the effect of treatment across the three capture periods, repeated measure ANOVAs were performed to determine differences in body mass, leg length and the Scaled Mass Index. The main factor was treatment (albendazole administered or untreated control), and the repeated factor was captures. Mauchly’s test of sphericity indicated that the assumption of sphericity was violated in all cases (body mass: χ22 = 9.86, P = 0.007; leg: χ22 = 10.93, P = 0.004; body condition: χ22 = 13.97, P = 0.001), so a Greenhouse–Geisser correction was used. Analysis of the effect of treatment on haematological and serum biochemical parameters was carried out using independent sample t-tests or Mann–Whitney U tests in the cases where data were non-normal.
Power analyses were performed for each growth and blood parameter, using G*Power (Erdfelder et al., 1996). The magnitude of effects for most of the parameters we tested is rarely reported in the literature. The magnitude of body mass effects reported for livestock hosts are extremely variable. When compared to parasitised controls, unparasitised heifers gained approximately 18% more weight (Mejia et al., 1999), whereas in sheep, the difference ranged from 74% (McLeod and Wolff, 1968) to 81% (Anderson et al., 1980). Consequently, we used both 20% and 80% differences between the treatment and control groups as the effect sizes in these calculations.
All other statistical analyses were carried out using SPSS Version 21 (IBM Corporation, Armonk, New York, USA). The assumptions of parametric statistic analyses (normality and equality of variances) were tested for all sets of data. Normality was assessed using the Kolmogorov–Smirnov statistic with α > 0.05, and Levene’s test for homogeneity of variances was used to test equality of variances, with α > 0.05. We did not apply sequential Bonferroni adjustments on the basis that we report power analyses for all our tests, and had already selected a subset of blood and growth parameters for analysis (Moran, 2003).
3. Results
The overall disappearance rate of juveniles from the beginning to the end of the experiment was 39%. Neither treatment (β = −1.12, χ21 = 2.30, P = 0.13) nor kangaroo sex (β = 0.21, χ21 = 0.08, P = 0.77) accounted for variation in disappearance.
Mean (±SE) faecal egg counts of treated kangaroos (36 ± 24 epg) were much lower than for control kangaroos (885 ± 251 epg, F1,25.6 = 19.23, P < 0.001; Fig. 1) 12–33 days following treatment, resulting in a 99% reduction in faecal egg counts. There was no difference in the faecal egg counts within each group in either time period (F1,3.1 = 2.41, P = 0.22). However, there was a significant interaction between time and treatment (F1,3.4 = 32.36, P = 0.007), such that faecal egg counts in the treated group increased more than those in the control group. No cestode eggs were detected in the faecal flotations. Eimeria oocysts were present in some samples but at very low numbers.
Fig. 1.
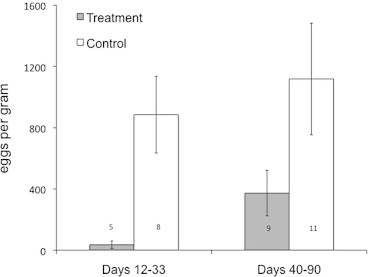
Mean faecal egg counts for control and anthelmintic-treated juvenile eastern grey kangaroos in two periods post-capture (12–33 days) and initial treatment (40–90 days) at the Anglesea Golf Club, Victoria, Australia, from March to May 2012. Bars indicate standard errors. It was not always possible to sample each individual in each period; numbers on columns indicate sample size for each time period.
Body mass of control and treated juvenile kangaroos did not differ at the time of first capture, (t25 = 0.19, P = 0.85), nor did skeletal size, measured as leg length (t25 = 0.01, P = 0.99). Mass gain was explained by time (F1.49,37.39 = 21.22, P < 0.001) but not treatment (F1,25 = 0.04, P = 0.84), and there was no interaction between the two (F1.49,37.39 = 0.44, P = 0.59). There was also no significant effect of treatment on the growth of leg length (F1,25 = 0.08, P = 0.93). Instead, time explained leg growth (F1.46,36.61 = 71.39, P < 0.001), and there were no interactions between time and treatment (F1.46,36.61 = 0.24, P = 0.72). The body condition of individual juvenile kangaroos increased during the study (F1.39,34.69 = 21.60, P < 0.001; Table 1) but there was no effect of treatment (F1,25 = 0.01, P = 0.91) nor any interaction between treatment and time (F1.39,34.69 = 0.32, P = 0.65). With the estimated 80% effect size, power was high for leg measurements (>0.8, Table 1) and moderate for body mass and body condition (0.3–0.8, Table 1). With the estimated 20% effect size, power was low (<0.3, Table 1) for all of the ecological parameters.
Table 1.
Repeated measures ANOVA of treatment effects on selected ecological growth parameters for control and anthelmintic-treated juvenile eastern grey kangaroos at the Anglesea Golf Club, Victoria, Australia, from May to September 2012. The summaries show the sample size (n), the mean (±SE) increase (first to final capture) and the P-value for each group. The statistical power of this experiment to detect a significant difference between treatment and control groups was calculated for effect sizes of 20% and 80%.
| Parameter | Control | Treated | P-Value | Power | |||
|---|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | 20% | 80% | ||
| Body mass (kg) | 15 | 0.97 ± 0.25 | 12 | 1.33 ± 0.37 | 0.84 | 0.11 | 0.50 |
| Leg (mm) | 15 | 17.30 ± 2.17 | 12 | 19.50 ± 2.58 | 0.93 | 0.26 | 0.99 |
| Body condition (scaled mass index) | 15 | 0.95 ± 1.14 | 12 | 1.22 ± 0.74 | 0.91 | 0.13 | 0.63 |
Serum albumin levels were 8% higher in treated juveniles than in controls, and this difference was significant (P = 0.01, Table 2). While there were no significant differences in the concentrations of total protein, globulin or haemoglobin, the red cell count or the haematocrit, all of these parameters showed trends in the predicted directions, with lower levels in the parasitised juveniles. Power was high for all the blood parameters (>0.8, Table 2) for both 20% and 80% effect sizes.
Table 2.
Summary of the sample size (n), mean (±SE) (at the final capture) and test statistics of selected serum chemistry and haematological parameters for control and anthelmintic-treated juvenile eastern grey kangaroos at the Anglesea Golf Club, Victoria, Australia, from May to September 2012. The statistical power of this experiment to detect a significant difference between treatment and control groups was calculated for effect sizes of 20% and 80%.
| Parameter | Control | Treated | Test-statistic | df | P-Value | Power | |||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | 20% | 80% | ||||
| Total protein (g/L) | 15 | 61.20 ± 1.39 | 11 | 62.64 ± 1.67 | t = 0.67 | 24 | 0.51 | 0.99 | 1.0 |
| Albumin (g/L) | 11 | 27.82 ± 0.57⁎ | 6 | 30.17 ± 0.47⁎ | t = 2.75 | 15 | 0.01 | 0.99 | 1.0 |
| Globulin (g/L) | 11 | 31.00 ± 1.14 | 5 | 30.20 ± 1.88 | t = −0.38 | 14 | 0.71 | 0.86 | 1.0 |
| Red blood cells (×1012/L) | 15 | 4.48 ± 0.15 | 10 | 4.75 ± 0.14 | t = 1.25 | 23 | 0.22 | 0.98 | 1.0 |
| Haemoglobin (g/L) | 15 | 131.53 ± 4.43 | 10 | 139.10 ± 4.03 | U = 57.0 | – | 0.33 | 0.99 | 1.0 |
| Haematocrit (L/L) | 15 | 0.37 ± 0.01 | 10 | 0.38 ± 0.01 | U = 64.5 | – | 0.57 | 0.99 | 1.0 |
Denotes a significant difference between groups.
4. Discussion
Contrary to our predictions, experimental removal of parasites in juvenile kangaroos had no effect on their body condition, mass gain or limb growth. Juvenile kangaroos treated with anthelmintics had significantly higher albumin levels than control juveniles, but showed no differences in any other blood parameters. There was no evidence of parasite-induced mortality at our site, and the disappearance rate of juveniles (39%) was comparable to that seen in another eastern grey kangaroo population subject to predation by red foxes (Banks et al., 2000). Our results suggest that juvenile eastern grey kangaroos are largely unaffected by gastrointestinal parasitism in populations where burdens of G. trifidospicularis are low, despite carrying high burdens of other gastrointestinal helminth species.
Defence against parasites incurs a cost, which may lead to resources being partitioned into immune response rather than growth (Zuk and Stoehr, 2002). As resources are limited, animals undergoing rapid growth must partition and prioritize them appropriately (partitioning framework; Coop and Kyriazakis, 1999). Accordingly, a growing animal encountering parasites for the first time would be expected to prioritise immunity over growth (Coop and Kyriazakis, 1999). We therefore predicted that untreated (control) juvenile kangaroos, which were encountering nematode parasite larvae for the first time, would have a reduced allocation to growth and energy reserves, and this would be reflected in their blood parameters. Field experiments such as ours are rare in wildlife hosts, but have mostly shown significant subclinical effects on hosts (e.g. Hillegass et al., 2010; Stien et al., 2002). It was therefore surprising that the removal of parasites had little effect on growth or the haematological variables examined, particularly as the faecal egg counts were high in the parasitised kangaroos. However, when compared to untreated controls, anthelmintic-treated juveniles tended to gain more weight, and tended to have a longer leg and pes. Although not significant, the power analysis suggests that perhaps with larger sample sizes, greater impacts of parasitism in juvenile grey kangaroos might have be seen. If individuals were investing extra energy and nutrients in several areas at once (such as both skeletal and muscular growth), it may have made it difficult to detect changes in each parameter alone (Munger and Karasov, 1989), leading to low power.
Haematological parameters can be altered by parasites directly through haematophagy (blood-feeding) and indirectly by limiting the digestion and absorption of essential nutrients, such as amino acids and protein (Colditz, 2008). Lowered albumin levels in kangaroos could be directly caused by blood loss and/or inflammation in the gastrointestinal tract (Rothschild et al., 1988; Arundel et al., 1990). In juvenile eastern grey kangaroos, heavy infections of G. trifidospicularis have been associated with severe anaemia and clinical disease (Arundel et al., 1990; I. Beveridge pers. obs), although burdens (from post-mortems) at our site were more than five times lower than levels known to cause disease (400–1500, Arundel et al., 1990), perhaps explaining why we observed only an 8% difference in albumin levels between the two groups of juveniles. The nematode M. baylisi may also feed on blood (Arundel et al., 1990), however clinical impacts have never been confirmed. Larvae of a third species, R. rosemariae, can cause severe lesions on the gastric mucosa, yet hosts can carry large burdens without any obvious effects on health (Beveridge and Presidente, 1978). As in all eastern grey kangaroo populations, helminth coinfection was ubiquitous in kangaroos at our site, and juveniles were infected with between 5 and 8 different helminth species (post-mortem data, Cripps, unpublished data). However, due to the inability to morphologically distinguish the eggs of the various taxa in the faeces, it was impossible to determine which combination of species were present in any individual in our study at any one time, and therefore hypoalbuminaemia could not be attributed to any helminth species in particular. Severe blood loss should also reduce concentrations of haemoglobin and total protein, and lower the haematocrit, but we found no differences in any of these despite our high statistical power. We are confident our experimental study would have detected even small changes in any of the other blood parameters. Alternatively, the hypoalbuminaemia we observed could have resulted from reduced food intake and consequent malnutrition, which is a common cause of reduced albumin synthesis (Rothschild et al., 1972). Voluntary reductions in food intake are common during parasitic infections in livestock (Holmes, 1987; Kyriazakis et al., 1998), but whether parasite-infected kangaroos also exhibit anorexia is unknown. Our study demonstrates that when levels of G. trifidospicularis are low, kangaroo hosts are able to tolerate their helminth community and exhibit few subclinical effects from infection.
Increasing resource acquisition may be pivotal in allowing hosts to reduce the potential costs of parasitism. Livestock hosts on high-protein diets show reduced pathophysiological responses to parasitism (reviewed by Van Houtert and Sykes, 1996). For example, infected sheep maintained on high protein diets increase their live weight gain by around 85% compared with control sheep (Van Houtert et al., 1995). Similarly, juvenile kangaroos could compensate for the costs of parasitism by using high-quality resources that offset nutrient and resource depletion. The best predictors of body condition in free-ranging kangaroos are the biomass and quality of forage (Shepherd, 1987; Moss and Croft, 2009), and juvenile kangaroos cannot sustain their growth on a poor-quality diet, even when they are still suckling (Munn and Dawson, 2003, 2006). The climate at Anglesea is mild, and the fairways are irrigated, fertilized and regularly mown, which encourages new foliage with high protein content (Jarman, 1974; Mattson, 1980). Furthermore, although the juveniles in our study should have been weaned, maternal care in kangaroos is a variable trait that can be influenced by a number of factors, including a mother’s age and/or body condition, and environmental conditions (Stuart-Dick and Higginbottom, 1989). Differential maternal investment among individual mothers could explain the high variability in the growth parameters we measured, leading to a low power to detect change. The combination of resources from lactation and protein-rich pasture could have allowed infected juveniles to maintain their growth and body condition in spite of parasitic infections.
We have strong experimental evidence that juvenile kangaroos experience few subclinical effects from parasitism, contrary to our predictions. While parasites clearly have subclinical effects in many herbivorous hosts, data on free-ranging wildlife can be difficult to obtain and experimental field manipulations like ours are imperative for investigating such relationships. Importantly, our study is one of the first to combine both growth parameters and haematological parameters, and supports the suggestion by Budischak et al. (2012) that haematological parameters are more sensitive to the subclinical effects of parasitism. In our study, where levels of G. trifidospicularis were relatively low, gastrointestinal helminths had minimal subclinical effects on the juvenile eastern grey kangaroos. However, even small differences in growth and blood parameters could be biologically meaningful, and may have implications for individuals later in life, particularly for life-history traits (Metcalfe and Monaghan, 2001). Future studies should take a longitudinal, individual-based approach toward examining the cumulative effects of parasites over time.
Conflicts of Interest
There are no known conflicts of interest.
Acknowledgements
This work was supported by the Holsworth Wildlife Research Endowment. We thank Rachel Kane and staff at Anglesea Golf Club for logistic support, and the many volunteers who assisted with fieldwork. Special thanks to Marco Festa-Bianchet and two anonymous reviewers for perceptive comments on the manuscript, Christine Andersen for technical assistance and to Rachel Sore and Paul Carnell for statistical advice. This research was carried out with approval from The University of Melbourne’s Animal Ethics Committee (Project 1011709) and the Department of Sustainability and Environment (research permit 10005557). There are no known conflicts of interest.
References
- Albon S.D., Stien A., Irvine R.J., Langvatn R., Ropstad E., Halvorsen O. The role of parasites in the dynamics of a reindeer population. Proc. R. Soc. Lond. B Biol. Sci. 2002;269:1625–1632. doi: 10.1098/rspb.2002.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S., Nunn C.L., Thrall P.H., Gittleman J.L., Antonovics J., Cunningham A.A., Dobson A.P., Ezenwa V., Jones K.E., Pedersen A.B., Poss M., Pulliam J.R.C. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu. Rev. Ecol. Evol. Syst. 2003;34:517–547. [Google Scholar]
- Anderson N., Laby R., Prichard R., Hennessy D. Controlled release of anthelmintic drugs: a new concept for prevention of helminthosis in sheep. Res. Vet. Sci. 1980;29:333–341. [PubMed] [Google Scholar]
- Arundel J., Beveridge I., Presidente P. Parasites and pathological findings in enclosed and free-ranging populations of Macropus rufus (Demarest) (Marsupialia) at Menindee, New South Wales. Wildl. Res. 1979;6:361–379. [Google Scholar]
- Arundel J.H., Dempster K.J., Harrigan K.E., Black R. Epidemiological observations on the helminth parasites of Macropus giganteus (Shaw) in Victoria. Aust. Wildl. Res. 1990;17:39–51. [Google Scholar]
- Banks P., Newsome A., Dickman C. Predation by red foxes limits recruitment in populations of eastern grey kangaroos. Austral Ecol. 2000;25:283–291. [Google Scholar]
- Beveridge I., Presidente P. Rugopharynx rosemariae new species (Nematoda: Pharyngostrongylidae) from grey kangaroos (Macropus giganteus and M. fuliginosus) with life cycle stages and associated pathology. Int. J. Parasitol. 1978;8:379–387. [Google Scholar]
- Beveridge I., Arundel J.H. Helminth parasites of grey kangaroos, Macropus giganteus (Shaw) and M. fuliginosus (Desmarest), in eastern Australia. Aust. Wildl. Res. 1979;6:69–79. [Google Scholar]
- Beveridge I., Chilton N.B. Co-evolutionary relationships between the nematode subfamily Cloacininae and its macropodid marsupial hosts. Int. J. Parasitol. 2001;31:976–996. doi: 10.1016/s0020-7519(01)00200-4. [DOI] [PubMed] [Google Scholar]
- Bordes F., Morand S. The impact of multiple infections on wild animal hosts: a review. Infect. Ecol. Epidemiol. 2011;1:7346. doi: 10.3402/iee.v1i0.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budischak S.A., Jolles A.E., Ezenwa V.O. Direct and indirect costs of co-infection in the wild: linking gastrointestinal parasite communities, host hematology, and immune function. Int. J. Parasitol. Parasites Wildl. 2012;1:2–12. doi: 10.1016/j.ijppaw.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A. University of Sydney; 2003. Reso for excel V.4.0 – Faecal egg count reduction test analysis. AusVet Animal Health Services. [Google Scholar]
- Chiejina S.N.C., Sewell M. Worm burdens, acquired resistance and live weight gains in lambs during prolonged daily infections with Trichostrongylus colubriformis (Giles, 1892) Loos, 1905. Parasitology. 1974;69:315–327. doi: 10.1017/s0031182000063010. [DOI] [PubMed] [Google Scholar]
- Colditz I.G. Six costs of immunity to gastrointestinal nematode infections. Parasite Immunol. 2008;30:63–70. doi: 10.1111/j.1365-3024.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- Coop R., Kyriazakis I. Nutrition–parasite interaction. Vet. Parasitol. 1999;84:187–204. doi: 10.1016/s0304-4017(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Coulson G. Behavioural ecology of red and grey kangaroos: Caughley’s insights into individuals, associations and dispersion. Wildl. Res. 2009;36:57–69. [Google Scholar]
- Cripps J., Beveridge I., Coulson G. The efficacy of anthelmintic drugs against nematodes infecting free-ranging eastern grey kangaroos, Macropus giganteus. J. Wildl. Dis. 2013;49:535–544. doi: 10.7589/2012-06-151. [DOI] [PubMed] [Google Scholar]
- Erdfelder E., Faul F., Buchner A. GPOWER: a general power analysis program. Behav. Res. Methods Instrum. Comput. 1996;28:1–11. [Google Scholar]
- Green A.J. Mass/length residuals: measures of body condition or generators of spurious results? Ecology. 2001;82:1473–1483. [Google Scholar]
- Gulland F.M. The role of nematode parasites in Soay sheep (Ovis aries) mortality during a population crash. Parasitology. 1992;105:493–503. doi: 10.1017/s0031182000074679. [DOI] [PubMed] [Google Scholar]
- Gunn A., Irvine R.J. Subclinical parasitism and ruminant foraging strategies: a review. Wildl. Soc. Bull. 2003;31:117–126. [Google Scholar]
- Hillegass M.A., Waterman J.M., Roth J.D. Parasite removal increases reproductive success in a social African ground squirrel. Behav. Ecol. 2010;21:696–700. [Google Scholar]
- Holmes P. Pathophysiology of nematode infections. Int. J. Parasitol. 1987;17:443–451. doi: 10.1016/0020-7519(87)90120-2. [DOI] [PubMed] [Google Scholar]
- Hudson P.J., Dobson A.P., Newborn D. Do parasites make prey vulnerable to predation? Red grouse and parasites. J. Anim. Ecol. 1992;61:681–692. [Google Scholar]
- Hudson P.J., Newborn D., Dobson A.P. Regulation and stability of a free-living host–parasite system: Trichostrongylus tenuis in red grouse. I. Monitoring and parasite reduction experiments. J. Anim. Ecol. 1992;61:477–486. [Google Scholar]
- Inwood D., Catanchin H., Coulson G. Roo town slow down: a community-based kangaroo management plan for Anglesea, Victoria. In: Lunney D., Munn A., Meikle W., editors. Too Close for Comfort: Contentious Issues in Human-wildlife Encounters. Royal Zoological Society of New South Wales; Mosman, Australia: 2008. pp. 1–8. [Google Scholar]
- Jarman P.J. The social organisation of antelope in relation to their ecology. Behaviour. 1974;48:215–267. [Google Scholar]
- Johnson C.N., Jarman P.J., Southwell C.J. Macropod studies at Wallaby Creek V. Patterns of defaecation by eastern grey kangaroos and red-necked wallabies. Aust. Wildl. Res. 1987;14:133–138. [Google Scholar]
- King W.J., Wilson M.E., Allen T., Festa-Bianchet M., Coulson G. A capture technique for free-ranging eastern grey kangaroos (Macropus giganteus) habituated to humans. Aust. Mammal. 2011;33:47–51. [Google Scholar]
- Kyriazakis I., Tolkamp B.J., Hutchings M.R. Towards a functional explanation for the occurrence of anorexia during parasitic infections. Anim. Behav. 1998;56:265–274. doi: 10.1006/anbe.1998.0761. [DOI] [PubMed] [Google Scholar]
- Loyacano A., Williams J., Gurie J., DeRosa A. Effect of gastrointestinal nematode and liver fluke infections on weight gain and reproductive performance of beef heifers. Vet. Parasitol. 2002;107:227–234. doi: 10.1016/s0304-4017(02)00130-9. [DOI] [PubMed] [Google Scholar]
- Mattson W.J. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 1980;11:119–161. [Google Scholar]
- McLeod C., Wolff J. Increased live weight gain and wool weight from anthelmintic drenching of ewe hoggets in South Canterbury. N.Z. J. Agric. Res. 1968;11:407–419. [Google Scholar]
- Mejia M., Gonzalez-Iglesias A., Diaz-Torga G., Villafane P., Formia N., Libertun C., Becu-Villalobos D., Lacau-Mengido I. Effects of continuous ivermectin treatment from birth to puberty on growth and reproduction in dairy heifers. J. Anim. Sci. 1999;77:1329. doi: 10.2527/1999.7761329x. [DOI] [PubMed] [Google Scholar]
- Metcalfe N.B., Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Milner J.M., Stien A., Irvine R.J., Albon S.D., Langvatn R., Ropstad E. Body condition in Svalbard reindeer and the use of blood parameters as indicators of condition and fitness. Can. J. Zool. 2003;81:1566–1578. [Google Scholar]
- Moore I.T., Hopkins W.A. Interactions and trade-offs among physiological determinants of performance and reproductive success. Integr. Comp. Biol. 2009;49:441–451. doi: 10.1093/icb/icp081. [DOI] [PubMed] [Google Scholar]
- Moran M.D. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos. 2003;100:403–405. [Google Scholar]
- Moss G., Croft D. Body condition of the red kangaroo (Macropus rufus) in arid Australia: the effect of environmental condition, sex and reproduction. Aust. J. Ecol. 2009;24:97–109. [Google Scholar]
- Munger J.C., Karasov W.H. Sublethal parasites and host energy budgets: tapeworm infection in white-footed mice. Ecology. 1989;40:904–921. [Google Scholar]
- Munn A., Dawson T. How important is milk for near-weaned red kangaroos (Macropus rufus) fed different forages? J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2003;173:141–148. doi: 10.1007/s00360-002-0319-3. [DOI] [PubMed] [Google Scholar]
- Munn A.J., Dawson T.J. The ecophysiology of survival in juvenile red kangaroos Macropus rufus: greater demands and higher costs. Aust. Mammal. 2004;26:161–168. [Google Scholar]
- Munn A.J., Dawson T.J. Forage fibre digestion, rates of feed passage and gut fill in juvenile and adult red kangaroos Macropus rufus Desmarest: why body size matters. J. Exp. Biol. 2006;209:1535–1547. doi: 10.1242/jeb.02137. [DOI] [PubMed] [Google Scholar]
- O’Kelly J.C., Post T.B., Bryan R.P. The influence of parasitic infestations on metabolism, puberty and first mating performance of heifers grazing in a tropical area. Anim. Reprod. Sci. 1988;16:177–189. [Google Scholar]
- Peig J., Green A.J. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos. 2009;118:1883–1891. [Google Scholar]
- Poole W., Carpenter S., Wood J. Growth of grey kangaroos and the reliability of age determination from body measurements. I. The eastern grey kangaroo Macropus giganteus. Aust. Wildl. Res. 1982;9:9–20. [Google Scholar]
- Poole W.E. Reproduction in the two species of grey kangaroos, Macropus giganteus Shaw and M. fuliginosus (Desmarest). II. Gestation, parturition and pouch life. Aust. J. Zool. 1975;23:333–353. [Google Scholar]
- Poole W.E. Breeding in the grey kangaroo, Macropus giganteus, from widespread locations in eastern Australia. Wildl. Res. 1983;10:453–466. [Google Scholar]
- Riney T. Evaluating condition of free-ranging red deer (Cervus elaphus), with special reference to New Zealand. NZ J Sci Technol. 1955;36:429–463. [Google Scholar]
- Rothschild M.A., Oratz M., Schreiber S.S. Albumin synthesis. New Eng. J. Med. 1972;286:748–757. doi: 10.1056/NEJM197204062861404. [DOI] [PubMed] [Google Scholar]
- Rothschild M.A., Oratz M., Schreiber S.S. Serum albumin. Hepatology. 1988;8:385–401. doi: 10.1002/hep.1840080234. [DOI] [PubMed] [Google Scholar]
- Scantlebury M., Waterman J., Hillegass M., Speakman J., Bennett N. Energetic costs of parasitism in the Cape ground squirrel Xerus inauris. Proc. R. Soc. Biol. Sci. Ser., B. 2007;274:2169. doi: 10.1098/rspb.2007.0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R.L., Hibler C.P., Spraker T.R., Rutherford W.H. An evaluation of drug treatment for lungworm in bighorn sheep. J. Wildl. Manage. 1979;43:461–467. [Google Scholar]
- Schulte-Hostedde A.I., Zinner B., Millar J.S., Hickling G.J. Restitution of mass-size residuals: validating body condition indices. Ecology. 2005;86:155–163. [Google Scholar]
- Seitz A., Ratte H. Aquatic ecotoxicology: on the problems of extrapolation from laboratory experiments with individuals and populations to community effects in the field. Comp. Biochem. Physiol. C: Comp. Pharmacol. 1991;100:301–304. [Google Scholar]
- Shepherd N. Condition and recruitment of kangaroos. In: Caughly G., Shepard N., Short J., editors. Kangaroos: Their Ecology and Management in the Sheep Rangelands of Australia. Cambridge University Press; Cambridge: 1987. pp. 135–158. [Google Scholar]
- Stien A., Irvine R.J., Ropstad E., Halvorsen O., Langvatn R., Albon S.D. The impact of gastrointestinal nematodes on wild reindeer: experimental and cross-sectional studies. J. Anim. Ecol. 2002;71:937–945. [Google Scholar]
- Stuart-Dick R.I., Higginbottom K.B. Strategies of parental investment in Macropodoids. In: Grigg G., Jarman P., Hume I., editors. Kangaroos. Wallabies and Rat-Kangaroos. Surrey Beatty & Sons Pty Limited; Chipping Norton, Australia: 1989. pp. 571–592. [Google Scholar]
- Sykes A.R. Endoparasites and herbivore nutrition. In: Hacker J., Ternouth J., editors. Nutrition of Herbivores. Academic Press; New South Wales: 1987. pp. 211–232. [Google Scholar]
- Van Houtert M., Barger I., Steel J. Dietary protein for young grazing sheep: interactions with gastrointestinal parasitism. Vet. Parasitol. 1995;60:283–295. doi: 10.1016/0304-4017(95)00864-8. [DOI] [PubMed] [Google Scholar]
- Van Houtert M.F.J., Sykes A.R. Implications of nutrition for the ability of ruminants to withstand gastrointestinal nematode infections. Int. J. Parasitol. 1996;26:1151–1167. doi: 10.1016/s0020-7519(96)00120-8. [DOI] [PubMed] [Google Scholar]
- Watson M.J. The costs of parasites – What drives population-level effects? Meta-analysis meets life-history. Int. J. Parasitol. Parasites Wildl. 2013;2:190–196. doi: 10.1016/j.ijppaw.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood I., Amaral N., Bairden K., Duncan J., Kassai T., Malone J., Pankavich J., Reinecke R., Slocombe O., Taylor S. World Association for the Advancement of Veterinary Parasitology (WAAVP) of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine) Vet. Parasitol. 1995;58:181–213. doi: 10.1016/0304-4017(95)00806-2. [DOI] [PubMed] [Google Scholar]
- Zuk M., Stoehr A.M. Immune defense and host life history. Am. Nat. 2002;160:9–22. doi: 10.1086/342131. [DOI] [PubMed] [Google Scholar]


