Keywords: Toxoplasmosis, Epidemiology, Feral cat, Felis catus, Population decline
Highlights
-
•
Seroprevalence of Toxoplasma gondii was investigated in eastern quolls.
-
•
Seroprevalence of IgG antibodies was higher at sites where quolls were declining.
-
•
T. gondii infection did not reduce quoll survival or reproduction.
-
•
Higher seroprevalence signals higher exposure to feral cats at declining sites.
Abstract
Disease is often considered a key threat to species of conservation significance. For some, it has resulted in localised extinctions and declines in range and abundance. However, for some species, the assertion that a disease poses a significant threat of extinction is based solely on correlative or anecdotal evidence, often inferred from individual clinical case reports. While a species’ susceptibility to a disease may be demonstrated in a number of individuals, investigations rarely extend to measuring the impact of disease at the population level and its contribution, if any, to population declines. The eastern quoll (Dasyurus viverrinus) is a medium-sized Australian marsupial carnivore that is undergoing severe and rapid decline in Tasmania, its last refuge. Reasons for the decline are currently not understood. Feral cats (Felis catus) may be undergoing competitive release following the ongoing decline of the Tasmanian devil (Sarcophilus harrisii), with cats suppressing eastern quolls through increased predation, competition, exclusion or exposure to diseases such as toxoplasmosis. To investigate the effects of Toxoplasma gondii infection, eastern quoll populations at four sites were regularly screened for the seroprevalence of T. gondii-specific IgG antibodies. Seroprevalence was approximately five times higher at sites with declining quoll populations, and there was a negative association between seroprevalence and quoll abundance. However, T. gondii infection did not reduce quoll survival or reproduction. Despite a high susceptibility to T. gondii infection, eastern quoll populations do not appear to be limited by the parasite or its resultant disease. Significantly higher seroprevalence is a signal of greater exposure to feral cats at sites where eastern quolls are declining, suggesting that increased predation, competition or exclusion by feral cats may be precipitating population declines.
1. Introduction
Pathogens, parasites and their associated diseases can have significant negative impacts on wildlife populations, causing reduced abundance (Muths et al., 2003; Leroy et al., 2004; Hawkins et al., 2006), range (van Riper et al., 1986; Scott, 1988) or even extinction of populations (Thorne and Williams, 1988; Cunningham and Daszak, 1998; Blaustein et al., 2012). Deterministic extinction may result where disease holds mortality rates above replacement rates (Satō et al., 1994; Jones et al., 2008). Alternatively, disease may suppress fecundity, growth rates or population size, thereby increasing vulnerability to extinction through demographic stochasticity or Allee effects (Caughley, 1994; McCallum, 1994; Lafferty and Gerber, 2002; De Castro and Bolker, 2005). Emerging infectious diseases and ‘spill-overs’ from reservoir animal populations to sympatric wildlife species have increased in recent decades (Daszak et al., 1999; Daszak et al., 2000; Hawkins et al., 2006; Rhyan and Spraker, 2010) and are recognised as a key threatening process for many species. However, while infectious disease has been considered among the top five causes of species extinction in the United States (Wilcove et al., 1998), it is thought to have contributed to less than 4% of species extinctions worldwide since 1500 (Smith et al., 2006). For some of these species, the role of disease in decline or extinction is inferred solely from correlative or anecdotal evidence (Huijbregts et al., 2003; Walsh et al., 2003; Abbott, 2006; Smith et al., 2006; Smith et al., 2008; Wyatt et al., 2008).
To determine the effects of a disease in natural populations, the relationship of disease to survival or fecundity should be established (McCallum and Dobson, 1995). While individual clinical case studies may demonstrate a species’ susceptibility to a disease (e.g. Canfield and Cunningham, 1993; Blanchard et al., 2001; Sleeman et al., 2009; Eleni et al., 2014; Howe et al., 2014), correlation between the prevalence of disease or pathogen and population decline does not establish causality. For example, six viruses are known to infect lions (Panthera leo) in the Serengeti, but only one, canine distemper virus, clearly decreases lion abundance (Packer et al., 1999). Even the presence of a pathogen or parasite in a dying or dead animal provides only circumstantial evidence without demonstrating cause of death (McCallum, 1994). In some declining populations, equilibrium prevalence of a benign infection may be high, while some other factor is responsible for the deaths (McCallum and Dobson, 1995). However, many studies do not progress beyond establishing the prevalence of a disease or pathogen in a host population (Gauthier-Clerc et al., 2002; Cabello et al., 2013; Chadwick et al., 2013; Cross et al., 2013).
The eastern quoll is a medium-sized Australian marsupial carnivore that is presumed extinct on the Australian mainland, and survives only on the island of Tasmania (McKnight, 2008). Numbers in Tasmania are declining rapidly, with statewide declines of more than 50% in the 10 years to 2009 (Fancourt et al., 2013). Population declines are continuing with no sign of recovery (B. Fancourt, unpublished data). The cause(s) of the decline are not currently known. The Tasmanian devil is also in steep decline, due to the spread of the fatal Devil Facial Tumour Disease (DFTD) (Hawkins et al., 2006). Devil declines may allow mesopredators such as feral cats to increase in abundance, possibly leading to suppression of eastern quoll populations through increased predation, competition, exclusion or exposure to diseases such as toxoplasmosis.
Toxoplasma gondii is an intracellular coccidian microparasite with a worldwide distribution (Hill et al., 2005; Dubey, 2010). Infection by T. gondii can result in overt clinical disease (Dubey and Frenkel, 1972; Innes, 1997; Dubey, 2010), with fatalities observed in many wildlife species (Work et al., 2000; Szabo et al., 2004; Jokelainen and Nylund, 2012; Howe et al., 2014). Some Australian marsupials are especially susceptible to toxoplasmosis (Obendorf and Munday, 1983; Canfield et al., 1990; Innes, 1997; Bettiol et al., 2000). In Australia, feral, stray and domestic cats are the only definitive host that can excrete the environmentally persistent oocysts that are the major source of infection for many intermediate hosts (Dubey et al., 2004). For around 1 week following infection, cats shed millions of oocysts in their faeces (Hutchison, 1965; Dubey et al., 1970; Frenkel et al., 1970; Miller et al., 1972; Lukešová and Literák, 1998), which can remain infective in the environment for at least 18 months under optimal climatic conditions (Yilmaz and Hopkins, 1972; Frenkel et al., 1975). Potential intermediate hosts of T. gondii include all birds and mammals, which typically acquire the parasite through eating food, soil or water contaminated with the parasite (Miller et al., 1972; Attwood et al., 1975; Aramini et al., 1999; Hill and Dubey, 2002). Once eaten, the sporozoites excyst and rapidly multiply as tachyzoites (Frenkel, 1973), leading to clinical toxoplasmosis in some hosts. Acutely infected individuals may exhibit a range of clinical signs or symptoms, including lymphadenopathy, anorexia, lethargy, incoordination, apparent blindness, disorientation, ataxia, dyspnea, icterus, fever, abortion or death (Desmonts and Couvreur, 1974; Attwood et al., 1975; Tenter et al., 2000; Hill and Dubey, 2002; Burns et al., 2003; Pereira-Bueno et al., 2004; Dubey, 2010), although pathogenicity and clinical signs vary between individuals and species. However, many immunocompetent individuals remain subclinical (Dubey et al., 1988; Hill and Dubey, 2002). For individuals that survive acute infection, bradyzoites form latent tissue cysts predominantly in the neural and muscular tissues (Attwood et al., 1975; Dubey and Frenkel, 1976; Canfield et al., 1990). Tissue cysts rarely cause harm and remain in situ for the life of the host (Ekanayake et al., 2004; Eymann et al., 2006), although latent infection has been associated with increases in certain risky behaviours in some species (Hay et al., 1984; Webster et al., 1994; Berdoy et al., 2000; Vyas et al., 2007). While infection is commonly acquired through the faecal–oral route, many intermediate host species can transmit the parasite through eating infected animal tissues (Attwood et al., 1975; Burns et al., 2003), sexually (Arantes et al., 2009; de Moraes et al., 2010; Santana et al., 2013) or congenitally (Beverley, 1959; Parameswaran et al., 2009).
The hypothesis that toxoplasmosis is contributing to declines of the eastern quoll is plausible for several reasons. First, many aspects of eastern quoll ecology, such as foraging for ground-dwelling invertebrates and scavenging carrion, increases the likelihood of exposure to infective T. gondii oocysts and tissue cysts. Second, disease has been implicated in the demise of the eastern quoll on the mainland and in a sudden decline in thylacine, devil and quoll populations in Tasmania in the early 1900s (Wood Jones, 1923; Guiler, 1961; Green, 1967; Peacock and Abbott, submitted for publication), with some proposing toxoplasmosis as a candidate disease (Cross, 1990; Freeland, 1993; Recher et al., 1993). Third, while feral cats have been in Tasmania for over 200 years (Abbott, 2002) with no obvious negative effect on eastern quoll populations, several stressors such as drought or habitat loss over recent years may have triggered recrudescence of any latent infections into overt disease. However, despite toxoplasmosis posing a significant threat to some Tasmanian mammals (Obendorf and Munday, 1983; Skerratt et al., 1997; Bettiol, 2000) and a high prevalence of T. gondii infection in feral cats throughout Tasmania (Fancourt and Jackson, In review), there has been no research investigating the prevalence of T. gondii in eastern quolls, nor its effect on population dynamics.
In this study, we address the following four questions. First, is seroprevalence of T. gondii associated with population decline of eastern quolls? To answer this, seroprevalence of T. gondii-specific IgG antibodies was compared between sites with declining quoll populations and a site with a non-declining population. Individual quolls were screened for clinical signs indicating clinical toxoplasmosis. Seroprevalence was also regressed against quoll captures within a site to identify any negative correlation between seroprevalence and quoll abundance. Second, does survival differ between seropositive and seronegative quolls? We compared recapture data and survival trajectories of seropositive and seronegative individuals within a population. Third, are there indirect effects of T. gondii infection on reproduction? We compared annual production of pouch young in females and testicular volume in males during the mating season for seropositive and seronegative quolls. Fourth, which variables that influence exposure to T. gondii are associated with differences in seroprevalence within and among populations? We investigated if seroprevalence within a population differed by age or sex of quoll, and if seroprevalence among quoll populations differed with estimated seroprevalence in and activity of feral cats.
2. Materials and methods
2.1. Study sites
Eastern quolls were surveyed at four study sites in Tasmania: Cradoc (43°06′13″S, 147°02′40″E), Judbury (43°01′24″S, 146°54′50″E), Cradle Mountain (41°38′35″S, 145°57′32″E) and Bruny Island (43°09′48″S, 147°21′17″E) (Fig. 1). Mean annual rainfall for Cradoc, Judbury and Bruny Island sites ranged from 650 to 740 mm; mean daily minimum and maximum temperatures were 2 and 13 °C respectively in winter, and 10 and 22 °C in summer. Mean annual rainfall at the Cradle Mountain site was 2830 mm, and mean daily minimum and maximum temperatures were −1 and 5 °C respectively in winter, and 4 and 17 °C in summer (Australian Bureau of Meteorology 2013 data). Sites were categorised as declining sites (Cradoc and Judbury) or a non-declining site with a high density, stable quoll population (Bruny Island). The population at the Cradle Mountain site fluctuated throughout the study. The population status for each site was determined during a pilot study undertaken in 2010 by comparing current capture rates to historic studies at each site (Fancourt, 2010; Fancourt et al., 2013).
Fig. 1.
Map of Tasmania showing location of study sites used for blood collection.
2.2. Quoll surveys, screening and blood sampling
Eastern quolls were surveyed at each site using live capture and release. Sites were surveyed usually every second month from May 2011 to July 2013, although Bruny Island was also surveyed in September 2013 and some prior survey data were available from a pilot study conducted in 2010 at all sites except Judbury. Quolls were captured using standard PVC pipe traps baited with raw lamb heart. All bait was frozen for a minimum of one month at −20 °C, then thawed prior to use in traps. This protocol aimed to eliminate the risk of captured quolls acquiring the parasite through eating infected baits (Dubey, 1988; Kotula et al., 1991; Burns et al., 2003). Samples were collected from individual quolls only on their first capture in each sampling period, and were re-sampled if recaptured in subsequent periods. All captured quolls were examined for signs of clinical toxoplasmosis, such as dyspnea, icterus, hind leg paresis, ataxia, and apparent ophthalmic problems. Approximately 300 μL of whole blood was collected from the peripheral ear vein of captured quolls and was kept on ice until processed later the same day. Once clotted, blood was centrifuged for at least 5 min and serum frozen at −20 °C until processed (within 12 months of collection).
2.3. Feral cat surveys and blood sampling
Remote camera surveys were performed to assess feral cat activity at each site. Three replicate surveys were undertaken at each site in February/March 2012, June/July 2012 and December 2012/January 2013, using 20 passive RECONYX™ PC-800 infrared motion detector cameras for a minimum of 21 nights. Each camera was fastened to a tree approximately 1.5 m above the ground, with a muttonbird (Puffinus tenuirostris) oil scent lure positioned 2–3 m in front of each camera. Cameras were programmed to take three pictures in rapid succession following each trigger, with images taken continuously in groups of three until all movement ceased. An infrared flash was used to illuminate images at night. All images were stamped with the time, date, site and camera number.
Blood was collected from 55 feral cats trapped, euthanased and frozen on Bruny Island under control programs conducted by the Tasmanian Parks & Wildlife Service, and from an additional six cats trapped and immediately euthanased at the Judbury study site as part of this study. For 23 of the Bruny Island cats, samples were collected from cats defrosted up to 2 years later. For the Judbury cats and 32 of the Bruny Island cats, blood was collected using cardiac puncture soon after death. All blood samples were processed and stored as outlined in Section 2.2.
2.4. Testing for T. gondii IgG antibodies
Serum samples were defrosted and tested for the presence of T. gondii-specific IgG antibodies using a commercial modified agglutination test (MAT) (Toxo-Screen DA, bioMérieux, Marcy-l’Etoile, France). IgG antibodies are usually detectable within 2 weeks of initial infection and remain detectable for the life of the host (Remington et al., 2004; Dubey, 2010). Accordingly, MAT-derived titres are not indicative of recency of infection or clinical status (Dubey, 2010) but rather an exposure to the parasite at some time at least 2 weeks before sampling. Of the agglutination tests that do not require species-specific reagents, MAT is considered to be the most sensitive for detecting T. gondii specific-IgG antibodies in marsupials (Munday, 1972; Dubey, 2010). Haemolysis does not interfere with the test, so it can be used with serum, blood plasma or even whole blood (Dubey, 2010).
Samples were treated with 2-mercaptoethanol to denature any IgM antibodies and suppress any non-specific agglutination (Desmonts and Remington, 1980; Dubey and Desmonts, 1987). Each sample was tested at serial fourfold dilutions of 1/16, 1/64 and 1/256 together with positive and negative controls supplied in the MAT kit. A positive reaction was observed when agglutination of toxoplasma formed a mat covering about half of the well base. Titres were expressed as the inverse of the highest dilution at which a positive reaction was observed. A titre of ⩾64 was used for determining a sample as positive for T. gondii infection (Dubey and Desmonts, 1987).
To validate the results obtained using these protocols, a sub-sample of sera underwent retesting by the Tasmanian government Animal Health Laboratories. Where longitudinal samples were collected from individual quolls over multiple sampling periods, further validation was obtained by checking that seroconversion occurred only once in each quoll’s life, and that seroconversion occurred only in one direction (from seronegative to seropositive).
To validate the reliability of results using blood from frozen cats, 20 samples were collected from cats at the time of death in 2012, and matched to samples from the same cats after the body had been frozen for around 12 months.
2.5. Data analysis
All statistical analyses were performed using R (ver. 3.0.1, R Development Core Team, 2013).
2.5.1. Seroprevalence
Quolls were classified as adults by May of the year following birth (when they were 10–11 months old), as both sexes reach sexual maturity by this age (Bryant, 1986). Seroprevalence was calculated as the proportion of quolls tested in each sampling period that were seropositive. We used a Fisher’s exact test to determine if adult seroprevalence at the declining sites was significantly different from that at the non-declining site. As several individual quolls were sampled in multiple periods (but not every quoll in every period), seroprevalence was calculated and compared for each sampling period separately. Any increase in type I error resulting from multiple comparisons was considered unimportant due to the highly significant P-values in every period, and was unavoidable due to the non-independence of individual quolls between sampling periods. Because of the high number of periods with 100% prevalence at the declining sites and the resultant infinite odds ratios in each period, a generalised linear mixed model could not be used for this analysis. Only those sampling periods between May 2011 and July 2013 where both declining and non-declining sites were sampled were included in the analysis.
To identify whether seroprevalence in juveniles and the rate of seroconversion differed between declining sites and the non-declining site, seroprevalence was compared and assessed graphically for each annual juvenile cohort (2011 and 2012 emergence), from time of first emergence in November until September of the following year.
To investigate whether increased seroprevalence was correlated with decreased quoll abundance within a site, seroprevalence for each sampling period was regressed against the number of quolls captured in the subsequent survey period (2 months later) as an index of abundance. This analysis was restricted to data from Cradle Mountain as it was the only site where seroprevalence fluctuated throughout the study, enabling the number of captures to be compared at differing levels of seroprevalence in different periods. Seroprevalence for each period was taken as the number of seropositive quolls captured in that period plus the number of quolls known to be seropositive at that time (but not captured in that period) divided by the total number of quolls known to be alive in that period. Eastern quoll capture data were square root transformed to stabilise the variance, and linear regression was used to model seroprevalence against the square root of the number of quolls captured in the subsequent survey period.
2.5.2. Recapture and survival
Data from the Bruny Island site were used to assess recapture likelihood and survivorship. This was the only site with sufficient numbers of both seropositive and seronegative individuals captured in every sampling period, and allowed the effect of serological status to be assessed without involvement of other confounding variables that might be contributing to declines at other sites and that might differ among sites.
The proportion of individuals recaptured was compared between serological groups to identify any effect of serological status on recapture likelihood. All individuals were included in the analysis except those first captured in the final trapping session in September 2013 as there was no possibility of recapture data. Juveniles first captured between November and March each year and not recaptured were also excluded as a high rate of juvenile dispersal is typical in this species soon after first emergence from the den in summer each year (Godsell, 1982; Bryant, 1986), so failure to recapture these individuals could be due to dispersal rather than death. The proportion of seropositive and seronegative individuals recaptured was compared using a Fisher’s exact test.
All individuals first captured between August 2010 and October 2012 were included in the survival analysis, with recapture data up to September 2013 used to assess survival of each individual.
Quolls first captured after October 2012 were excluded due to insufficient time to ascertain robust survival data between first capture and the end of the study in September 2013. Juvenile quolls that were first captured during the period of juvenile emergence and not subsequently recaptured were also excluded. The number of days known to be alive was used as a measure of quoll survival time, and was calculated from the date of birth (estimated from 1 July in the year of birth) to the most recent capture for each quoll. As the ultimate fate of each individual was not known, analysis was performed on censored data, with failure to recapture an individual assumed to be failure to survive at the date of last capture. Mean survival time was compared between seropositive and seronegative quolls using a one-way ANOVA, and Kaplan Meier (KM) survival curves were used to compare the survival of seropositive and seronegative individuals throughout the study period. A log-rank test was used to identify differences between KM survival curves, with an average hazard ratio calculated to provide an overall comparison of the two serological groups. To quantify effects of serological status on mean longevity, survival time for the oldest cohort (all quolls born in 2009 or earlier) was compared between serological groups using a one-way ANOVA.
2.5.3. Reproduction
The mean number of pouch young (PY) in July was compared between seropositive and seronegative females using a three-way ANOVA incorporating site and quoll age. Females from all sites and all years were included in the analysis. Only 2 quolls were captured in July in more than one year; data from their second year were excluded. Females at Cradle Mountain bred around 2 months later than other sites in most years, so were assessed in either July or September, depending on when PY first appeared at that site.
Testicular volume (TV) was calculated for each male quoll captured in May (the mating season) using the formula for a prolate spheroid: TV (cm3) = 0.5236 × TL × TW2 (Bailey et al., 1998; Power et al., 2009). Mean TV was compared between seropositive and seronegative males using a two-way ANOVA incorporating age of quoll at the time of assessment. Males from all years were included in the analysis. Where individual quolls were captured in May in more than one year, data were included only from the first (for seropositive males) or second year (for seronegative males). As the likelihood of infection increases with age (due to increased exposure over time), excluding data from the first capture for seronegative males reduced the likelihood of inadvertently biasing younger males in the seronegative sample set. Males from the Cradle Mountain site were excluded due to an unpredictable delay in breeding at this site in some years, meaning assessment of May TV did not indicate breeding condition in some years.
A body condition index (BCI) was calculated for each female and male at the same time reproductive condition was assessed. Body mass was regressed against maximum head width for each sex, and the regression was used to predict body mass from the observed head width for each individual. BCI was calculated for each quoll as the ratio of observed to predicted body mass (Krebs and Singleton, 1993). BCI was compared between seropositive and seronegative individuals, separately for each sex, using a three-way ANOVA incorporating site and quoll age (females) and a two-way ANOVA incorporating quoll age (males).
2.5.4. Exposure variables
To investigate whether seroprevalence differed between sexes, we used a Fisher’s exact test to compare seroprevalence between adult male and female quolls for each sampling period. Only quolls from the non-declining site (Bruny Island) were used in this analysis as it was the only site with both seropositive and seronegative individuals of both sexes in most periods.
To determine if T. gondii infection was affected by quoll age, we used a generalised linear mixed model (GLMM) fit by maximum likelihood with a binomial error distribution and logit link function using R package lme4 (Bates et al., 2013). Individual ID was treated as a random factor to account for non-independence of individual quolls between sampling periods. The model was fit with site (non-declining, declining or Cradle Mountain) and quoll age as fixed effects. All quolls of all ages were included in the analysis, with probability by age plotted for each site.
An index of cat activity was calculated for each site by dividing the number of feral cat detections by the number of camera nights for each camera survey. The mean cat detection rate per 100 camera nights across all 3 surveys was then compared using a two-tailed t-test to identify any difference in cat activity among sites.
Seroprevalence in cats was compared using a Fisher’s exact test to identify if infection rates differed between sites.
3. Results
No signs of overt toxoplasmosis were observed in any of the 290 quolls captured and examined on 1138 occasions between March 2010 and September 2013.
3.1. Seroprevalence
Declining sites had significantly higher seroprevalence (range: 77.3–100.0%) than the non-declining site (range: 9.4–29.4%) in every period throughout the study (P < 0.005 for all periods) (Fig. 2). There were no differences among sampling periods. Seroconversion of newly emerged juveniles occurred earlier and more rapidly at declining sites than at the non-declining site (Fig. 3), being evident by January for both cohorts at the declining sites, but not evident at the non-declining site until July 2012 or May 2013 (2011 and 2012 cohorts respectively). All juveniles were seropositive at the declining sites by May 2012 or July 2013 (2011 and 2012 cohorts respectively) while seroprevalence at the non-declining site was still below 10% almost a year after emergence for both cohorts. There was a significant negative association between seroprevalence and the number of quolls captured two months later at the Cradle Mountain site (adjusted R2 = 0.393, F1,10 = 8.128, P = 0.017) (Fig. 4).
Fig. 2.
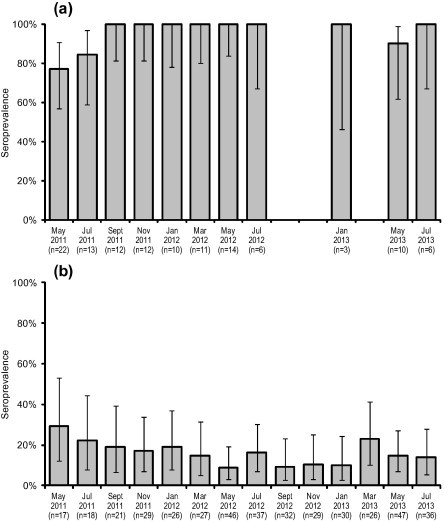
Seroprevalence of T. gondii IgG antibodies in adult eastern quolls at (a) declining sites (Cradoc and Judbury) and (b) non-declining site (Bruny Island). Declining sites were not surveyed in September or November 2012 or March 2013. Vertical axis shows proportion of quolls tested that were seropositive at titres ⩾ 64. Error bars represent 95% confidence intervals calculated using the Jeffreys interval estimation for a small sample size with binomial distribution (Brown et al., 2001).
Fig. 3.
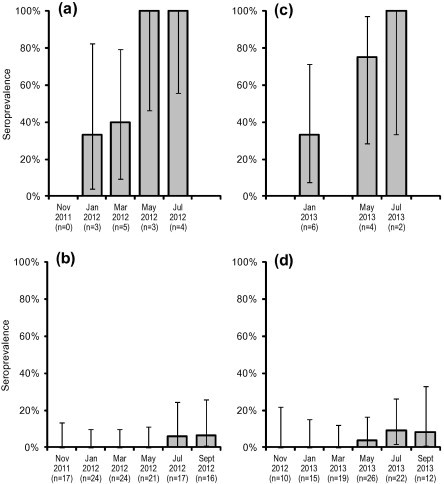
Seroprevalence of T. gondii IgG antibodies in juvenile eastern quolls from time of emergence for 2011 cohort ((a) declining sites (Cradoc and Judbury) and (b) non-declining site (Bruny Island)) and 2012 cohort ((c) declining sites and (d) non-declining site). Declining sites were not surveyed in September 2012, November 2012, March 2013 or September 2013. Vertical axis shows proportion of quolls tested that were seropositive at titres ⩾ 64. Error bars represent 95% confidence intervals calculated using the Jeffreys interval estimation for a small sample size with binomial distribution (Brown et al., 2001).
Fig. 4.
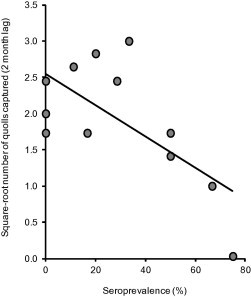
Association between seroprevalence of T. gondii IgG antibodies in eastern quolls at Cradle Mountain and the square-root transformed number of eastern quolls captured 2 months later (y = 2.552–0.022×). Each data point represents a single trapping/sampling session between May 2011 and July 2013.
3.2. Recapture and survival
The serological status of the 151 quolls captured at the non-declining site had no effect on the likelihood of recapture (P = 1.000): 61.9% (95% CI: 40.1–83.7%) of seropositive quolls and 60.8% (95% CI: 52.0–69.5%) of seronegative quolls were recaptured in at least one subsequent trapping session. Mean survival time did not differ significantly with serological status (F1,85 = 2.018, P = 0.159). For first-captures prior to October 2012, seropositive quolls (n = 11) survived 890.5 ± 92.8 (mean ± s.e.) days compared to 758.1 ± 32.8 days for seronegative quolls (n = 76). Mean longevity of the oldest cohort did not differ between serological groups (seropositive: 949.0 ± 149.6 days, seronegative: 953.6 ± 52.4 days; P = 0.972). KM curves indicated a similar survival trajectory for both serological groups (P = 0.261) (Fig. 5). The mean hazard ratio (or conditional failure rate) comparing seronegative to seropositive quolls of 1.17 indicated no relationship between serological status and survival.
Fig. 5.
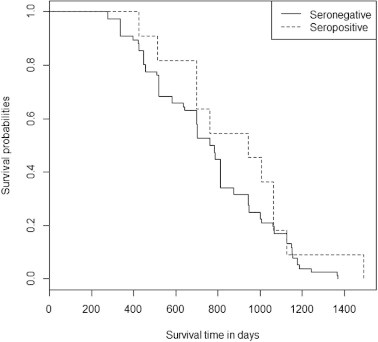
Kaplan Meier survival curves comparing survival trajectories for seronegative (solid line) and seropositive (broken line) eastern quolls. Curves show survival of all quolls first captured at the non-declining site between August 2010 and October 2012.
3.3. Reproduction
Seropositive females had significantly more pouch-young (6.0 ± 0.0) than seronegative females (4.0 ± 0.4) (F1,30 = 7.101, P = 0.012). There was no effect of site (F2,30 = 2.002, P = 0.153) or age of mother (F1,30 = 0.839, P = 0.367) on number of pouch-young. Body condition did not differ with serological status (F1,21 = 0.680, P = 0.419) or age of mother at time of PY assessment (F1,21 = 0.471, P = 0.500), however BCI was significantly higher at the non-declining site than other sites (F2,21 = 4.517, P = 0.023).
Mean testicular volume (F1,87 = 9.473, P = 0.003) and body condition (F1,87 = 9.945, P = 0.002) were both significantly higher in seropositive males. While age had no effect on mean TV (F1,87 = 0.126, P = 0.723), BCI was significantly higher in older males (F1,87 = 8.328, P = 0.005).
3.4. Exposure variables
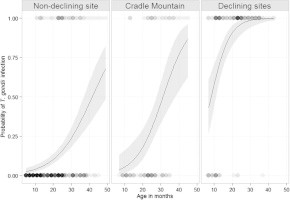
Seroprevalence in male quolls (range: 11.1–35.7 %) was higher than in females (range: 0.0–3.7 %) in all periods, however differences were not significant in any period except May 2013 (P = 0.010). The probability of T. gondii infection increased with age, with a significant interaction between age and site (P < 0.001) (Fig. 6). At any given age, the probability of infection was significantly higher for quolls at the declining sites than either the non-declining site or Cradle Mountain (P = 0.034).
Fig. 6.
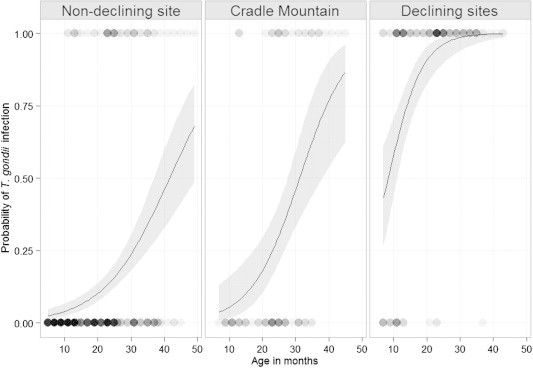
Comparison of the probability of T. gondii infection with quoll age, by site. Non-declining site = Bruny Island, Cradle Mountain = fluctuating site, Declining sites = pooled data from Cradoc and Judbury sites. Circles represent individual observations of seronegative (probability = 0) and seropositive (probability = 1) quolls at a given age, with darker circles indicating a higher number of quolls with the same combination of age and serological status. Curve illustrates the fitted data, with grey shading representing 95% confidence intervals.
The mean rate of cat detections (per 100 camera nights) of 1.9 ± 0.3 was significantly higher at the declining sites than the 0.1 ± 0.1 detections at the non-declining site (T4 = 6.457, P = 0.003). One cat was detected at the Cradle Mountain site in the March 2012 camera survey (0.2 detections per 100 camera nights) but no cats were detected in either the July 2012 or January 2013 surveys.
Seroprevalence of feral cats from Bruny Island (non-declining) was 80% (44/55; 95% CI: 68.1–88.9%) compared with 100% (6/6; 95% CI: 67.0–100.0%) of cats captured at the Judbury (declining) site, although differences were not significant (P = 0.577). No cats were captured at either the Cradoc or Cradle Mountain sites. Titres obtained from frozen cats matched titres from fresh samples collected at the time of death prior to freezing, indicating no difference in results due to sample type (fresh or frozen).
4. Discussion
We found no evidence that T. gondii infection reduces survival or reproduction of eastern quolls. Seroprevalence of T. gondii antibodies was higher at sites with declining quoll populations than in the non-declining population, and there was a negative association between seroprevalence and the number of quolls captured. While this might suggest a causal link between T. gondii infection and quoll declines, our epidemiological studies suggest no such link. High prevalence per se is a poor indicator of the impact of disease on a population (McCallum and Dobson, 1995). On the one hand, highly virulent diseases remove infected individuals soon after exposure, either through rapid death or predation of symptomatic individuals, leaving only unexposed individuals to be detected and a low observed prevalence (e.g. Obendorf et al., 1996). On the other hand, if a disease is benign, infected individuals remain to be detected and the observed prevalence of the disease will be relatively high (McCallum, 1994). This is evidently the case for T. gondii in the eastern quoll.
The nonpathogenicity of T. gondii in eastern quolls is also supported by the absence of clinical signs in any of the quolls captured and examined in this study. Tasmanian government Animal Health Laboratory records also have no cases of histopathology indicating clinical toxoplasmosis in any seropositive quolls examined (B. Jackson, Department of Primary Industries, Parks, Water & Environment (DPIPWE), personal communication), although we acknowledge that given the unspecific clinical signs associated with the disease, diagnosis is challenging to reach both ante mortem and post mortem in many host species. An extensive search of the literature (this study; D. Peacock and I. Abbott, personal communication) uncovered only one suspected case of toxoplasmosis in what was probably an eastern quoll (‘native cat’ Dasyurus quoll: Carne (1950) unpublished, cited in Seddon (1952)). However this was based on necropsy findings of “toxoplasms” in the lung of a moribund individual, probably bradyzoites associated with dormant tissue cysts and not diagnostic of clinical disease. Reasonably high seroprevalence has been recorded in many marsupial carnivore species, including spotted-tailed quolls (Dasyurus maculatus) (Hollings et al., 2013), western quolls (Dasyurus geoffroii) (Haigh et al., 1994) and Tasmanian devils (Phillips, 2009; Hollings et al., 2013), but with no known confirmed cases of clinical toxoplasmosis. Therefore, while the high seroprevalence indicates that the larger marsupial carnivores are highly susceptible to T. gondii infection, they are evidently less likely to succumb to acute disease than other marsupial guilds.
The lower pathogenicity of T. gondii in marsupial carnivores than in other marsupial species may be partly related to the route of infection. The primary source of T. gondii in carnivores is probably through the consumption of bradyzoites in tissue cysts of infected prey or carrion. While transmission of bradyzoites is the most infective form of the parasite for the cat as the definitive host (Dubey and Frenkel, 1976), circumstantial evidence suggests that oocyst-transmitted infections can be more clinically severe in intermediate hosts (Bowie et al., 1997; Hill and Dubey, 2002; Dubey, 2004). This could partly explain the occurrence of clinical disease in a range of herbivore and insectivore species, including eastern barred bandicoots (Perameles gunnii) (Obendorf et al., 1996), Tasmanian pademelons (Thylogale billardierii) and Bennett’s wallabies (Macropus rufogriseus rufogriseus) (Obendorf and Munday, 1983), Tammar wallabies (Macropus eugenii) (Reddacliff et al., 1993), koalas (Phascolarctos cinereus) (Hartley et al., 1990) and wombats (Vombatus ursinus) (Skerratt et al., 1997), while clinical cases in the larger marsupial carnivores are notably absent. Herbivores ingest oocyst-contaminated vegetation while grazing where cats have defaecated, while bandicoots could acquire the parasite through eating soil-dwelling invertebrates that can transport oocysts mechanically on their bodies (Wallace, 1971, 1972; Saitoh and Itagaki, 1990). Eastern quolls consume both invertebrate and vertebrate prey (Blackhall, 1980; Godsell, 1983), but infection would be less severe if initial T. gondii infection occurred through carnivory, and the subsequent immune response could protect against subsequent exposure to oocysts. Experimental feeding trials could reveal the relative pathogenicity of different parasite sources to the eastern quoll.
The absence of clinical cases in the current study does not prove that clinical cases never occur. If eastern quolls were highly susceptible to acute toxoplasmosis, infected animals could die rapidly before serological or clinical evidence of overt disease could be observed, as occurs in eastern barred bandicoots (Bettiol et al., 2000). In wild populations, rapid predation of infected individuals and scavenging of carcasses would mean illness is rarely detected. In that case, reduced survival of seronegative quolls would be expected, as rapid death or predation would result in loss from the population before seroconversion could be detected. However, we found that seronegative quolls had similar survivorship to seropositive quolls, and the high numbers of seropositive quolls in the population shows that many eastern quolls survive the initial infection. Hence, while individual occurrences may occur, the eastern quoll is unlikely to be highly susceptible to acute disease, and the high seroprevalence indicates a benign infection in this species (McCallum, 1994).
The similarity in longevity of seronegative and seropositive quolls could in principle be explained by equivalent reduction in survival for both classes. While seropositive quolls survive the initial acute infection, they may then be vulnerable to predation due to risky behaviours associated with latent infection, as observed in seropositive rats (Rattus norvegicus) and mice (Mus musculus) that not only lost their fear of cats, but were attracted to them (Berdoy et al., 2000; Vyas et al., 2007). The predation of seropositive quolls may cause a reduction in survival of similar magnitude to the sudden death or predation due to acute infection that removes susceptible seronegative quolls. However, the mean longevity of 2.6 years observed in both serological categories is comparable to survival rates measured in Tasmania before the species went into decline (Godsell, 1983). Accordingly, there is no evidence that a simultaneous pathogen-caused reduction in survival of both seropositive and seronegative quolls could explain the recently observed decline in quoll populations by more than 50% across Tasmania (Fancourt et al., 2013).
The current strain(s) of T. gondii at the declining sites may be more virulent than those at the non-declining site, or strains historically resident in quoll populations at the same site 20–30 years ago. Molecular epidemiological studies of T. gondii infections have shown significant genetic diversity, particularly in wildlife populations (Wendte et al., 2011; Pan et al., 2012; Dubey et al., 2013). Strains differ in their virulence and their propensity to form cysts (Carruthers and Suzuki, 2007), leading to different impacts on individuals or populations (Blader and Saeij, 2009). The majority of marsupial T. gondii infections are caused by atypical strains, with several novel alleles (Parameswaran et al., 2010). We did not undertake molecular identification of T. gondii strains, but our evidence highlights that molecular investigations should form an important part of future research into the effects of T. gondii infections in marsupials.
Another possibility is that the observed decline in eastern quolls may have resulted from recrudescence of latent T. gondii infection with environmental stressors throughout that period. The physiological effects of stress due to factors such as poor nutrition, increased predation risk and competition for food and resources, co-infection with other pathogens or the effects of habitat loss, may contribute to an increased host susceptibility and severity of infection (McCallum and Dobson, 1995; Davey et al., 2006; Pedersen and Greives, 2008). Such stressors could lead to immunosuppression of eastern quolls, thereby allowing any latent disease to recrudesce into overt clinical disease, as occurs in immunocompromised humans, including AIDS patients (Luft et al., 1984) and organ transplant recipients (Wendum et al., 2002). If this were occurring in quoll populations, for example in response to the millennium drought (2001–2009) (Tasmanian Planning Commission, 2009), it would be evident in a reduced survival time for quolls with latent infection during this period, but not when the drought broke (2010–2013). However, given mean survival time for seropositive quolls was equivalent to uninfected quolls, and quoll populations have continued to decline in the post-drought period (B. Fancourt, unpublished data), this scenario is unlikely.
Notwithstanding the apparent inability for T. gondii to affect eastern quoll survival, such a highly prevalent infection can often have the greatest impact on a host population through reduced fecundity (McCallum, 1994). However, the mean number of pouch young produced by seropositive mothers was 50% higher than by uninfected mothers. While we were unable to assess the relative fitness of these pouch young, we found no evidence that T. gondii negatively affected the number of offspring produced. All the seropositive mothers captured in July came from the declining sites, so the higher reproductive output may be a function of reduced population densities and reduced competition for resources at these sites, with more nutrition available for investment in offspring. However, female body condition was actually lower at the declining sites, suggesting that the number of offspring was not driven by more favourable resource levels. Alternatively, seropositive mothers might give birth to more sons, as observed in mice (Kaňková et al., 2007a) and humans (Kaňková et al., 2007b). Such mechanisms would result in a loss of reproductive capacity as fewer females are born over successive generations. However, we were not able to test this hypothesis due to the low number of seropositive mothers captured in July and the inability to sex pouch young at this immature stage of development. No evidence was found for T. gondii infection having adverse effects on male reproduction, with mean testicular volume and body condition of seropositive males both higher than those of seronegative males. Better body condition and increased testicular volume in infected males may allow them to out-compete their uninfected cohorts for mates, however, the evolutionary mechanisms driving such differences are currently not understood and warrant further investigation.
While the combined weight of evidence suggests that T. gondii infection is not contributing to population declines in the eastern quoll, the highly significant difference in seroprevalence between the declining sites and the non-declining site cannot be ignored. Higher seroprevalence indicates higher levels of T. gondii contamination in the environment at the declining sites. Under cool, moist environmental conditions, oocysts are known to be infective for at least 18 months (Yilmaz and Hopkins, 1972; Frenkel et al., 1975). However, the similar climatic conditions at both the declining and non-declining sites suggest similar oocyst persistence in the environment at these sites. A lower number of oocysts at the non-declining site, therefore, would suggest either a lower prevalence of T. gondii in cats, or lower cat activity.
The high seroprevalence detected in cats across Bruny Island indicates that T. gondii oocysts would be prevalent in environments occupied by those cats. Therefore, the low observed prevalence in eastern quolls suggests low cat activity locally at the study site. Camera surveys confirmed that cat activity at the Bruny Island site was lower than at the declining sites. The occurrence and continued prevalence of T. gondii is usually dependent on the presence of cats, and prevalence is generally higher where cats are present than where they are absent (Wallace, 1969; Frenkel, 1974; Wallace, 1976), even though transmission of cysts between intermediate hosts is possible (Tenter et al., 2000). The higher exposure to feral cats at the declining sites that is indicated by high prevalence of T. gondii infection suggests that feral cats may be contributing to suppression of quoll populations at these sites, through non-T. gondii related mechanisms such as predation, competition or exclusion. Future experimental manipulation of cat and quoll populations could enable evaluation of the relative impact that each of these mechanisms may have on eastern quoll populations.
5. Conclusions
While individual clinical cases or deaths cannot be completely ruled out, the absence of any signs of clinical toxoplasmosis in either live or dead quolls is noteworthy. When combined with the high number of seropositive individuals persisting in the population and in the absence of adverse effects on either survival or fecundity, the weight of evidence from the current study suggests that T. gondii infection is nonpathogenic in eastern quolls. While further research into the relative pathogenicity of different transmission modes and T. gondii strains is required, the eastern quoll could be considered a sentinel species for the threat of toxoplasmosis in susceptible wildlife, livestock and humans. Further research investigating the impact of feral cats on eastern quoll populations through mechanisms such as predation, competition and exclusion is needed.
Acknowledgements
We are grateful to Ken Rowe, Arnie Woolley, Tasmanian Parks & Wildlife Service (Cradle Mountain-Lake St. Clair National Park) and Bruce and Lynne Michael (Murrayfield Bruny Island) for providing regular access to study sites over 4 years, and the 86 volunteers who kindly assisted with all aspects of field work. We also thank the following people: Bernard Edwards, PWS Bruny Island (collecting feral cat blood samples on Bruny Island); Pat Statham, Animal Health Laboratories (assistance with testing protocols, retesting samples); Bruce Jackson, DPIPWE (providing unpublished data on necropsied quolls); James Harris, Mayfair Veterinary Clinic (euthanasing and collecting samples from feral cats); Leon Barmuta and Shannon Troy (statistical assistance). Our thanks to Elissa Cameron, Scott Carver and two anonymous reviewers for providing comments on an earlier draft of the manuscript. Funding was provided by the Norman Wettenhall Foundation, Wildlife Disease Association-Australasian Section, Holsworth Wildlife Research Endowment, Foundation for National Parks & Wildlife, the Australian Research Council and the National Environmental Research Program. The funders had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. This research was carried out under the University of Tasmania Animal Ethics Permits #A11017 and #A11655 with permission from DPIPWE under scientific permits FA10042, FA11208, FA12048 and FA13060. The authors declare that no actual or potential conflicts of interests exist.
References
- Abbott I. Origin and spread of the cat, Felis catus, on mainland Australia, with a discussion of the magnitude of its early impact on native fauna. Wildl. Res. 2002;29:51–74. [Google Scholar]
- Abbott I. Mammalian faunal collapse in Western Australia, 1875–1925: the hypothesised role of epizootic disease and a conceptual model of its origin, introduction, transmission, and spread. Aust. Zool. 2006;33:530–561. [Google Scholar]
- Aramini J.J., Stephen C., Dubey J.P., Engelstoft C., Schwantje H., Ribble C.S. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiol. Infect. 1999;122:305–315. doi: 10.1017/s0950268899002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes T.P., Lopes W.D.Z., Ferreira R.M., Pieroni J.S.P., Pinto V.M.R., Sakamoto C.A., daCosta A.J. Toxoplasma gondii: evidence for the transmission by semen in dogs. Exp. Parasitol. 2009;123:190–194. doi: 10.1016/j.exppara.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Attwood H.D., Woolley P.A., Rickard M.D. Toxoplasmosis in dasyurid marsupials. J. Wildl. Dis. 1975;11:543–551. doi: 10.7589/0090-3558-11.4.543. [DOI] [PubMed] [Google Scholar]
- Bailey T.L., Hudson R.S., Powe T.A., Riddell M.G., Wolfe D.F., Carson R.L. Caliper and ultrasonographic measurements of bovine testicles and a mathematical formula for determining testicular volume and weight in vivo. Theriogenology. 1998;49:581–594. doi: 10.1016/s0093-691x(98)00009-0. [DOI] [PubMed] [Google Scholar]
- Bates, D., Maechler, M., Bolker, B., Walker, S., 2013. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0-5.
- Berdoy M., Webster J.P., MacDonald D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. B. 2000;267:1591–1594. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettiol, S.S., 2000. Diseases of the Eastern Barred Bandicoot (Perameles gunnii) with special reference to toxoplasmosis and the marsupial immune system. Ph.D. Thesis, University of Tasmania, Hobart.
- Bettiol S.S., Obendorf D.L., Nowarkowski M., Goldsmid J.M. Pathology of experimental toxoplasmosis in eastern barred bandicoots in Tasmania. J. Wildl. Dis. 2000;36:141–144. doi: 10.7589/0090-3558-36.1.141. [DOI] [PubMed] [Google Scholar]
- Beverley J.K.A. Congenital transmission of toxoplasmosis through successive generations of mice. Nature. 1959;183:1348–1349. doi: 10.1038/1831348a0. [DOI] [PubMed] [Google Scholar]
- Blackhall S. Diet of the Eastern Native-Cat, Dasyurus viverrinus (Shaw), in southern Tasmania. Aust. Wildl. Res. 1980;7:191–197. [Google Scholar]
- Blader I.J., Saeij J.P. Communication between Toxoplasma gondii and its host: Impact on parasite growth, development, immune evasion, and virulence. APMIS. 2009;117:458–476. doi: 10.1111/j.1600-0463.2009.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard T.W., Santiago N.T., Lipscomb T.P., Garber R.L., McFee W.E., Knowles S. Two novel alphaherpesviruses associated with fatal disseminated infections in Atlantic bottlenose dolphins. J. Wildl. Dis. 2001;37:297–305. doi: 10.7589/0090-3558-37.2.297. [DOI] [PubMed] [Google Scholar]
- Blaustein A.R., Gervasi S.S., Johnson P.T.J., Hoverman J.T., Belden L.K., Bradley P.W., Xie G.Y. Ecophysiology meets conservation: understanding the role of disease in amphibian population declines. Philos. Trans. R. Soc. B. 2012;367:1688–1707. doi: 10.1098/rstb.2012.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie W.R., King A.S., Werker D.H., Isaac-Renton J.L., Bell A., Eng S.B., Marion S.A. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997;350:173–177. doi: 10.1016/s0140-6736(96)11105-3. [DOI] [PubMed] [Google Scholar]
- Brown L.D., Cai T.T., DasGupta A. Interval estimation for a binomial proportion. Stat. Sci. 2001;16:101–117. [Google Scholar]
- Bryant S.L. Seasonal variation of plasma testosterone in a wild population of male eastern quoll, Dasyurus viverrinus (Marsupialia: Dasyuridae), from Tasmania. Gen. Comp. Endocrinol. 1986;64:75–79. doi: 10.1016/0016-6480(86)90030-4. [DOI] [PubMed] [Google Scholar]
- Burns R., Williams E.S., O’Toole D., Dubey J.P. Toxoplasma gondii infections in captive black-footed ferrets (Mustela nigripes), 1992–1998: clinical signs, serology, pathology, and prevention. J. Wildl. Dis. 2003;39:787–797. doi: 10.7589/0090-3558-39.4.787. [DOI] [PubMed] [Google Scholar]
- Cabello J., Altet L., Napolitano C., Sastre N., Hidalgo E., Dávila J.A., Millán J. Survey of infectious agents in the endangered Darwin’s fox (Lycalopex fulvipes): High prevalence and diversity of hemotrophic mycoplasmas. Vet. Microbiol. 2013;167:448–454. doi: 10.1016/j.vetmic.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Canfield P.J., Hartley W.J., Dubey J.P. Lesions of toxoplasmosis in Australian marsupials. J. Comp. Pathol. 1990;103:159–166. doi: 10.1016/s0021-9975(08)80172-7. [DOI] [PubMed] [Google Scholar]
- Canfield P.J., Cunningham A.A. Disease and mortality in Australasian marsupials held at London Zoo, 1872–1972. J. Zoo Wildl. Med. 1993;24:158–167. [Google Scholar]
- Carruthers V.B., Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr. Bull. 2007;33:745–751. doi: 10.1093/schbul/sbm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughley G. Directions in conservation biology. J. Anim. Ecol. 1994;63:215–244. [Google Scholar]
- Chadwick E., Cable J., Chinchen A., Francis J., Guy E., Kean E., Paul S., Perkins S., Sherrard-Smith E., Wilkinson C., Forman D. Seroprevalence of Toxoplasma gondii in the Eurasian otter (Lutra lutra) in England and Wales. Parasites Vectors. 2013;6:75. doi: 10.1186/1756-3305-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross J. Charles Sturt University; Riverina: 1990. The Feral Cat – Justification for its Control. [Google Scholar]
- Cross T.A., Arsnoe D., Minnis R., King D., Swafford S., Pedersen K., Owen J. Prevalence of avian paramyxovirus 1 and avian influenza virus in double-crested cormorants (Phalacrocorax auritus) in eastern North America. J. Wildl. Dis. 2013;49:965–977. doi: 10.7589/2012-06-164. [DOI] [PubMed] [Google Scholar]
- Cunningham A.A., Daszak P. Extinction of a species of land snail due to infection with a microsporidian parasite. Conserv. Biol. 1998;12:1139–1141. [Google Scholar]
- Daszak P., Berger L., Cunningham A., Hyatt A., Green D., Speare R. Emerging infectious diseases and amphibian population declines. Emerg. Infect. Dis. 1999;5:735–748. doi: 10.3201/eid0506.990601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak P., Cunningham A.A., Hyatt A.D. Emerging infectious diseases of wildlife – threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- Davey C., Sinclair A.R.E., Pech R.P., Arthur A.D., Krebs C.J., Newsome A.E., Hik D., Molsher R., Allcock K. Do exotic vertebrates structure the biota of Australia? An experimental test in New South Wales. Ecosystems. 2006;9:992–1008. [Google Scholar]
- De Castro F., Bolker B. Mechanisms of disease-induced extinction. Ecol. Lett. 2005;8:117–126. [Google Scholar]
- de Moraes É.P.B.X., Batista A.M., Faria E.B., Freire R.L., Freitas A.C., Silva M.A.R., Braga V.A., Mota R.A. Experimental infection by Toxoplasma gondii using contaminated semen containing different doses of tachyzoites in sheep. Vet. Parasitol. 2010;170:318–322. doi: 10.1016/j.vetpar.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Desmonts G., Couvreur J. Congenital Toxoplasmosis. N. Engl. J. Med. 1974;290:1110–1116. doi: 10.1056/NEJM197405162902003. [DOI] [PubMed] [Google Scholar]
- Desmonts G., Remington J.S. Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J. Clin. Microbiol. 1980;11:562–568. doi: 10.1128/jcm.11.6.562-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Miller N.L., Frenkel J.K. The Toxoplasma gondii oocyst from cat faeces. J. Exp. Med. 1970;132:636–662. doi: 10.1084/jem.132.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Frenkel J.K. Cyst-induced toxoplasmosis in cats. J. Protozool. 1972;19:155–177. doi: 10.1111/j.1550-7408.1972.tb03431.x. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Frenkel J.K. Feline toxoplasmosis from acutely infected mice and the development of toxoplasma cysts. J. Protozool. 1976;23:537–546. doi: 10.1111/j.1550-7408.1976.tb03836.x. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Desmonts G. Serological responses of equids fed Toxoplasma gondii oocysts. Equine Vet. J. 1987;19:337–339. doi: 10.1111/j.2042-3306.1987.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Long-term persistence of Toxoplasma gondii in tissues of pigs inoculated with T. gondii oocysts and effect of freezing on viability of tissue cysts in pork. Am. J. Vet. Res. 1988;49:910–913. [PubMed] [Google Scholar]
- Dubey J.P., Ott-Joslin J., Torgerson R.W., Topper M.J., Sundberg J.P. Toxoplasmosis in black-faced kangaroos (Macropus fuliginosus melanops) Vet. Parasitol. 1988;30:97–105. doi: 10.1016/0304-4017(88)90156-2. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. Toxoplasmosis – a waterborne zoonosis. Vet. Parasitol. 2004;126:57–72. doi: 10.1016/j.vetpar.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Navarro I.T., Sreekumar C., Dahl E., Freire R.L., Kawabata H.H., Vianna M.C.B., Kwok O.C.H., Shen S.K., Thulliez P., Lehmann T. Toxoplasma gondii infections in cats from Paraná, Brazil: seroprevalence, tissue distribution, and biologic and genetic characterization of isolates. J. Parasitol. 2004;90:721–726. doi: 10.1645/GE-382R. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. CRC Press; Boca Raton: 2010. Toxoplasmosis of Animals and Humans. [Google Scholar]
- Dubey J.P., Hill D., Zarlenga D., Choudhary S., Ferreira L.R., Oliveira S., Verma S.K., Kwok O.C.H., Driscoll C.P., Spiker H., Su C. Isolation and characterization of new genetic types of Toxoplasma gondii and prevalence of Trichinella murrelli from black bear (Ursus americanus) Vet. Parasitol. 2013;196:24–30. doi: 10.1016/j.vetpar.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Ekanayake D.K., Rajapakse R.P.V.J., Dubey J.P., Dittus W.P.J. Seroprevalence of Toxoplasma gondii in wild toque macaques (Macaca sinica) at Polonnaruwa, Sri Lanka. J. Parasitol. 2004;90:870–871. doi: 10.1645/GE-291R. [DOI] [PubMed] [Google Scholar]
- Eleni C., De Liberato C., Azam D., Morgan E.R., Traversa D. Angiostrongylus vasorum in wolves in Italy. Int. J. Parasitol. 2014;3:12–14. doi: 10.1016/j.ijppaw.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eymann J., Herbert C.A., Cooper D.W., Dubey J.P. Serologic survey for Toxoplasma gondii and Neospora caninum in the common brushtail possum (Trichosurus vulpecula) from urban Sydney, Australia. J. Parasitol. 2006;92:267–272. doi: 10.1645/GE-709R.1. [DOI] [PubMed] [Google Scholar]
- Fancourt, B.A., 2010. Spatial and Temporal Variation in Declining Eastern Quoll (Dasyurus viverrinus) Populations in Tasmania. B.Sc.(Hons) Thesis, University of Tasmania, Hobart, School of Zoology.
- Fancourt B.A., Nicol S.C., Hawkins C.E. Evidence of rapid population decline of the eastern quoll (Dasyurus viverrinus) in Tasmania. Aust. Mammal. 2013;35:195–205. [Google Scholar]
- Fancourt, B.A., Jackson, R.B., In review. Regional seroprevalence of Toxoplasma gondii antibodies in feral and stray cats (Felis catus) from Tasmania. Aust. J. Zool.
- Freeland W.J. Parasites, pathogens and the impacts of introduced organisms on the balance of nature in Australia. In: Moritz C., Kikkawa J., editors. Conservation Biology in Australia and Oceania. Surrey Beatty and Sons; Chipping Norton, NSW: 1993. pp. 171–180. [Google Scholar]
- Frenkel J.K., Dubey J.P., Miller N.L. Toxoplasma gondii in cats: fecal stages identified as coccidian oocysts. Science. 1970;167:893–896. doi: 10.1126/science.167.3919.893. [DOI] [PubMed] [Google Scholar]
- Frenkel J.K. Toxoplasmosis: parasite life cycle, pathology and immunology. In: Hammond D.A., Long P.L., editors. The Coccidia. Baltimore University Park Press; Baltimore: 1973. pp. 343–410. [Google Scholar]
- Frenkel J.K. Breaking the transmission chain of Toxoplasma: a program for the prevention of human toxoplasmosis. Bull. N. Y. Acad. Med. 1974;50:228–235. [PMC free article] [PubMed] [Google Scholar]
- Frenkel J.K., Ruiz A., Chinchilla M. Soil survival of Toxoplasma oocysts in Kansas and Costa Rica. Am. J. Trop. Med. Hyg. 1975;24:439–443. doi: 10.4269/ajtmh.1975.24.439. [DOI] [PubMed] [Google Scholar]
- Gauthier-Clerc M., Eterradossi N., Toquin D., Guittet M., Kuntz G., Le Maho Y. Serological survey of the king penguin, Aptenodytes patagonicus, in Crozet Archipelago for antibodies to infectious bursal disease, influenza A and Newcastle disease viruses. Polar Biol. 2002;25:316–319. [Google Scholar]
- Godsell J. The population ecology of the Eastern Quoll Dasyurus viverrinus (Dasyuridae, Marsupialia), in southern Tasmania. In: Archer M., editor. Carnivorous Marsupials. Royal Zoological Society of New South Wales; Sydney: 1982. pp. 199–207. [Google Scholar]
- Godsell, J., 1983. Ecology of the Eastern Quoll, Dasyurus viverrinus (Dasyuridae: Marsupialia). Ph.D. Thesis, Australian National University, Canberra.
- Green R.H. Notes on the devil (Sarcophilus harrisi) and the quoll (Dasyurus viverrinus) in north-eastern Tasmania. Rec. Queen Victoria Mus. 1967;27:1–13. [Google Scholar]
- Guiler E.R. The former distribution and decline of the thylacine. Aust. J. Sci. 1961;23:207–210. [Google Scholar]
- Haigh, S.A., Gaynor, W.T., Morris, K.D., 1994. A health monitoring program for captive, wild and translocated chuditch (Dasyurus geoffroii). Proceedings of the 1994 Conference of the Australian Association of Veterinary Conservation Biologists, pp. 52–66.
- Hartley W.J., Dubey J.P., Spielman D.S. Fatal toxoplasmosis in koalas (Phascolarctos cinereus) J. Parasitol. 1990;76:271–272. [PubMed] [Google Scholar]
- Hawkins C.E., Baars C., Hesterman H., Hocking G.J., Jones M.E., Lazenby B., Mann D., Mooney N., Pemberton D., Pyecroft S., Restani M., Wiersma J. Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biol. Conserv. 2006;131:307–324. [Google Scholar]
- Hay J., Aitken P., Hair D., Hutchison W., Graham D. The effect of congenital Toxoplasma infection on mouse activity and relative preference for exposed areas over a series of trials. Ann. Trop. Med. Parasitol. 1984;78:611–618. doi: 10.1080/00034983.1984.11811872. [DOI] [PubMed] [Google Scholar]
- Hill D., Dubey J. Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002;8:634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- Hill D.E., Chirukandoth S., Dubey J.P. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 2005;6:41–61. doi: 10.1079/ahr2005100. [DOI] [PubMed] [Google Scholar]
- Hollings T., Jones M., Mooney N., McCallum H. Wildlife disease ecology in changing landscapes: mesopredator release and toxoplasmosis. Int. J. Parasitol. 2013;2:110–118. doi: 10.1016/j.ijppaw.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe L., Hunter S., Burrows E., Roe W. Four cases of fatal toxoplasmosis in three species of endemic New Zealand birds. Avian Dis. 2014;58:171–175. doi: 10.1637/10625-080413-Case.1. [DOI] [PubMed] [Google Scholar]
- Huijbregts B., De Wachter P., Sosthene L., Obiang N., Akou M.E. Ebola and the decline of gorilla Gorilla gorilla and chimpanzee Pan troglodytes populations in Minkebe Forest, north-eastern Gabon. Oryx. 2003;37:437–443. [Google Scholar]
- Hutchison W.M. Experimental transmission of Toxoplasma gondii. Nature. 1965;206:961–962. doi: 10.1038/206961a0. [DOI] [PubMed] [Google Scholar]
- Innes E.A. Toxoplasmosis: Comparative species susceptibility and host immune response. Comp. Immunol. Microbiol. Infect. Dis. 1997;20:131–138. doi: 10.1016/s0147-9571(96)00038-0. [DOI] [PubMed] [Google Scholar]
- Jokelainen P., Nylund M. Acute fatal toxoplasmosis in three Eurasian red squirrels (Sciurus vulgaris) caused by genotype II of Toxoplasma gondii. J. Wildl. Dis. 2012;48:454–457. doi: 10.7589/0090-3558-48.2.454. [DOI] [PubMed] [Google Scholar]
- Jones M.E., Cockburn A., Hamede R., Hawkins C., Hesterman H., Lachish S., Mann D., McCallum H., Pemberton D. Life-history change in disease-ravaged Tasmanian devil populations. Proc. Nat. Acad. Sci. U.S.A. 2008;105:10023–10027. doi: 10.1073/pnas.0711236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaňková Š., Kodym P., Frynta D., Vavřinová R., Kuběna A., Flegr J. Influence of latent toxoplasmosis on the secondary sex ratio in mice. Parasitology. 2007;134:1709–1717. doi: 10.1017/S0031182007003253. [DOI] [PubMed] [Google Scholar]
- Kaňková Š., Šulc J., Nouzová K., Fajfrlík K., Frynta D., Flegr J. Women infected with parasite Toxoplasma have more sons. Naturwissenschaften. 2007;94:122–127. doi: 10.1007/s00114-006-0166-2. [DOI] [PubMed] [Google Scholar]
- Kotula A.W., Dubey J.P., Sharar A.K., Andrews C.D., Shen S.K., Lindsay D.S. Effect of freezing on infectivity of Toxoplasma gondii tissue cysts in pork. J. Food Prot. 1991;54:687–690. doi: 10.4315/0362-028X-54.9.687. [DOI] [PubMed] [Google Scholar]
- Krebs C., Singleton G. Indexes of Condition for Small Mammals. Aust. J. Zool. 1993;41:317–323. [Google Scholar]
- Lafferty K.D., Gerber L.R. Good medicine for conservation biology: the intersection of epidemiology and conservation theory. Conserv. Biol. 2002;16:593–604. [Google Scholar]
- Leroy E.M., Rouquet P., Formenty P., Souquiere S., Kilbourne A., Froment J.-M., Bermejo M., Smit S., Karesh W., Swanepoel R., Zaki S.R., Rollin P.E. Multiple Ebola virus transmission events and rapid decline of Central African wildlife. Science. 2004;303:387–390. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- Luft B.J., Brooks R.G., Conley F.K., McCabe R.E., Remington J.S. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. J. Am. Med. Assoc. 1984;252:913–917. [PubMed] [Google Scholar]
- Lukešová D., Literák I. Shedding of Toxoplasma gondii oocysts by Felidae in zoos in the Czech Republic. Vet. Parasitol. 1998;74:1–7. doi: 10.1016/s0304-4017(97)00155-6. [DOI] [PubMed] [Google Scholar]
- McCallum H. Quantifying the impact of disease on threatened species. Pac. Conserv. Biol. 1994;1:107–117. [Google Scholar]
- McCallum H., Dobson A. Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol. Evol. 1995;10:190–194. doi: 10.1016/s0169-5347(00)89050-3. [DOI] [PubMed] [Google Scholar]
- McKnight, M., 2008. Dasyurus viverrinus, IUCN 2013. IUCN Red List of Threatened Species. Version 2013.2.
- Miller N.L., Frenkel J.K., Dubey J.P. Oral infections with Toxoplasma cysts and oocysts in felines, other mammals and in birds. J. Parasitol. 1972;58:928–937. [PubMed] [Google Scholar]
- Munday B.L. A serological study of some infectious diseases of Tasmanian wildlife. J. Wildl. Dis. 1972;8:169–175. doi: 10.7589/0090-3558-8.2.169. [DOI] [PubMed] [Google Scholar]
- Muths E., Stephen Corn P., Pessier A.P., Earl Green D. Evidence for disease-related amphibian decline in Colorado. Biol. Conserv. 2003;110:357–365. [Google Scholar]
- Obendorf D., Statham P., Driessen M. Detection of agglutinating antibodies to Toxoplasma gondii in sera from free-ranging eastern barred bandicoots (Perameles gunnii) J. Wildl. Dis. 1996;32:623–626. doi: 10.7589/0090-3558-32.4.623. [DOI] [PubMed] [Google Scholar]
- Obendorf D.L., Munday B.L. Toxoplasmosis in wild Tasmanian wallabies. Aust. Vet. J. 1983;60:62. doi: 10.1111/j.1751-0813.1983.tb05867.x. [DOI] [PubMed] [Google Scholar]
- Packer C., Altizer S., Appel M., Brown E., Martenson J., O’Brien S.J., Roelke-Parker M., Hofmann-Lehmann R., Lutz H. Viruses of the Serengeti: patterns of infection and mortality in African lions. J. Anim. Ecol. 1999;68:1161–1178. [Google Scholar]
- Pan S., Thompson R.A., Grigg M.E., Sundar N., Smith A., Lymbery A.J. Western Australian marsupials are multiply infected with genetically diverse strains of Toxoplasma gondii. PLoS One. 2012;7:e45147. doi: 10.1371/journal.pone.0045147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N., O’Handley R.M., Grigg M.E., Wayne A., Thompson R.C. Vertical transmission of Toxoplasma gondii in Australian marsupials. Parasitology. 2009;136:939–944. doi: 10.1017/S0031182009006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N., Thompson R.C.A., Sundar N., Pan S., Johnson M., Smith N.C., Grigg M.E. Non-archetypal Type II-like and atypical strains of Toxoplasma gondii infecting marsupials of Australia. Int. J. Parasitol. 2010;40:635–640. doi: 10.1016/j.ijpara.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock, D.E., Abbott, I., submitted for publication. When the “native cat” would “plague”: historical hyper-abundance in the quoll (Marsupialia: Dasyuridae) and the role of disease, cats and foxes in its curtailment. Aust. J. Zool.
- Pedersen A.B., Greives T.J. The interaction of parasites and resources cause crashes in a wild mouse population. J. Anim. Ecol. 2008;77:370–377. doi: 10.1111/j.1365-2656.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- Pereira-Bueno J., Quintanilla-Gozalo A., Pérez-Pérez V., Álvarez-García G, Collantes-Fernández E., Ortega-Mora L.M. Evaluation of ovine abortion associated with Toxoplasma gondii in Spain by different diagnostic techniques. Vet. Parasitol. 2004;121:33–43. doi: 10.1016/j.vetpar.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Phillips, A., 2009. Helminth parasites and Toxoplasma gondii in Tasmanian Devils (Sarcophilus harrisii). University of Sydney.
- Power V., Lambert C., Matson P. Reproduction of the numbat (Myrmecobius fasciatus): observations from a captive breeding program. Aust. Mammal. 2009;31:25–30. [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2013. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Recher H., Hutchings P., Rosen S. The biota of the Hawkesbury-Nepean catchment: reconstruction and restoration. Aust. Zool. 1993;29:3–41. [Google Scholar]
- Reddacliff G.L., Hartley W.J., Dubey J.P., Cooper D.W. Pathology of experimentally-induced, acute toxoplasmosis in macropods. Aust. Vet. J. 1993;70:4–6. doi: 10.1111/j.1751-0813.1993.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Remington J.S., Thulliez P., Montoya J.G. Recent developments for diagnosis of toxoplasmosis. J. Clin. Microbiol. 2004;42:941–945. doi: 10.1128/JCM.42.3.941-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyan J.C., Spraker T.R. Emergence of diseases from wildlife reservoirs. Vet. Pathol. Online. 2010;47:34–39. doi: 10.1177/0300985809354466. [DOI] [PubMed] [Google Scholar]
- Saitoh Y., Itagaki H. Dung beetles Onthophagus spp. as potential transport hosts of feline coccidia. Jpn. J. Vet. Sci. 1990;52:293–297. doi: 10.1292/jvms1939.52.293. [DOI] [PubMed] [Google Scholar]
- Santana L.F., Rossi G.A.M., Gaspar R.C., Pinto V.M.R., Oliveira G.P.D., Costa A.J.D. Evidence of sexual transmission of Toxoplasma gondii in goats. Small Ruminant Res. 2013;115:130–133. [Google Scholar]
- Satō K., Matsuda H., Sasaki A. Pathogen invasion and host extinction in lattice structured populations. J. Math. Biol. 1994;32:251–268. doi: 10.1007/BF00163881. [DOI] [PubMed] [Google Scholar]
- Scott M.E. The impact of infection and disease on animal populations: implications for conservation biology. Conserv. Biol. 1988;2:40–56. [Google Scholar]
- Seddon H. A.H. Pettifer, Government Printer; Sydney: 1952. Diseases in Domestic Animals in Australia. Part 4: Protozoan and Viral Diseases. [Google Scholar]
- Skerratt L.F., Phelan J., McFarlane R., Speare R. Serodiagnosis of toxoplasmosis in a common wombat. J. Wildl. Dis. 1997;33:346–351. doi: 10.7589/0090-3558-33.2.346. [DOI] [PubMed] [Google Scholar]
- Sleeman J.M., Manning E.J.B., Rohm J.H., Sims J.P., Sanchez S., Gerhold R.W., Keel M.K. Johne’s disease in a free-ranging white-tailed deer from Virginia and subsequent surveillance for Mycobacterium avium subspecies paratuberculosis. J. Wildl. Dis. 2009;45:201–206. doi: 10.7589/0090-3558-45.1.201. [DOI] [PubMed] [Google Scholar]
- Smith A., Clark P., Averis S., Lymbery A.J., Wayne A.F., Morris K.D., Thompson R.C.A. Trypanosomes in a declining species of threatened Australian marsupial, the brush-tailed bettong Bettongia penicillata (Marsupialia: Potoroidae) Parasitology. 2008;135:1329–1335. doi: 10.1017/S0031182008004824. [DOI] [PubMed] [Google Scholar]
- Smith K.F., Sax D.F., Lafferty K.D. Evidence for the role of infectious disease in species extinction and endangerment. Conserv. Biol. 2006;20:1349–1357. doi: 10.1111/j.1523-1739.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- Szabo K.A., Mense M.G., Lipscomb T.P., Felix K.J., Dubey J.P. Fatal toxoplasmosis in a bald eagle (Haliaeetus leucocephalus) J. Parasitol. 2004;90:907–908. doi: 10.1645/GE-270R. [DOI] [PubMed] [Google Scholar]
- Tasmanian Planning Commission . Tasmanian Planning Commission; Hobart, Tasmania: 2009. State of the Environment Tasmania 2009. [Google Scholar]
- Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne E.T., Williams E.S. Disease and endangered species: the black-footed ferret as a recent example. Conserv. Biol. 1988;2:66–74. [Google Scholar]
- van Riper C., III, van Riper S.G., Goff M.L., Laird M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol. Monogr. 1986;56:327–344. [Google Scholar]
- Vyas A., Kim S.K., Giacomini N., Boothroyd J.C., Sapolsky R.M. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc. Nat. Acad. Sci. U.S.A. 2007;104:6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace G.D. Serologic and epidemiologic observations on toxoplasmosis on three Pacific atolls. Am. J. Epidemiol. 1969;90:103–111. doi: 10.1093/oxfordjournals.aje.a121054. [DOI] [PubMed] [Google Scholar]
- Wallace G.D. Experimental transmission of Toxoplasma gondii by filth-flies. Am. J. Trop. Med. Hyg. 1971;20:411–413. doi: 10.4269/ajtmh.1971.20.411. [DOI] [PubMed] [Google Scholar]
- Wallace G.D. Experimental transmission of Toxoplasma gondii by cockroaches. J. Infect. Dis. 1972;126:545–547. doi: 10.1093/infdis/126.5.545. [DOI] [PubMed] [Google Scholar]
- Wallace G.D. The prevalence of toxoplasmosis on Pacific Islands, and the influence of ethnic group. Am. J. Trop. Med. Hyg. 1976;25:48–53. doi: 10.4269/ajtmh.1976.25.48. [DOI] [PubMed] [Google Scholar]
- Walsh P.D., Abernethy K.A., Bermejo M., Beyers R., De Wachter P., Akou M.E., Huijbregts B., Mambounga D.I., Toham A.K., Kilbourn A.M., Lahm S.A., Latour S., Maisels F., Mbina C., Mihindou Y., Ndong Obiang S., Effa E.N., Starkey M.P., Telfer P., Thibault M., Tutin C.E.G., White L.J.T., Wilkie D.S. Catastrophic ape decline in western equatorial Africa. Nature. 2003;422:611–614. doi: 10.1038/nature01566. [DOI] [PubMed] [Google Scholar]
- Webster J.P., Brunton C.F.A., Macdonald D.W. Effect of Toxoplasma gondii upon neophobic behaviour in wild brown rats, Rattus norvegicus. Parasitology. 1994;109:37–43. doi: 10.1017/s003118200007774x. [DOI] [PubMed] [Google Scholar]
- Wendte J.M., Gibson A.K., Grigg M.E. Population genetics of Toxoplasma gondii: new perspectives from parasite genotypes in wildlife. Vet. Parasitol. 2011;182:96–111. doi: 10.1016/j.vetpar.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendum D., Carbonell N., Svrcek M., Chazouillères O., Flejou J. Fatal disseminated toxoplasmosis in a toxoplasma seropositive liver transplant recipient. J. Clin. Pathol. 2002;55:637. doi: 10.1136/jcp.55.8.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcove D.S., Rothstein D., Dubow J., Phillips A., Losos E. Quantifying threats to imperiled species in the United States. Bioscience. 1998;48:607–615. [Google Scholar]
- Wood Jones F. Government printer; Adelaide: 1923. The Mammals of South Australia – Part I. [Google Scholar]
- Work T., Massey J., Rideout B., Gardiner C., Ledig D., Kwok O., Dubey J. Fatal toxoplasmosis in free-ranging endangered ‘Alala from Hawaii. J. Wildl. Dis. 2000;36:205–212. doi: 10.7589/0090-3558-36.2.205. [DOI] [PubMed] [Google Scholar]
- Wyatt K.B., Campos P.F., Gilbert M.T.P., Kolokotronis S.-O., Hynes W.H., DeSalle R., Daszak P., MacPhee R.D.E., Greenwood A.D. Historical mammal extinction on Christmas Island (Indian Ocean) correlates with introduced infectious disease. PLoS One. 2008;3:1–9. doi: 10.1371/journal.pone.0003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz S.M., Hopkins S.H. Effects of different conditions on duration of infectivity of Toxoplasma gondii oocysts. J. Parasitol. 1972;58:938–939. [PubMed] [Google Scholar]


