Abstract
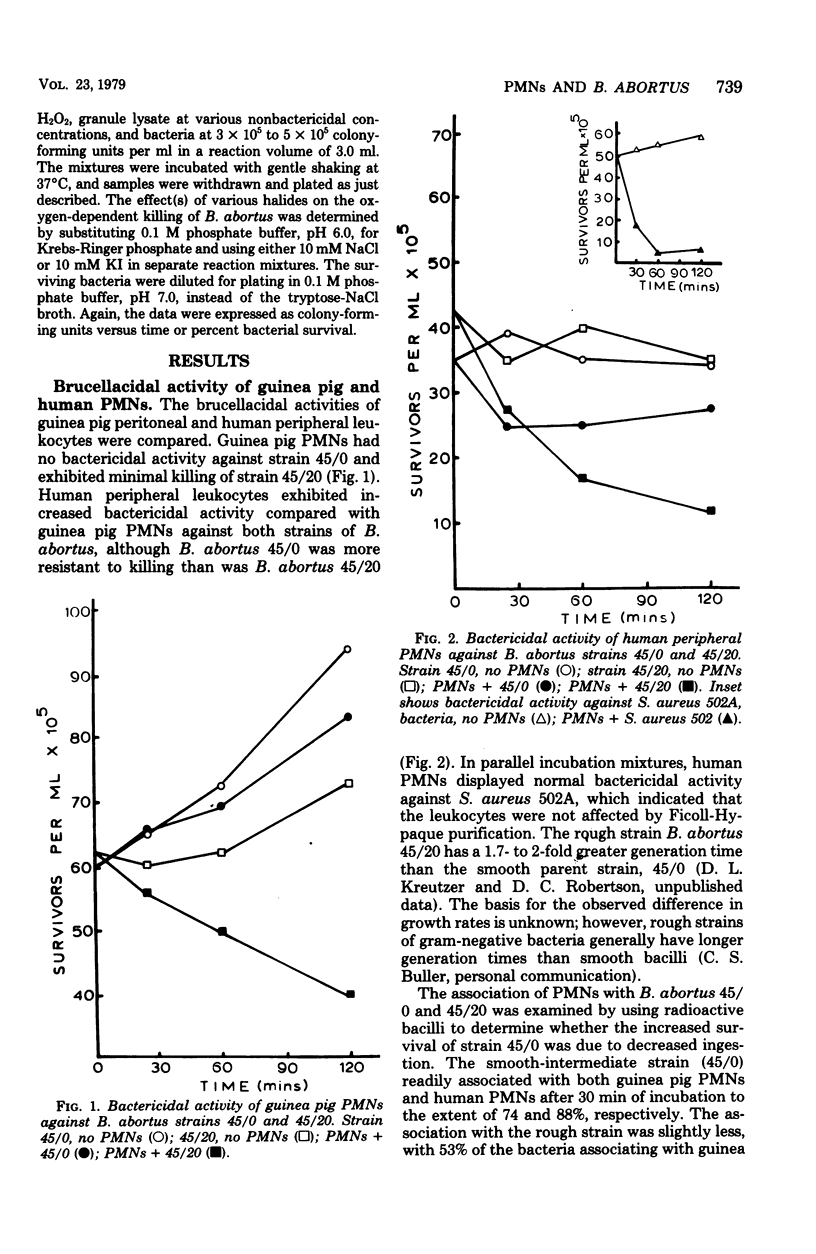
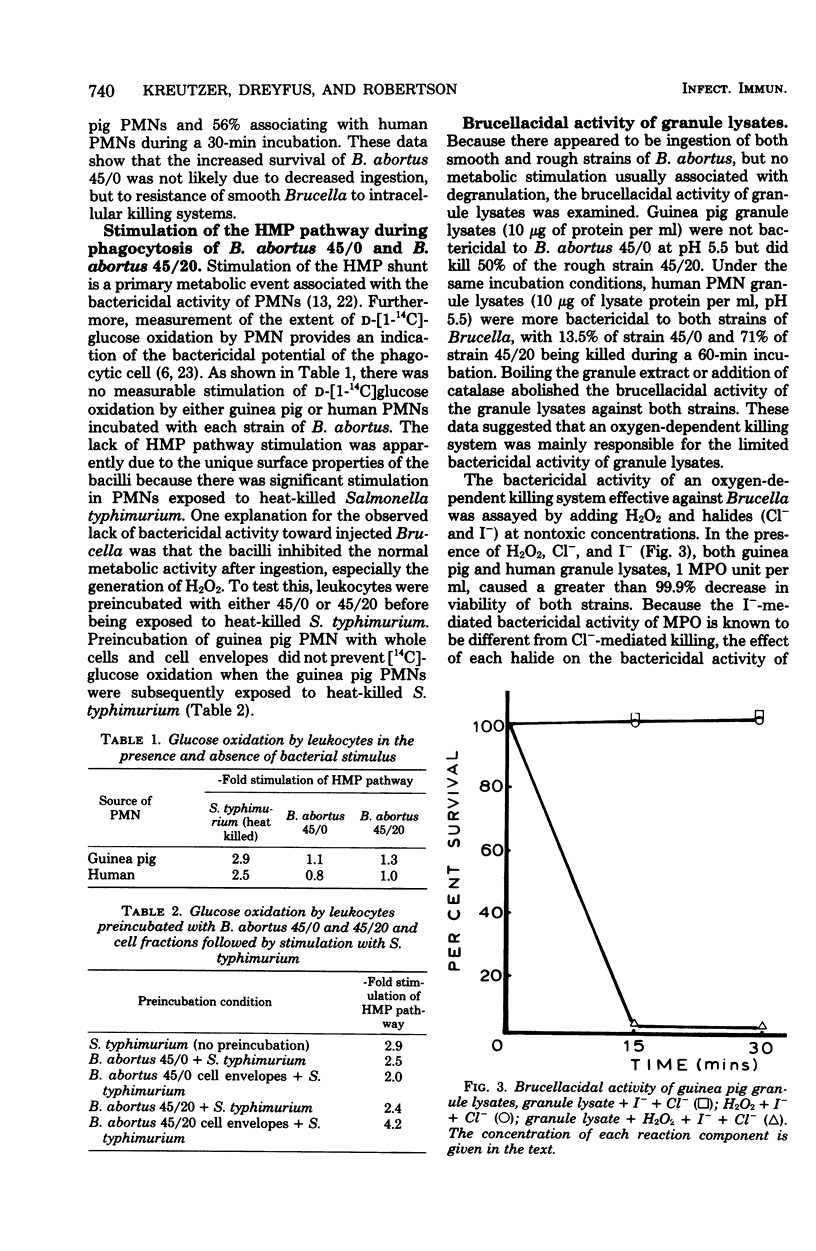
The bactericidal activity of guinea pig and human polymorphonuclear leukocytes (PMNs) against a smooth-intermediate strain (45/0) and a rough strain (45/20) of Brucella abortus has been examined. After incubation for 120 min, guinea pig PMNs incubated with either the smooth strain 45/0 or the rough strain 45/20 exhibited no bactericidal activity against the former and caused only a 34% decrease in viability of the latter. Human PMNs were more bactericidal than guinea pig PMNs to both strains; however, the killing of strain 45/20 by human PMNs was less than that observed in control experiments with S. aureus strain 502A. Both strains of B. abortus readily associated with guinea pig and human PMNs, and the bacteria were apparently ingested without stimulation of the hexose monophosphate pathway. Lysates (10 micrograms/ml, pH 5.5), prepared from guinea pig or human granules, were not particularly toxic to either strain unless supplemented with H2O2 and a halide (I- or Cl-). An oxygen-dependent killing system appeared to be lethal against both strains of B. abortus, with I- being more active than Cl- in the presence of H2O2 and granule lysate. The data suggest that degranulation after ingestion of Brucella by phagocytes does not occur due to the lack of a proper stimulus or possibly the baccilli actively inhibit the degranulation process thereby protecting the microbe from killing systems normally effective against extracellular parasites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUN W., POMALES-LEBRON A., STINEBRING W. R. Interactions between mononuclear phagocytes and Brucella abortus strains of different virulence. Proc Soc Exp Biol Med. 1958 Feb;97(2):393–397. doi: 10.3181/00379727-97-23752. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The isolation and properties of the specific cytoplasmic granules of rabbit polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:983–1004. doi: 10.1084/jem.112.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R. Oxidative bactericidal mechanisms of polymorphonuclear leukocytes. J Infect Dis. 1975 Mar;131(3):295–303. doi: 10.1093/infdis/131.3.295. [DOI] [PubMed] [Google Scholar]
- FREEMAN B. A., VANA L. R. Host-parasite relationships in brucellosis. I. Infection of normal guinea pig macrophages in tissue culture. J Infect Dis. 1958 May-Jun;102(3):258–267. doi: 10.1093/infdis/102.3.258. [DOI] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B. Phagocyte lysosomes: interactions with infectious agents, phagosomes, and experimental perturbations in function. Annu Rev Microbiol. 1977;31:507–533. doi: 10.1146/annurev.mi.31.100177.002451. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., PICKETT M. J. A cellular basis of immunity in experimental Brucella infection. J Exp Med. 1958 Sep 1;108(3):343–360. doi: 10.1084/jem.108.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A. Strain virulence and the lysosomal response in macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1974 Oct;10(4):742–746. doi: 10.1128/iai.10.4.742-746.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- Kreutzer D. L., Buller C. S., Robertson D. C. Chemical characterization and biological properties of lipopolysaccharides isolated from smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):811–818. doi: 10.1128/iai.23.3.811-818.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., Robertson D. C. Surface macromolecules and virulence in intracellular parasitism: comparison of cell envelope components of smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):819–828. doi: 10.1128/iai.23.3.819-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie D. B., Jackett P. S., Ratcliffe N. A. Mycobacterium microti may protect itself from intracellular destruction by releasing cyclic AMP into phagosomes. Nature. 1975 Apr 17;254(5501):600–602. doi: 10.1038/254600a0. [DOI] [PubMed] [Google Scholar]
- MACRAE R. M., SMITH H. THE CHEMICAL BASIS OF THE VIRULENCE OF BRUCELLA ABORTUS. VI. STUDIES ON IMMUNITY AND INTRACELLULAR GROWTH. Br J Exp Pathol. 1964 Dec;45:595–603. [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Freeman B. A. Osmotically sensitive Brucella in infected normal and immune macrophages. Infect Immun. 1970 Feb;1(2):146–150. doi: 10.1128/iai.1.2.146-150.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Intracellular parasitism: life in an extreme environment. J Infect Dis. 1974 Sep;130(3):300–306. doi: 10.1093/infdis/130.3.300. [DOI] [PubMed] [Google Scholar]
- Renshaw H. W., Davis W. C., Fudenberg H. H., Padgett G. A. Leukocyte dysfunction in the bovine homologue of the Chediak-Higashi syndrome of humans. Infect Immun. 1974 Oct;10(4):928–937. doi: 10.1128/iai.10.4.928-937.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SMITH H., FITZGEORGE R. B. THE CHEMICAL BASIS OF THE VIRULENCE OF BRUCELLA ABORTUS. V. THE BASIS OF INTRACELLULAR SURVIVAL AND GROWTH IN BOVINE PHAGOCYTES. Br J Exp Pathol. 1964 Apr;45:174–186. [PMC free article] [PubMed] [Google Scholar]
- STINEBRING W. R., KESSEL R. Continuous growth of Brucella abortus in mononuclear phagocytes of rats and guinea pigs. Proc Soc Exp Biol Med. 1959 Jul;101(3):412–415. doi: 10.3181/00379727-101-24962. [DOI] [PubMed] [Google Scholar]
- Sbarra A. J., Paul B. B., Jacobs A. A., Strauss R. R., Mitchell G. W., Jr Biochemical aspects of phagocytic cells as related to bactericidal function. J Reticuloendothel Soc. 1972 May;11(5):492–502. [PubMed] [Google Scholar]
- Sperry J. F., Robertson D. C. Erythritol catabolism by Brucella abortus. J Bacteriol. 1975 Feb;121(2):619–630. doi: 10.1128/jb.121.2.619-630.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagesson C., Stendahl O. Influence of the cell surface lipopolysaccharide structure of Salmonella typhimurium on resistance to intracellular bactericidal systems. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Aug;81(4):473–480. doi: 10.1111/j.1699-0463.1973.tb02232.x. [DOI] [PubMed] [Google Scholar]



