Abstract
Patients treated with whole-brain irradiation often develop cognitive deficits that are presumed to result from normal tissue injury. Age is a risk factor for these side effects. We compared the cognitive effects of fractionated whole-brain irradiation (300 kV X rays) in rats irradiated either as young adults or in middle age. A deficit in object memory was apparent at 3 months in rats irradiated as young adults, however, no comparable deficit was apparent in rats irradiated in middle age. In addition, the deficit in object memory in young adults was no longer apparent at 6 and 12 months after fractionated whole-brain irradiation and no radiation-induced deficit was detectable in a spatial memory task at any time, regardless of age at time of irradiation. Thus, clinically relevant fractionated whole-brain irradiation in adult rats resulted in early-delayed cognitive changes that were heterogeneous, transient and age-dependent. The results of the current and previous studies of radiation-induced cognitive changes support the continued investigation and validation of rodent models of radiation-induced brain injury, which are critical for developing and testing new therapies for treatment-induced cognitive dysfunction in cancer survivors.
INTRODUCTION
More than 1.6 million patients will be diagnosed with cancer this year. A small percentage of these patients will have primary brain tumors and 20–40% of the other patients will develop or are at high risk for brain metastases (1, 2). As a result, hundreds of thousands of patients will be treated with large-field partial or whole-brain irradiation (WBI). Cranial irradiation is efficacious, but dose is limited by damage to surrounding normal tissues and even with careful radiation treatment planning, many patients develop treatment-induced neural problems (3, 4). Radiation-induced neural dysfunction may include acute changes and “early-delayed” effects that occur within a few weeks or months and generally resolve spontaneously or with treatment, as well as “late-delayed” effects that are progressive and may lead to dementia (5–9). Age is a risk factor for the development and severity of cognitive dysfunction after WBI (6, 10–12), presumably because age influences the neurobiological response to radiation exposure. Understanding the neurobiological mechanisms of radiation-induced brain injury and developing therapeutic approaches is increasingly important as more patients survive long enough for cognitive dysfunction to develop and impact their quality of life (13).
Radiation-induced deficits in the clinic involve many cognitive domains [e.g., mood, memory, attention and executive function (6, 14–22)], suggesting that the normal tissue injury associated with these cognitive defects involves diverse neural regions and systems. There is significant variation among patients in the cognitive effects of cranial irradiation, and functional deficits can occur without degenerative changes detectable by standard imaging or with typical pathological evaluation (4, 23). Since the underlying neurobiological mechanisms remain unclear, experimental studies in animal models continue to be critically important. Although these preclinical studies of neurobiological effects of irradiation are most powerful in animals in which cognitive effects of irradiation are well characterized, the effects of clinically relevant brain irradiation on cognitive function in laboratory rodents remain poorly understood.
As part of our ongoing studies of the effects of brain irradiation in laboratory rodents, we exposed young adult and middle-aged rats to a clinically relevant regimen of fractionated whole-brain irradiation (fWBI) and evaluated their cognitive function at 3, 6 and 12 months after completion of irradiation. Two spontaneous object memory tasks with comparable sensory and motor demands were used. Novel object recognition (NOR) is a measure of declarative memory that is sensitive to damage to the perirhinal cortex (24, 25), and novel object location (NOL) recognition is a measure of spatial memory that is sensitive to damage to the hippocampus (26).
MATERIALS AND METHODS
Animal Husbandry and Irradiation
Details of animal husbandry, irradiation procedures and dosimetry have been published previously (27). In brief, male Fischer 344 × Brown Norway F1 hybrid rats (F×BN) were housed on a reverse light cycle (off 9:00 a.m. and on 9:00 p.m.) and irradiation/sham irradiation was begun when rats were 3 months of age (young adult) or 18 months of age (middle-aged) (N =24 irradiated and 24 sham-irradiated rats at each age). Irradiations were performed under anesthesia with ketamine/xylazine. Sham-irradiated (control) rats were anesthetized but not irradiated, to control for any possible effects of anesthesia on later cognitive performance. Irradiated rats received fWBI with a Precision X-ray X-RAD 320 orthovoltage X-ray unit (300 kV, 10 mA, 1.25 Gy/min). An 11 × 16 mm aperture was positioned laterally to permit irradiation of the brain from the apical pole of the cerebral cortex posteriorly though the cerebellum. A dose of 40 Gy fWBI was achieved by 2 fractions of 5 Gy each week for 4 weeks. Each 5 Gy fraction was given by consecutive doses of 2.5 Gy per lateral field, one from each side and separated by <10 min. The biologically effective dose (BED) calculated for 8 × 5 Gy is 106.7 Gy based on the linear quadratic model (28) and assuming an α/β ratio of 3 Gy for late delayed effects in the brain. Rats were maintained for 34 or 62 weeks after completion of fWBI. All young adult rats survived through completion of the study. Eight rats were lost from the middle-aged cohort (4 fWBI and 4 sham-irradiated control) prior to cognitive testing at 3 months after treatment (6 failed to recover from anesthesia and 2 were humanely euthanized for problems unrelated to irradiation), leaving N = 20 rats per treatment at the start of cognitive testing. An additional 2 sham-irradiated and 2 fWBI rats in the middle-aged cohort were euthanized (presumed aging-related morbidity) prior to cognitive testing at 6 months after treatment (leaving N =18 rats per treatment). Five middle-aged rats and 8 young adult rats from each treatment group (sham/fWBI) were euthanized and tissue was collected for a mid-study end point group after completion of the 6 month cognitive testing, leaving N = 16 young adult and N = 12 middle aged rats per treatment. An additional 3 sham-irradiated and 1 fWBI rat were euthanized (presumed aging-related morbidity) prior to cognitive testing at 12 months after treatment, leaving N= 9 sham-irradiated and N = 11 fWBI rats for testing at 12 months after treatment. Those rats were euthanized after testing for the final end point group and brain tissue was collected for later histological and biochemical analyses.
Cognitive Testing
Rats were tested for object recognition memory and spatial object location memory using the NOR and NOL, respectively, at 3, 6 and 12 months after completion of fWBI. The behavioral methods were similar to versions of the NOR and NOL reported in the literature but differed in important details, and these methods are described in detail in the supplementary material (http://dx.doi.org/10.1667/RR13362.1.S1) and summarized here. For the NOR task, rats explored two identical objects in a test arena for 6 or 8 min (sample) and then returned to the home cage for a 6-min (NOR 6) or 30-min (NOR 30) intertrial delay (ITD) period. After the delay, the rats were returned to the arena in which one object had been replaced with an identical copy and the other replaced with a novel object. The rats then explored for a 3-min test period. For NOL testing, the 4-min sample period included two identical objects, the ITD was 6 min, and the test period involved two identical copies of the objects present during the sample period, one of which was displaced from the original position. Each animal’s behavior in the arena was video recorded and exploratory behavior during the sample and test periods was analyzed using computer-based methods (Ethovision XT, Noldus Information Technology, Leesburg, VA), with relative exploration of novel/moved versus familiar/unmoved objects providing the primary measures of object/object–location recognition memory. Rats completed all phases of trials regardless of performance, although rats that failed to explore objects in the sample phase or the critical portion of the test phase were excluded from analysis. Failure to explore objects occurred more frequently in middle-aged rats, occurred in random individuals per test and did not vary by treatment. Across 3 tests for the 3 time points (9 measures), the average number of rats excluded was: young sham irradiated 0.44 ± 1.01; young fWBI 0.44 ± 0.53; middle-aged sham irradiated 1.00 ± 1.00; and middle-aged fWBI 1.11 ± 1.17. All animals also were evaluated for basic movement parameters in each trial as indicators of capacity to perform, and this included analysis of time spent exploring objects and total distance moved.
At the final time point in the experiment, 12 months after treatment, rats treated at 3 months of age were also tested with an additional NOR protocol (NOR 1) to provide a better comparison to previous studies (29–32) (see Discussion section). For the NOR 1, rats were tested with: 1. The single pair of objects used in the earlier studies (29–32) rather than with the array of objects used in our NOR 6 and NOR 30 testing, and 2. a very short ITD. The previous studies reported an ITD of 1 min, while the current study used an ITD of 90 s, which was the shortest possible with our methods.
Statistical Analyses
Preference for novel objects or object locations in the test period was determined by a discrimination ratio (DR) calculated as novel object exploration minus familiar object exploration divided by the sum of both exploration times. Exploration times during the first 2 min for NOR test trials and during the first minute for the NOL trials were evaluated (33). A DR of zero indicates equal preference between novel and familiar objects/object locations (chance outcome), whereas a positive DR score that is significantly greater than chance indicates preference for novel objects/object locations (interpreted as memory of familiar). Discrimination ratio scores for each treatment group were tested for outcomes different from chance using a one-sample t test comparing average DR to zero. A modified DR was calculated for exploration in the sample phase to confirm that side-bias in the arena did not contribute to test results (see Supplementary materials, Methods section; http://dx.doi.org/10.1667/RR13662.1.S1). Effects of treatment (sham irradiated vs. fWBI) and of longitudinal age (at 3, 6 and 12 months after treatment) on DR scores and on the performance metrics of exploration time and path length were tested using two-way analysis of variance (ANOVA) (separate analyses for rats treated at 3 vs. 18 months of age, results shown in Table 1). Post-hoc paired comparisons were made using Tukey’s multiple comparisons tests (results indicated in figures). A significance level of P < 0.05 was used for all tests.
TABLE 1.
Results of ANOVAs Testing for Effects of Age and fWBI on Discrimination Ratios (DR), Exploration Time (Expl) and Distance Moved (Path) in the Sample (Samp) and Test Phases of Each Test at Each Time Point for Rats Treated as Young Adults or in Middle Age
| DR | Samp Expl | Test Expl | Samp Path | Test Path | ||
|---|---|---|---|---|---|---|
| Treatment at 3 months of age | ||||||
| NOR 30 | ||||||
| fWBI | F(1, 113) | 0.052 | 11.080 | 0.631 | 0.090 | 19.99 |
| P | 0.82 | 0.001 | 0.43 | 0.76 | <0.0001 | |
| Age | F(2, 113) | 1.642 | 7.942 | 7.081 | 7.099 | 21.81 |
| P | 0.20 | 0.0006 | 0.001 | 0.001 | <0.0001 | |
| Interaction | F(2, 113) | 4.653 | 0.801 | 0.43 | 0.534 | 4.601 |
| P | 0.01 | 0.45 | 0.65 | 0.588 | 0.01 | |
| NOR 6 | ||||||
| fWBI | F(1, 121) | 0.388 | 1.383 | 7.576 | 0.455 | 8.812 |
| P | 0.53 | 0.24 | 0.007 | 0.50 | 0.004 | |
| Age | F(2, 121) | 0.461 | 23.73 | 12.90 | 2.036 | 46.91 |
| P | 0.63 | <0.0001 | <0.0001 | 0.14 | <0.0001 | |
| Interaction | F(2, 121) | 0.309 | 0.421 | 1.065 | 1.687 | 2.573 |
| P | 0.73 | 0.66 | 0.35 | 0.19 | 0.08 | |
| NOL | ||||||
| fWBI | F(1, 121) | 0.323 | 0.301 | 0.411 | 0.826 | 25.09 |
| P | 0.57 | 0.58 | 0.52 | 0.37 | <0.0001 | |
| Age | F(1, 121) | 7.686 | 12.30 | 0.075 | 8.598 | 17.78 |
| P | 0.007 | <0.0001 | 0.93 | 0.0003 | <0.0001 | |
| Interaction | F(1, 121) | 0.122 | 4.33 | 0.238 | 3.990 | 0.702 |
| P | 0.89 | 0.02 | 0.79 | 0.02 | 0.50 | |
| Treatment at 18 months of age | ||||||
| NOR 30 | ||||||
| fWBI | F(1, 72) | 0.001 | 1.374 | 0.468 | 0.272 | 7.029 |
| P | 0.98 | 0.24 | 0.50 | 0.60 | 0.01 | |
| Age | F(2, 72) | 1.85 | 0.574 | 0.613 | 2.239 | 6.499 |
| P | 0.16 | 0.57 | 0.54 | 0.11 | 0.002 | |
| Interaction | F(2, 72) | 0.054 | 0.110 | 0.606 | 0.014 | 1.530 |
| P | 0.95 | 0.90 | 0.55 | 0.99 | 0.22 | |
| NOR 6 | ||||||
| fWBI | F(1, 84) | 0.911 | 8.860 | 0.033 | 1.657 | 3.558 |
| P | 0.34 | 0.004 | 0.86 | 0.20 | 0.06 | |
| Age | F(2, 84) | 0.181 | 7.848 | 8.306 | 3.115 | 2.964 |
| P | 0.83 | 0.0007 | 0.0005 | 0.049 | 0.06 | |
| Interaction | F(2, 84) | 0.71 | 0.476 | 2.132 | 0.867 | 0.110 |
| P | 0.49 | 0.62 | 0.13 | 0.42 | 0.90 | |
| NOL | ||||||
| fWBI | F(1, 85) | 1.019 | 0.751 | 2.674 | 1.198 | 11.98 |
| P | 0.32 | 0.39 | 0.11 | 0.82 | 0.0008 | |
| Age | F(1, 85) | 2.021 | 1.175 | 4.297 | 9.085 | 0.345 |
| P | 0.14 | 0.31 | 0.02 | 0.0003 | 0.71 | |
| Interaction | F(1, 85) | 0.288 | 0.756 | 1.777 | 0.203 | 1.935 |
| P | 0.75 | 0.47 | 0.18 | 0.82 | 0.15 | |
Note. Significant effects of fWBI and/or age and significant interactions are in boldface.
RESULTS
Transient Radiation-Induced Deficit in Object Recognition Memory
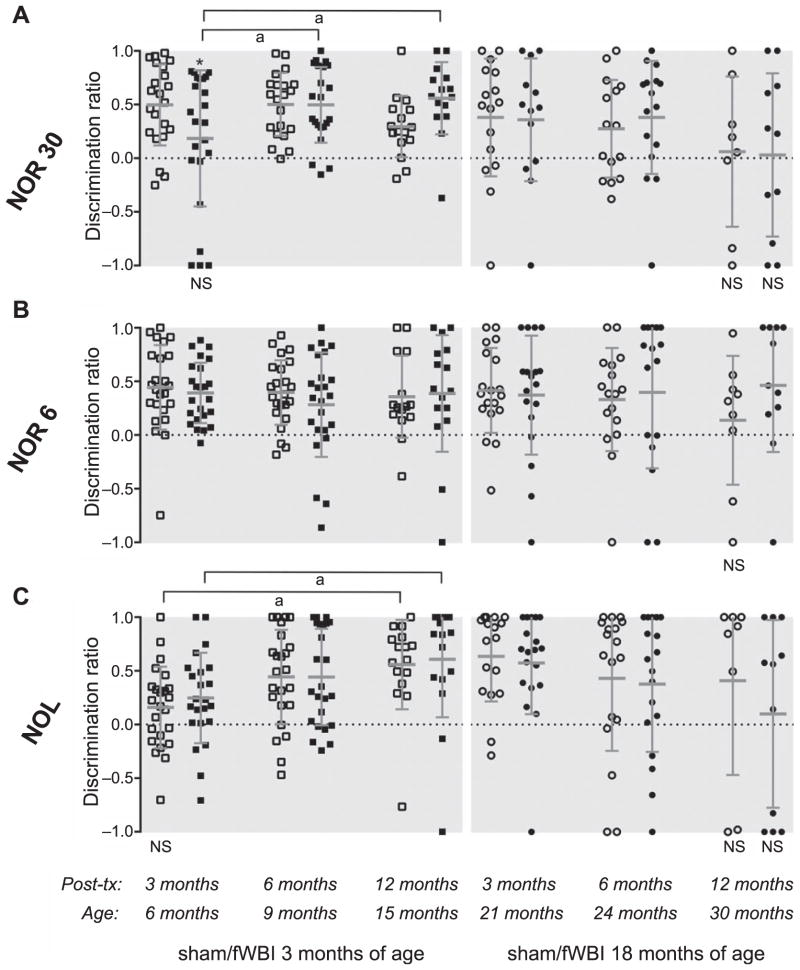
When tested 3 months after treatment, rats irradiated at 3 months of age performed poorly on the NOR 30 task compared to control rats that received sham irradiation at the same age (Fig. 1A). Consistent with that conclusion, the average DR was significantly different from chance for sham-irradiated control rats but not for fWBI rats, indicating that irradiated rats could not differentiate between the familiar and novel objects at 3 months after treatment, and the average DR was significantly lower in fWBI rats than in sham-irradiated, control rats. Rats irradiated at 18 months of age did not exhibit an object recognition deficit in the NOR 30 at 3 months after fWBI. The average DR for both the sham-irradiated and fWBI rats was significantly different from chance, there was no difference in average DR (P > 0.4) and the distributions of DR values appeared almost identical between the middle-aged fWBI and sham-irradiated control groups (Fig. 1A).
FIG. 1.
Recognition memory. Discrimination ratios for novelty preference are plotted for individual rats irradiated (closed symbols) or sham-irradiated (open symbols) at either 3 or 18 months of age and tested in the NOR 30, NOR 6 and NOL at 3, 6 and 12 months after treatment. The group mean (with standard deviation) also is indicated; groups of subjects for which the mean discrimination ratio was not significantly different from chance are indicated (NS = P > 0.05). *P < 0.05 vs. age-matched, sham-irradiated control; aP < 0.05 for indicated age comparison within treatment group.
The radiation-induced decrease in object recognition memory in the NOR 30 in rats irradiated as young adults was transient. At 6 months after treatment, the average DR value in the NOR 30 was significantly greater than zero regardless of treatment or age at time of treatment (Fig. 1A), and there was no significant difference between the fWBI and sham-irradiated control rats in the young adult or middle-aged groups. At 12 months after treatment, the average DR values for both fWBI and control rats in the groups treated as young adult were significantly greater than zero. Among the middle-aged rats, neither the fWBI nor control group performed successfully in the NOR 30 at 12 months after treatment (when the rats were 30 months old); the average DR for each group was not significantly different from zero.
The transient, radiation-induced deficit in object recognition memory in young adult rats was limited to the 30-min ITD version of the task. At 3 and 6 months after treatment, irradiated and sham-irradiated control rats in both age groups performed successfully in the NOR 6 task, with average DR values significantly greater than zero and no differences between fWBI and control groups (Fig. 1B). At 12 months after treatment, fWBI and control rats in the group treated at 3 months of age performed the NOR 6 well and comparably. In contrast, sham-irradiated control rats in the group treated middle age did not perform above chance levels at 12 months after treatment, at 30 months of age, indicating an aging related decline in performance (see below). Somewhat surprisingly and in contrast to the sham-irradiated control animals, fWBI rats did perform above chance levels at 12 months after treatment in middle age (Fig. 1B).
The absence of evidence for a radiation-induced deficit in object recognition memory in young adult rats tested 6 months after fWBI stood in contrast to previous reports of a late-delayed deficit in the NOR that appears by 6 months after 8 × 5 Gy fWBI (29–32). Therefore, one week prior to scheduled testing with the NOR 6, NOR 30 and NOL tasks at 12 months after treatment, we tested rats treated as young adults using a NOR protocol (NOR 1) that replicated several features of the earlier studies, including the use of a very short (~1 min) ITD and testing with the same objects used in the earlier studies (see Supplementary material, Methods sections; http://dx.doi.org/10.1667/RR13662.1.S1). Considering all trials together, without respect to which object was novel in the test phase, the average DR value was not significantly different from chance performance for the young adult sham-irradiated control or fWBI rats (each P > 0.05). The rats’ performance in the NOR 1 was affected greatly, however, by which object was novel. When object X (a plastic jar) was the novel object, sham-irradiated control and fWBI rats performed well and comparably, with average DR values significantly different from chance (P < 0.001). In contrast, when object Y (a metal can) was the novel object, the average DR for sham-irradiated control rats was not different from chance (P > 0.05), and the average DR for fWBI rats was significantly less than chance (P < 0.005), indicating greater exploration of the familiar object during the test phase (Supplementary Fig. S1A; http://dx.doi.org/10.1667/RR13662.1.S1).
No Radiation-Induced Deficit in Object Location Memory
Average DR values for object location recognition in the NOL task did not differ between irradiated and sham-irradiated control rats in either age group at any time point after treatment (Fig. 1C). Excluding the oldest rats (12 months after treatment at 18 months of age), sham-irradiated control and fWBI rats of each age and at each time point performed above chance levels, except the average DR for sham-irradiated control rats at 3 months after treatment as young adults was not quite significantly different from zero (P = 0.05). At 12 months after treatment at 18 months of age, neither the sham-irradiated control nor fWBI rats successfully performed in the NOL (average DR not significantly different from zero, P > 0.05) (Fig. 1C).
Effects of fWBI on Object Exploration and Movement
Qualitative observation suggested that behavior within the testing arena differed between fWBI and sham-irradiated control rats (and also changed as rats aged after treatment, see below). Two quantitative measures, object exploration time and total path length, were assessed to test for effects of fWBI and to determine whether such factors could have contributed to poor performance by fWBI rats in the NOR 30 and by fWBI and sham-irradiated control rats in the NOL at 3 months after treatment.
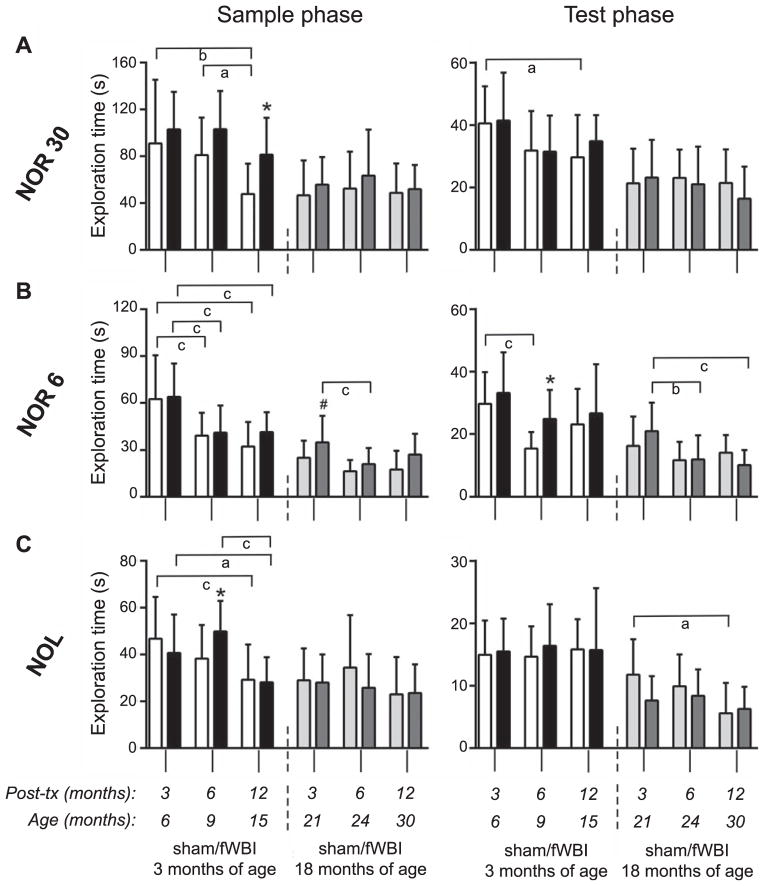
There were small and limited effects of radiation on the time spent exploring objects (Fig. 2). Among rats treated as young adults, fWBI rats tended to exhibit greater exploration, on average, than sham-irradiated controls. The difference reached statistical significance during the sample period of the NOR 30 at 12 months after treatment, the sample period of the NOL at 6 months after treatment, and the test phase of the NOR 6 at 6 months after treatment. Among the rats treated in middle age, object exploration time appeared unaffected by fWBI, although a small increase in the average for fWBI rats in the sample phase of the NOR 6 at 3 months after treatment was almost significant (P = 0.05, Fig. 2B). Analysis of the index of global habituation (IGH the difference in time spent exploring during the sample phase and time spent exploring during the test phase), proposed as a measure of habituation of exploratory behavior (24), revealed no significant effects of fWBI (Supplementary Fig. S2; http://dx.doi.org/10.1667/RR13662.1.S1).
FIG. 2.
Exploration. Exploration times (mean + SD) in the sample and test phase of each task are plotted for groups of rats irradiated (black and dark gray bars) or sham-irradiated (open and light gray bars) at either 3 or 18 months of age and tested in the NOR 30, NOR 6 and NOL at 3, 6 and 12 months after treatment. Y-axis scaling is relative to the total duration of the task phase. *P < 0.05 and #P = 0.05 vs. age-matched, sham-irradiated control; aP < 0.05,bP < 0.01 and cP < 0.005 for indicated age comparison within treatment group.
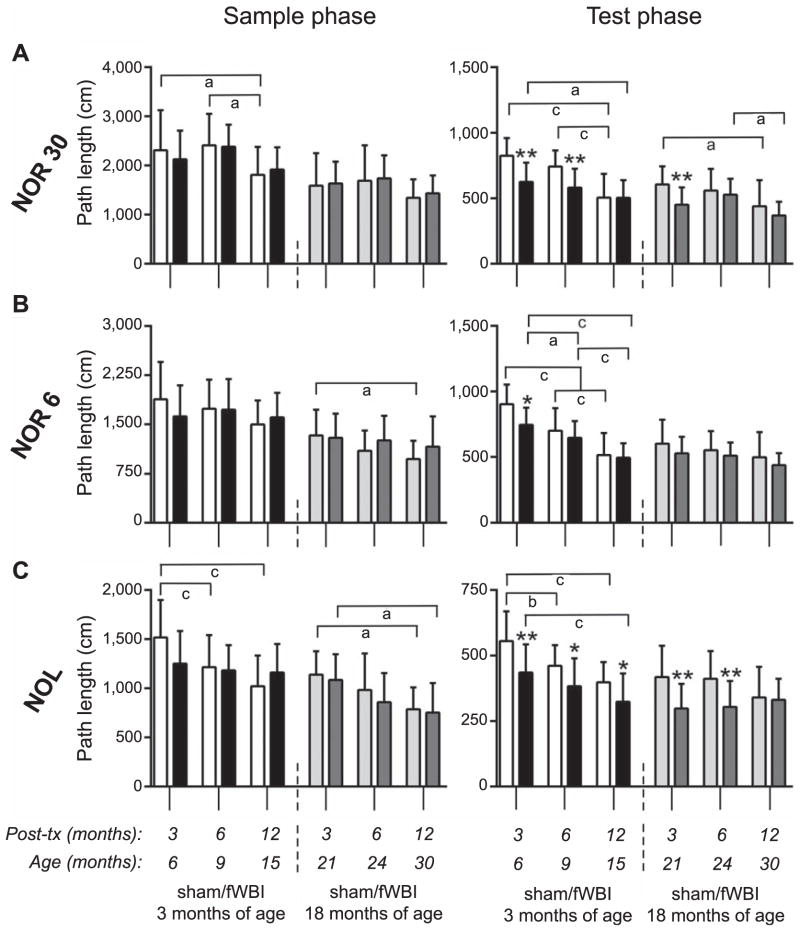
The effect of radiation on the extent of movement by rats within the test arena was greater than any effect on object exploration, as indicated by a reduction in average total path length for fWBI rats in all tests and at multiple times after treatment (Fig. 3). These effects on movement were evident primarily in the test phase and most commonly reached statistical significance in the group treated as young adults.
FIG. 3.
Movement. The length of the path (mean + SD) traversed by rats in the sample and test phase of each task are plotted for groups of rats irradiated (black and dark gray bars) or sham-irradiated (open and light gray bars) at either 3 or 18 months of age and tested in the NOR 30, NOR 6 and NOL at 3, 6 and 12 months after treatment. Y-axis scaling is relative to the total duration of the task phase. *P < 0.05 and **P < 0.01 vs. age-matched, sham-irradiated control; aP < 0.05, bP < 0.01 and cP < 0.005 for indicated age comparison within treatment group.
The observed effects of fWBI on object exploration and total path length were unrelated to recognition memory task performance. The fWBI rats exposed as young adults exhibited both an object recognition deficit and reduced movement (test phase) in the NOR 30 task at 3 months after treatment. These effects of fWBI on recognition memory and movement were independent, however, since fWBI also decreased movement in the NOR 30 in young adult animals at 6 months after treatment and in middle-aged animals at 3 months after treatment but did not affect object recognition in those groups at those times. Similarly, fWBI-induced changes in average object exploration and/or movement in the NOR 6 and NOL were not associated with changes in object or object location memory (compare Figs. 1–3). Attempts to relate individual performance (DR value) in each task to movement measures by correlation analysis also failed to reveal significant relationships (data not shown).
Effects of Aging on Performance in Spontaneous Recognition Tasks
The design of this study permitted testing for effects of normal aging on performance in the NOR and NOL and on exploration- and movement-related measures in these tasks. Rats were treated at 3 or 18 months of age and were evaluated at 3, 6 and 12 months after treatment, which allowed quantitative comparisons of rats at ~6, 9 and 15 months of age within the young adult cohort and at ~21, 24 and 30 months of age within the middle-aged cohort (only qualitative comparisons were made across the two cohorts). For the NOR 6 and NOR 30, the DR scores indicated increased variance in the oldest rats, and average performance also declined numerically with age, although the change was not statistically significant given the high variance in old animals and the lower N associated with euthanizing one cohort of rats at 6 months after treatment (Fig. 1A, B). For the NOL, performance improved and became more consistent across individuals between 6 and 15 months of age (Fig. 1C). Performance in the NOL appeared more variable in the oldest rats but even at 30 months of age half of the sham-irradiated control rats had high DR scores in the NOL (above 0.5, Fig. 1C).
The total time exploring objects generally appeared greater in the youngest rats compared to older rats. Among the rats that entered the study as young adults, 6-month-old rats explored more than older rats in the sample and test phases of the NOR 6 and NOR 30 and in the sample phase of the NOL (Fig. 2). Exploration time appeared stable across the latter half of the adult lifespan (sham-irradiated control rats treated at 18 months of age) except for a decline in exploration time in the test phase of the NOR 6 (fWBI rats) and the NOL (sham-irradiated control rats).
There were modest effects of aging/time after treatment on the IGH in the rats irradiated as young adults (Supplementary Fig. S2; http://dx.doi.org/10.1667/RR13662.1.S1). For the NOR 6, NOR 30 and NOL, the IGH was lower at 12 months after treatment than at earlier time points for sham-irradiated control rats treated at 3 months; for the NOL the IGH also was lower at 12 months than at earlier time points in rats irradiated at 3 months. The IGH was unchanged across ages/time after treatment in both sham-irradiated control and fWBI rats treated at 18 months.
The average distance covered by rats during testing also declined with age (Fig. 3), with evidence for an aging related decline in movement during the sample and/or test phase of each test (NOR 6, NOR 30 and NOL) across the first half of the lifespan (group treated at 3 months) and more limited evidence for additional declines in the latter half of the lifespan (group treated at 18 months).
DISCUSSION
The current study indicates that rats treated with clinically relevant fWBI as young adults develop an early, transient deficit in a perirhinal cortex-dependent object recognition memory task while sustaining normal function in a hippocampal-dependent object location memory task. Rats irradiated in middle age showed no radiation-induced memory changes.
The observation that only a subset of the young adult, fWBI treated rats appeared to be compromised in performance in the NOR may be consistent with clinical observations that only some patients develop cognitive dysfunction after cranial radiation therapy. It should be recognized, however, that single-trial versions of spontaneous object and object–location recognition tasks (the NOR and NOL) used here and in other studies of whole-brain irradiation in rodents do not establish whether differences in discrimination measures for individuals represent sustained differences in performance capacity [see e.g., conclusion in ref. (34)]. To differentiate between a subset of irradiated rats with consistently impaired performance indicating a greater individual response to fWBI versus an effect of fWBI on performance that is modest and appears in a varied subset of animals in any given testing session would require studies with multiple testing of each animal at the affected time point(s).
The presence of an object memory deficit in rats irradiated as young adults but not in rats irradiated in middle age may seem counterintuitive, given evidence that older brains are more vulnerable than young adult brains to some types of neural injury [discussed in ref. (35)], but it is consistent with previously reported data demonstrating that multiple neural and systemic effects of fWBI were greater in the rats irradiated as young adults (27). These effects included radiation-induced reductions in: brain weight and the weight of multiple neural regions, pituitary growth hormone, plasma insulin-like growth factor-I (IGF-I) and serum brain-derived neurotrophic factor (BDNF). The relationships among such changes and the presence (or absence) of cognitive deficits remain unknown and represent important areas for future investigation.
These data add to the growing and complex literature on radiation-induced cognitive dysfunction in animal models. Previous studies of fWBI have not examined performance in the NOR and NOL longitudinally, but several laboratories have tested rodents using the NOR and/or NOL at single postirradiation survival times similar to those examined here, with mixed results.
Effects of Radiation on Object Recognition Memory
Among previous studies of the NOR after brain irradiation, some report findings consistent with our observation that brain irradiation produces a deficit in the NOR in the first weeks to a few months after treatment (36–38), whereas other studies report no effects (39–42). Comparison of the current and previous studies is complicated by significant variation in irradiation (as well as differences in species/strains and age at time of irradiation). In addition to a wide range in doses, the majority of previous studies examined effects of single-fraction WBI. Because of the substantial differences in the biological response to single-fraction vs. fractionated WBI, the latter rather than single-dose WBI is typically used clinically. These differences complicate comparisons and also limit the translation of experimental findings based on single-dose WBI to the clinical problem of radiation-induced cognitive dysfunction.
Even experiments delivering fWBI at nominally the same dose have demonstrated different effects on performance in the NOR. The current study demonstrated early-delayed but no late-delayed deficits after an 8 × 5 Gy dose of fWBI, whereas earlier experiments delivering the same dose found no early-delayed but significant late-delayed deficits in the NOR [deficits at 6 and 12 months but not at 3 months (29–32)]. Perhaps significantly, the previous and current studies delivered the same BED but utilized different radiation sources (cesium vs. X ray) and dose rates (~4 Gy/min vs. 1.25 Gy/min). The current study also involved less irradiation of the brain stem, olfactory bulb and extracranial structures. Given the limited understanding of the mechanisms of radiation-induced brain injury, the significance of these differences remains unclear.
In addition to differences in irradiation, methods for what are nominally similar NOR or NOL tasks also vary greatly among laboratories. Virtually every aspect of NOR and NOL procedures (e.g., lighting, habituation, object characteristics and variety, and delays between sample and testing periods) varies among laboratories and studies. This likely contributes to the mixed results in the literature and, more specifically, to differences between the current results and previous studies reporting that 8 × 5 Gy fWBI produces a late-delayed deficit in the NOR (29–32). Importantly, neural processes other than memory likely impact performance in object preference tasks, may be affected by radiation, and may be differentially revealed by different protocols for behavioral testing. Such factors merit greater consideration in rodent models of radiation-induced cognitive dysfunction and are discussed below.
One key difference in NOR protocols used in the current versus previous testing after fWBI was the ITD interposed between the sample and test phases, which was 1 min in previous studies (29–32) versus 6 or 30 min in the current study (except for our attempt to replicate results with the short ITD at 12 months after fWBI at 3 months of age). If the DR in the NOR is purely a measure of object memory, one might expect the DR to be highest with the shortest ITD. Evidence suggests, however, that a 1-min delay in the NOR provides a less robust measure of object memory than delays of several minutes. In one of the earliest studies using the NOR (43), ITDs from 1 min to 24 h were compared directly using otherwise identical protocols. The discrimination index for normal, young adult rats was higher with a 15-min delay than with a 60-min delay (as one would predict), but the discrimination index was significantly lower with a 1-min delay than with either a 15- or 60-min delay (~40% lower with 1-min than with 15-min delay). This suggests that with a very short ITD, factors are involved that impact memory or the demonstration of memory by object preference. The same study (43) demonstrated: 1. That the discrimination index was highly correlated with the amount of exploration during the sample phase for the 1-min, but not 15- or 60-min, version of the NOR, and 2. that exploration during the test phase was reduced almost 50% with a 1-min ITD compared to a 15-min, 60-min or 24-h ITD; that is, rats exhibited significantly less exploration when returned to the arena with only a 1-min delay. The correlation of discrimination with exploration in the sample phase may indicate that with the very short ITD there are factors that disrupt consolidation and/or maintenance of weaker memory traces (resulting from less exploration). Reduced exploration during the test phase indicates that some factor(s) limit the animals’ exploration when they are removed from and then quickly returned to the testing arena, reducing the power of the task to demonstrate object preference. Intriguingly, in a version of the object recognition task that does not require animals to be removed from and then returned to an open arena, discrimination with a 1-min delay was not worse than with longer delays, and instead, the discrimination measure decreased progressively as the ITD was increased from 1 min to 5 min to 13 min and then to 21 min (44). These observations suggest that factors beyond memory mechanisms, which likely include activity, exploration and anxiety, impact performance in the NOR and that the effects of these factors are exacerbated with a very short ITD in common versions of the NOR.
The current and previous studies involved additional differences in behavioral protocols that likely influence activity and anxiety and therefore impact any secondary effects on testing for object and object location memory. Given the longitudinal design of the current study, the repeated experience of the rats in earlier testing periods could have influenced performance in later testing periods, with greater familiarity with testing decreasing any effects of anxiety mechanisms in later testing. In addition, in the current study, rats were maintained on a reverse light cycle and tested during the active (dark) phase of their diurnal cycle and under very-low-light conditions to maximize activity and minimize anxiety. In previous studies, rats were tested during the less active (light) phase of their diurnal cycle and under typical room lighting (29–32). The latter represents conditions that likely are more anxiogenic, may decrease activity and exploration, and therefore, may be more likely to reveal indirect (i.e., nonmemory) effects of fWBI on measures of spontaneous object/location “memory”. There have been several studies of the effects on anxiety measures of either single-dose WBI or of fractionated irradiation focused on the hippocampus. The results of those studies are mixed, but some report increases in anxiety measures after brain irradiation in rodents [e.g., see refs. (45, 46)] consistent with evidence that patients receiving cranial radiation therapy exhibit increased levels of anxiety several years after treatment (47). To our knowledge, there is no study on the long-term effects of clinically relevant, fWBI on anxiety measures in rodent models or, critically, of possible secondary contributions of anxiety mechanisms on other cognitive domains.
In addition to possible effects of anxiety mechanisms, there is an increasing recognition that novel object discrimination tasks are sensitive to habituation and dishabituation and to neophilic/neophobic responses to objects (34). Our attempt to replicate previous studies reporting a late-delayed deficit in the NOR after a 1-min ITD revealed a substantial difference in exploration attraction between the two objects used in the earlier studies (retested in the current study). In the NOR 1, both fWBI and sham-irradiated control rats demonstrated strongly positive object discrimination memory in trials in which a specific, preferred object was novel in the test phase. When that preferred object was familiar in the test phase, however, neither group showed novelty discrimination, but instead, each showed greater exploration of the familiar object (negative average DR). Moreover, the perseverative exploration of the familiar object was amplified in fWBI rats (DR negative and significantly greater than chance). It is not possible to determine whether and to what extent the previously published experiments may have been impacted by this difference in response to the two objects, since typically only average DR values have been reported. However, in one study the authors reported that the radiation-induced decrease in average DR in fWBI rats was the result of increased exploration of the familiar object during the test phase, not decreased exploration of the novel object (31). This is consistent with the amplified perseveration response seen in the fWBI rats tested here. In the current study, the design of the NOR protected against differences in the “attractiveness” of objects by using multiple pairs of objects and balancing their presentation across conditions.
Effects of Radiation on Object Location Recognition Memory
In contrast to the transient deficit in object recognition memory, the current study revealed no effect of fWBI on location recognition memory. The results of published analyses of performance in the NOL after brain irradiation are as mixed as the results of experiments using the NOR. Two studies reported no effect on the NOL in mice at 3 months after 10 Gy WBI (36, 39), whereas others reported deficits in the NOL at 3 months after 10 Gy irradiation in mice (41), at 1 and 5 months after 5 × 4 Gy fWBI in mice (38), and at 1 and 4 months after 10 Gy irradiation in rats (48–50). The current study revealed no radiation-induced deficit in the NOL at 3, 6 or 12 months after fWBI of rats at 3 or 18 months of age. It is important to note, however, that at 3 months after treatment, the time when some previous studies reported radiation-induced deficits in the NOL, the young, sham-irradiated control rats did not successfully perform (or barely performed) the task in the current study (P = 0.05, average DR vs. chance performance). It may be significant that the NOL task at 3 months after treatment was the first of all longitudinal testing performed and that, in that first testing period, the sham-irradiated control rats in the young adult cohort appeared unusually active, presumably due to their age and being less habituated than in subsequent tests. It appears that, just as normal, aging-related changes may limit the ability to detect radiation-induced deficits in old animals in some memory tasks (51), developmental changes in some neural functions and/or sensitivity of test design may limit assessment of radiation-induced changes in young adults.
Effects of Aging and Interactions with Brain Irradiation
In addition to the observed improvement in location memory across early adulthood in control rats, the latter part of the lifespan was characterized by greater inter-individual variance in the performance of all tasks, with old rats more likely to exhibit poor performance in a given trial. Aging-related changes in exploration and movement were modest but significant, and movement was sensitive to irradiation as well as to age. Intriguingly, although neither sham-irradiated control nor fWBI rats performed the NOR 30 successfully at the oldest age tested (12 months after treatment at 18 months of age), irradiated but not control rats performed well in the NOR 6. This indicates that for some cognitive functions brain irradiation may not exacerbate aging-related dysfunction but rather may ameliorate normal aging-related changes. Given our current, limited understanding of the neurobiological mechanisms underlying aging-related and radiation-induced cognitive changes, it should not be surprising that aging and radiation interact in complex ways. It is critical to consider aging-related changes in performance on cognitive tasks, since they may impact strategies and performance in behavioral tests, may not be limited to the end of the lifespan, and represent sites of interaction between aging-related and radiation-induced changes. This issue is particularly important in rodent models, since the time over which radiation-induced cognitive dysfunction is expected to develop represents a substantial fraction of the lifespan.
CONCLUSION
The centrality of changes in hippocampal neurogenesis in mechanistic models of radiation-induced brain injury and associated experimental studies in rodents [e.g., see refs. (39, 52–55)] are leading toward significant changes in clinical practice. Well-demonstrated links between radiation-induced changes in hippocampal neurogenesis and cognitive deficits in rodents [e.g., see refs. (39, 42, 56)] have been cited as the foundation for clinical treatment involving hippocampal sparing, that is, conforming radiation dose plans to greatly reduce the dose to the neurogenic zone of the hippocampus with the expectation of preventing or ameliorating treatment-induced cognitive deficits [e.g., see refs. (57–59)]. Significantly, however, data linking radiation-induced deficits in neurogenesis to cognitive changes, particularly to hippocampal-dependent learning and memory, come largely from experiments in which developing or very young adult animals were irradiated with single dose, not fractionated, WBI and were tested cognitively just a few months after irradiation (whereas cognitive deficits develop clinically many months or even years after treatment). These details indicate the need for circumspection in translating the experimental data to the clinic. The current study suggests that effects of clinically relevant, fractionated irradiation on hippocampal-dependent and other cognitive processes not only remain unclear, any such effects likely are age-dependent, even within adults. The data call for continued and expanded investigation and validation of rodent models of radiation-induced brain injury, which are critical for developing and testing new therapies for any potential the cognitive dysfunction that diminishes the quality of life in thousands of cancer survivors.
Supplementary Material
Supplementary Fig. S1. NOR 1 recognition memory and exploration. Panel A: Discrimination ratios for novel preference are plotted for individual rats treated at 3 months of age and tested in the NOR 1 at 12 months after fWBI (closed symbols) or sham irradiated (open symbols). The mean ratio also is indicated (with SD). DR values are plotted for all trials (black symbols) and then separately plotted for trials in which object X was the novel object (jar, red symbols) and for trials in which object Y was the novel object (can, blue symbols). Groups for which the mean discrimination ratio was not significantly different from chance (NS) are indicated. Panel B: Mean (+ SD) exploration times during the test period of the NOR 1 are shown for all trials and then for the trials separated based on which object was novel in the test phase. The open bar in each pair represents sham-irradiated control rats and the filled bar fWBI rats. In the test phase, irradiated rats spent significantly more time than sham-irradiated control rats exploring object X when it was the familiar object, indicating a perseverative response specific to fWBI rats and to object X. Panel C: The greater exploration of object X by fWBI rats was not limited to the test phase, in the sample phase irradiated rats but not sham-irradiated control rats spent significantly more time exploring object X than object Y. *P < 0.05 for indicated comparison.
Supplementary Fig. S2. Index of global habituation. The mean (+ sem) normalized values for the index of global habituation (IGH) are plotted for sham-irradiated control (open and light gray bars) and fWBI rats (black and dark gray bars) treated at 3 or 18 months of age (moa) and tested in the NOR 30 (panel A), NOR 6 (panel B) and NOL (panel C). Values for each test are presented as percentage of the maximum possible IGH value (duration of the sample phase minus duration of the analyzed portion of the test phase). ANOVA revealed an effect of time after treatment in rats treated as young adults but not in rats treated in middle age. #To simplify the presentation of the IGH values for the other groups, the normalized IGH in the NOR 6 for sham-irradiated rats treated at 18 months of age, −0.59 + 2.13, is not shown aP < 0.05, bP < 0.01 and cP < 0.005 for indicated comparison.
Acknowledgments
This work was supported in part by NIH grant R01CA133483. We thank Dr. Linda Metheny-Barlow and Dr. Dana Greene-Schloesser for helpful comments on the manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533–40. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 3.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357–70. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Belka C, Budach W, Kortmann RD, Bamberg M. Radiation induced CNS toxicity–molecular and cellular mechanisms. Br J Cancer. 2001;285:1233–9. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818–22. doi: 10.1002/ana.410280614. [DOI] [PubMed] [Google Scholar]
- 6.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–42. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 7.Johannesen TB, Lien HH, Hole KH, Lote K. Radiological and clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother Oncol. 2003;69:169–76. doi: 10.1016/s0167-8140(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 8.Meyers CA, Smith JA, Bezjak A, Mehta MP, Liebmann J, Illidge T, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. Journal Clin Oncol. 2004;22:157–65. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 9.Hahn CA, Zhou SM, Raynor R, Tisch A, Light K, Shafman T, et al. Dose-dependent effects of radiation therapy on cerebral blood flow, metabolism, and neurocognitive dysfunction. Int J Radiat Oncol Biol Phys. 2009;73:1082–7. doi: 10.1016/j.ijrobp.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 10.Brandes AA, Rigon A, Monfardini S. Radiotherapy of the brain in elderly patients. Contra Eur J Cancer (Oxford, England: 1990) 2000;36:447–51. doi: 10.1016/s0959-8049(99)00322-6. Discussion 51–2. [DOI] [PubMed] [Google Scholar]
- 11.Stylopoulos LA, George AE, de Leon MJ, Miller JD, Foo SH, Hiesiger E, et al. Longitudinal CT study of parenchymal brain changes in glioma survivors. AJNR Am J Neuroradiol. 1988;9:517–22. [PMC free article] [PubMed] [Google Scholar]
- 12.Asai A, Matsutani M, Kohno T, Nakamura O, Tanaka H, Fujimaki T, et al. Subacute brain atrophy after radiation therapy for malignant brain tumor. Cancer. 1989;63:1962–74. doi: 10.1002/1097-0142(19890515)63:10<1962::aid-cncr2820631016>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 14.Lee PW, Hung BK, Woo EK, Tai PT, Choi DT. Effects of radiation therapy on neuropsychological functioning in patients with nasopharyngeal carcinoma. J Neurol Neurosurg Psychiatry. 1989;52:488–92. doi: 10.1136/jnnp.52.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correa DD, DeAngelis LM, Shi W, Thaler H, Glass A, Abrey LE. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62:548–55. doi: 10.1212/01.wnl.0000109673.75316.d8. [DOI] [PubMed] [Google Scholar]
- 16.Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol. 2006;7:517–23. doi: 10.1007/s11864-006-0026-5. [DOI] [PubMed] [Google Scholar]
- 17.Shaw EG, Rosdhal R, D’Agostino RB, Jr, Lovato J, Naughton MJ, Robbins ME, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415–20. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 18.Calabrese P, Schlegel U. Neurotoxicity of treatment. Recent Results Cancer Res. 2009;171:165–74. doi: 10.1007/978-3-540-31206-2_10. [DOI] [PubMed] [Google Scholar]
- 19.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–44. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 20.Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, Aaronson NK, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8:810–8. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao KY, Yeh SA, Chang CC, Tsai PC, Wu JM, Gau JS. Cognitive function before and after intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma: a prospective study. Int J Radiat Oncol Biol Phys. 2010;77:722–6. doi: 10.1016/j.ijrobp.2009.06.080. [DOI] [PubMed] [Google Scholar]
- 22.Correa DD, Shi W, Abrey LE, Deangelis LM, Omuro AM, Deutsch MB, et al. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro Oncol. 2012;14:101–8. doi: 10.1093/neuonc/nor186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodges H, Katzung N, Sowinski P, Hopewell JW, Wilkinson JH, Bywaters T, et al. Late behavioural and neuropathological effects of local brain irradiation in the rat. Behav Brain Res. 1998;91:99–114. doi: 10.1016/s0166-4328(97)00108-3. [DOI] [PubMed] [Google Scholar]
- 24.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 25.Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neuroscience. 2004;24:5901–8. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neuroscience. 2011;31:10721–31. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forbes ME, Paitsel M, Bourland JD, Riddle DR. Systemic effects of fractionated, whole-brain irradiation in young adult and aging rats. Radiat Res. 2013;180:326–33. doi: 10.1667/RR3313.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowler F. The linear quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–94. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 29.Zhao W, Payne V, Tommasi E, Diz DI, Hsu FC, Robbins ME. Administration of the peroxisomal proliferator-activated receptor gamma agonist pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2007;67:6–9. doi: 10.1016/j.ijrobp.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 30.Robbins ME, Payne V, Tommasi E, Diz DI, Hsu FC, Brown WR, et al. The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2009;73:499–505. doi: 10.1016/j.ijrobp.2008.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee TC, Greene-Schloesser D, Payne V, Diz DI, Hsu FC, Kooshki M, et al. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat Res. 2012;178:46–56. doi: 10.1667/rr2731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atwood T, Payne VS, Zhao W, Brown WR, Wheeler KT, Zhu JM, et al. Quantitative magnetic resonance spectroscopy reveals a potential relationship between radiation-induced changes in rat brain metabolites and cognitive impairment. Radiat Res. 2007;168:574–81. doi: 10.1667/RR0735.1. [DOI] [PubMed] [Google Scholar]
- 33.Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- 34.Gaskin S, Tardif M, Cole E, Piterkin P, Kayello L, Mumby DG. Object familiarization and novel-object preference in rats. Behav Processes. 2010;83:61–71. doi: 10.1016/j.beproc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Schindler MK, Forbes ME, Robbins ME, Riddle DR. Aging-dependent changes in the radiation response of the adult rat brain. Int J Radiat Oncol Biol Phys. 2008;70:826–34. doi: 10.1016/j.ijrobp.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raber J, Villasana L, Rosenberg J, Zou Y, Huang TT, Fike JR. Irradiation enhances hippocampus-dependent cognition in mice deficient in extracellular superoxide dismutase. Hippocampus. 2011;21:72–80. doi: 10.1002/hipo.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2012;22:1188–201. doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao AA, Ye H, Decker PA, Howe CL, Wetmore C. Therapeutic doses of cranial irradiation induce hippocampus-dependent cognitive deficits in young mice. J Neuro Oncol. 2011;105:191–8. doi: 10.1007/s11060-011-0582-9. [DOI] [PubMed] [Google Scholar]
- 39.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 40.Belarbi K, Jopson T, Arellano C, Fike JR, Rosi S. CCR2 deficiency prevents neuronal dysfunction and cognitive impairments induced by cranial irradiation. Cancer Res. 2013;73:1201–10. doi: 10.1158/0008-5472.CAN-12-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acevedo SE, McGinnis G, Raber J. Effects of 137Cs gamma irradiation on cognitive performance and measures of anxiety in Apoe–/– and wild-type female mice. Radiat Res. 2008;170:422–8. doi: 10.1667/rr1494.1. [DOI] [PubMed] [Google Scholar]
- 42.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 43.Ennaceur A, Meliani K. A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behav Brain Res. 1992;51:83–92. doi: 10.1016/s0166-4328(05)80315-8. [DOI] [PubMed] [Google Scholar]
- 44.Albasser MM, Chapman RJ, Amin E, Iordanova MD, Vann SD, Aggleton JP. New behavioral protocols to extend our knowledge of rodent object recognition memory. Learn Mem. 2010;17:407–19. doi: 10.1101/lm.1879610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiskova J, Smajda B. Open field behavior and habituation in rats irradiated on the head with gamma-rays. Acta Physiol Hung. 2008;95:307–12. doi: 10.1556/APhysiol.95.2008.3.6. [DOI] [PubMed] [Google Scholar]
- 46.Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R, Gass P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PloS One. 2010;5 doi: 10.1371/journal.pone.0012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang Y, Luo D, Rong X, Shi X, Peng Y. Psychological disorders, cognitive dysfunction and quality of life in nasopharyngeal carcinoma patients with radiation-induced brain injury. PloS One. 2012;7(6):e36529. doi: 10.1371/journal.pone.0036529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acharya MM, Christie LA, Lan ML, Limoli CL. Comparing the functional consequences of human stem cell transplantation in the irradiated rat brain. Cell Transplant. 2013;22:55–64. doi: 10.3727/096368912X640565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acharya MM, Christie LA, Lan ML, Giedzinski E, Fike JR, Rosi S, et al. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011;71:4834–45. doi: 10.1158/0008-5472.CAN-11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acharya MM, Christie LA, Lan ML, Donovan PJ, Cotman CW, Fike JR, et al. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc Natl Acad Sci USA. 2009 Nov 10;106(45):19150–5. doi: 10.1073/pnas.0909293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi L, Olson J, D’Agostino R, Jr, Linville C, Nicolle MM, Robbins ME, et al. Aging masks detection of radiation-induced brain injury. Brain Res. 2011;1385:307–16. doi: 10.1016/j.brainres.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–34. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 53.Andres-Mach M, Rola R, Fike JR. Radiation effects on neural precursor cells in the dentate gyrus. Cell Tissue Res. 2008;331:251–62. doi: 10.1007/s00441-007-0480-9. [DOI] [PubMed] [Google Scholar]
- 54.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–95. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 55.Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res. 2013;19:2294–300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Oehler J, Brachwitz T, Wendt TG, Banz N, Walther M, Wiezorek T. Neural stem cell sparing by linac based intensity modulated stereotactic radiotherapy in intracranial tumors. Radiation Oncol. 2013;8:187. doi: 10.1186/1748-717X-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gondi V, Hermann BP, Mehta MP, Tome WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85:348–54. doi: 10.1016/j.ijrobp.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 59.Prokic V, Wiedenmann N, Fels F, Schmucker M, Nieder C, Grosu AL. Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: a planning study on treatment concepts. Int J Radiat Oncol Biol Phys. 2013;85:264–70. doi: 10.1016/j.ijrobp.2012.02.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. NOR 1 recognition memory and exploration. Panel A: Discrimination ratios for novel preference are plotted for individual rats treated at 3 months of age and tested in the NOR 1 at 12 months after fWBI (closed symbols) or sham irradiated (open symbols). The mean ratio also is indicated (with SD). DR values are plotted for all trials (black symbols) and then separately plotted for trials in which object X was the novel object (jar, red symbols) and for trials in which object Y was the novel object (can, blue symbols). Groups for which the mean discrimination ratio was not significantly different from chance (NS) are indicated. Panel B: Mean (+ SD) exploration times during the test period of the NOR 1 are shown for all trials and then for the trials separated based on which object was novel in the test phase. The open bar in each pair represents sham-irradiated control rats and the filled bar fWBI rats. In the test phase, irradiated rats spent significantly more time than sham-irradiated control rats exploring object X when it was the familiar object, indicating a perseverative response specific to fWBI rats and to object X. Panel C: The greater exploration of object X by fWBI rats was not limited to the test phase, in the sample phase irradiated rats but not sham-irradiated control rats spent significantly more time exploring object X than object Y. *P < 0.05 for indicated comparison.
Supplementary Fig. S2. Index of global habituation. The mean (+ sem) normalized values for the index of global habituation (IGH) are plotted for sham-irradiated control (open and light gray bars) and fWBI rats (black and dark gray bars) treated at 3 or 18 months of age (moa) and tested in the NOR 30 (panel A), NOR 6 (panel B) and NOL (panel C). Values for each test are presented as percentage of the maximum possible IGH value (duration of the sample phase minus duration of the analyzed portion of the test phase). ANOVA revealed an effect of time after treatment in rats treated as young adults but not in rats treated in middle age. #To simplify the presentation of the IGH values for the other groups, the normalized IGH in the NOR 6 for sham-irradiated rats treated at 18 months of age, −0.59 + 2.13, is not shown aP < 0.05, bP < 0.01 and cP < 0.005 for indicated comparison.