Abstract
The floating beads have been employed to make a sustained release of the drug in the stomach and to decrease the dose of the drug and hence overcome its side effects. The common benefits of the floating beads were it is easy preparation, without the need of a high temperature, and high percentage of the drug entrapment. In the present work, the Ketorolac tromethamine (KT) floating beads were prepared by extrusion congealing method utilizing calcium carbonate as a gas forming agent. The physical characters of the produced beads were investigated such as KT yield, KT loading, and entrapment efficiency of the drug. In addition, floating behavior, swelling, particle size, morphology and KT stability were also evaluated. In vitro drug release study was carried out, and the kinetics of the release was evaluated using the linear regression method. Furthermore, the in vivo analgesic effect of KT after oral administration of the selected formula of floating beads (F10) was carried out using hot plate and tail flick methods. Oral commercial KT tablets and KT solution were used for the comparison. The prepared beads remained floated for more than 8 h. The optimized formulation (F10) exhibited prolonged drug release (more than 8 h) and the drug release follows the Higuchi kinetic model, with a Fickian diffusion mechanism according to Korsmeyer-Peppas (n = 0.466). Moreover, F10 showed a sustained analgesic effect as compared to the commercial tablet.
Keywords: GRDFS, Floating alginate beads, Gastric residence time, Ketorolac tromethamine, Hot plate and tail flick methods
1. Introduction
Gastric residence time is an important parameter for different dosage forms and the prolongation and control of this time, epically for the dosage forms which remain in the stomach for a longer period of time result in better absorption and enhanced bioavailability. Gastro-retentive drug delivery systems are systems that can reside in the gastric region for several hours and can significantly prolong and control the gastric residence time of drugs. Prolonged gastric retention enhances bioavailability and improves solubility for drugs that are less soluble in high pH environment. Moreover, gastro-retention has application for local drug delivery to the stomach and proximal small intestines and also has substantial advantages for patients (Alexander and Juergen, 2006; Arora et al., 2005). Floating drug delivery systems are low density, matrix type systems designed by the addition of some polymers such as cellulose derivatives, polysaccharides, carbopol and chitosan and different effervescent components like sodium bicarbonate, calcium carbonate, citric acid or tartaric acid. These floating dosage forms are formulated in such a way that when they come in contact with gastric content, carbon dioxide is released and is entrapped in the hydrocolloids which makes the dosage forms to float (Gangadharappa et al., 2007). When the system is floating on the gastric juice, the drug is released slowly and this results in an increased gastric residence time and a better control of the variability in plasma drug concentration. Alginate beads have been designed in the present study as vehicle for drug delivery systems. Alginate is a hydrophilic polymer, stable in acidic media and easily depredated in alkaline media. These properties have enabled widespread use of alginate beads in gastro-retentive dosage forms (GRDFs) for sustained release of drugs (Gadad et al., 2009). Ketorolac tromethamine (KT) is a potent non-steroidal anti inflammatory drug with biological half-life ranged from 4 to 6 h and it is mainly absorbed in the proximal part of the small intestine (Sinha and Trehan, 2005). KT is 800 times more potent than aspirin and produces strongly analgesic and moderate anti-inflammatory activity (Padma et al., 2000). The main principle of floating beads can be applied to decrease the irritant effect of KT on the stomach by avoiding the direct contact with the gastric mucosa and obtaining a low dosage for prolonged periods (Thanoo et al., 1993). The aim of the present work is to prepare KT in different floating calcium alginate beads to control the drug release using calcium carbonate as a gas forming agent. The influence of hydroxylpropyl cellulose, sodium carboxymethyl cellulose, methyl cellulose and pectin as hydrophilic co-polymers on percentage loading of drug, entrapment efficiency, swelling, floating, particle size and in vitro drug release was studied. Furthermore, the analgesic effect of the drug using tail flick and hot plate methods was investigated.
2. Materials and methods
2.1. Materials
Ketorolac tromethamine (KT) was purchased from (Varda Biotech Ltd., India). Sodium alginate, pectin, calcium carbonate and glacial acetic acid were obtained from (BDH, UK). HPMC, MC, HPC and Na CMC were obtained from (DOW Chemicals, UK). Ketoral® was purchased from (Al-Arab Drug Company, Egypt). All other reagents used were of analytical grade.
2.2. Methods
2.2.1. Formulation of KT floating beads
Stock solution of KT was prepared by dissolving 40 mg in 5 ml of water. The KT solution then was added to 95 ml sodium alginate solution, (3% w/v) containing methyl cellulose (MC), hydroxypropyl cellulose (HPC), sodium carboxymethyl cellulose (Na CMC), and pectin as co-polymers in ratio of (alginate: copolymer, 9:1, 7:1, and 5:1 w/w). Calcium carbonate was added to sodium alginate solution in a ratio of 1:1 and the mixture dropped using 24 G syringe needle into 100 ml of calcium chloride solution (1% w/v) which contains glacial acetic acid (10% v/v). Then the beads formed in the solution were stirred for 10 min to improve its hardness. After that the beads were collected and washed with distilled water, then dried for 48 h. The different formulations of KT are presented in Table 1.
Table 1.
Composition of different KT floating beads.
| Formulae code | Formulae composition (% w/w) |
|||||||
|---|---|---|---|---|---|---|---|---|
| KT (mg) | Sodium alginate (SA) | MC | Na CMC | HPC | Pectin | CaCO3 | CaCl2 | |
| F1 | 40 | 3.000 | – | – | – | – | 3.00 | 1.00 |
| F2 | 40 | 2.700 | 0.300 | – | – | – | 3.00 | 1.00 |
| F3 | 40 | 2.625 | 0.375 | – | – | – | 3.00 | 1.00 |
| F4 | 40 | 2.500 | 0.500 | – | – | – | 3.00 | 1.00 |
| F5 | 40 | 2.700 | – | 0.300 | – | – | 3.00 | 1.00 |
| F6 | 40 | 2.625 | – | 0.375 | – | – | 3.00 | 1.00 |
| F7 | 40 | 2.500 | – | 0.500 | – | – | 3.00 | 1.00 |
| F8 | 40 | 2.700 | – | – | 0.300 | – | 3.00 | 1.00 |
| F9 | 40 | 2.625 | – | – | 0.375 | – | 3.00 | 1.00 |
| F10 | 40 | 2.500 | – | – | 0.500 | – | 3.00 | 1.00 |
| F11 | 40 | 2.700 | – | – | – | 0.300 | 3.00 | 1.00 |
| F12 | 40 | 2.625 | – | – | – | 0.375 | 3.00 | 1.00 |
| F13 | 40 | 2.500 | – | – | – | 0.500 | 3.00 | 1.00 |
2.3. Characterization of KT floating beads
2.3.1. Determination of percentage yield, drug loading (DL) and encapsulation efficiency of the prepared beads
-
-
Percentage yield for the formulated beads was calculated from the following equation (Prasad et al., 2011):
-
-
Determination of drug loading
Twenty-five milligrams were weighed of the KT prepared beads and were dissolved in 50 ml of buffer pH 1.2 centrifuged, filtered and then the filterate was analyzed at 322 nm using a UV/visible spectrophotometer (Thermospectronic, USA). The experiment was carried out in triplicate and drug loading was calculated from the following equation (Prasad et al., 2011):
Percentage encapsulation efficiency was calculated using the following formula:
-
-
The encapsulation efficiency was determined from the following equation = actual amount of drug (AQ)/theoretical amount of drug (TQ) × 100 Prasad et al., 2011.
2.3.2. Scanning electron microscope (SEM)
The prepared floated beads were examined for its surface morphology by SEM (Metler Toledo, Tokyo, Japan). The samples were fixed in individual stubs and coated uniformly with gold.
2.3.3. Determination of particle size
Average particle size of beads was determined using sieve analysis method. Twenty gram beads were weighed carefully and placed on the first screen and shaken for certain time. Each fraction is then taken and weighed. The particle size was determined as follows:
where, dave. is the average diameter of beads, n is percent of each fraction retained on each sieve and d is the arithmetic mean size of sieve opening (Parrott et al., 1986). The experiment was performed in triplicate.
2.3.4. Determination of swelling properties
Known weight (50 mg) of floated beads was soaked in 100 ml of buffer 1.2 pH at 37 ± 0.5 °C. Then the beads were removed at specified time intervals, dried and weighed to determine the swelling index. Swelling ratio was determined from the following equation:
Swelling ratio = (weight of swollen beads – initial weight of beads)/initial weight of beads (Yagnesh et al., 2006). The experiment was performed in triplicate and the average results were taken.
2.3.5. Determination of lag and buoyant time
Specified weight (50 mg) of floated beads was placed in a beaker containing 100 ml of buffer 1.2 pH and shaked at 50 rpm in a water bath 37 ± 0.5 °C. The time taken by beads to float on the surface was determined (lag time). The layer of the floated beads was removed and the layer at the bottom was separated by filtration. The upper and the lower layers were dried at 40 °C until constant weight. Buoyant time was calculated by the following equation:
(Stops et al., 2008). All the determinations were made in triplicate.
2.3.6. In-vitro release study
The dissolution rate of the prepared beads was studied using USP rotating paddle dissolution apparatus II (Erweka DT-600 GmbH, Germany). Known weight of beads containing equivalent amount of 10 mg of KT was placed in the dissolution medium (900 ml of buffer pH 1.2). The temperature was adjusted to 37 ± 0.5 °C at 50 rpm. 5 ml of samples was withdrawn at specified time intervals and replaced with fresh dissolution medium. The samples were filtered with 0.45 μm Whatman membrane filter and the quantity of the drug released was determined using spectrophotometrically at 322 nm. The release rate of 10 mg of KT powder and KT commercial tablets (Ketoral® 10 mg) was determined. The experiment was carried out in triplicate and the average values of the released amount were calculated and plotted versus time. The results were expressed as the percentage of cumulative amount released as a function of time.
2.3.7. Kinetic study
The release data were analyzed using different kinetic equations which include Zero-order, First-order kinetics, Higuchi diffusion and Korsmeyer-Peppas models (Martin et al., 1993).
2.3.8. Stability study
The stability of Kt floating beads was studied at 25 °C and 60% relative humidity in closed high-density polyethylene bottles for 6 months. The drug content and the floating behavior were determined at different time intervals such as 1, 2, 3, and 6 months.
2.3.9. Analgesic effect study
The in vivo analgesic effect of selected KT floating beads’ formula (F10) was investigated. It was assessed by two different methods; hot plate and tail flick models.
2.3.9.1. Animals
Five groups of Swiss-albino male and female mice weighing 30–40 g were used for each formula; each group consists of six mice. Group I was used as a control, which received 1 ml saline, group II received KT solution, while group III received KT commercial tablets (Ketoral®) and, finally groups IV and V were given the selected KT floating beads (F10) in fast and fed state respectively. Food was withdrawn 12 h before starting the experiment for all groups except for the fifth group; which was kept on standard laboratory diet and tap water and the animals were kept at room temperature. All the experiments were carried out according to the ethical guidelines established and approved by the committee on the use and care of laboratory animals of our university (Saleem et al., 2011).
2.3.9.2. Calculation of KT dose
For the solution of KT, commercial KT tablets (Ketoral®) and selected KT floating beads (F10), the dose level of 1.1 mg/kg of the drug corresponding to 10 mg human dose was used. This equivalent dose for mice was calculated by the aid of surface area ratio as; the therapeutic dose of human was multiplied by a certain mathematical factor obtained from a special table for surface area ratios of some common laboratory species and man (Ghosh, 1971). The following equation was used: Dm = Dh (Wm/Wh)3/4 where, Dm: mice dose; Dh: human dose; Wm: mice weight; Wh: human weight (Geoffrey and James, 2005).
2.3.9.3. Hot plate method
The mice were placed on hot plate analgesiameter (MK-350 D, Japan) adjusted to temperature 51 ± 1 °C, where the surface is hot enough to cause discomfort without tissue damage. The mice were received orally the calculated KT dose (1.1 mg/kg) thirty minutes before the beginning of the test. The time taken till the mice begin to jump or lick forepaw was determined and this time was called the reaction time. Each trial was not taking more than 25 s to avoid tissue damage. The reaction time of the analgesic effect was measured at 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 h and the changes in the behavior of mice in the presence of KT were detected in fast and fed mice. The effect of food on the duration of analgesic effect was also determined (Amberkar et al., 2011).
2.3.9.4. Tail flick method
Mice were placed on tail flick apparatus, analgesia meter (MK-350 D, Japan) using simple restrain device to fix the animal for testing. The tail was placed into a sensing groove and a photo-sensor was located under this groove. The beam that produces the radiant heat stimulus was focused on the area of the distal part of the tail and the strength of the radiant heat lamp was kept constant. The time taken by mice to withdraw the tail was measured and this time was called the reaction time of the analgesic effect. Cut-off reaction time was 10 s to avoid any tissue injury. Then the same procedure of the hot plate method was carried out (Ibrahim et al., 2010).
2.4. Statistical analysis
All results were expressed as mean values ± standard deviation (SD) for in vitro results and as means ± SE for in vivo results. One-way analysis of variance (ANOVA) test followed by the Duncan’s multiple comparison tests were used. A probability value less than 0.05 was considered to be a significant value (Bolton and Bon, 2004).
3. Results and discussion
The data of physicochemical characterization include percentage yield, drug loading, drug encapsulation efficiency, mean particle size, floating lag time and % floating after 8 h of all KT formulations and are represented in Table 2. The percentage yield of floating beads ranged from 80% to 94.5% for F1 and F10 respectively. Results showed that; yield increased by increasing co-polymer concentration and with respect to co-polymer type; beads composed of sodium alginate/HPC (F10) showed the highest yield (94.5%) while those composed of sodium alginate/pectin (F13) showed the lowest one(83%).
Table 2.
Percentage yield, drug loading %, drug encapsulation efficiency %, mean particle size, density, floating lag time and floating % after 8 h of KT floating beads.
| Formulae code | Yield (%) | Drug loading (%) | Drug encapsulation efficiency (%) | Mean particle size (μm) | Floating lag time (s) | Floating (%) after 8 (h) |
|---|---|---|---|---|---|---|
| Physicochemical characterization | ||||||
| F1 | 80.0 ± 4.0 | 56.9 ± 3.6 | 45.00 ± 1.7 | 711 ± 14.50 | 41.0 ± 1.70 | 95 ± 5.60 |
| F2 | 84.5 ± 2.3 | 88.5 ± 4.8 | 70.25 ± 2.1 | 825 ± 19.90 | 86.0 ± 4.40 | 92.5 ± 6.8 |
| F3 | 86.5 ± 2.0 | 73.2 ± 6.3 | 73.25 ± 2.0 | 907 ± 17.00 | 95.0 ± 6.00 | 90.4 ± 7.6 |
| F4 | 87.0 ± 2.6 | 86.1 ± 5.9 | 85.50 ± 1.5 | 1005 ± 22.9 | 120 ± 10.5 | 91 ± 6.60 |
| F5 | 83.5 ± 2.5 | 78.5 ± 3.6 | 64.25 ± 2.1 | 860 ± 25.00 | 90.0 ± 5.60 | 93.6 ± 2.5 |
| F6 | 84.0 ± 3.6 | 82.3 ± 7.1 | 67.50 ± 1.5 | 975 ± 22.90 | 110 ± 9.50 | 91.2 ± 2.3 |
| F7 | 85.0 ± 1.7 | 82.3 ± 6.5 | 69.00 ± 1.7 | 1112 ± 13.1 | 140 ± 12.5 | 92.1 ± 3.5 |
| F8 | 89.0 ± 2.7 | 88.5 ± 3.6 | 78.00 ± 1.8 | 1000 ± 21.8 | 83.0 ± 6.10 | 88.5 ± 4.0 |
| F9 | 90.0 ± 3.6 | 90.0 ± 5.7 | 80.00 ± 1.0 | 1130 ± 13.2 | 95.0 ± 7.0 | 86 ± 4.00 |
| F10 | 94.5 ± 1.5 | 92.3 ± 4.4 | 86.75 ± 3.2 | 1260 ± 22.7 | 125 ± 9.5 | 86.5 ± 2.9 |
| F11 | 81.0 ± 2.0 | 73.1 ± 3.2 | 58.25 ± 1.1 | 710 ± 18.00 | 45.0 ± 4.40 | 96 ± 1.00 |
| F12 | 82.5 ± 1.5 | 74.6 ± 4.8 | 61.00 ± 1.0 | 780 ± 31.20 | 53.0 ± 4.40 | 95.5 ± 2.5 |
| F13 | 83.0 ± 2.5 | 77.0 ± 3.1 | 63.25 ± 1.2 | 801 ± 18.40 | 50.0 ± 2.00 | 95 ± 2.00 |
All values are mean ± SD of three determinations.
3.1. Drug loading and encapsulation efficiency
The% drug loading of various beads was ranged from 56.9% to 92.3% for F1 and F10, respectively, while the % encapsulation efficiency was 45.0% for (F1) and 86.75% for (F10). Results indicated that the drug loading and encapsulation efficiency were low for beads composed of sodium alginate (F1), while it was increased significantly by the addition of another co-polymer to alginate, which increased its ability to retain the drug by the formation of two types of protective layers in beads and delayed the diffusion of the drug more effectively than a single protective layer formed by sodium alginate (Ajit et al., 2006). In addition the use of pectin showed the lowest KT loading and encapsulation efficiency when compared with the other used co-polymers which may be due to that pectin did not increase the viscosity of hydrogel matrix and the calcium pectinate layer did not prove effective in prevention of the diffusion of the drug (Yang et al., 2000). Furthermore, the results showed that the drug loading and encapsulation efficiency were dependent on the type and concentration of co-polymer used in the formulation (Sriamornsak et al., 2007). Co-polymers could be arranged according to its ability to encapsulate the drug in the following order: HPC > MC > Na CMC > pectin (Table 2). These results depend on the viscosity of the polymer used, as the viscosity increased, the diffusion of the drug from the beads decreased.
3.2. SEM of floating KT beads
The SEM pictures for various preparations of KT floating beads are given in Fig. 1. SEM photographs of beads showed that beads had oval, disk or spherical shape with a uniform texture and smooth surface. The smooth surface was due to the use of optimum concentration ratio of calcium carbonate to sodium alginate (1:1 w/w) as a gas forming agent for the bead formation. The result coincident with the report of Shishu et al., 2007 who studied the effect of various concentration ratios of calcium carbonate and sodium alginate on the morphology of the surface of beads (Shishu et al., 2007).
Figure 1.
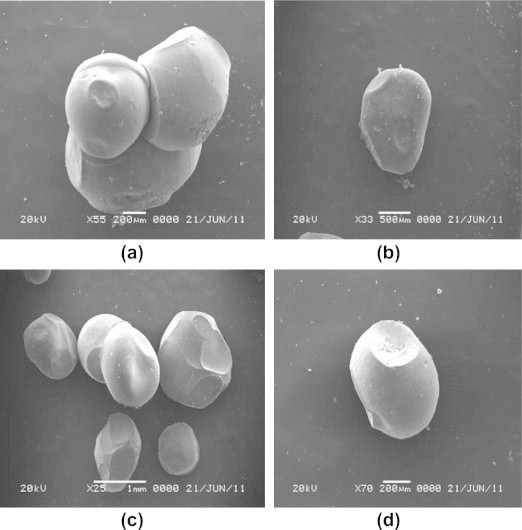
Scanning electron microphotographs of different formulations of KT floating beads. (a) F1, (b) F2, (c) F5, and (d) F10.
3.3. Study of particle size
Particle size of the floating KT beads was affected by the content of co-polymer used and its ratio in relation to alginate in the formulation. The average particle size of the KT floating beads markedly increased with increasing co-polymer concentration and it was in the range from 711 to 1260 μm (Table 2). With respect to the effect of co-polymer type on size of beads, co-polymers could be arranged as follows: HPC > Na CMC > MC > pectin. This may be related to differences in the molecular weight and structure of co-polymers which results in polymeric solutions of different viscosities (Gattani et al., 2009).
3.4. Swelling characteristics of KT floating beads
The swelling behavior of beads as expressed in terms of swelling ratio was done by incubating beads in 1.2 pH buffer at 37 °C for 8 h. All formulated KT beads remained intact in 1.2 pH buffer during the experimental time and appeared more transparent due to the conversion of ca-alginate into alginic acid gel. A reduction in gel strength appeared following the conversion of ca-alginate into alginic acid gel. This may be due to the weaker hydrogen bonds cross linking in alginic acid which leads to the absorption of high quantity of water, swelling and increasing in size (Draget et al., 2005). In general, it was evident that, the swelling ratios increased by increasing co-polymer concentration but this increase was not statistically significant (p > 0.05) for all co-polymers used (Figs. 2–5). F11 showed the lowest equilibrium swelling ratio, 2.5 while F10 showed the highest swelling ratio value about 5.5 at the end of 8 h. Beads containing HPC showed the highest swelling ratio than the other beads due to the hydrophilic nature of the cellulose derivative polymers, also the presence of hydroxyl group in the molecules which plays a significant role in the water uptake and in the matrix integrity of swollen polymer (Borkar et al., 2010). From Fig. 6, the co-polymers used could be arranged in the following ranked order according to their swelling ratios after 8 h in 0.1 N HCl as follows: HPC > Na CMC > MC > pectin.
Figure 2.
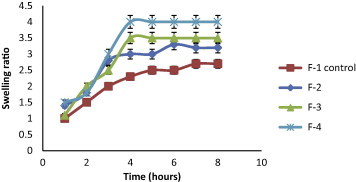
Swelling ratios of KT floating beads, SA/MC (F2–F4) in 0.1 N HCl (pH 1.2).
Figure 3.
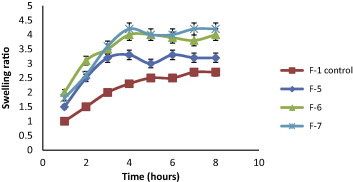
Swelling ratios of KT floating beads-SA/Na CMC-(F5–F7) in 0.1 N HCl (pH 1.2).
Figure 4.
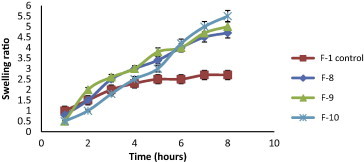
Swelling ratios of KT floating beads, SA/HPC (F8–F10) in 0.1 N HCl (pH 1.2).
Figure 5.
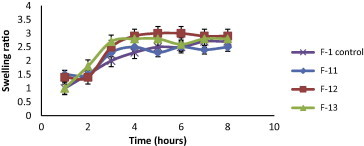
Swelling ratios of KT floating beads of SA/Pectin (F11–F13) in 0.1 N HCl (pH 1.2).
Figure 6.
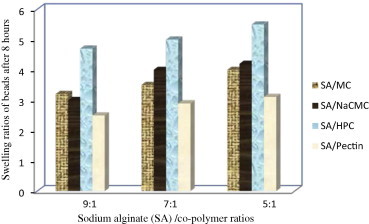
Swelling ratios of KT floating beads in 0.1 N HCl after 8 h.
3.5. Floating studies
The results showed that all prepared beads had lag time from 41 to 140 s and also from 86.4% to 96% of beads remained floated for 8 h. According to the percentage of beads floated after 8 h; co-polymers, could be arranged in the following rank order: pectin > Na CMC > MC > HPC (Table 2).
3.6. In vitro release studies
In vitro drug release experiments were done to examine the probability of employing sodium alginate in combination with the different co-polymers as matrix for intra-gastric floating drug delivery, also, to evaluate the applying of four different co-polymers in three different concentrations on drug release. Release profiles are represented in (Figs. 7–11) by plotting the percent cumulative amount of drug released in 1.2 pH buffer against time. The influence of co-polymer concentration on the release rate of KT may be due to the decrease in total porosity of the matrices (initial porosity plus porosity due to drug dissolution) (Boza et al., 1999) and hence an increase in the hydrophobicity which decreases the diffusion of the drug from this matrix (Tiwari et al., 2003). 100% of KT was released from the KT powder and commercial KT tablets (Ketoral®) in about 50 and 85 min, respectively, while the release of KT from floating beads sustained for a long time depending on co-polymer type and its concentration in each formula. From the obtained data it was concluded that, the lower the concentration of sodium alginate in floating beads, the lower is the rate of drug release from the beads (except for pectin containing beads, F11–F13). It was observed that 100% of drug was released from F1 within five hours (Fig. 7); this may be due to the large mesh size of sodium alginate matrix, which allows the rapid diffusion of the dissolution media into gel matrix, dissolving drug and outward release of the dissolved drug (Borkar et al., 2010). So F1 did not show the expected sustained release characteristics and drug was released within five hours. In order to sustain the release of the drug; co-polymers were added to increase the encapsulation efficiency and modifying the mode of release of KT from the beads.
Figure 7.
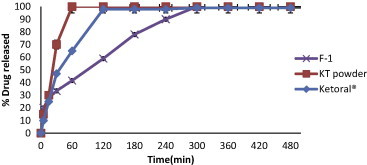
Release profiles of KT from floating beads (F-1), KT powder and Ketoral® in 0.1 N HCl (pH 1.2).
Figure 8.
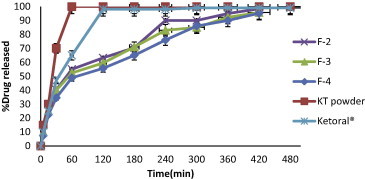
Release profiles of KT from floating beads (F2–F4), KT powder and Ketoral® in 0.1 N HCl (pH 1.2).
Figure 9.
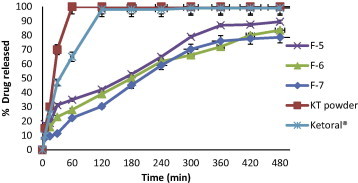
Release profiles of KT from floating beads (F5–F7), KT powder and Ketoral® in 0.1 N HCl (pH 1.2).
Figure 10.
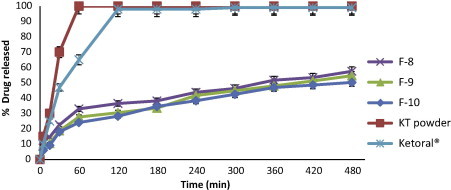
Release profiles of KT from floating beads (F8-F10), KT powder and Ketoral® in 0.1 N HCl (pH 1.2).
Figure 11.
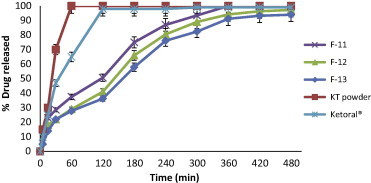
Release profiles of KT from floating beads (F11–F13), KT powder and Ketoral® in 0.1 N HCl (pH 1.2).
F2, F3 and F4 containing (9:1, 7:1, and 5:1) ratios of sodium alginate: methylcellulose respectively (Fig. 8) showed only slight prolongation of drug release when compared to F1 with a non significant difference in retardation of the release (p > 0.05). Also there was no significant (p > 0.05) retardation in drug release in F4 when compared with F3 and F2. Methylcellulose (MC) is a cellulose ether in which the methyl group has been substituted for the hydroxyl group on the 2-glucopyranose residues and it has been reported that the MC has the ability to increase the degree of agglomeration of alginate beads (Raju et al., 2010; Chan et al., 1997). This may result in the formation of more rigid gel structure around the drug particles leading to more encapsulation efficiency while in acidic media protonation of ether linkage of MC leads to decrease in the rigidity of MC molecules. These results were in agreement with those obtained by El-Kamel et al. (2003) who found that the addition of MC to alginate resulted in a non significant difference in the release rate of diltiazem in 0.1 N HCl.
Fig. 9. shows that F5, F6 and F7 containing sodium alginate/Na CMC in ratios 9:1, 7:1 and 5:1, respectively, have more retardation in drug release when compared with F1, and the retardation was non significant (p > 0.05) when compared F6 and F7 with F5. F6 and F7 showed retardation of drug release, about 85% and 80% of the drug were released within 8 h from the two formulae, respectively. The increase in polymer concentration; increases the viscosity and swelling of the polymer matrix as well as the formation of the water-swollen gel with longer diffusion path length that could substantially reduce the penetration of the dissolution medium into the beads and so the drug release was retarded (Kamel et al., 2008).
In the case of F8, F9 and F10, the replacement of part of sodium alginate with HPC showed a significant decrease in drug release rate when compared to F1 and about 50–60% of a drug released within 8 h (Fig. 10). This can be attributed to the low solubility of HPC, which is soluble in water only at a temperature below 38 °C, also HPC produces water-swollen gel that could substantially reduce the penetration of dissolution medium into the beads and so the drug release was retarded. Moreover, by increasing the amount of HPC (F9 & F10) the rate of drug release decreased but this decrease was not significant at p < 0.05 when compared to F8.
The use of combinations of sodium alginate with pectin in F11, F12 and F13 (Fig. 11) showed no retardation in the drug release when compared to F1 and more than 95% of drug was released within 5 h. Pectin has the ability to form gel with divalent cations and has been used in the production of calcium pectinate which used for retardation of drug release, but in this study the incorporation of pectin together with alginate appears to influence the degree of cross-linking of alginate with calcium ions and no retardation in drug release was obtained (Pillay and Fassihi, 1999). From the results; it was concluded that floating beads containing HPC (F10) gave the highest swelling ratio (5.5) after 8 h (Fig. 2) and also gave the slowest in vitro release of KT (Fig. 7).
Hence, the release rate of the drug from the floating beads can be governed by the type and concentration of co-polymer employed in the preparation of the beads. All formulated beads except F1, F11, F12 and F13 showed retardation in the release of drug from beads with different extents depending on the co-polymer used. The rank order for the rate of drug released from the floating beads in 0.1 N HCl (pH 1.2) according to the co-polymer type was as follows: HPC < Na CMC < MC < Pectin, when compared with alginate beads.
3.7. Kinetic studies of drug release
In order to determine the release mechanism that provides the best description to the pattern of drug release; data of the in vitro release of all formulae (F1–F13) were fitted to different kinetic models. The kinetic models used were zero, first-order, Higuchi diffusion and Korsmeyer-Peppas models. The kinetic rate constant (k) and the determination coefficient (R2) were calculated and are presented in Table 3. The best fit with the highest determination coefficient (R2) for all formulae was shown with Higuchi diffusion model followed by Peppas release model and then zero-order equation. These results revealed that the kinetic release pattern was best fitted to Higuchi equation which describes drug release from a polymeric system by diffusion mechanism. Fickian diffusion mechanism of release (n < 0.5) was the drug release controlling mechanism for all formulae; except for F4, F7 and pectin containing formulae (F11–F13) having n values >0.5. Increase in the value of the exponent n indicates that the release mechanism shifted from Fickian diffusion controlled to non-Fickian diffusion mechanism.
Table 3.
Kinetic parameters of KT release data according to different kinetic models.
| Formulae code | Zero-order |
First-order |
Higuchi model |
Korsmeyer-Peppas |
||||
|---|---|---|---|---|---|---|---|---|
| R2 | K0 (% min−1) | R2 | K1 (min−1) | R2 | K (% min−1) | R2 | n | |
| F1 | 0.880 | 0.178 | 0.905 | 0.002 | 0.988 | 5.378 | 0.974 | 0.398 |
| F2 | 0.974 | 0.174 | 0.871 | 0.001 | 0.974 | 3.958 | 0.948 | 0.388 |
| F3 | 0.965 | 0.151 | 0.822 | 0.001 | 0.995 | 3.789 | 0.994 | 0.471 |
| F4 | 0.951 | 0.166 | 0.848 | 0.002 | 0.973 | 4.126 | 0.960 | 0.573 |
| F5 | 0.946 | 0.170 | 0.800 | 0.001 | 0.996 | 4.289 | 0.988 | 0.433 |
| F6 | 0.934 | 0.174 | 0.702 | 0.001 | 0.993 | 4.407 | 0.916 | 0.433 |
| F7 | 0.945 | 0.174 | 0.743 | 0.001 | 0.995 | 4.390 | 0.987 | 0.569 |
| F8 | 0.895 | 0.085 | 0.755 | 0.001 | 0.970 | 2.178 | 0.969 | 0.342 |
| F9 | 0.920 | 0.089 | 0.760 | 0.001 | 0.981 | 2.277 | 0.975 | 0.413 |
| F10 | 0.907 | 0.087 | 0.711 | 0.001 | 0.983 | 2.233 | 0.987 | 0.466 |
| F11 | 0.957 | 0.249 | 0.792 | 0.002 | 0.987 | 5.463 | 0.986 | 0.521 |
| F12 | 0.923 | 0.198 | 0.765 | 0.001 | 0.973 | 5.015 | 0.961 | 0.575 |
| F13 | 0.936 | 0.195 | 0.770 | 0.002 | 0.976 | 4.985 | 0.960 | 0.638 |
3.8. Stability study
KT floating beads were subjected to stability studies at 25 °C with a relative humidity (RH) of 60% for a period of 6 months. After storage under these conditions, beads were analyzed for the change in the physical appearance, drug content and floating behavior. Storage of the beads under the studied conditions did not result in appreciable changes in the physical properties of the all tested formulae regarding color and shape via the visual inspection. Also, drug content of the floating beads did not change till the end of six months after storage. For floating behavior; it was observed that the lag time was increased for F11, F12 and F13 after storage which may be due to the matrix of these formulae; sodium alginate and pectin which were not impact enough to entrap carbon dioxide under storage conditions and a part of carbon dioxide was escaped which led to elongation in the lag time but this elongation was not significant (p > 0.05). Also it was observed from the results that there was no change in the duration of floating and all formulae showed floating for more than 8 h. F1 containing sodium alginate showed also a good stability within the time of storage with respect to the physical and chemical changes.
3.9. Analgesic effect using hot plate method
The KT floating beads’ formula (F10) containing sodium alginate/hydroxypropyl cellulose (5:1) was selected to evaluate the in vivo analgesic effect of KT in the comparison with both the commercial KT tablets (ketoral®) and KT solution. F10 was selected on the basis of its in vitro release profile that it showed a significant retardation of drug release and about 60% of the drug was released within 8 h. In addition, F10 remained buoyant for >8 h and showed the highest % drug loading and also the highest drug encapsulation efficiency (Table 2).
The hot plate method was described by Woolfe and Donalod (1944) and Adams et al. (1969). This test was found to be suitable for evaluating the centrally and peripherally acting analgesics. Group I mice did not receive any medication and considered as a control for the evaluation of analgesic effect in the other groups. Three parameters have been utilized to assess the analgesic effect of KT. These parameters are: the maximum response (MR) to KT in terms of the reaction time in seconds which reflects the intensity of drug action, the time of the maximum response (TMR) and the duration of drug action (DA) which is the time through which the effect is maintained.
Fig. 12 and Table 5 illustrated the effect of oral administration of KT solution, commercial KT tablets (Ketoral®) and the selected KT floating beads (F10) on the change of animal’s behavior toward pain stimulant. From Fig. 12 and Table 5 it was observed that KT solution (1.1 mg/kg) showed a significant increase of the analgesic effect (p < 0.05) in mice after 0.5 h of oral drug administration as compared to a control and the maximum analgesic response, MR (12.6 ± 0.55 s.) was observed after 2 h, then it decreased gradually till disappearance after 5 h.
Figure 12.
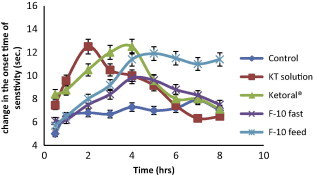
Analgesic effect of the selected formula of KT floating beads (F10) in fast and fed mice compared with KT solution and Ketoral® tablets using hot plate method. All values are means ± SE of six determinations.
Table 5.
Parameters of analgesic effect of KT solution, Ketoral® tablets and KT floating beads (F10) in mice using hot plate and tail flick methods.
| KT in | Parameters of analgesic effect using hot plate method |
Parameters of analgesic effect using tail flick method |
||||
|---|---|---|---|---|---|---|
| MR (s) ± SE | TMR (h) | DA (h) | MR (s) ± SE | TMR (h) | DA (h) | |
| Solution | 12.6 ± 0.55 | 2 | 5 | 8.2 ± 0.31 | 2 | 4 |
| Ketoral® | 13.0 ± 0.37 | 4 | 5 | 8.4 ± 0.14 | 3 | 6 |
| F10 (fast) | 9.80 ± 0.67 | 4 | 6 | 7.5 ± 0.15 | 4 | 7 |
| F10 (fed) | 11.5 ± 0.67 | 4 | >8 | 7.9 ± 0.10 | 4 | >8 |
All values are means ± SE of six determinations. MR is the maximum analgesic response, TMR is the time of maximum analgesic response and DA is the duration of analgesic action.
Also Ketoral® (1.1 mg/kg) showed a significant increase of the analgesic effect (p < 0.05) in mice after 0.5 h of oral drug administration as compared to a control and the maximum analgesic response, MR (13.0 ± 0.37 s.) was observed after 4 h, then it decreased gradually till disappearance after 5 h.
On the other hand, oral administration of selected KT floating beads (F10) containing HPC (sodium alginate/HPC, 5:1) showed a significant increase of the analgesic effect (p < 0.05) in mice after 0.5 h of oral drug administration as compared to a control and the maximum analgesic response, MR (9.8 ± 0.67 s.) was seen after 4 h then it decreased gradually till disappearance after 6 h. In fed mice, F10 showed also significant analgesic effect (p < 0.05) after 0.5 h of oral administration and a maximum analgesic response (11.5 ± 0.67 s.) was observed after 4 h. The duration of drug action was maintained for more than 8 h (Fig. 12 & Table 5). This sustained action may be due to the lower ability of HPC to absorb fluid and swell, therefore, a long time is taken for fluid to penetrate the system, dissolve the drug then for the drug solution to diffuse outwards (Borkar et al., 2010). From the results it was observed that food intake had significant effect on improving analgesic profile of the drug in mice. This phenomenon can be explained as follows, longer residence of beads at a favorable site of absorption and kept it floated in the stomach for a period of time sufficient to release the drug from dense structure beads which lead to increase in the absorption of the drug. On the other hand in the fast mice there was rapid emptying of beads from the stomach before complete release of the drug from dense structure of beads and this led to decrease in the absorption of the drug (Singh and Kim, 1991).
3.10. Analgesic effect using tail flick method
For further confirmation of the results of analgesic effect, tail flick method was performed. Tail withdrawal time (time passed till mice withdraw its tail from the radiant heat source) was taken as the end point. A cut off time of 10 s was used to prevent any injury to the tail. Also group I did not receive any medication, and considered as a control for the evaluation of analgesic effect in other groups (Tables 4 and 5). From the tables it was observed that oral administration of KT (1.1 mg/kg) solution induced a significant increase of the analgesic effect (p < 0.05) in mice after 0.5 h (5.2 ± 0.19 s.) and the time of maximum analgesic effect was seen after 2 h. All measures until 4 h showed a significant increase (p < 0.05) in the analgesic effect when compared to a control.
Table 4.
Analgesic effect of KT floating beads (F10), KT solution and commercial KT tablets (Ketoral®) in mice using tail-flick method.
| Tested groups | Time of tail flick test (h) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 |
1.0 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
| Reaction time of analgesic effect in sec. (mean ± SE) | |||||||||
| Group І (Control) | 3.5 ± 0.13 | 3.2 ± 0.14 | 4.0 ± 0.25 | 3.7 ± 0.14 | 3.3 ± 0.11 | 3.5.±0.23 | 3.4 ± 0.14 | 3.3 ± 0.22 | 3.1 ± 0.18 |
| Group II (KT solution) | ⁎5.2 ± 0.19 | ⁎7.8 ± 0.20 | ⁎8.2 ± 0.31 | ⁎7.7 ± 0.28 | ⁎5.0 ± 0.20 | 3.7 ± 0.29 | 3.2 ± 0.22 | 3.4 ± 0.18 | 3.5 ± 0.11 |
| Group III (Ketoral®) | ⁎4.1 ± 0.20 | ⁎5.5 ± 0.21 | ⁎7.3 ± 0.11 | ⁎8.4 ± 0.14 | ⁎6.6 ± 0.13 | ⁎6.3 ± 0.12 | ⁎4.6 ± 0.15 | 3.2 ± 0.15 | 3.0 ± 0.21 |
| Group ІV (F10 fast) | ⁎4.3 ± 0.10 | ⁎4.6 ± 0.13 | ⁎5.7 ± 0.16 | ⁎6.3 ± 0.13 | ⁎7.5 ± 0.15 | ⁎7.3 ± 0.14 | ⁎5.1 ± 0.14 | ⁎4.5 ± 0.16 | 4.1 ± 0.13 |
| Group V (F10 fed) | ⁎4.4 ± 0.22 | ⁎4.2 ± 0.19 | ⁎6.4 ± 0.15 | ⁎7.5 ± 0.28 | ⁎7.9 ± 0.10 | ⁎7.9 ± 0.26 | ⁎7.5 ± 0.22 | ⁎7.5 ± 0.12 | ⁎7.0 ± 0.18 |
All values are means ± SE of six determinations.
Indicates that a significant increase in the analgesic effect was obtained (p < 0.05) as compared to a control.
Commercial oral KT tablets (Ketoral®) showed the analgesic effect in mice for 6 h. Analgesic effect started after 0.5 h of the drug administration (4.1 ± 0.20 s.) and time of maximum analgesic effect was observed after 3 h. All measures until 6 h showed a significant increase in the analgesic effect (p < 0.05) as compared to a control (Tables 4 and 5).
Oral administration of the selected KT (1.1 mg/kg) floating beads (F10) in fast mice induced significant (p < 0.05) analgesic effect after 0.5 h (4.3 ± 0.10) with a maximum analgesic effect after 4 h, while a maximum analgesic effect was observed after 5 h in fed mice and its effect continued for more than 8 h (Tables 4 and 5). From the results, it was revealed that, duration of analgesic effect induced by F10 in fed mice was doubled compared to that induced by KT solution (group II) and it was about 1.5 times higher than the duration of analgesic effect induced by commercial KT tablets (group III). These results obtained from the tail flick test showed a good agreement with those obtained from hot plate test.
4. Conclusion
In the present study floating beads of KT were formulated to achieve sustained release of the drug. The sodium alginate beads prepared with hydroxypropyl cellulose showed better loading and floating characteristics and the release studies revealed that the beads exhibited sustained drug release. The results obtained from tail flick method showed a good agreement with those obtained from the hot plate method and revealed the sustained analgesic effect of the drug for more than 8 h.
Acknowledgement
This research project was supported by a grant from the “Research Center for Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adams W., Yehur S., Woods L., Mitchell C. Drug interaction as a factor in the development of tolerance to the analgesic effect of morphine. J. Pharm. Exp. Ther. 1969;168:251–257. [PubMed] [Google Scholar]
- Ajit P., Sunil A., Sangamesh A., Nadagouda N. Semi-interpenetrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine. J. Carbohydr. Polym. 2006;65:243–252. [Google Scholar]
- Alexander S., Juergen S. Gastroretentive drug delivery systems. Expert Opin. Drug Delivery. 2006;3:217–233. doi: 10.1517/17425247.3.2.217. [DOI] [PubMed] [Google Scholar]
- Amberkar M., Tara S., Meena K., Smita S. Evaluation of anti-inflammatory and analgesic activities of alcoholic extract of kaempferia galangal in rats. Indian J. Physiol. Pharmacol. 2011;55:13–24. [PubMed] [Google Scholar]
- Arora S., Ali J., Ahuja A. Floating drug delivery system: a review. AAPS Pharm. Sci. Tech. 2005;6:372–390. doi: 10.1208/pt060347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton S., Bon C. fourth ed. Marcel Dekker Inc.; New York: 2004. Pharmaceutical statistics and practical and clinical applications; pp. 96–146. (Rev. and Expanded Ed.). [Google Scholar]
- Borkar S., Suresh R., Sawant V. An approach to formulate bilayered gastroretentive floating drug delivery system of cefpodoxime proxetil. Int. J. Chem. Tech. Res. 2010;2:1229–1242. [Google Scholar]
- Borkar S., Suresh R., Sawant V. An approach to formulate bilayered gastroretentive floating drug delivery system of cefpodoxime proxetil. Int. J. Chem. Tech. Res. 2010;2:1229–1242. [Google Scholar]
- Boza A., Caraballo I., Alvarez-Fuentes J., Rabasco A. Evaluation of Eduragit RSPO and ethocel 100 matrices for the controlled release of Lobenzarit disodium. Drug Dev. Ind. Pharm. 1999;25:229–233. doi: 10.1081/ddc-100102164. [DOI] [PubMed] [Google Scholar]
- Chan L., Heng P., Wan L. Effect of cellulose derivatives on alginate microspheres prepared by emulsification. J. Microencapsul. 1997;14:545–555. doi: 10.3109/02652049709006808. [DOI] [PubMed] [Google Scholar]
- Draget K., Smidsrod O., Skjak-Braek G. In: Steinbuchel A., Rhee S.K., editors. Wiley; Winheim: 2005. pp. 1–30. (Alginates Polysaccharides and Polyamides in the Food Industry). [Google Scholar]
- El-Kamel A., Gohary O., Hosny E. Alginate-diltiazem hydrochloride beads: optimization of formulation factors, in vitro and in vivo availability. J. Microencapsul. 2003;20:211–225. [PubMed] [Google Scholar]
- Gadad A.P., Patil M.B., Naduvinamani S.N., Mastiholimath V.S., Dandagi P.M., Kulkarni A.R. Sodium alginate polymeric floating beads for the delivery of cefpodoxime proxetil. J. Appl. Polym. Sci. 2009;114:1921–1926. [Google Scholar]
- Gangadharappa H.V., Pramod Kumar T.M., Shiva Kumar H.G. Gastric floating drug delivery systems. Indian J. Pharm. Educ. Res. 2007;41(4):295–306. [Google Scholar]
- Gattani Y., Kawtikwar P., Sakarkar D. Formulation and evaluation of gastro retentive multi particulate drug delivery system of aceclofenac. Int. J. Chem. Tech. Res. 2009;1:1–10. [Google Scholar]
- Geoffrey B., James H. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J. Exp. Biol. 2005;208:1575–1592. doi: 10.1242/jeb.01589. [DOI] [PubMed] [Google Scholar]
- Ghosh, M.N., 1971. Toxicity studies. In: Fundamentals of Experimental Pharmacology. Scientific Book Agency, Calcultta, pp. 85–96.
- Ibrahim M., Amin M., Fetih G., Abou Ela A. Formulation and evaluation of ketorolac tromethamine-Eudragit solid dispersions of potential sustained release properties. J. Pharm. Partiques. 2010;20:189–200. [Google Scholar]
- Kamel S., Ali N., Jahangir K., Shah S., El-Gendy A. Pharmaceutical significance of cellulose: a review. J. Express Polym. Lett. 2008;2:758–778. [Google Scholar]
- Martin A., Bustamante P., Chun A.H.C. fourth ed. Lea and Febiger; Philadelphia: 1993. Diffusion and dissolution; pp. 284–288. (Physical Pharmacy). (Chapter 13) [Google Scholar]
- Padma V., Subhash P., Sunil V. HPTLC determination of ketorolac tromethamine. J. Pharm. Biomed. Anal. 2000;22:679–683. doi: 10.1016/s0731-7085(99)00296-4. [DOI] [PubMed] [Google Scholar]
- Milling Parrott E.L. In: third ed. Lachman L., Liberman H., Kanig J., editors. Lea and Febiger; Philadelphia: 1986. pp. 23–25. (The Theory and Practice of Industrial Pharmacy). [Google Scholar]
- Pillay V., Fassihi R. In vitro release modulation from cross linked pellets for site-specific drug delivery to the gastrointestinal tract. II. Physico-chemical characterization of calcium–alginate, calcium–pectinate and calcium–alginate pectinate pellets. J. Control. Rel. 1999;59:243–256. doi: 10.1016/s0168-3659(98)00197-7. [DOI] [PubMed] [Google Scholar]
- Prasad S., Mukesh T., Ashok S., Monit S., Ashish S. Preparation and optimization of oral floating alginate gel beads of famotidine. J. Pharmakin. 2011;3:1–15. [Google Scholar]
- Raju D., Kiran S., Varma M. Design development and evaluation of extended release tablets of Alfuzosin hydrochloride. J. Chem. Pharm. Res. 2010;2:90–96. [Google Scholar]
- Saleem M., Darbar S., Mahesh G., Rani S. Analgesic, anti-pyretic and anti-inflammatory activity of dietary sesame oil in experimental animal models. J. Pharm. 2011;2:172–177. [Google Scholar]
- Shishu, Gupta N., Aggarwal N. Stomach specific drug delivery of 5-Fluorouracil using floating alginate beads. AAPS Pharm. Sci. Tech. 2007;8:E143–E149. doi: 10.1208/pt0802048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Kim K. Floating drug delivery systems: an approach to oral controlled sustained-release characteristics with p-aminobenzoic acid and isosorbide dinitrate as model drugs. J. Pharm. Sci. 1991;80:1153–1156. doi: 10.1002/jps.2600801212. [DOI] [PubMed] [Google Scholar]
- Sinha V., Trehan A. Formulation, characterization, and evaluation of ketorolac tromethamine-loaded biodegradable microspheres. Drug Delivery. 2005;12:133–139. doi: 10.1080/10717540590925726. [DOI] [PubMed] [Google Scholar]
- Sriamornsak P., Thirawong N., Puttipipatkhachorn S. Emulsion gel beads of calcium pectinate capable of floating on the gastric fluid: effect of some additives, hardening agent or coating on release behavior of metronidazole. Eur. J. Pharm. 2007;24:363–373. doi: 10.1016/j.ejps.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Stops F., Fell J., Collett J., Martini L. Floating dosage forms to prolong gastro-retention-the characterization of calcium alginate beads. Int. J. Pharm. 2008;350:301–311. doi: 10.1016/j.ijpharm.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Thanoo B., Sunny M., Jayakrishnan A. Oral sustained release drug delivery systems using polycarbonate microspheres capable of floating on the gastric fluid. J. Pharm. Pharmacol. 1993;45:21–24. doi: 10.1111/j.2042-7158.1993.tb03672.x. [DOI] [PubMed] [Google Scholar]
- Tiwari S., Murthy T., Pai M., Mehta P., Chowdary P. Controlled release formulation of tramadol hydrochloride using hydrophilic and hydrophobic matrix system. J. AAPS Pharm. Sci. Tech. 2003;4:18–23. doi: 10.1208/pt040331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfe G., Donalod A. The evaluation of analgesic action of pethadine hydrochloride. J. Pharmacol. Exp. 1944;80:300–304. [Google Scholar]
- Yagnesh L., Praveen S., Atmaram P. The effect of drug concentration and curing time on processing and properties of calcium alginate beads containing metronidazole by response surface methodology. J. AAPS Pharm. Sci. Tech. 2006;7:86–92. doi: 10.1208/pt070486. [DOI] [PubMed] [Google Scholar]
- Yang Y., Chung T., Bai X., Chan W. Effect of preparation conditions on morphology and release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion method. J. Chem. Eng. Sci. 2000;55:2223–2236. [Google Scholar]



