Abstract
Methotrexate (MTX) is an antifolate cytotoxic medication used to treat certain types of cancer and at lower doses for rheumatic diseases. MTX has many serious adverse effects, such as myelosuppression, hepatic, renal and pulmonary disorders. For safe and effective use of high dose methotrexate (HDMTX) certain precautions should be followed. We present this case study with short review to briefly summarize the important practical issues related to HDMTX therapy.
Keywords: Methotrexate, Chemotherapy, Drug interaction
1. Case
The case concerns a 21-year-old male patient with osteosarcoma of the right tibia (Fig. 1), admitted to the oncology department to receive chemotherapy in the form of 12 g/m2 high-dose methotrexate with leucovorin rescue. His current medications include: 40 mg pantoprazole for a peptic ulcer, 160/800 mg trimethoprim–sulfamethoxazole (TMP–SMX) for a urinary tract infection, 100 mg tramadol as needed for bone pain, and 500 mg aspirin added by his dentist for dental pain.
Figure 1.
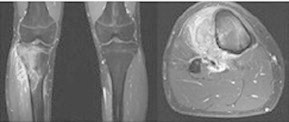
Magnetic resonance imaging of the proximal right tibia osteosarcoma.
The patient has shortness of breath secondary to pleural effusion, as revealed by a chest X-ray. His vital signs are stable with performance status of 1 and his kidney and liver functions are normal.
2. Questions
-
1.
Is there any contraindication to methotrexate (MTX) therapy in this case?
-
2.
What are the potential major drug interactions found in this case, and why?
-
3.
What are the key precautions to prevent toxicity from HDMTX therapy?
3. Answers
-
1.
Third-space compartments such as pleural effusion and ascites are considered as contraindication to the HDMTX therapy.
-
2.
Many major drug–drug interactions can be found in this case. Clinically relevant drug interactions include MTX and pantoprazole, MTX with aspirin, and MTX with TMP–SMX.
-
3.
The general approach to prevent MTX toxicity includes frequent MTX plasma level monitoring, urine alkalanization, adequate hydration, and leucovorin rescue.
4. Discussion
Methotrexate (MTX) is an antifolate cytotoxic agent used to treat certain types of hematological cancers, solid tumors, and rheumatoid arthritis. HDMTX has many serious toxic effects, such as myelosuppression, hepatic, renal, and pulmonary disorders (Bleyer, 1977). HDMTX, defined as MTX dose of >1 g/m2, is given in combination with doxorubicin and a platinum agent in most osteosarcoma protocols (Ferguson and Goorin, 2001).
The presence of a third-space fluid such as pleural effusions or ascites is an important contraindication to the administration of HDMTX. Third-space fluids lead to a prolonged MTX plasma half-life and subsequently to prolonged exposure to MTX, increasing the risk of toxicity. Drainage of third-space fluids before HDMTX is recommended to prevent toxicity (Fox, 1979).
Many drug–drug interactions can compromise the safety of HDMTX therapy by either delaying MTX elimination or augmenting its adverse effects. One major drug interaction is the concurrent use of TMP–SMX with MTX; this is a serious interaction that can be fatal in some cases. TMP–SMX is a synergistic fixed combination of trimethoprim and sulfamethoxazole in a 1:5 ratio respectively (Kielhofner, 1990). Both sulfamethoxazole and trimethoprim are synthetic folate antagonists. It is recommended to avoid such combination or to stop TMP–SMX at least 3 days before starting MTX (Al-Quteimat and Al-Badaineh, 2013).
Clinical evidence suggests that concomitant use of MTX (primarily at high doses) with proton pump inhibitors (PPIs) such as omeprazole, esomeprazole, and pantoprazole may decrease methotrexate clearance, leading to elevated serum levels of methotrexate, which in turn potentiate the risk of MTX toxicity. Thus, combining MTX with PPIs such as pantoprazole, in our case, is not recommended; if necessary, H2-blockers such as ranitidine should be used alternatively (Bezabeh et al., 2012).
NSAIDs can decrease renal perfusion and cause a rise in serum MTX levels with the potential for toxicity (Baxter, 2011). MTX is also strongly protein bound and may be displaced by NSAIDs (Kavanaugh and Broide, 2009).
According to a newly published systematic review, using NSAID with HDMTX appears to be safe, provided appropriate monitoring is performed. However, the use of anti-inflammatory doses of aspirin should be avoided (Colebatch et al., 2012).
Many case reports have indicated reduction in the MTX clearance when NSAIDs are used concurrently. Ketoprofen, indomethacin, salicylate, ibuprofen, naproxen and diclofenac were shown to produce different levels of toxicities in patients treated with MTX (El-Sheikh et al., 2007). In this case, the patient is receiving tramadol so it is reasonable to discontinue aspirin to avoid this interaction.
HDMTX can result in severe toxicity if used inappropriately, so certain precautions should be applied to promote safe and effective use of HDMTX. These precautions include the following:
5. Frequent monitoring of MTX level
Monitoring of plasma MTX level is very important to improve the safety of HDMTX therapy. MTX levels should be followed until the plasma level is less than 0.1 μM. Plasma MTX levels are usually measured at 24, 48 and 72 h after starting the MTX infusion (Gaies et al., 2012).
6. Elimination of third space fluids before starting MTX therapy
The accumulation of third-space fluids, as seen in pleural effusion and ascites, will prolong MTX plasma half-life and increase the risk of HDMTX toxicity. Drainage of third-space fluids before HDMTX is recommended to avoid toxicity (Fox, 1979).
7. Urine alkalanization
MTX and its metabolite 7-OH-MTX show, respectively, 20- and 12-fold increased solubility when pH increases from 5 to 7 (Jacobs et al., 1976). Renal tubular precipitation of MTX and 7-OH-MTX occurs when pH is lower than 5.7 (Fox, 1979). So it is recommended to keep the urine pH ⩾ 7.0 and to maintain it in this range until plasma MTX levels decline to less than 0.1 μM.
8. Using leucovorin rescue
Leucoverin rescue should be started within 24–36 h of the start of the MTX infusion. Dose and frequency of leucoverin depend on the HDMTX protocol used. Commonly used doses of leucoverin are in the range of 10–15 mg/m2, given every 6 h until plasma MTX levels are less than 0.2 μM (Olsen, 1991).
9. Adequate hydration
Adequate hydration is an essential part of HDMTX therapy to promote diuresis and to prevent intratubular precipitation of MTX. Intravenous fluids of at least 2.5–3.5 L/m2 of IV per day are recommended by most HDMTX regimens, starting 4–12 h before HDMTX infusion (Olsen, 1991; Widemann and Adamson, 2006).
10. Using glucarpidase in patients with renal dysfunction and delayed MTX clearance
Coadministration of drugs having the potential to displace MTX from serum proteins and/or to reduce MTX clearance should be avoided (Green, 2012). The concurrent use of TMP–SMX and NSAIDs with MTX therapy has a well-documented major effect on MTX safety and should be avoided. Delayed clearance of MTX was also reported with pyrazoles, aminoglycosides, probenecid, some penicillins, macrolides, omeprazole, mezocillin, piperacillin, amphotericin B, and ciprofloxacin (Gaies et al., 2012).
11. Conclusion
MTX has many serious and sometimes life-threatening side effects that can be avoided by following special precautions during HDMTX administration and post-treatment management guidelines. Close monitoring, adequate hydration and avoidance of interacting drugs are general principles applied to prevent HDMTX toxicity that are common to all regimens.
Disclosure
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Quteimat O.M., Al-Badaineh M.A. Methotrexate and trimethoprim–sulphamethoxazole: extremely serious and life-threatening combination. J. Clin. Pharm. Ther. 2013;38:203–205. doi: 10.1111/jcpt.12060. [DOI] [PubMed] [Google Scholar]
- Bezabeh S., Mackey A.C., Kluetz P., Jappar D., Korvick J. Accumulating evidence for a drug–drug interaction between methotrexate and proton pump inhibitors. Oncologist. 2012;17:550–554. doi: 10.1634/theoncologist.2011-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter K. Stockley’s drug interactions. 9th ed and Stockley’s drug interactions 2010 pocket companion. J. Med. Libr. Assoc. 2011;99:174–175. [Google Scholar]
- Bleyer W.A. Methotrexate: clinical pharmacology, current status and therapeutic guidelines. Cancer Treat. Rev. 1977;4:87–101. doi: 10.1016/s0305-7372(77)80007-8. [DOI] [PubMed] [Google Scholar]
- Colebatch A.N., Marks J.L., van der Heijde D.M., Edwards C.J. Safety of nonsteroidal antiinflammatory drugs and/or paracetamol in people receiving methotrexate for inflammatory arthritis: a Cochrane systematic review. J. Rheumatol. Suppl. 2012;90:62–73. doi: 10.3899/jrheum.120345. [DOI] [PubMed] [Google Scholar]
- El-Sheikh A.A., van den Heuvel J.J., Koenderink J.B., Russel F.G. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J. Pharmacol. Exp. Ther. 2007;320:229–235. doi: 10.1124/jpet.106.110379. [DOI] [PubMed] [Google Scholar]
- Ferguson W.S., Goorin A.M. Current treatment of osteosarcoma. Cancer Invest. 2001;19:292–315. doi: 10.1081/cnv-100102557. [DOI] [PubMed] [Google Scholar]
- Fox R.M. Methotrexate nephrotoxicity. Clin. Exp. Pharmacol. Physiol. Suppl. 1979;5:43–45. [PubMed] [Google Scholar]
- Gaies E., Jebabli N., Trabelsi S., Salouage I., Charfi R. Methotrexate side effects: review article. J. Drug Metab. Toxicol. 2012;3:125. [Google Scholar]
- Green J.M. Glucarpidase to combat toxic levels of methotrexate in patients. Ther. Clin. Risk Manag. 2012;8:403–413. doi: 10.2147/TCRM.S30135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S.A., Stoller R.G., Chabner B.A., Johns D.G. 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. J. Clin. Invest. 1976;57:534–538. doi: 10.1172/JCI108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielhofner M.A. Trimethoprim–sulfamethoxazole: pharmacokinetics, clinical uses, and adverse reactions. Tex. Heart Inst. J. 1990;17:86–93. [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh A., Broide D.H. Middleton’s Allergy: Principles and Practice. seventh ed. Mosby Elsevier; US: 2009. Immunomodulators. [Google Scholar]
- Olsen E.A. The pharmacology of methotrexate. J. Am. Acad. Dermatol. 1991;25:306–318. doi: 10.1016/0190-9622(91)70199-c. [DOI] [PubMed] [Google Scholar]
- Widemann B.C., Adamson P.C. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]



