Abstract
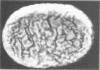
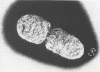
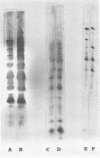
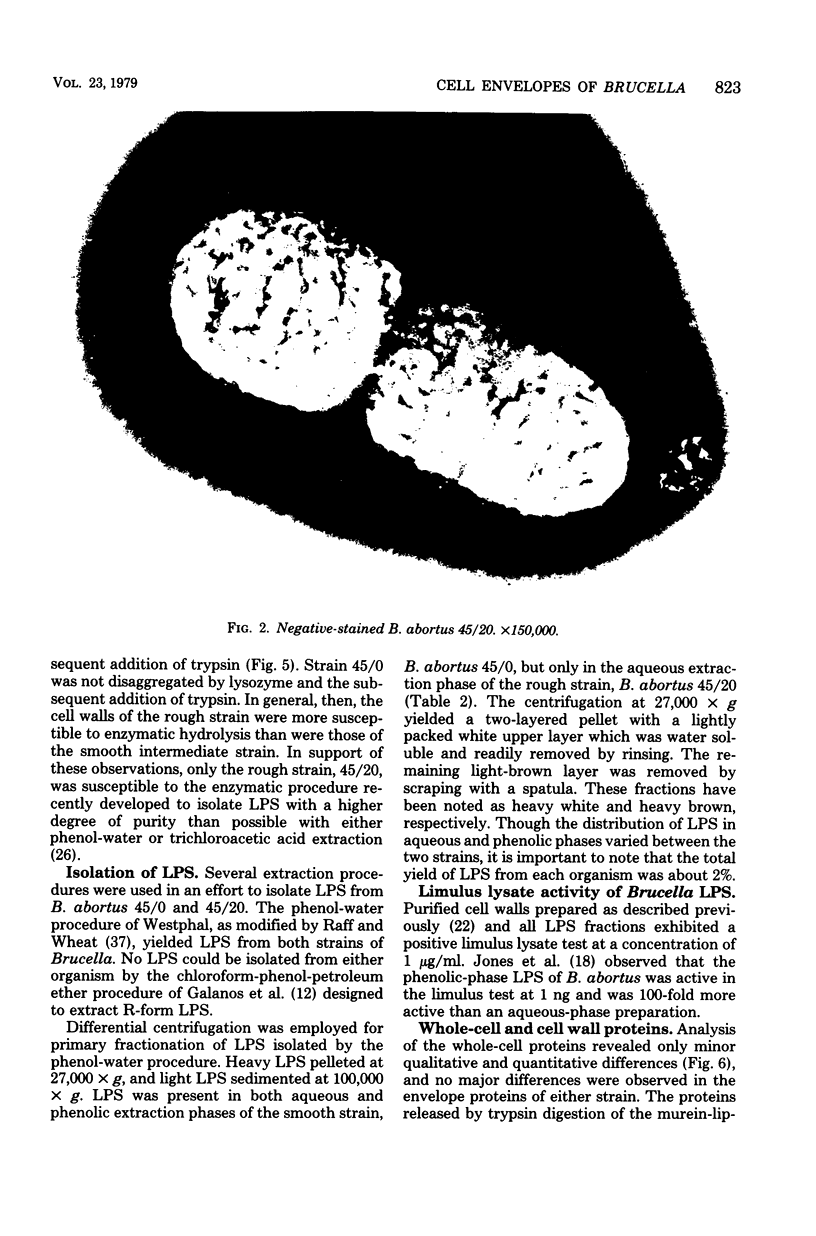
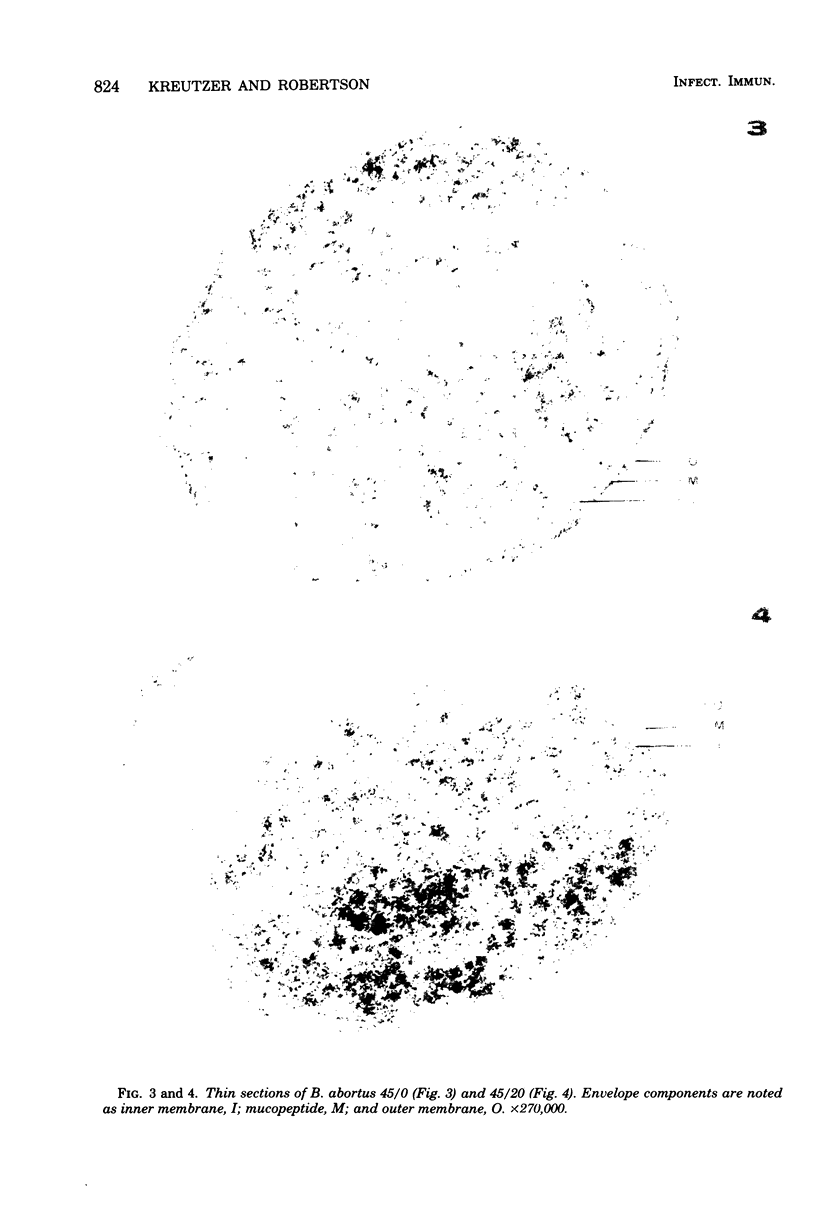
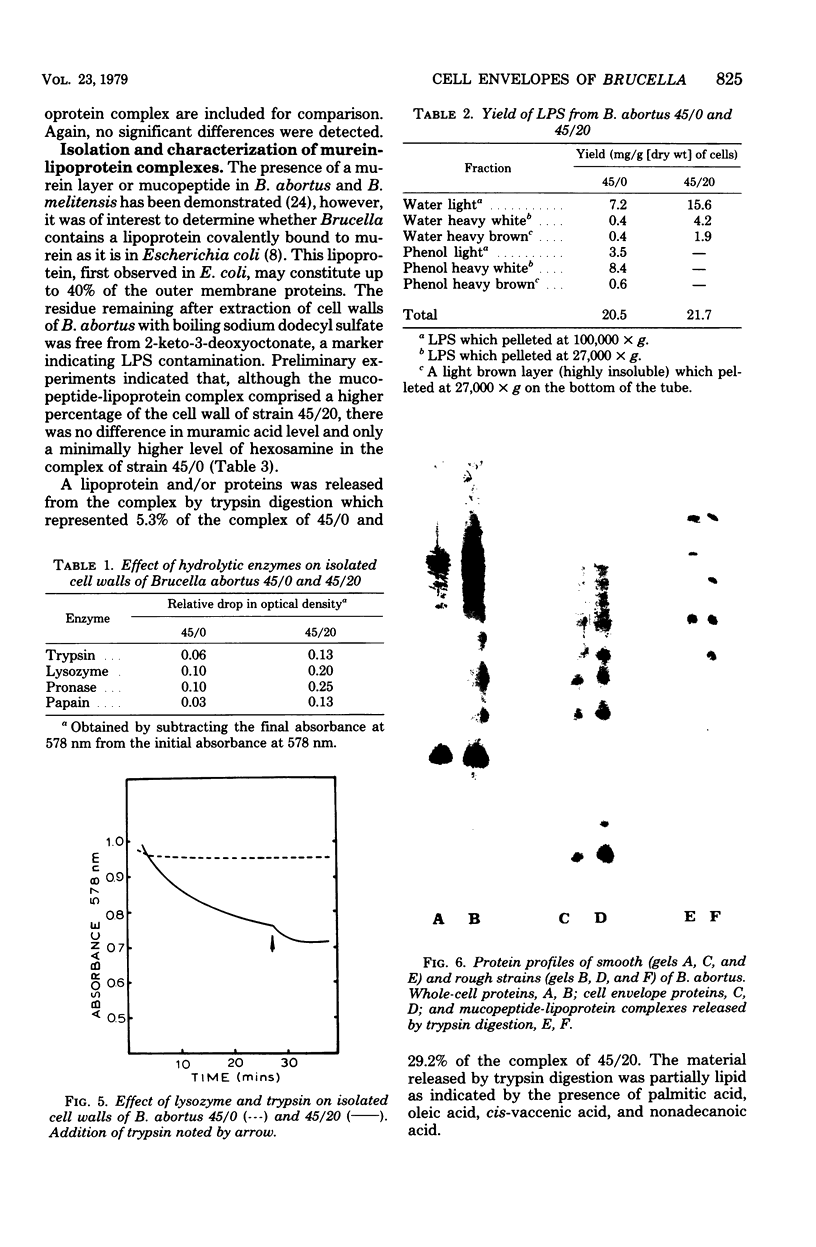
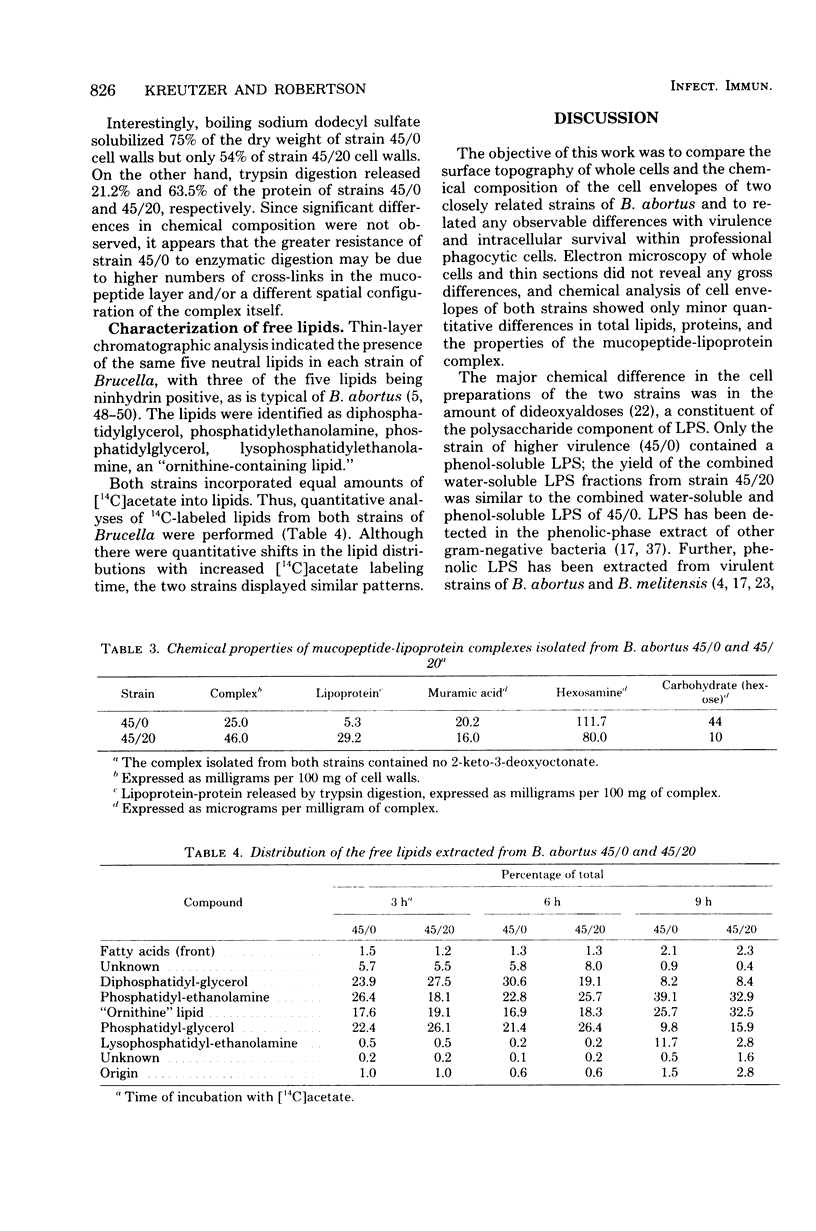
The surface topography of whole cells and the chemical composition of cell envelopes of a smooth-intermediate strain (45/0) and a rough strain (45/20) of Brucella abortus was examined. Electron microscopy of whole cells and thin sections did not reveal any gross surface difference(s). Only minor quantitative differences were observed in total lipids, proteins, and the murein layer. However, the lipopolysaccharide composition of the two strains was quite different. Both phenol- and water-soluble lipopolysaccharide fractions were obtained from the strain of higher virulence (45/0), whereas only aqueous lipopolysaccharide could be isolated from the rough strain. In addition to being toxic, the phenol-soluble lipopolysaccharide may be a key virulence factor in intracellular survival of B. obortus within phagocytic cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOBO R. A., FOSTER J. W. THE EFFECT OF ENZYME TREATMENTS ON BRUCELLA ABORTUS CELL WALLS. J Gen Microbiol. 1964 Jan;34:1–8. doi: 10.1099/00221287-34-1-1. [DOI] [PubMed] [Google Scholar]
- BRAUN W., POMALES-LEBRON A., STINEBRING W. R. Interactions between mononuclear phagocytes and Brucella abortus strains of different virulence. Proc Soc Exp Biol Med. 1958 Feb;97(2):393–397. doi: 10.3181/00379727-97-23752. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Wilson J. B. Chemical composition and biological properties of the endotoxin of Brucella abortus. J Bacteriol. 1965 Oct;90(4):895–902. doi: 10.1128/jb.90.4.895-902.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Corbel M. J. The immune response to Brucella abortus 45/20 adjuvant vaccine in terms of immunoglobin class. Dev Biol Stand. 1976;31:141–144. [PubMed] [Google Scholar]
- Cunningham B. Vaccination of cattle with killed 45-20 adjuvant vaccine. Effects on serological and Milk Ring Tests when used in cattle previously exposed to infection or vaccinated with S.19. Vet Rec. 1970 Jan 3;86(1):2–7. doi: 10.1136/vr.86.1.2. [DOI] [PubMed] [Google Scholar]
- DE PETRIS S., KARLSBAD G., KESSEL R. W. THE ULTRASTRUCTURE OF S AND R VARIANTS OF BRUCELLA ABORTUS GROWN ON A LIFELESS MEDIUM. J Gen Microbiol. 1964 Jun;35:373–382. doi: 10.1099/00221287-35-3-373. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hadzija O. A simple method for the quantitative determination of muramic acid. Anal Biochem. 1974 Aug;60(2):512–517. doi: 10.1016/0003-2697(74)90261-9. [DOI] [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. I. Chemical and immunoelectrophoretic characterization. J Bacteriol. 1967 Feb;93(2):544–549. doi: 10.1128/jb.93.2.544-549.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES A. T. Qualitative and quantitative determination of the fatty acids by gas-liquid chromatography. Methods Biochem Anal. 1960;8:1–59. doi: 10.1002/9780470110249.ch1. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Berman D. T. Studies of Brucella lipopolysaccharide. Dev Biol Stand. 1976;31:62–67. [PubMed] [Google Scholar]
- Jones L. M., Diaz R., Berman D. T. Endotoxic activity of rough organisms of Brucella species. Infect Immun. 1976 Jun;13(6):1638–1641. doi: 10.1128/iai.13.6.1638-1641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARLSBAD G., KESSEL R. W., DE PETRIS S., MONACO L. ELECTRON MICROSCOPE OBSERVATIONS OF BRUCELLA ABORTUS GROWN WITHIN MONOCYTES IN VITRO. J Gen Microbiol. 1964 Jun;35:383–390. doi: 10.1099/00221287-35-3-383. [DOI] [PubMed] [Google Scholar]
- Kellerman G. D., Foster J. W., Badakhsh F. F. Comparison of Chemical Components of Cell Walls of Brucella abortus Strains of Low and High Virulence. Infect Immun. 1970 Sep;2(3):237–243. doi: 10.1128/iai.2.3.237-243.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., Buller C. S., Robertson D. C. Chemical characterization and biological properties of lipopolysaccharides isolated from smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):811–818. doi: 10.1128/iai.23.3.811-818.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., Scheffel J. W., Draper L. R., Robertson D. C. Mitogenic activity of cell wall components from smooth and rough strains of Brucella abortus. Infect Immun. 1977 Mar;15(3):842–845. doi: 10.1128/iai.15.3.842-845.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACAVE C., ROUX J. COMPOSITION CHIMIQUE DES PAROIS DE BRUCELLA. GLYCOSAMINOPEPTIDE DE BRUCELLA ABORTUS ET BRUCELLA MELITENSIS. C R Hebd Seances Acad Sci. 1965 Feb 1;260:1514–1517. [PubMed] [Google Scholar]
- Lacave C., Asselineau J., Serre A., Roux J. Comparaison de la composition chimique d'une fraction lipopolysaccharidique et d'une fraction polysaccharidique isolées de Brucella melitensis. Eur J Biochem. 1969 Jun;9(2):189–198. doi: 10.1111/j.1432-1033.1969.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Langworthy T. A., Buller C. S. The effect of phage infection on the synthesis of lipid A by Escherichia coli. Virology. 1973 Jul;54(1):80–89. doi: 10.1016/0042-6822(73)90116-5. [DOI] [PubMed] [Google Scholar]
- Lehrer S., Nowotny A. Isolation and purification of endotoxin by hydrolytic enzymes. Infect Immun. 1972 Dec;6(6):928–933. doi: 10.1128/iai.6.6.928-933.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D., Diaz R., Milner K., Rudbach J., Wilson J. B. Some structural and biological properties of Brucella endotoxin. Infect Immun. 1970 Feb;1(2):174–182. doi: 10.1128/iai.1.2.174-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. A. The use of polyacrylamide gel electrophoresis in taxonomy of Brucella. J Gen Microbiol. 1973 May;76(1):231–237. doi: 10.1099/00221287-76-1-231. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Intracellular parasitism: life in an extreme environment. J Infect Dis. 1974 Sep;130(3):300–306. doi: 10.1093/infdis/130.3.300. [DOI] [PubMed] [Google Scholar]
- Nelson E. T., Buller C. S. Phospholipase activity in bacteriophage-infected Escherichia coli. I. Demonstration of a T4 bacteriophage-associated phospholipase. J Virol. 1974 Sep;14(3):479–484. doi: 10.1128/jvi.14.3.479-484.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff R. A., Wheat R. W. Carbohydrate composition of the phenol-soluble lipopolysaccharides of Citrobacter freundii. J Bacteriol. 1968 Jun;95(6):2035–2043. doi: 10.1128/jb.95.6.2035-2043.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoux G., Renoux M., Tinelli R. Phenol-water fractions from smooth Brucella abortus and Brucella melitensis: immunochemical analysis and biologic behavior. J Infect Dis. 1973 Feb;127(2):139–148. doi: 10.1093/infdis/127.2.139. [DOI] [PubMed] [Google Scholar]
- Robertson D. C., McCullough W. G. The glucose catabolism of the genus Brucella. I. Evaluation of pathways. Arch Biochem Biophys. 1968 Sep 20;127(1):263–273. doi: 10.1016/0003-9861(68)90225-7. [DOI] [PubMed] [Google Scholar]
- Robertson D. C., McCullough W. G. The glucose catabolism of the genus Brucella. II. Cell-free studies with B. abortus (S-19). Arch Biochem Biophys. 1968 Sep 20;127(1):445–456. doi: 10.1016/0003-9861(68)90249-x. [DOI] [PubMed] [Google Scholar]
- Roerink J. H. Investigation into the usefulness of the non-agglutinogenic Brucella abortus adjuvant vaccine duphavac N.A. in the control of bovine brucellosis. Vet Rec. 1967 Jun 24;80(25):727–733. doi: 10.1136/vr.80.25.727. [DOI] [PubMed] [Google Scholar]
- Rowley D. Endotoxins and bacterial virulence. J Infect Dis. 1971 Mar;123(3):317–327. doi: 10.1093/infdis/123.3.317. [DOI] [PubMed] [Google Scholar]
- Smith H. Biochemical challenge of microbial pathogenicity. Bacteriol Rev. 1968 Sep;32(3):164–184. doi: 10.1128/br.32.3.164-184.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Thiele O. W., Busse D., Hoffmann K. Die "freien" Lipide aus Brucella abortus Bang. 4. Die Natur der Phosphatide und ihre Fettsäurezusammensetzung. Eur J Biochem. 1968 Sep 24;5(4):513–519. doi: 10.1111/j.1432-1033.1968.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Thiele O. W., Busse D. The 'free' lipids of Brucella abortus Bang, II. The positional distribution of the phospholipid fatty acids. Experientia. 1968 Feb 15;24(2):112–112. doi: 10.1007/BF02146926. [DOI] [PubMed] [Google Scholar]
- Thiele O. W., Kehr W. Die "freien" Lipide aus Brucella abortus Bang. Uber die Neutrallipide. Eur J Biochem. 1969 Jun;9(2):167–175. doi: 10.1111/j.1432-1033.1969.tb00591.x. [DOI] [PubMed] [Google Scholar]
- WILSON J. B., DASINGER B. L. Biochemical properties of virulent and avirulent strains of brucellae. Ann N Y Acad Sci. 1960 Nov 21;88:1155–1166. doi: 10.1111/j.1749-6632.1960.tb20106.x. [DOI] [PubMed] [Google Scholar]